Teucrium polium (L.): Phytochemical Screening and Biological Activities at Different Phenological Stages
Abstract
:1. Introduction
2. Results and Discussion
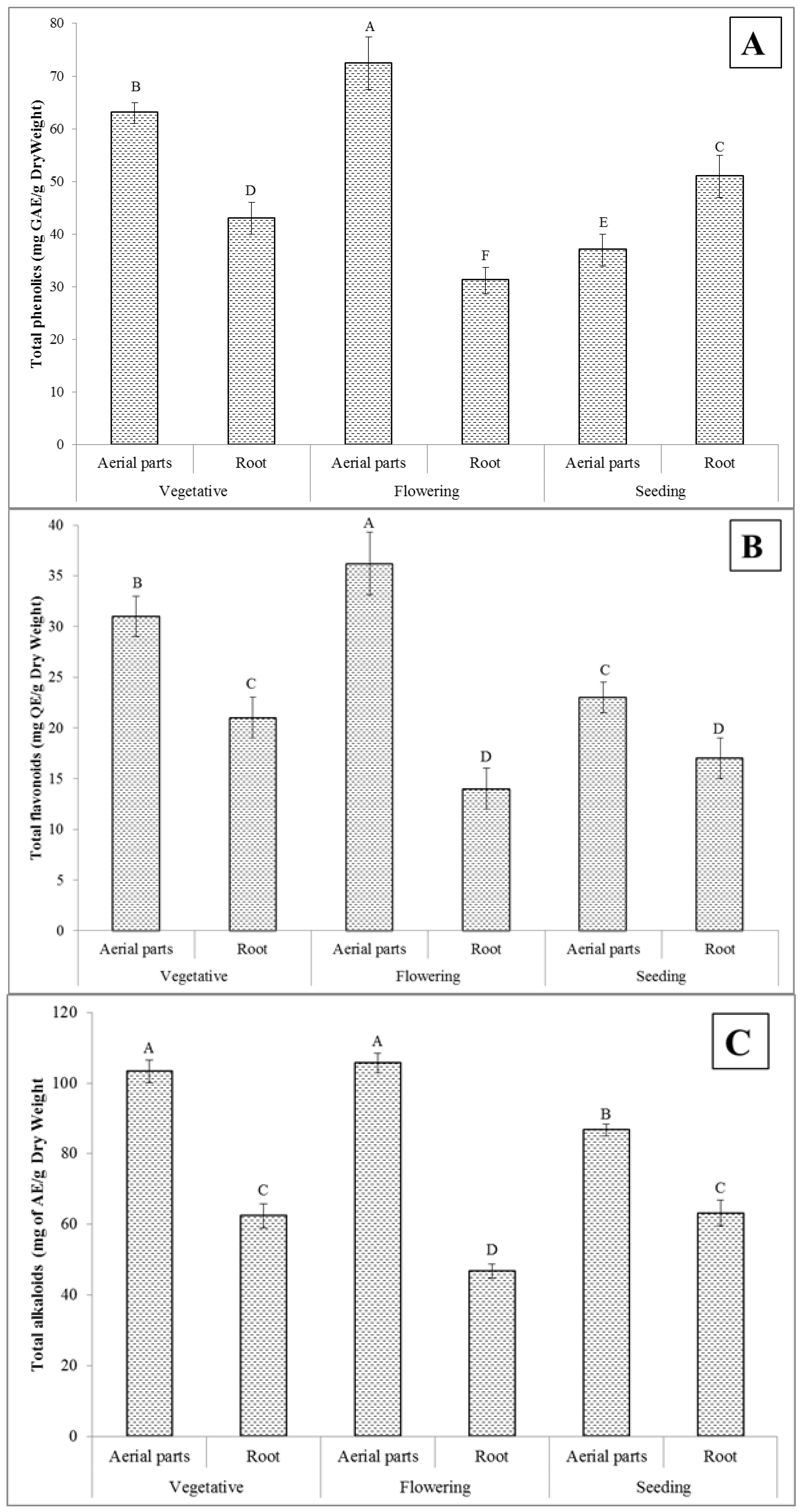
2.1. Total Phenolics Content
2.2. Total Flavonoids Content
2.3. Total Alkaloids Content
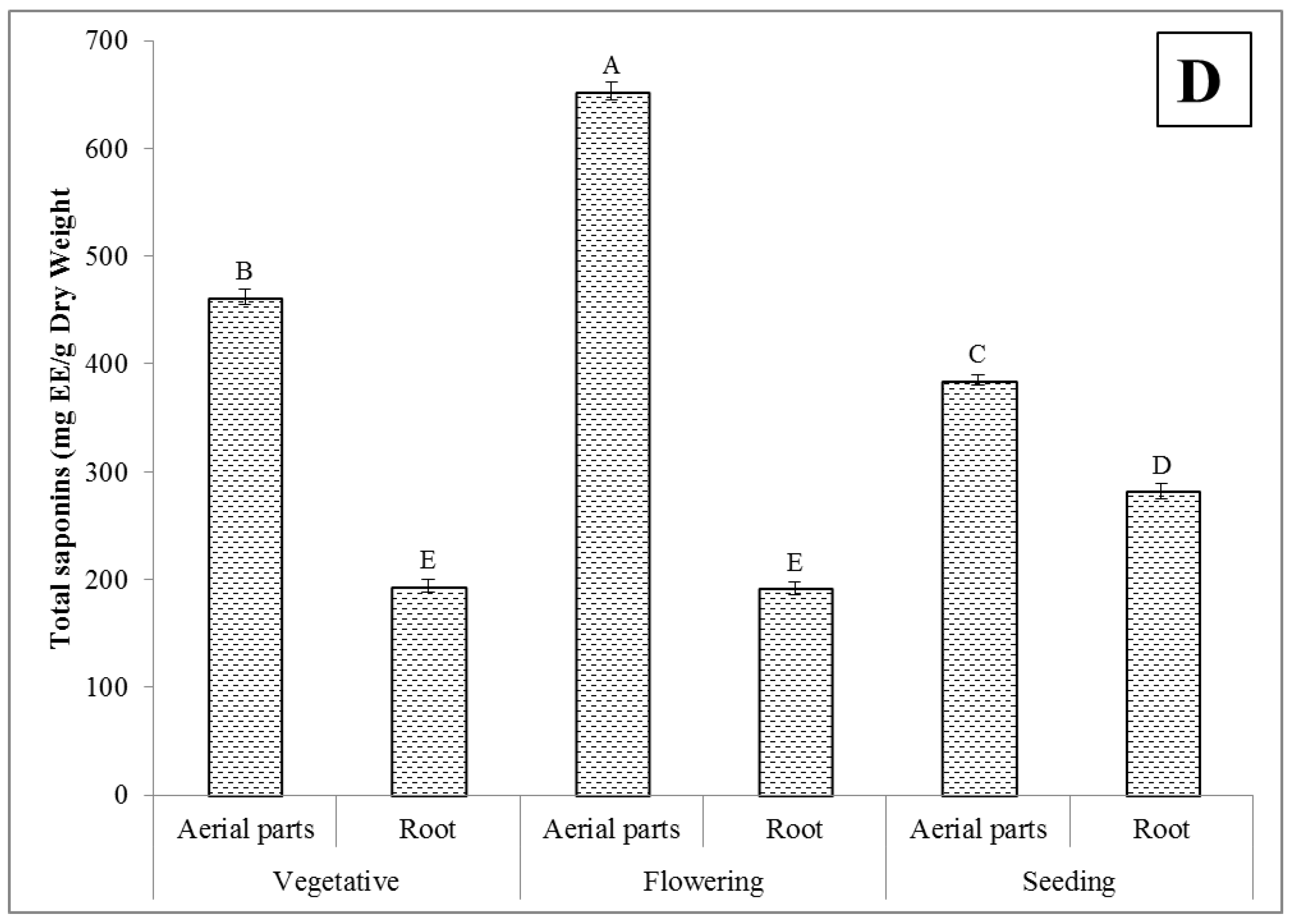
2.4. Total Saponins Content
2.5. Gas Chromatography–Mass Spectrometry Analysis
2.6. Antioxidant Activity
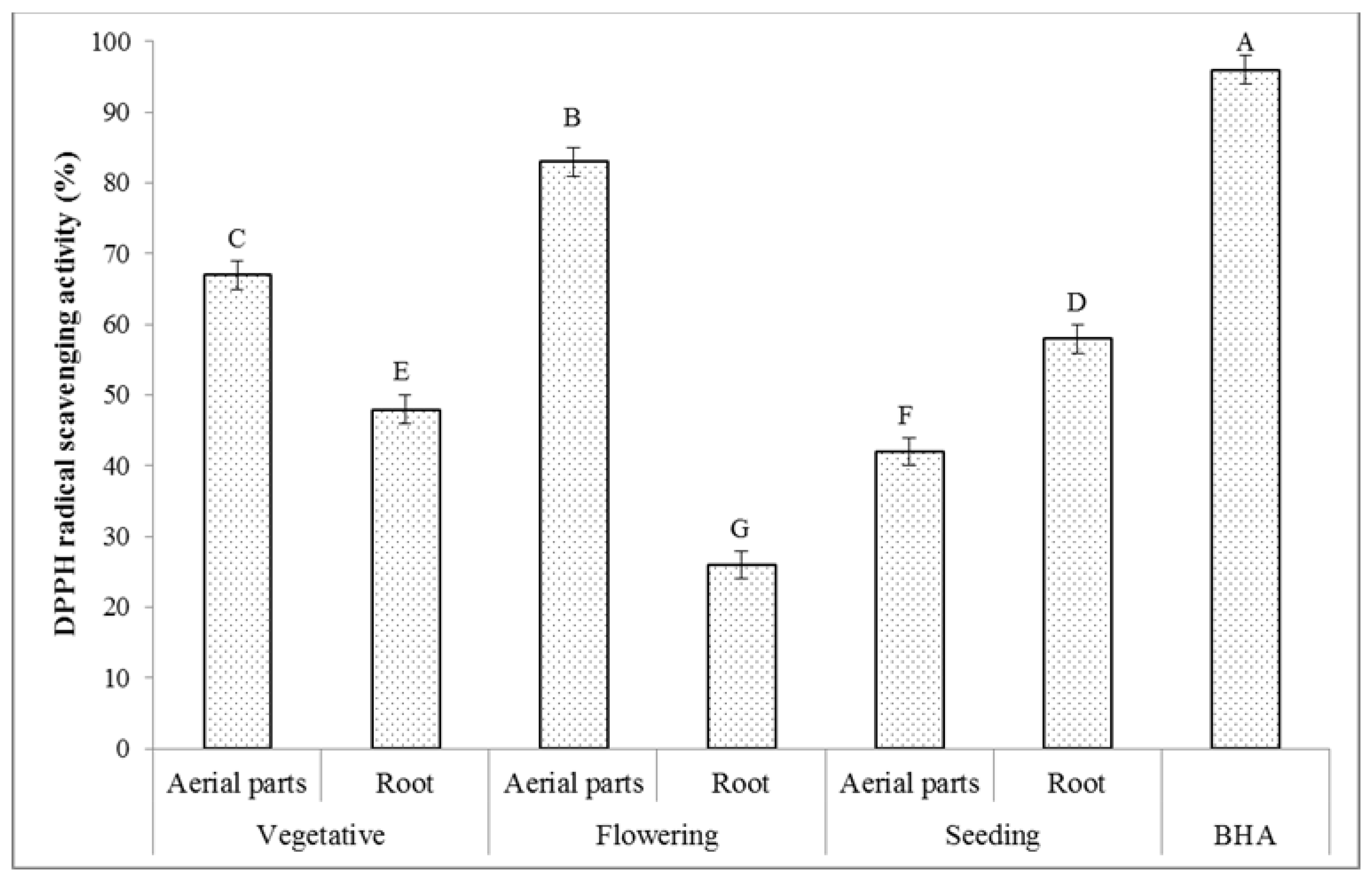
2.6.1. DPPH Radical Scavenging Activity
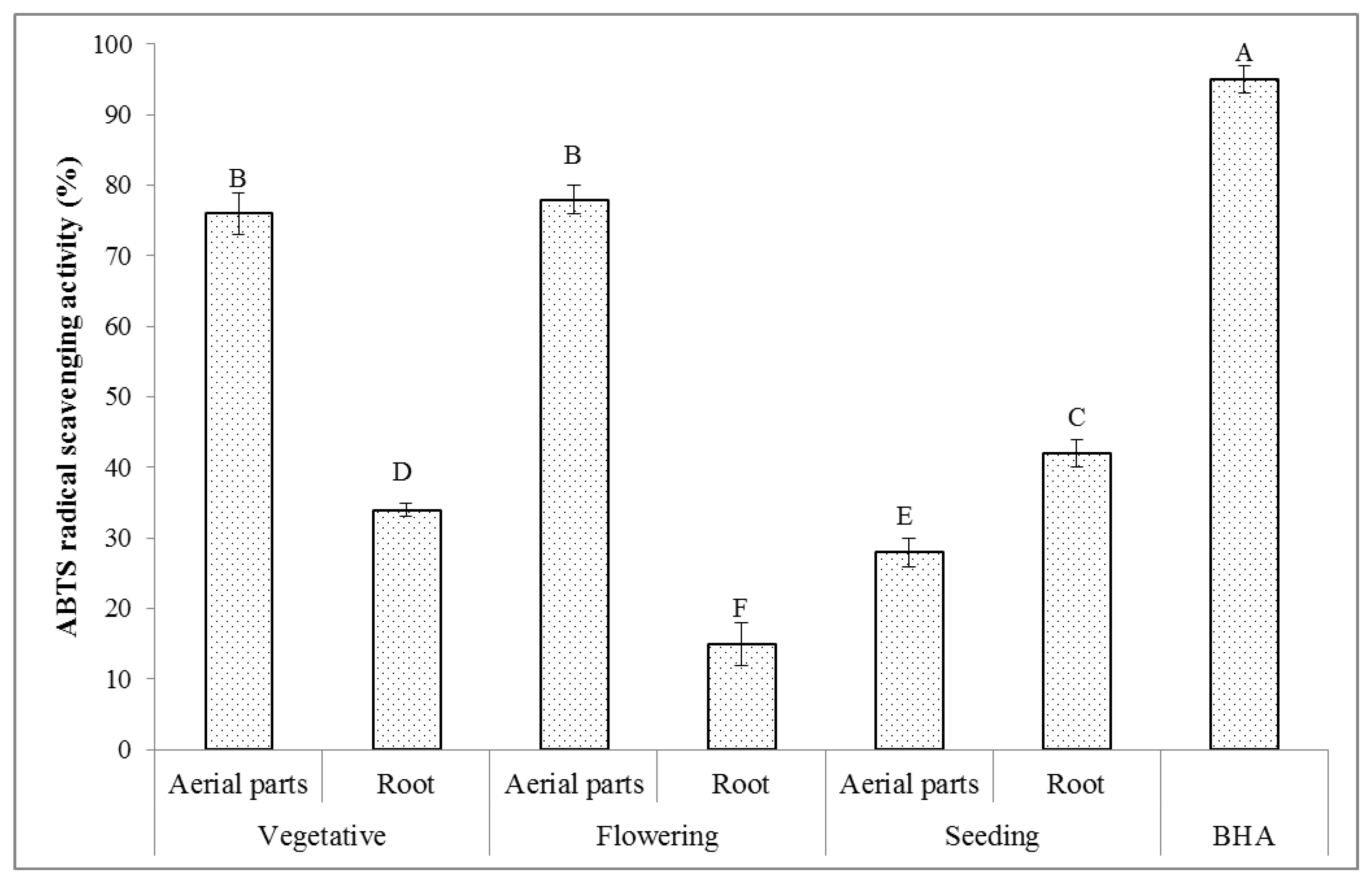
2.6.2. ABTS Radical Scavenging Activity
2.6.3. Nitric Oxide Radical Scavenging Activity
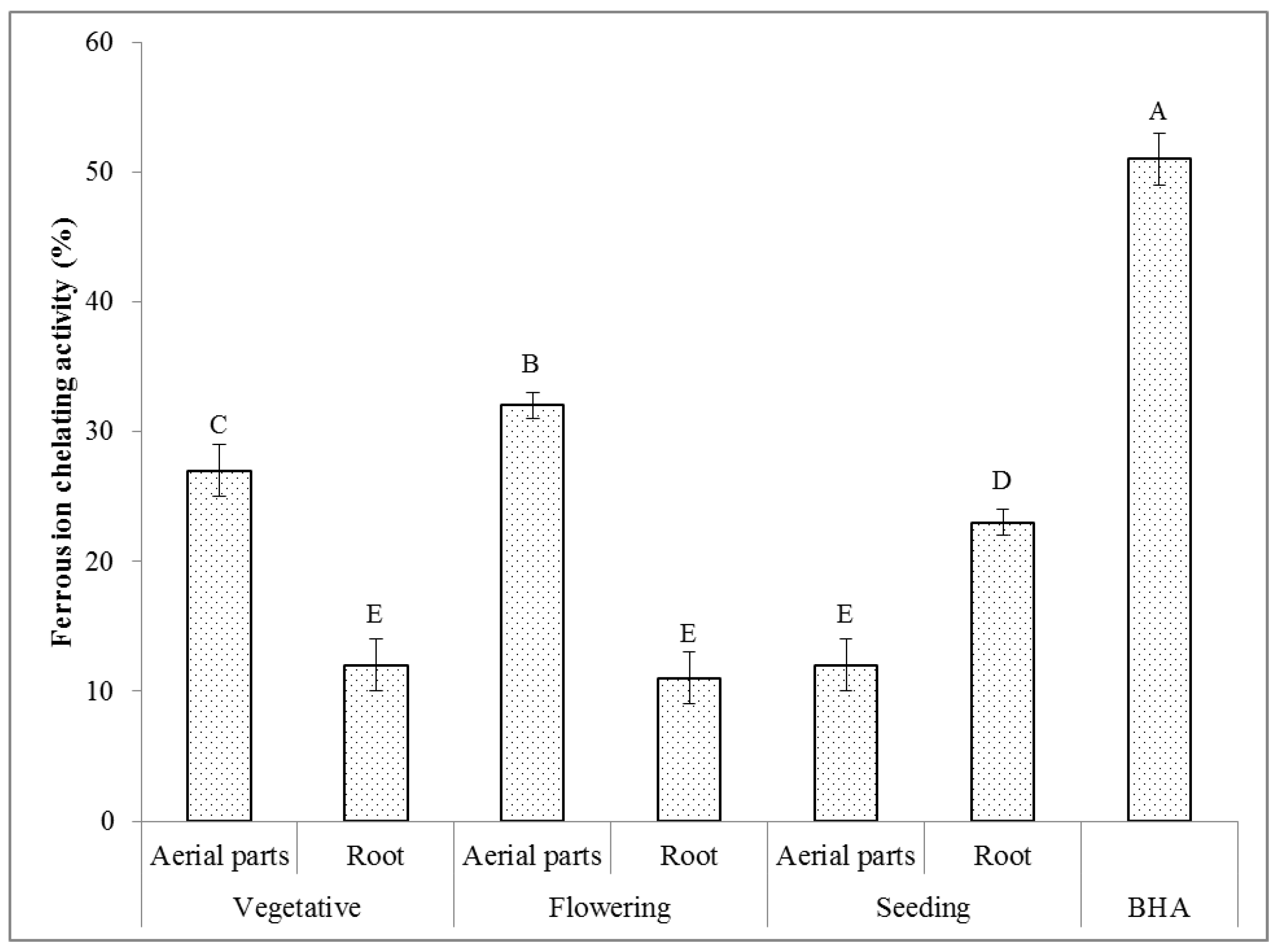
2.6.4. Ferrous Ion Chelating Activity
2.7. Antibacterial Activity
2.7.1. Disc Diffusion Method
2.7.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.8. Anti-Inflammatory Activity
Human Red Blood Cell Stabilization
3. Materials and Methods
3.1. The Plant Extract Preparation
3.2. Phytochemical Analysis
3.2.1. The Quantification of the Total Contents of Phenolics
3.2.2. The Quantification of Total Content of Flavonoids
3.2.3. The Quantification of Total Content of Alkaloids
3.2.4. The Quantification of the Total Content of Saponins
3.2.5. Gas Chromatography–Mass Spectrometry Analysis
3.3. Antioxidant Activity
3.3.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity Method
3.3.2. 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic Acid) (ABTS) Radical Scavenging Activity Method
3.3.3. Nitric Oxide (NO) Radical Scavenging Activity
3.3.4. Ferrous Ion (Fe2+) Chelating Activity
3.4. Antibacterial Activity
3.4.1. Disc Diffusion Assay
3.4.2. The Minimum Inhibitory Concentration (MIC) Determination
3.4.3. The Minimum Bactericidal Concentration (MBC) Determination
3.5. Anti-Inflammatory Activity
Human Red Blood Cell Stabilization Assay
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sharifi-Rad, M.; Epifano, F.; Fiorito, S.; Álvarez-Suarez, J.M. Phytochemical analysis and biological investigation of Nepeta juncea Benth. different extracts. Plants 2020, 9, 646. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Duh, P.D.; Yen, G.C.; Yen, W.J.; Chang, L.W. Antioxidant effects of water extracts from barley (Hordeum vulgare L.) prepared under different roasting temperatures. J. Agric. Food Chem. 2001, 49, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, S.S.; Amarowicz, R.; Shahidi, F. Antioxidant activity of almonds and their by-products in food model systems. J. Am. Oil Chem. Soc. 2006, 83, 223–230. [Google Scholar] [CrossRef]
- Sharma, A.; del Carmen Flores-Vallejo, R.; Cardoso-Taketa, A.; Villarreal, M.L. Antibacterial activities of medicinal plants used in Mexican traditional medicine. J. Ethnopharmacol. 2017, 208, 264–329. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashley, N.T.; Weil, Z.M.; Nelson, R.J. Inflammation: Mechanisms, costs, and natural variation. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 385–406. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018, 9, 143. [Google Scholar] [CrossRef] [Green Version]
- Ghasemian, M.; Owlia, S.; Owlia, M.B. Review of anti-inflammatory herbal medicines. Adv. Pharmacol. Sci. 2016, 2016, 9130979. [Google Scholar] [CrossRef] [Green Version]
- Yatoo, M.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Tufani, N.A.; Chakraborty, S.; Tiwari, R.; Dhama, K.; Iqbal, H. Anti-inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders-A review. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 39–58. [Google Scholar] [CrossRef]
- Bahramikia, S.; Yazdanparast, R. Phytochemistry and medicinal properties of Teucrium polium L. (Lamiaceae). Phytother. Res. 2012, 26, 1581–1593. [Google Scholar] [CrossRef]
- Abdollahi, M.; Karimpour, H.; Monsef-Esfehani, H.R. Antinociceptive effects of Teucrium polium L. total extract and essential oil in mouse writhing test. Pharmacol. Res. 2003, 48, 31–35. [Google Scholar] [CrossRef]
- Esmaeili, M.A.; Yazdanparast, R. Hypoglycaemic effect of Teucrium polium: Studies with rat pancreatic islets. J. Ethnopharmacol. 2004, 95, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Mossa, J.S.; Al-Yahya, M.A.; Al-Meshal, I.A. Medicinal Plants of Saudi Arabia; King Saud University Press: Riyadh, Saudi Arabia, 2000. [Google Scholar]
- Nawash, O.; Shudiefat, M.; Al-Tabini, R.; Al-Khalidi, K. Ethnobotanical study of medicinal plants commonly used by local bedouins in the badia region of Jordan. J. Ethnopharmacol. 2013, 148, 921–925. [Google Scholar] [CrossRef]
- Bulut, G.; Tuzlaci, E. An ethnobotanical study of medicinal plants in Bayramiç (Canakkale-Turkey). Marmara Pharm. J. 2015, 19, 268–282. [Google Scholar] [CrossRef]
- Akin, M.; Oguz, D.; Saracoglu, H. Antibacterial activity of essential oil from Thymbra spicata var. spicata L. and Teucrium polium (Stapf Brig.). Interventions 2010, 8, 53–58. [Google Scholar]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Lyoussi, B.; Taleb, M.; Abdellaoui, A. Total phenolic and flavonoid contents and antioxidant activities of extracts from Teucrium polium growing wild in Morocco. Mater. Today Proc. 2019, 13, 777–783. [Google Scholar] [CrossRef]
- Ait Chaouche, F.S.; Mouhouche, F.; Hazzit, M. Antioxidant capacity and total phenol and flavonoid contents of Teucrium polium L. grown in Algeria. Mediterr. J. Nutr. Metab. 2018, 11, 135–144. [Google Scholar] [CrossRef]
- Jaradat, N.A.; Ayesh, O.I.; Anderson, C. Ethnopharmacological survey about medicinal plants utilized by herbalists and traditional practitioner healers for treatments of diarrhea in the West Bank/Palestine. J. Ethnopharmacol. 2016, 182, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ali-Shtayeh, M.S.; Jamous, R.M.; Jamous, R.M. Traditional Arabic Palestinian ethnoveterinary practices in animal health care: A field survey in the West Bank (Palestine). J. Ethnopharmacol. 2016, 182, 35–49. [Google Scholar] [CrossRef]
- Šarić-Kundalić, B.; Dobeš, C.; Klatte-Asselmeyer, V.; Saukel, J. Ethnobotanical study on medicinal use of wild and cultivated plants in middle, south and west Bosnia and Herzegovina. J. Ethnopharmacol. 2010, 131, 33–55. [Google Scholar] [CrossRef]
- Pieroni, A.; Dibra, B.; Grishaj, G.; Grishaj, I.; Maçai, S.G. Traditional phytotherapy of the Albanians of Lepushe, Northern Albanian Alps. Fitoterapia 2005, 76, 379–399. [Google Scholar] [CrossRef] [PubMed]
- Gras, A.; Garnatje, T.; Ibanez, N.; Lopez-Pujol, J.; Nualart, N.; Valles, J. Medicinal plant uses and names from the herbarium of Francesc Bolòs (1773–1844). J. Ethnopharmacol. 2017, 204, 142–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dall’Acqua, S.; Cervellati, R.; Loi, M.C.; Innocenti, G. Evaluation of in vitro antioxidant properties of some traditional Sardinian medicinal plants: Investigation of the high antioxidant capacity of Rubus ulmifolius. Food Chem. 2008, 106, 745–749. [Google Scholar] [CrossRef]
- Khazaei, M.; Nematollahi-Mahani, S.N.; Mokhtari, T.; Sheikhbahaei, F. Review on Teucrium polium biological activities and medical characteristics against different pathologic situations. J. Contemp. Med. Sci. 2018, 4, 1–6. [Google Scholar]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Lyoussi, B.; Oumokhtar, B.; Abdellaoui, A. Phytochemistry, antioxidant and antibacterial activities of two Moroccan Teucrium polium L. subspecies: Preventive approach against nosocomial infections. Arab. J. Chem. 2020, 13, 3866–3874. [Google Scholar] [CrossRef]
- Guetat, A.; Al-Ghamdi, F.A. Analysis of the essential oil of the germander (Teucrium polium L.) aerial parts from the northern region of Saudi Arabia. Int. J. Appl. Biol. Pharm. Technol. 2014, 5, 128–135. [Google Scholar]
- Jaradat, N.A. Review of the taxonomy, ethnobotany, phytochemistry, phytotherapy and phytotoxicity of germander plant (Teucrium polium L.). Asian J. Pharm. Clin. Res. 2015, 8, 13–19. [Google Scholar]
- Alreshidi, M.; Noumi, E.; Bouslama, L.; Ceylan, O.; Veettil, V.N.; Adnan, M.; Danciu, C.; Elkahoui, S.; Badraoui, R.; Al-Motair, K.A. Phytochemical screening, antibacterial, antifungal, antiviral, cytotoxic, and anti-quorum-sensing properties of Teucrium polium L. aerial parts methanolic extract. Plants 2020, 9, 1418. [Google Scholar] [CrossRef]
- Noumi, E.; Snoussi, M.; Anouar, E.H.; Alreshidi, M.; Veettil, V.N.; Elkahoui, S.; Adnan, M.; Patel, M.; Kadri, A.; Aouadi, K. HR-LCMS-Based Metabolite Profiling, Antioxidant, and Anticancer Properties of Teucrium polium L. Methanolic Extract: Computational and In Vitro Study. Antioxidants 2020, 9, 1089. [Google Scholar] [CrossRef]
- Campêlo, M.C.S.; Medeiros, J.M.S.; Silva, J.B.A. Natural products in food preservation. Int. Food Res. J. 2019, 26, 41–46. [Google Scholar]
- Chepel, V.; Lisun, V.; Skrypnik, L. Changes in the content of some groups of phenolic compounds and biological activity of extracts of various parts of heather (Calluna vulgaris (L.) Hull) at different growth stages. Plants 2020, 9, 926. [Google Scholar] [CrossRef] [PubMed]
- Riipi, M.; Ossipov, V.; Lempa, K.; Haukioja, E.; Koricheva, J.; Ossipova, S.; Pihlaja, K. Seasonal changes in birch leaf chemistry: Are there trade-offs between leaf growth and accumulation of phenolics? Oecologia 2002, 130, 380–390. [Google Scholar] [CrossRef]
- Samanta, A.; Das, G.; Das, S.K. Roles of flavonoids in plants. Carbon 2011, 100, 12–35. [Google Scholar]
- Del Valle, J.C.; Buide, M.L.; Casimiro-Soriguer, I.; Whittall, J.B.; Narbona, E. On flavonoid accumulation in different plant parts: Variation patterns among individuals and populations in the shore campion (Silene littorea). Front. Plant Sci. 2015, 6, 939. [Google Scholar] [CrossRef] [Green Version]
- Jimoh, M.; Anthony, J.; Francis, B. Antioxidant and phytochemical activities of Amaranthus caudatus L. harvested from different soils at various growth stages. Sci. Rep. 2019, 9, 12965. [Google Scholar] [CrossRef]
- Cirak, C.; Radusiene, J.; Camas, N.; Caliskan, O.; Odabas, M.S. Changes in the contents of main secondary metabolites in two Turkish Hypericum species during plant development. Pharm. Biol. 2013, 51, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Baranauskienė, R.; Venskutonis, P.R.; Dambrauskienė, E.; Viškelis, P. Harvesting time influences the yield and oil composition of Origanum vulgare L., ssp. vulgare and ssp. hirtum. Ind. Crops Prod. 2013, 49, 43–51. [Google Scholar] [CrossRef]
- Towler, M.J.; Weathers, P.J. Variations in key artemisinic and other metabolites throughout plant development in Artemisia annua L. for potential therapeutic use. Ind. Crops Prod. 2015, 67, 185–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wang, Y.; Zhao, H.; Fu, X.; Fang, S. Localization and dynamic change of saponins in Cyclocarya paliurus (Batal.) Iljinskaja. PLoS ONE 2019, 14, e0223421. [Google Scholar] [CrossRef]
- Lim, J.G.; Park, H.M.; Yoon, K.S. Analysis of saponin composition and comparison of the antioxidant activity of various parts of the quinoa plant (Chenopodium quinoa Willd.). Food Sci. Nutr. 2020, 8, 694–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, G.; Kerem, Z.; Makkar, H.P.S.; Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.D.J.P.; Ferreira, O.O.; Antônio Barbosa de Moraes, Â.; Varela, E.L.P.; Nascimento, L.D.D.; Percário, S.; de Oliveira, M.S.; Andrade, E.H.D.A. Chemical Composition and Antioxidant Activity of Essential Oils from Eugenia patrisii Vahl, E. punicifolia (Kunth) DC., and Myrcia tomentosa (Aubl.) DC., Leaf of Family Myrtaceae. Molecules 2021, 26, 3292. [Google Scholar] [CrossRef] [PubMed]
- Bakar, A.; Yao, P.C.; Ningrum, V.; Liu, C.T.; Lee, S.C. Beneficial biological activities of Cinnamomum osmophloeum and its potential use in the alleviation of oral mucositis: A systematic review. Biomedicines 2020, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Popa, C.L.; Lupitu, A.; Mot, M.D.; Copolovici, L.; Moisa, C.; Copolovici, D.M. Chemical and Biochemical Characterization of Essential Oils and Their Corresponding Hydrolats from Six Species of the Lamiaceae Family. Plants 2021, 10, 2489. [Google Scholar] [CrossRef] [PubMed]
- Kerbouche, L.; Hazzit, M.; Ferhat, M.A.; Baaliouamer, A.; Miguel, M.G. Biological activities of essential oils and ethanol extracts of Teucrium polium subsp. capitatum (L.) Briq. and Origanum floribundum Munby. J. Essent. Oil Bear. Plants 2015, 18, 1197–1208. [Google Scholar] [CrossRef]
- Njoya, E.M. Medicinal Plants, Antioxidant Potential, and Cancer; Academic Press: San Diego, CA, USA, 2021. [Google Scholar]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Alam, A.K. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nafiu, M.O.; Ashafa, A.O.T. Antioxidant and inhibitory effects of saponin extracts from Dianthus basuticus Burtt Davy on key enzymes implicated in type 2 diabetes In vitro. Pharmacogn. Mag. 2017, 13, 576. [Google Scholar]
- Gruz, J.; Ayaz, F.A.; Torun, H.; Strnad, M. Phenolic acid content and radical scavenging activity of extracts from medlar (Mespilus germanica L.) fruit at different stages of ripening. Food Chem. 2011, 124, 271–277. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Li, L.S.; Chiroma, S.M.; Hashim, T.; Adam, S.K.; Moklas, M.A.M.; Yusuf, Z.; Rahman, S.A. Antioxidant and anti-inflammatory properties of Erythroxylum cuneatum alkaloid leaf extract. Heliyon 2020, 6, e04141. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Paré, P.W.; Zhang, J.; Kang, T.; Zhang, Z.; Yang, D.; Wang, K.; Xing, H. Antioxidant Capacity Connection with Phenolic and Flavonoid Content in Chinese Medicinal Herbs. Rec. Nat. Prod. 2018, 12, 239–250. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.I. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Yang, Q.; Zhu, X.; Lin, T.; Hao, D.; Xu, J. Antioxidant activities of Clerodendrum cyrtophyllum Turcz leaf extracts and their major components. PLoS ONE 2020, 15, e0234435. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Walker, R.B.; Everette, J.D. Comparative reaction rates of various antioxidants with ABTS radical cation. J. Agric. Food Chem. 2009, 57, 1156–1161. [Google Scholar] [CrossRef]
- Pan, F.; Su, T.J.; Cai, S.M.; Wu, W. Fungal endophyte-derived Fritillaria unibracteata var. wabuensis: Diversity, antioxidant capacities in vitro and relations to phenolic, flavonoid or saponin compounds. Sci. Rep. 2017, 7, 42008. [Google Scholar] [CrossRef] [Green Version]
- Hayat, J.; Akodad, M.; Moumen, A.; Baghour, M.; Skalli, A.; Ezrari, S.; Belmalha, S. Phytochemical screening, polyphenols, flavonoids and tannin content, antioxidant activities and FTIR characterization of Marrubium vulgare L. from 2 different localities of Northeast of Morocco. Heliyon 2020, 6, e05609. [Google Scholar] [CrossRef]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hartzfeld, P.W.; Riechel, T.L. High molecular weight plant polyphenolics (Tannins) as biological antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Tylor, B.S.; Kion, Y.M.; Wang, Q.I.; Sharpio, R.A.; Billiar, T.R.; Geller, D.A. Nitric oxide down regulates hepatocyte-inducible nitric oxide synthase gene expression. Arch. Surg. 1997, 132, 1177–1183. [Google Scholar] [CrossRef]
- Huie, R.E.; Padmaja, S. The reaction of NO with superoxide. Free Radic. Res. Commun. 1993, 18, 195–199. [Google Scholar] [CrossRef]
- Möller, M.N.; Rios, N.; Trujillo, M.; Radi, R.; Denicola, A.; Alvarez, B. Detection and quantification of nitric oxide–derived oxidants in biological systems. J. Biol. Chem. 2019, 294, 14776–14802. [Google Scholar] [CrossRef] [Green Version]
- Kontoghiorghes, G.J.; Kontoghiorghe, C.N. Iron and chelation in biochemistry and medicine: New approaches to controlling iron metabolism and treating related diseases. Cells 2020, 9, 1456. [Google Scholar] [CrossRef]
- Liang, T.; Yue, W.; Li, Q. Comparison of the phenolic content and antioxidant activities of Apocynum venetum L. (Luo-Bu-Ma) and two of its alternative species. Int. J. Mol. Sci. 2010, 11, 4452–4464. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Tepe, B. Biological activity and phytochemistry of firethorn (Pyracantha coccinea MJ Roemer). J. Funct. Foods 2015, 19, 669–675. [Google Scholar] [CrossRef]
- Sudan, R.; Bhagat, M.; Gupta, S.; Singh, J.; Koul, A. Iron (FeII) chelation, ferric reducing antioxidant power, and immune modulating potential of Arisaema jacquemontii (Himalayan Cobra Lily). BioMed Res. Int. 2014, 2014, 179865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, M.A.; Palmeira, J.D.; Melo, D.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Alves, R.C.; Ferreira, H.; Oliveira, M.B.P.P. Chemical Composition and Antimicrobial Activity of a New Olive Pomace Functional Ingredient. Pharmaceuticals 2021, 14, 913. [Google Scholar] [CrossRef]
- Lehman, K.M.; Grabowicz, M. Countering gram-negative antibiotic resistance: Recent progress in disrupting the outer membrane with novel therapeutics. Antibiotics 2019, 8, 163. [Google Scholar] [CrossRef] [Green Version]
- Giessler, A.J.; Bekemeier, H.; Hischelamann, R.; Bakatheir, H.A. Pharmacology, Biochemistry and Immunology of Inflammatory Reaction; Martin Luther University Halle-Wittenberg: Halle (Saale), Germany, 1982. [Google Scholar]
- Sadique, J.; Al-Rqobahs, W.A.; Bughaith, E.I.; Gindi, A.R. The bioactivity of certain medicinal plants on the stabilization of RBC membrane system. Fitoterapia 1989, 60, 525–532. [Google Scholar]
- Govindappa, M.; Poojashri, M.N. Antimicrobial, antioxidant and in vitro anti-inflammatory activity of ethanol extract and active phytochemical screening of Wedelia trilobata (L.) Hitchc. J. Pharmacogn. Phytother. 2011, 3, 43–51. [Google Scholar]
- Parvin, M.S.; Das, N.; Jahan, N.; Akhter, M.A.; Nahar, L.; Islam, M.E. Evaluation of in vitro anti-inflammatory and antibacterial potential of Crescentia cujete leaves and stem bark. BMC Res. Notes 2015, 8, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P. In vitro anti-inflammatory properties of selected green leafy vegetables. Biomedicines 2018, 6, 107. [Google Scholar] [CrossRef] [Green Version]
- Yesmin, S.; Paul, A.; Naz, T.; Rahman, A.A.; Akhter, S.F.; Wahed, M.I.I.; Emran, T.B.; Siddiqui, S.A. Membrane stabilization as a mechanism of the anti-inflammatory activity of ethanolic root extract of Choi (Piper chaba). Clin. Phytosci. 2020, 6, 59. [Google Scholar] [CrossRef]
- Fialho, L.; Cunha-E-Silva, J.A.; Santa-Maria, A.F.; Madureira, F.A.; Iglesias, A.C. Comparative study of systemic early postoperative inflammatory response among elderly and non-elderly patients undergoing laparoscopic cholecystectomy. Rev. Col. Bras. Cir. 2018, 45, e1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, R.A.; El-Anssary, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. Herb. Med. 2019, 2, 11–30. [Google Scholar]
- Naji, E.; Luca, R. Comparative study of anthocyanins content of some selected Yemeni plants. J. Pharm. Phytother. 2013, 1, 5. [Google Scholar]
- Begashaw, B.; Mishra, B.; Tsegaw, A.; Shewamene, Z. Methanol leaves extract Hibiscus micranthus Linn exhibited antibacterial and wound healing activities. BMC Complement. Altern. Med. 2017, 17, 337. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Pohl, P.; Epifano, F. Phytofabrication of Silver Nanoparticles (AgNPs) with Pharmaceutical Capabilities Using Otostegia persica (Burm.) Boiss. Leaf Extract. Nanomaterials 2021, 11, 1045. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pohl, P.; Epifano, F.; Álvarez-Suarez, J.M. Green synthesis of silver nanoparticles using Astragalus tribuloides delile. root extract: Characterization, antioxidant, antibacterial, and anti-inflammatory activities. Nanomaterials 2020, 10, 2383. [Google Scholar] [CrossRef]
- Ajanal, M.; Gundkalle, M.B.; Nayak, S.U. Estimation of total alkaloid in Chitrakadivati by UV-Spectrophotometer. Anc. Sci. Life 2012, 31, 198. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Hirun, S.; Roach, P.D.; Bowyer, M.C.; Phillips, P.A.; Scarlett, C.J. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Herb. Med. 2013, 3, 104–111. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 1995. [Google Scholar]
- Sharifi-Rad, M.; Pohl, P. Synthesis of biogenic silver nanoparticles (Agcl-NPs) using a Pulicaria vulgaris gaertn. aerial part extract and their application as antibacterial, antifungal and antioxidant agents. Nanomaterials 2020, 10, 638. [Google Scholar] [CrossRef] [Green Version]
- Ko, M.J.; Nam, H.H.; Chung, M.S. Subcritical water extraction of bioactive compounds from Orostachys japonicus A. Berger (Crassulaceae). Sci. Rep. 2020, 10, 10890. [Google Scholar] [CrossRef]
- Kamble, S.; Humbare, R.; Sarkar, J.; Kulkarni, A. Assessment of Phytochemicals and Antioxidant Properties of Root Extracts of Rubia cordifolia L. in Different Solvent Systems. Biol. Life Sci. Forum 2021, 4, 100. [Google Scholar] [CrossRef]
- Dinis, T.C.; Madeira, V.M.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Iriti, M.; Gibbons, S.; Sharifi-Rad, J. Anti-methicillin-resistant Staphylococcus aureus (MRSA) activity of Rubiaceae, Fabaceae and Poaceae plants: A search for new sources of useful alternative antibacterials against MRSA infections. Cell. Mol. Biol. 2016, 62, 39–45. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard M7-A6; National Committee for Clinical Laboratory Standards: Wayne, PE, USA, 2012. [Google Scholar]
- Vane, J.R.; Botting, R.M. New insights into the mode of action of anti-inflammatory drugs. Inflamm. Res. 1995, 44, 1–10. [Google Scholar] [CrossRef] [PubMed]



| Vegetative | Flowering | Seeding | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | RI * | Molecular Formula | Aerial Parts (%) | Root (%) | Aerial Parts (%) | Root (%) | Aerial Parts (%) | Root (%) | |
| 1 | (E)-2-Hexenal | 860 | C6H10O | 0.1 ± 0.02 | 0.1 ± 0.00 | 0.1 ± 0.01 | - | - | - |
| 2 | 3-Heptanone | 885 | C7H14O | 0.1 ± 0.00 | - | - | - | - | - |
| 3 | α-Thujene | 924 | C10H16 | 0.2 ± 0.00 | 0.1 ± 0.01 | 0.4 ± 0.00 | 0.1 ± 0.00 | 0.1 ± 0.02 | 0.1 ± 0.00 |
| 4 | α-Pinene | 935 | C10H16 | 3.8 ± 0.04 | 1.9 ± 0.03 | 4.3 ± 0.00 | 0.7 ± 0.04 | 1.3 ± 0.05 | 2.5 ± 0.03 |
| 5 | Camphene | 950 | C10H16 | 0.2 ± 0.01 | - | 0.2 ± 0.00 | - | 0.1 ± 0.00 | - |
| 6 | β-Pinene | 975 | C10H16 | 7.2 ± 0.03 | 4.1 ± 0.05 | 9.4 ± 0.02 | 2.5 ± 0.05 | 3.6 ± 0.03 | 6.3 ± 0.02 |
| 7 | 1-Octen-3-ol | 984 | C8H16O | 0.3 ± 0.00 | - | - | - | - | - |
| 8 | β-Myrcene | 993 | C10H16 | 1.5 ± 0.00 | 0.5 ± 0.02 | 1.8 ± 0.03 | 0.2 ± 0.01 | 0.3 ± 0.00 | 0.8 ± 0.02 |
| 9 | α-Phellandrene | 1006 | C10H16 | - | - | 0.1 ± 0.00 | 0.1 ± 0.00 | - | - |
| 10 | α-Terpinene | 1015 | C10H16 | 0.3 ± 0.01 | - | - | - | - | - |
| 11 | p-Cymene | 1025 | C10H14 | 0.4 ± 0.00 | 0.1 ± 0.00 | 0.7 ± 0.02 | 0.1 ± 0.00 | - | 0.1 ± 0.01 |
| 12 | Limonene | 1030 | C10H16 | 3.6 ± 0.02 | 1.7 ± 0.01 | 4.2 ± 0.00 | 0.6 ± 0.03 | 1.0 ± 0.02 | 2.8 ± 0.06 |
| 13 | 1.8-Cineole | 1034 | C10H18O | 0.2 ± 0.00 | 0.1 ± 0.00 | 0.3 ± 0.01 | 0.1 ± 0.02 | - | - |
| 14 | cis-β-Ocimene | 1041 | C10H16 | - | 0.1 ± 0.01 | - | 0.1 ± 0.00 | - | - |
| 15 | trans-β-Ocimene | 1049 | C10H16 | 0.1 ± 0.00 | 0.1 ± 0.00 | - | - | - | - |
| 16 | γ-Terpinene | 1062 | C10H16 | 0.3 ± 0.01 | - | 0.5 ± 0.02 | 0.2 ± 0.00 | 0.2 ± 0.01 | 0.1 ± 0.00 |
| 17 | Terpinolene | 1094 | C10H16 | - | - | 0.2 ± 0.00 | - | - | - |
| 18 | Linalool | 1105 | C10H18O | 0.5 ± 0.02 | 0.1 ± 0.01 | 0.6 ± 0.00 | 0.2 ± 0.01 | 0.1 ± 0.00 | 0.2 ± 0.00 |
| 19 | 4-Acetyl-1-methylcyclohexene | 1118 | C9H14O | 0.1 ± 0.00 | - | 0.1 ± 0.01 | - | - | - |
| 20 | trans-Pinocarveol | 1125 | C10H16O | 1.5 ± 0.02 | 0.2 ± 0.00 | 0.1 ± 0.00 | 0.1 ± 0.02 | 0.1 ± 0.00 | - |
| 21 | Camphor | 1145 | C10H16O | 0.3 ± 0.00 | 0.1 ± 0.02 | 0.4 ± 0.03 | - | - | 0.3 ± 0.00 |
| 22 | Pinocarvone | 1160 | C10H14O | 0.4 ± 0.02 | 0.1 ± 0.02 | 0.6 ± 0.00 | 0.1 ± 0.03 | 0.1 ± 0.00 | 0.2 ± 0.01 |
| 23 | Borneol | 1168 | C10H18O | 0.3 ± 0.00 | 0.2 ± 0.00 | - | - | - | - |
| 24 | Terpinen-4-ol | 1178 | C10H18O | 0.2 ± 0.01 | 0.1 ± 0.00 | 0.5 ± 0.03 | - | - | 0.2 ± 0.00 |
| 25 | p-Cymen-8-ol | 1188 | C10H14O | - | - | 0.1 ± 0.00 | 0.1 ± 0.02 | 0.1 ± 0.00 | - |
| 26 | α-Terpineol | 1195 | C10H18O | 0.1 ± 0.00 | - | 0.1 ± 0.00 | - | 0.1 ± 0.01 | - |
| 27 | Myrtenol | 1197 | C10H16O | 0.5 ± 0.01 | 0.2 ± 0.01 | 0.7 ± 0.04 | - | - | 0.2 ± 0.00 |
| 28 | Verbenone | 1204 | C10H14O | - | 0.1 ± 0.00 | 0.1 ± 0.00 | 0.1 ± 0.01 | - | 0.1 ± 0.00 |
| 29 | Cuminaldehyde | 1215 | C10H12O | - | 0.1 ± 0.00 | - | 0.1 ± 0.01 | - | - |
| 30 | Bornyl acetate | 1285 | C12H20O2 | 0.1 ± 0.00 | - | 0.2 ± 0.00 | 0.1 ± 0.02 | 0.1 ± 0.00 | 0.1 ± 0.01 |
| 31 | α-Fenchyl acetate | 1294 | C12H20O2 | 0.1 ± 0.00 | - | - | - | - | - |
| 32 | Thymol | 1305 | C10H14O | 0.2 ± 0.01 | - | 0.4 ± 0.03 | - | 0.1 ± 0.00 | - |
| 33 | Carvacrol | 1319 | C10H14O | 6.2 ± 0.05 | 4.5 ± 0.03 | 8.2 ± 0.00 | 2.3 ± 0.05 | 3.8 ± 0.06 | 5.6 ± 0.02 |
| 34 | α-Cubebene | 1349 | C15H24 | 0.3 ± 0.00 | 0.2 ± 0.01 | - | - | - | - |
| 35 | α-Copaene | 1374 | C15H24 | 0.8 ± 0.03 | 0.2 ± 0.00 | 1.1 ± 0.02 | 0.1 ± 0.00 | 0.2 ± 0.00 | 0.5 ± 0.03 |
| 36 | β-Bourbonene | 1380 | C15H24 | 0.6 ± 0.01 | 0.1 ± 0.00 | 0.9 ± 0.04 | 0.1 ± 0.00 | - | 0.4 ± 0.05 |
| 37 | β-Elemene | 1395 | C15H24 | 0.1 ± 0.00 | - | 0.2 ± 0.01 | - | 0.1 ± 0.00 | 0.1 ± 0.01 |
| 38 | β-Caryophyllene | 1415 | C15H24 | 0.5 ± 0.00 | 0.2 ± 0.01 | 0.7 ± 0.03 | 0.1 ± 0.00 | 0.1 ± 0.02 | - |
| 39 | α-Bergamotene | 1435 | C15H24 | - | - | 0.1 ± 0.00 | 0.1 ± 0.00 | - | - |
| 40 | α-Humulene | 1454 | C15H24 | 0.1 ± 0.00 | - | 0.1 ± 0.01 | - | 0.1 ± 0.02 | - |
| 41 | Germacrene D | 1478 | C15H24 | 15.2 ± 0.07 | 11.3 ± 0.05 | 17.4 ± 0.08 | 7.6 ± 0.04 | 9.4 ± 0.06 | 13.4 ± 0.05 |
| 42 | Bicyclogermacrene | 1494 | C15H24 | 6.3 ± 0.05 | 4.1 ± 0.00 | 7.2 ± 0.05 | 2.1 ± 0.03 | 3.2 ± 0.02 | 5.5 ± 0.01 |
| 43 | γ-Cadinene | 1512 | C15H24 | 1.8 ± 0.04 | 0.8 ± 0.01 | 2.1 ± 0.03 | 0.3 ± 0.04 | 0.5 ± 0.02 | 1.4 ± 0.00 |
| 44 | δ-Cadinene | 1519 | C15H24 | 1.2 ± 0.01 | 0.4 ± 0.03 | 2.5 ± 0.02 | 0.1 ± 0.00 | 0.2 ± 0.01 | 0.8 ± 0.03 |
| 45 | α-Calacorene | 1530 | C15H20 | 0.1 ± 0.00 | 0.1 ± 0.00 | - | - | - | - |
| 46 | Spathulenol | 1571 | C15H24O | 0.7 ± 0.03 | 0.2 ± 0.00 | 1.5 ± 0.01 | 0.1 ± 0.00 | 0.1 ± 0.02 | 0.5 ± 0.00 |
| 47 | Viridiflorol | 1588 | C15H26O | 1.4 ± 0.06 | 0.6 ± 0.03 | 2.0 ± 0.04 | 0.2 ± 0.00 | 0.5 ± 0.02 | 0.9 ± 0.00 |
| 48 | t-Cadinol | 1638 | C15H26O | 12.3 ± 0.02 | 9.1 ± 0.04 | 14.5 ± 0.05 | 5.1 ± 0.03 | 7.5 ± 0.04 | 10.6 ± 0.00 |
| 49 | Nootkatone | 1775 | C15H22O | 0.1 ± 0.00 | - | 0.1 ± 0.00 | - | 0.1 ± 0.00 | - |
| Total identified compounds % | 70.3 | 41.8 | 84.7 | 23.7 | 33.1 | 53.7 | |||
| Phenological Stages | Plant Parts | Shigella flexneri | Escherichia coli | Bacillus cereus | Staphylococcus aureus | ||||
|---|---|---|---|---|---|---|---|---|---|
| MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | ||
| Vegetative | Aerial parts | 37.5 | 75 | 75 | 150 | 18.75 | 37.5 | 18.75 | 37.5 |
| Root | 75 | 150 | 150 | 300 | 37.5 | 75 | 37.5 | 75 | |
| Flowering | Aerial parts | 18.75 | 37.5 | 37.5 | 75 | 9.4 | 18.75 | 9.4 | 18.75 |
| Root | 300 | 600 | 300 | 600 | 150 | 300 | 150 | 300 | |
| Seeding | Aerial parts | 75 | 150 | 150 | 300 | 37.5 | 75 | 18.75 | 37.5 |
| Root | 150 | 300 | 300 | 600 | 75 | 150 | 75 | 150 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharifi-Rad, M.; Pohl, P.; Epifano, F.; Zengin, G.; Jaradat, N.; Messaoudi, M. Teucrium polium (L.): Phytochemical Screening and Biological Activities at Different Phenological Stages. Molecules 2022, 27, 1561. https://doi.org/10.3390/molecules27051561
Sharifi-Rad M, Pohl P, Epifano F, Zengin G, Jaradat N, Messaoudi M. Teucrium polium (L.): Phytochemical Screening and Biological Activities at Different Phenological Stages. Molecules. 2022; 27(5):1561. https://doi.org/10.3390/molecules27051561
Chicago/Turabian StyleSharifi-Rad, Majid, Pawel Pohl, Francesco Epifano, Gokhan Zengin, Nidal Jaradat, and Mohammed Messaoudi. 2022. "Teucrium polium (L.): Phytochemical Screening and Biological Activities at Different Phenological Stages" Molecules 27, no. 5: 1561. https://doi.org/10.3390/molecules27051561
APA StyleSharifi-Rad, M., Pohl, P., Epifano, F., Zengin, G., Jaradat, N., & Messaoudi, M. (2022). Teucrium polium (L.): Phytochemical Screening and Biological Activities at Different Phenological Stages. Molecules, 27(5), 1561. https://doi.org/10.3390/molecules27051561











