Investigating the Neurotoxic Impacts of Arsenic and the Neuroprotective Effects of Dictyophora Polysaccharide Using SWATH-MS-Based Proteomics
Abstract
:1. Introduction
2. Results
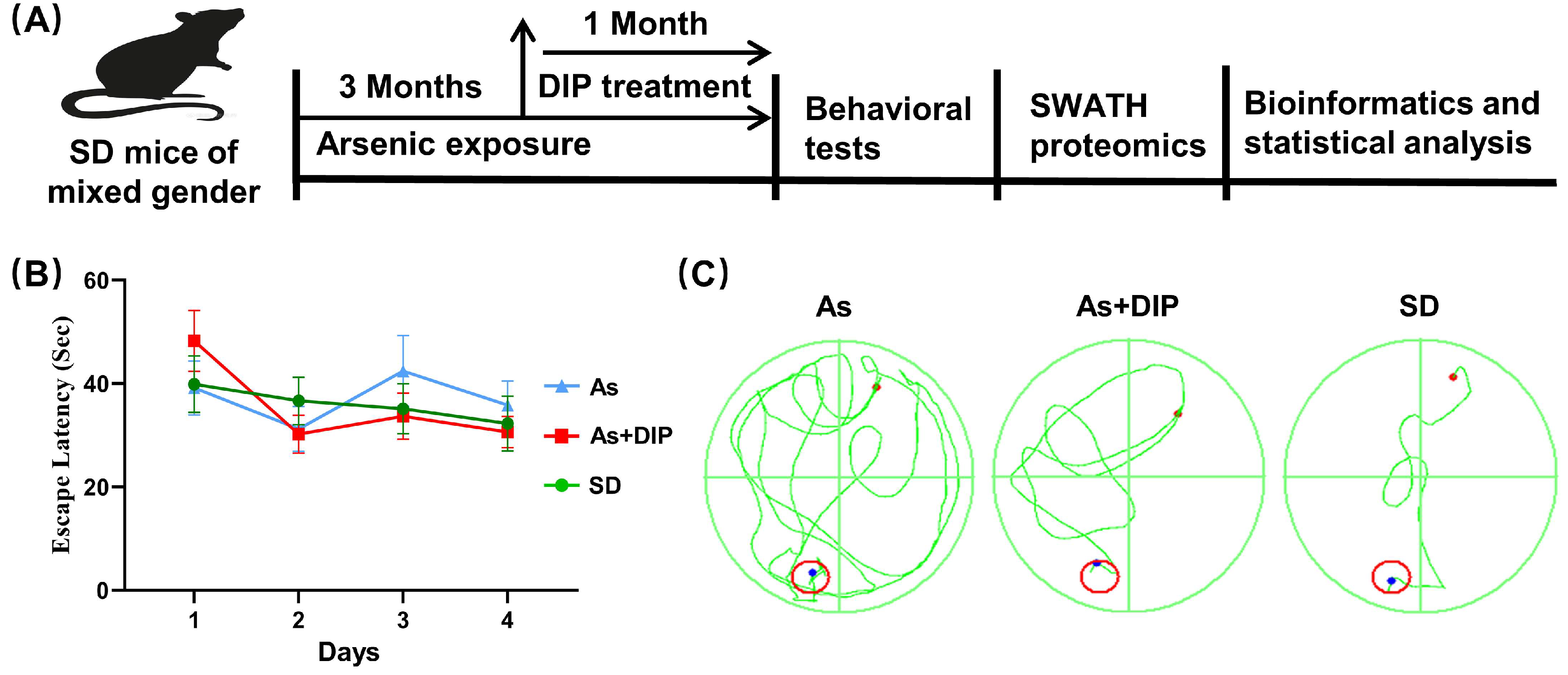
2.1. DIP Improved Spatial Learning and Memory of Arsenic Treated Rats
2.2. Overview of Protein Expression Characteristics of Different Groups
2.3. Differentially Expressed Proteins between NaAsO2-Treated Group and Control Group (As/Ctrl Group)
2.4. Differentially Expressed Proteins between DIP + As-Treated Group and As-Treated Group (DIP + As/As Group)
2.5. Differentially Expressed Proteins between DIP + As-Treated Group and Control Group (DIP + As/Ctrl Group)
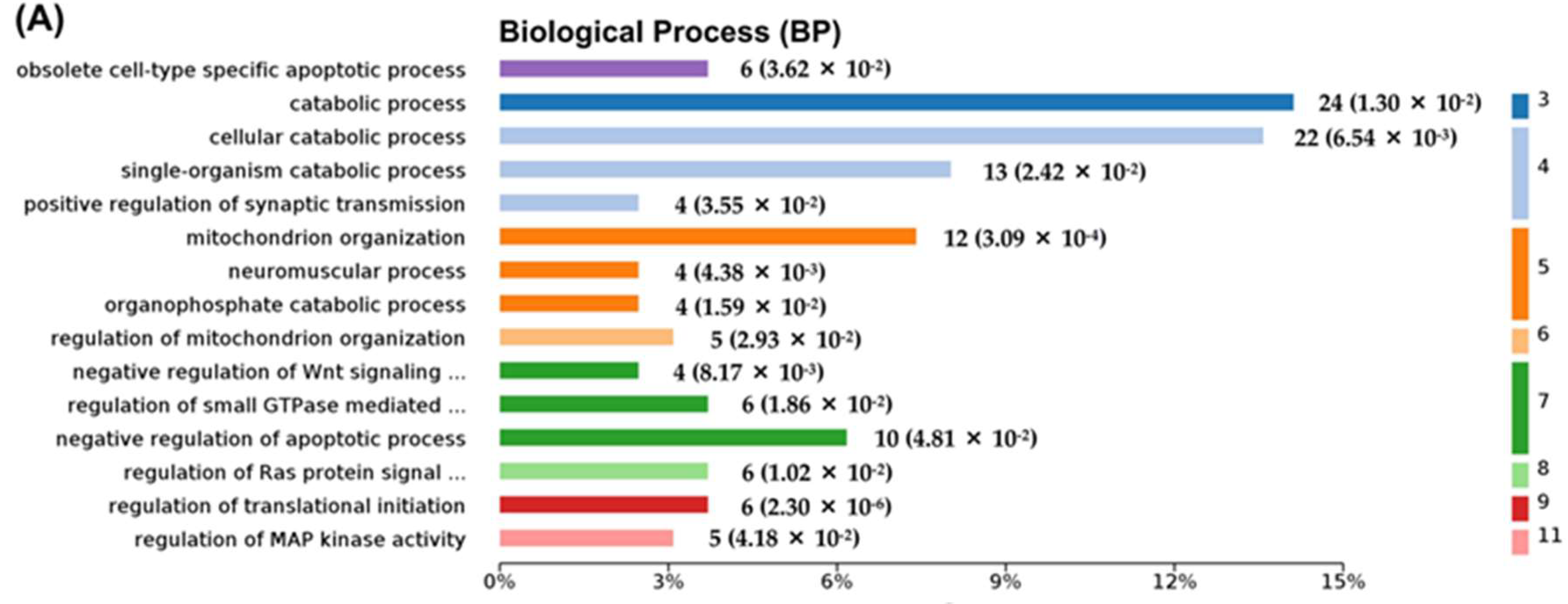
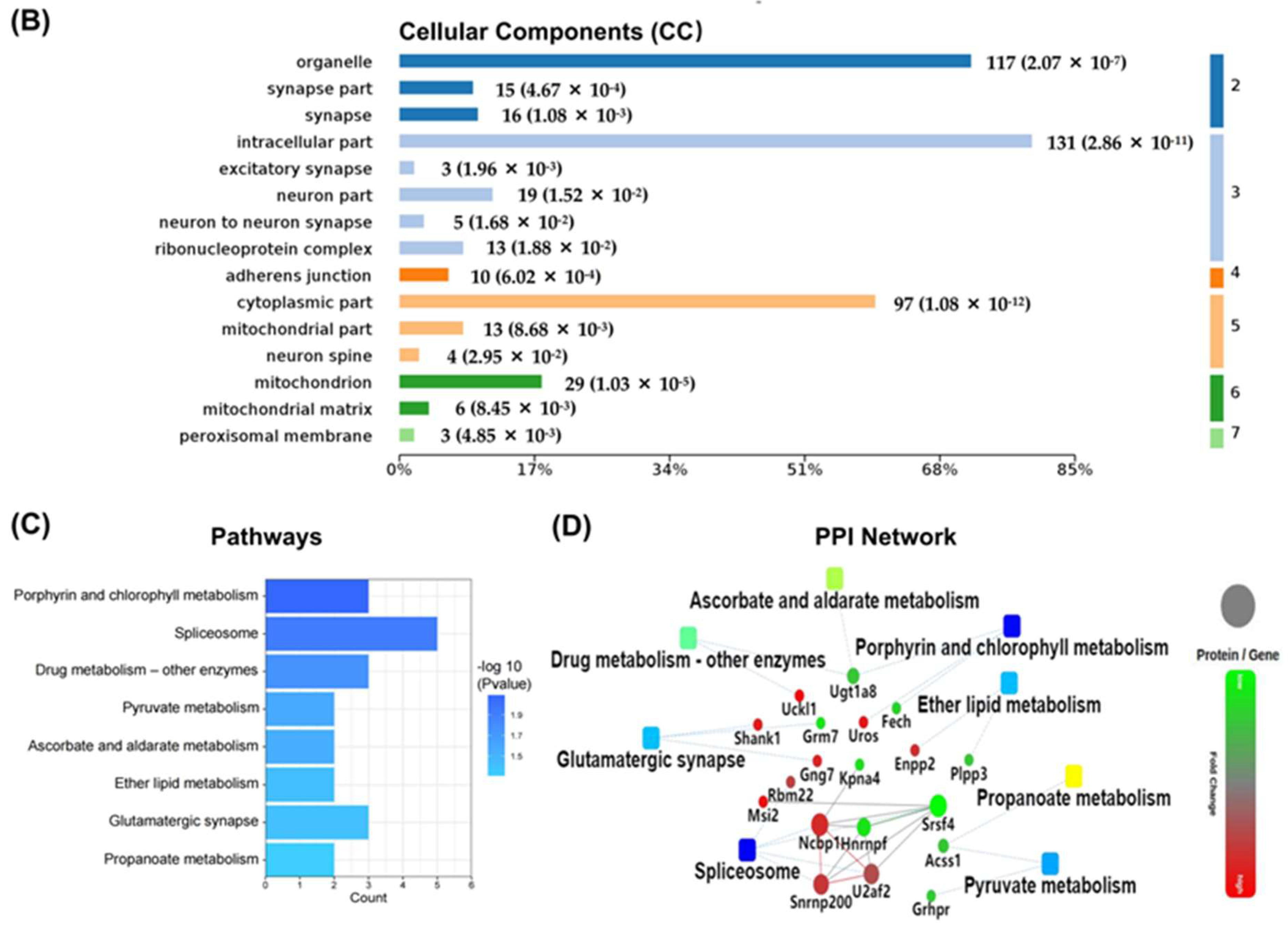
2.6. The Reversed Proteins between the As/Ctrl Group and DIP + As/As Group
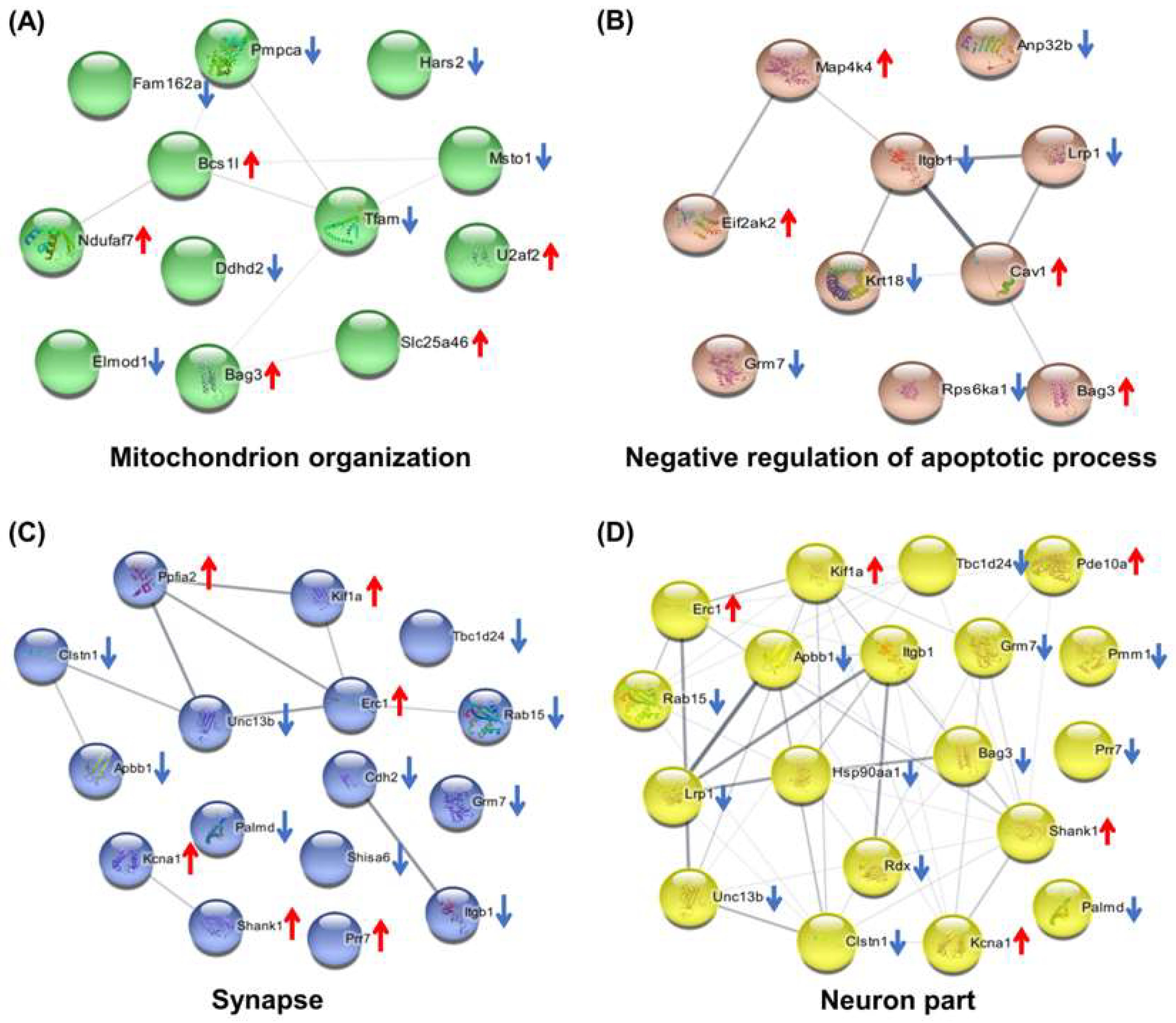
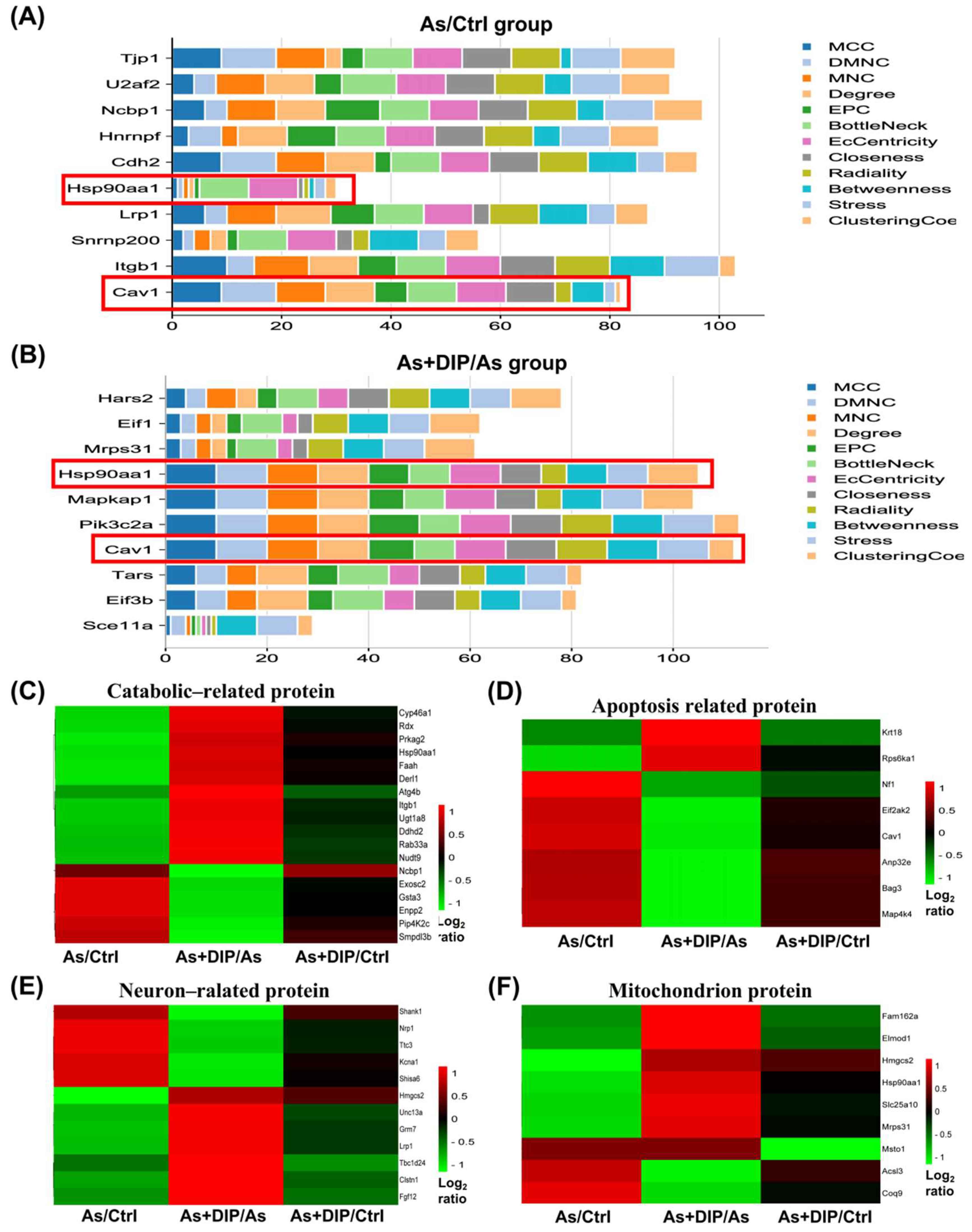
2.7. Hub Genes Analysis and Cluster Analysis of Differentially Expressed Proteins in Key Pathways
3. Discussion
4. Materials and Methods
4.1. Chemicals and Dictyophora Polysaccharide Preparation
4.2. Animals and Experimental Design
4.3. Morris Water Maze (MWM) Test
4.4. Sample Preparation for Proteomic Analysis
4.5. Generation of the Reference Spectral Library by Independent Data Analysis (IDA) Acquisition
4.6. Quantification of Proteins by SWATH Acquisition
4.7. Statistical Analysis
4.8. Bioinformatics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, T.; Hu, T.; Wu, C.; Yeh, Y.T.; Lu, J.; Zhang, Q.; Li, X.; Jian, W.; Luo, P. PINK1/Parkin-mediated mitophagy is involved in NaAsO2-induced apoptosis of human hepatic cells through activation of ERK signaling. Toxicol. Vitr. 2020, 66, 104857. [Google Scholar] [CrossRef] [PubMed]
- Kitchin, K.T. Recent advances in arsenic carcinogenesis: Modes of action, animal model systems, and methylated arsenic metabolites. Toxicol. Appl. Pharm. 2001, 172, 249–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, T.; Zhang, Y.F.; Murai, S.; Saito, H.; Nagahama, H.; Miyate, H.; Saito, Y.; Abe, E. The effect of arsenic trioxide on brain monoamine metabolism and locomotor activity of mice. Toxicol. Lett. 1990, 54, 345–353. [Google Scholar] [PubMed]
- Pandey, R.; Rai, V.; Mishra, J.; Mandrah, K.; Kumar, R.S.; Bandyopadhyay, S. From the Cover: Arsenic Induces Hippocampal Neuronal Apoptosis and Cognitive Impairments via an Up-Regulated BMP2/Smad-Dependent Reduced BDNF/TrkB Signaling in Rats. Toxicol. Sci. 2017, 159, 137–158. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Zhao, F.; Sun, D.; Zhong, Y.; Yu, X.; Li, G.; Lv, X.; Sun, G.; Jin, Y. Comparison of speciated arsenic levels in the liver and brain of mice between arsenate and arsenite exposure at the early life. Environ. Toxicol. 2014, 29, 797–803. [Google Scholar] [CrossRef]
- Kuzu, M.; Kandemir, F.M.; Yıldırım, S.; Çağlayan, C.; Küçükler, S. Attenuation of sodium arsenite-induced cardiotoxicity and neurotoxicity with the antioxidant, anti-inflammatory, and antiapoptotic effects of hesperidin. Environ. Sci. Pollut. Res. 2021, 28, 10818–10831. [Google Scholar] [CrossRef]
- Hua, Y.; Gao, Q.; Wen, L.; Yang, B.; Tang, J.; You, L.; Zhao, M. Structural characterisation of acid-and alkali-soluble polysaccharides in the fruiting body of Dictyophora indusiata and their immunomodulatory activities. Food Chem. 2021, 132, 739–743. [Google Scholar] [CrossRef]
- Han, S.; Ma, C.; Hu, M.; Wang, Y.; Ma, F.; Tao, N.; Qin, Z. A polysaccharide from Dictyophora indusiata inhibits the immunosuppressive function of cancer-associated fibroblasts. Cell Biochem. Funct. 2017, 35, 414–419. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Khantham, C.; Linsaenkart, P.; Chaitep, T.; Rachtanapun, P.; Jantanasakulwong, K.; Sringarm, K. Anti-inflammation of bioactive compounds from ethanolic extracts of edible bamboo mushroom (Dictyophora indusiata) as functional health promoting food ingredients. Int. J. Food. Sci. Technol. 2021, 57, 110–122. [Google Scholar] [CrossRef]
- Yu, W.X.; Lin, C.Q.; Zhao, Q.; Lin, X.J.; Dong, X.L. Neuroprotection against hydrogen peroxide-induced toxicity by Dictyophora echinovolvata polysaccharide via inhibiting the mitochondria-dependent apoptotic pathway. Biomed. Pharmacother. 2017, 88, 569–573. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, R.; Li, H.; Xiang, Y.; Xiao, L.; Hu, M.; Ma, F.; Ma, C.W.; Huang, Z. Antioxidant and neuroprotective effects of Dictyophora indusiata polysaccharide in Caenorhabditis elegans. J. Ethnopharmacol. 2016, 192, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Zheng, G.; Liu, M.; Shen, X.; Zhao, F.; Wang, J.; Zhang, J.; Huang, G.; Dai, P.; Chen, Y.; et al. Changes in the synaptic structure of hippocampal neurons and impairment of spatial memory in a rat model caused by chronic arsenite exposure. Neurotoxicology 2012, 33, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.H.; Qiu, Z.Q.; Shu, W.Q.; Zhang, Y.Y.; Zhang, L.; Chen, J.A. Effects of arsenic exposure from drinking water on spatial memory, ultra-structures and NMDAR gene expression of hippocampus in rats. Toxicol. Lett. 2009, 184, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.H.; Qiu, Z.Q.; Zhang, L.; Shu, W.Q. Arsenite exposure altered the expression of NMDA receptor and postsynaptic signaling proteins in rat hippocampus. Toxicol. Lett. 2012, 211, 39–44. [Google Scholar] [CrossRef]
- Betz, A.; Ashery, U.; Rickmann, M.; Augustin, I.; Neher, E.; Südhof, T.C.; Rettig, J.; Brose, N. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron 1998, 21, 123–136. [Google Scholar] [CrossRef] [Green Version]
- Song, J.M.; Kang, M.; Park, D.H.; Park, S.; Lee, S.; Suh, Y.H. Pathogenic GRM7 mutations associated with neurodevelopmental disorders impair axon outgrowth and presynaptic terminal development. J. Neurosci. 2021, 41, 2344–2359. [Google Scholar] [CrossRef]
- Fortier, A.M.; Asselin, E.; Cadrin, M. Keratin 8 and 18 loss in epithelial cancer cells increases collective cell migration and cisplatin sensitivity through claudin1 up-regulation. J. Biol. Chem. 2013, 288, 11555–11571. [Google Scholar] [CrossRef] [Green Version]
- Luanpitpong, S.; Wang, L.; Stueckle, T.A.; Tse, W.; Chen, Y.C.; Rojanasakul, Y. Caveolin-1 regulates lung cancer stem-like cell induction and p53 inactivation in carbon nanotube-driven tumorigenesis. Oncotarget 2014, 5, 3541. [Google Scholar] [CrossRef]
- Roy, S.; Narzary, B.; Ray, A.; Bordoloi, M. Arsenic-induced instrumental genes of apoptotic signal amplification in death-survival interplay. Cell Death Discov. 2016, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- TLu, T.H.; Tseng, T.J.; Su, C.C.; Tang, F.C.; Yen, C.C.; Liu, Y.Y.; Yang, C.Y.; Wu, C.C.; Chen, K.L.; Hung, D.Z.; et al. Arsenic induces reactive oxygen species-caused neuronal cell apoptosis through JNK/ERKmediated mitochondria-dependent and GRP 78/CHOPregulated pathways. Toxicol. Lett. 2014, 224, 130–140. [Google Scholar]
- Rezaei, M.; Keshtzar, E.; Khodayar, M.J.; Javadipour, M. SirT3 regulates diabetogenic effects caused by arsenic: An implication for mitochondrial complex II modification. Toxicol. Lett. 2019, 301, 24–33. [Google Scholar] [CrossRef]
- Chirumbolo, S.; Bjørklund, G. PERM hypothesis: The fundamental machinery able to elucidate the role of xenobiotics and hormesis in cell survival and homeostasis. Int. J. Mol. Sci. 2017, 18, 165. [Google Scholar] [CrossRef]
- Hu, T.; Shen, L.; Huang, Q.; Wu, C.; Zhang, H.; Zeng, Q.; Wang, G.; Wei, S.; Zhang, S.; Zhang, J.; et al. Protective Effect of Dictyophora Polysaccharides on Sodium arsenite-induced Hepatotoxicity: A Proteomics Study. Front. Pharmacol. 2021, 12, 749035. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, J.; Jin, X. iTRAQ-based quantitative proteomic analysis reveals important metabolic pathways for arsenic-induced liver fibrosis in rats. Sci. Rep. UK 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Moreno, A.; Isasa, M.; Bhanu, M.K.; Waterman, D.P.; Eapen, V.V.; Gygi, S.P.; Hanna, J. Proteomic analysis identifies ribosome reduction as an effective proteotoxic stress response. J. Biol. Chem. 2015, 290, 29695–29706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunier, E.; Antonio, S.; Regazzetti, A.; Auzeil, N.; Laprévote, O.; Shay, J.W.; Coumoul, X.; Barouki, R.; Benelli, C.; Huc, L.; et al. Resveratrol reverses the Warburg effect by targeting the pyruvate dehydrogenase complex in colon cancer cells. Sci. Rep. UK 2017, 7, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, C.; Hu, Z.; Fu, H.; Hu, M.; Xu, X.; Chen, J. Chemical analysis and antioxidant activity in vitro of a beta-D-glucan isolated from Dictyophora indusiata. Int. J. Biol. Macromol. 2012, 51, 70–75. [Google Scholar] [CrossRef]
- Panchal, K.; Tiwari, A.K. Mitochondrial dynamics, a key executioner in neurodegenerative diseases. Mitochondrion 2019, 47, 151–173. [Google Scholar] [CrossRef]
- Li, S.; Ren, Q. Effects of arsenic on wnt/β-catenin signaling pathway: A systematic review and meta-analysis. Chem. Res. Toxicol. 2020, 33, 1458–1467. [Google Scholar] [CrossRef]
- Stankiewicz, T.R.; Ramaswami, S.A.; Bouchard, R.J.; Aktories, K.; Linseman, D.A. Neuronal apoptosis induced by selective inhibition of Rac GTPase versus global suppression of Rho family GTPases is mediated by alterations in distinct mitogen-activated protein kinase signaling cascades. J. Biol. Chem. 2015, 290, 9363–9376. [Google Scholar] [CrossRef] [Green Version]
- Namgung, U.K.; Xia, Z. Arsenic induces apoptosis in rat cerebellar neurons via activation of JNK3 and p38 MAP kinases. Toxicol. Appl. Pharm. 2001, 174, 130–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.; Zhang, K.; Pan, D.; Pan, X.; Yang, H.; Xiao, J.; Shen, X.; Luo, P. Inhibition Effect of Dictyophora Polysaccharides on Human Hepatocellular Carcinoma Cell Line HCC-LM3. Med. Sci. Monitor. 2020, 26, e918870. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, Y.; Shao, H.; Sun, W.; Gu, M.; Wang, H.; Jiang, L.; Qu, L.; Sun, D.; Gao, Y. Sodium arsenite-induced learning and memory impairment is associated with endoplasmic reticulum stress-mediated apoptosis in rat hippocampus. Front. Mol. Neurosci. 2017, 10, 286. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Han, S.; Wu, C.; Liu, L.; Zhu, H.; Liu, A.; Lu, Q.; Huang, J.; Du, X.; Li, N.; et al. Bis (ethylmaltolato) oxidovanadium (iv) inhibited the pathogenesis of Alzheimer’ s disease in triple transgenic model mice. Metallomics 2020, 12, 474–490. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Shi, Q.; Zhao, Y.; Xie, Y.; Li, X.; Liu, Q.; Iqbal, J.; Zhang, H.; Liu, X.; Shen, L. Se-Methylselenocysteine (SMC) Improves Cognitive Deficits by Attenuating Synaptic and Metabolic Abnormalities in Alzheimer’s Mice Model: A Proteomic Study. ACS Chem. Neurosci. 2021, 12, 1112–1132. [Google Scholar] [CrossRef] [PubMed]


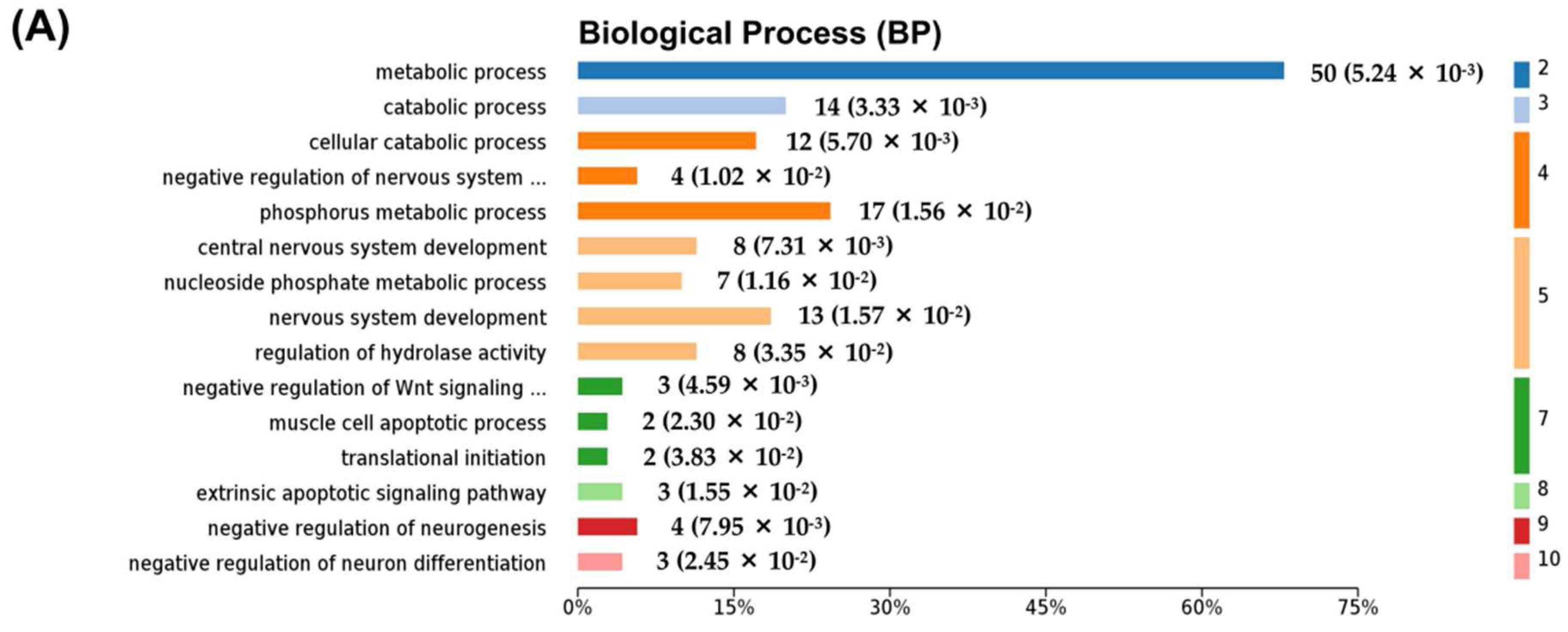
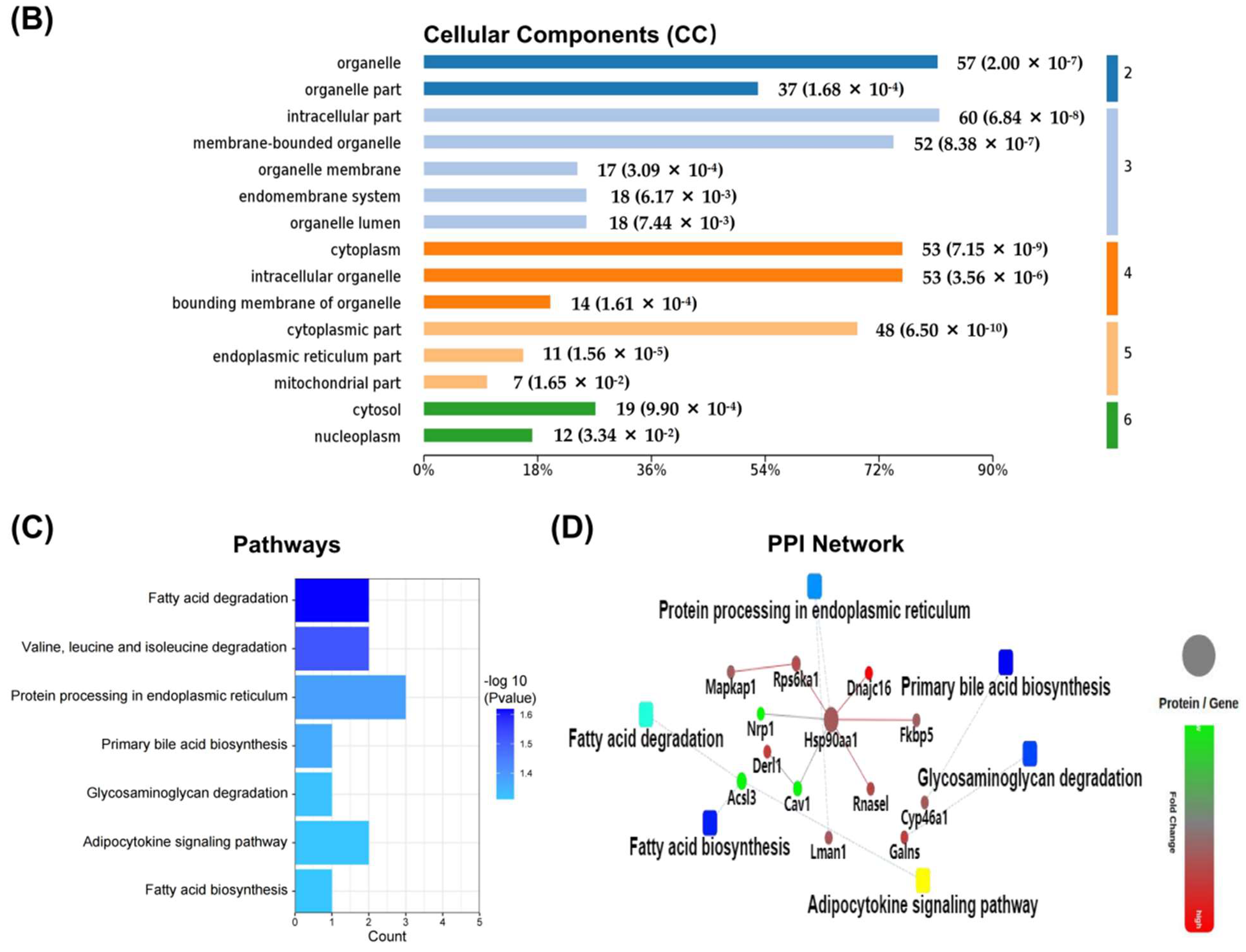
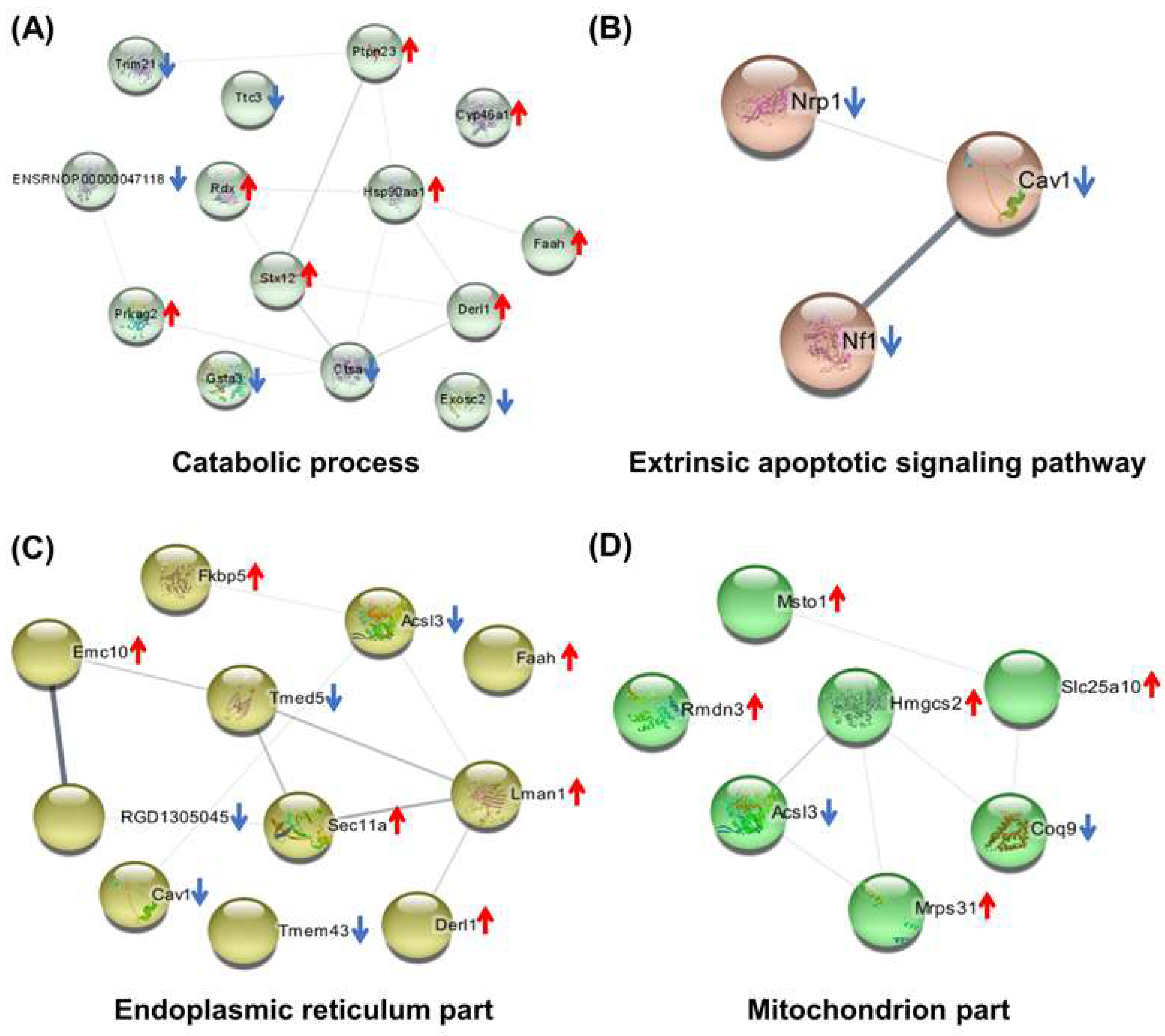
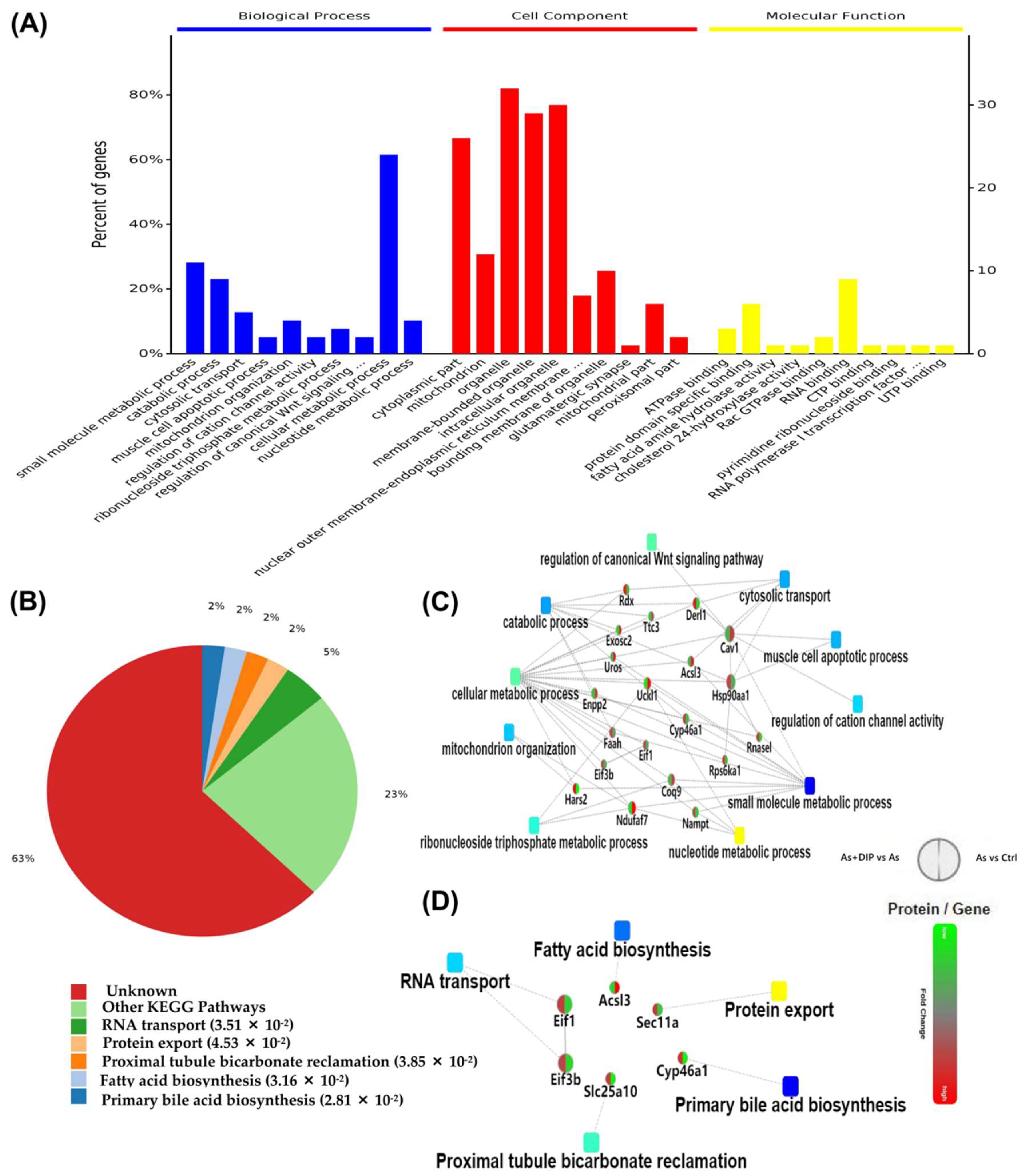

| No. | Protein Name | Gene Name | Uniprot Assention | As/Ctrl | As + DIP/As | As + DIP/Ctrl |
|---|---|---|---|---|---|---|
| 1 | Prefoldin 5 (Predicted), isoform CRA_a | Pfdn5 | B5DFN4 | 0.47a | 0.57a | 0.27a |
| 2 | Protein PRRC1 | Prrc1 | G3V834 | 0.21a | 2.87a | 0.61 |
| 3 | Histidine--tRNA ligase | Hars2 | F1M9C9 | 0.26a | 3.36a | 0.89 |
| 4 | Family with sequence similarity 219, member A | Fam219a | D4AAI7 | 0.33a | 1.82a | 0.60 |
| 5 | Ribonuclease L | Rnasel | G3V915 | 0.43a | 2.16a | 0.93 |
| 6 | Ribosomal protein S6 kinase | Rps6ka1 | F1LXV0 | 0.45a | 1.97a | 0.88 |
| 7 | Nicotinamide phosphoribosyltransferase | Nampt | A0A0G2K0I3 | 0.45a | 1.71a | 0.77 |
| 8 | Derlin | Derl1 | F7FNS3 | 0.46a | 2.55a | 1.17 |
| 9 | Protein misato homolog 1 | Msto1 | D3ZMW3 | 0.46a | 1.64a | 0.76 |
| 10 | 28S ribosomal protein S31, mitochondrial | Mrps31 | B0BN56 | 0.47a | 1.92a | 0.91 |
| 11 | Cyp46a1 protein | Cyp46a1 | F7EN52 | 0.50a | 1.71a | 0.85 |
| 12 | Radixin | Rdx | E9PT65 | 0.52a | 2.69a | 1.39 |
| 13 | Mitochondrial dicarboxylate carrier | Slc25a10 | O89035 | 0.53a | 1.62a | 0.86 |
| 14 | PiggyBac transposable element derived 5 (Predicted) | Pgbd5 | D3ZSZ4 | 0.53a | 1.67a | 0.89 |
| 15 | Sorbin and SH3 domain-containing protein 2 | Sorbs2 | F1LPM3 | 0.55a | 1.80a | 1.00 |
| 16 | BolA family member 2 | Bola2 | D4A9P7 | 0.60a | 1.56a | 0.93 |
| 17 | Protein Dr1 | Dr1 | Q5XI68 | 0.61a | 1.57a | 0.95 |
| 18 | Heat shock protein HSP 90-alpha | Hsp90aa1 | P82995 | 0.62a | 1.68a | 1.03 |
| 19 | Eukaryotic translation initiation factor 3 subunit B | Eif3b | Q4G061 | 0.62a | 1.54a | 0.96 |
| 20 | Eukaryotic translation initiation factor 1 | Eif1 | B0K008 | 0.64a | 1.59a | 1.02 |
| 21 | Fatty-acid amide hydrolase 1 | Faah | P97612 | 0.65a | 1.72a | 1.11 |
| 22 | Regulator of microtubule dynamics protein 3 | Rmdn3 | Q66H15 | 0.65a | 1.60a | 1.04 |
| 23 | Signal peptidase complex catalytic subunit SEC11 | Sec11a | Q6P9X2 | 0.66a | 1.61a | 1.07 |
| 24 | E3 ubiquitin-protein ligase TTC3 | Ttc3 | D3ZSP7 | 1.57a | 0.50a | 1.16 |
| 25 | Shisa family member 6 | Shisa6 | D4A4M0 | 1.60a | 0.66a | 1.06 |
| 26 | BCS1-like protein | Bcs1l | Q5XIM0 | 1.65a | 0.65a | 1.07 |
| 27 | LIM and calponin homology domains 1 | Limch1 | F1M392 | 1.69a | 0.52a | 0.88 |
| 28 | 4-trimethylaminobutyraldehyde dehydrogenase | Aldh9a1 | A0A0G2JSI1 | 1.71a | 0.62a | 1.07 |
| 29 | Ubiquinone biosynthesis protein COQ9, mitochondrial | Coq9 | Q68FT1 | 1.72a | 0.53a | 0.91 |
| 30 | MAP7 domain-containing 2 | Map7d2 | D4A4L4 | 1.72a | 0.54a | 0.92 |
| 31 | MTSS I-BAR domain-containing 2 | Mtss1l | D4A3S6 | 1.80a | 0.54a | 0.97 |
| 32 | Proline-rich transmembrane protein 3 | Prrt3 | D3ZWQ0 | 1.82a | 0.61a | 1.10 |
| 33 | PTPRF-interacting protein alpha 2 | Ppfia2 | F1M8A4 | 1.82a | 0.46a | 0.84 |
| 34 | Pleckstrin and Sec7 domain-containing 3 | Psd3 | D4A2Q3 | 1.83a | 0.66a | 1.21 |
| 35 | Caveolin | Cav1 | Q2IBC6 | 1.88a | 0.63a | 1.18 |
| 36 | ER membrane protein complex subunit 10 | Emc10 | Q6AYH6 | 1.89a | 0.66a | 1.24 |
| 37 | Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 | Enpp2 | Q64610 | 1.97a | 0.51a | 1.00 |
| 38 | Glutathione S-transferase alpha-3 | Gsta3 | P04904 | 2.07a | 0.49a | 1.02 |
| 39 | Ribosomal RNA-processing protein 4 | Exosc2 | D3ZBP3 | 2.11a | 0.44a | 0.94 |
| 40 | Potassium voltage-gated channel subfamily A member 1 | Kcna1 | P10499 | 2.30a | 0.53a | 1.21 |
| 41 | Hydroxymethylbilane hydrolyase [cyclizing] | Uros | Q5XIF2 | 2.41a | 0.62a | 1.51 |
| 42 | Long-chain-fatty-acid--CoA ligase 3 | Acsl3 | Q63151 | 2.50a | 0.63a | 1.57 |
| 43 | Uridine-cytidine kinase | Uckl1 | D3ZYQ8 | 2.95a | 0.33a | 0.96 |
| 44 | Protein arginine methyltransferase NDUFAF7, mitochondrial | Ndufaf7 | Q5XI79 | 3.71a | 0.37a | 1.38 |
| 45 | Uncharacterized protein | Dock2 | F7F7H4 | 0.15a | 2.56 | 0.38a |
| 46 | RAB33A, member RAS oncogene family | Rab33a | D3ZCU8 | 0.26a | 1.72 | 0.45a |
| 47 | Ubiquitin carboxyl-terminal hydrolase | Fam63b | D3ZWA1 | 0.27a | 1.15 | 0.31a |
| 48 | Mitogen-activated protein kinase kinase kinase 2 | Map4k2 | D3ZXB1 | 0.32a | 0.92 | 0.30a |
| 49 | Metallo-beta-lactamase domain-containing 2 | Mblac2 | D4A249 | 0.33a | 1.33 | 0.44a |
| 50 | Hypothetical protein MGC:15854 | RGD1302996 | G3V628 | 0.34a | 1.36 | 0.46a |
| 51 | Alpha-MPP | Pmpca | Q68FX8 | 0.37a | 1.34 | 0.50a |
| 52 | Microtubule associated protein 11 | RGD1305455 | A0A0G2KAX2 | 0.40a | 0.85 | 0.34a |
| 53 | Keratin, type I cytoskeletal 18 | Krt18 | Q5BJY9 | 0.43a | 1.05 | 0.45a |
| 54 | Calmin | Clmn | D4A626 | 0.44a | 1.02 | 0.45a |
| 55 | Tight junction protein ZO-1 | Tjp1 | A0A0G2K2P5 | 0.45a | 1.10 | 0.49a |
| 58 | Solute carrier family 7, member 14 | Slc7a14 | A0A0G2K1G8 | 0.47a | 1.28 | 0.61a |
| 59 | CD151 antigen | Cd151 | Q9QZA6 | 0.47a | 0.87 | 0.41a |
| 60 | Cysteine protease | Atg4b | A0A0G2QC33 | 0.48a | 1.14 | 0.54a |
| 61 | HECT-type E3 ubiquitin transferase | Ube3c | D3ZHB7 | 0.48a | 0.87 | 0.42a |
| 62 | Proline-rich protein 7 | Prr7 | P0C6T3 | 0.48a | 0.70 | 0.34a |
| 63 | Dystrophin | Dmd | Q7TPH4 | 0.50a | 1.04 | 0.52a |
| 64 | Inosine-5’-monophosphate dehydrogenase 2 | Impdh2 | E9PU28 | 0.52a | 1.20 | 0.62a |
| 65 | Amyloid-beta A4 precursor protein-binding family B member 1 | Apbb1 | P46933 | 0.56a | 1.09 | 0.61a |
| 66 | TBC1 domain family member 24 | Tbc1d24 | D4A3Z3 | 0.56a | 0.97 | 0.54a |
| 67 | Transmembrane protein 151A | Tmem151a | M0RAG0 | 0.56a | 1.08 | 0.61a |
| 68 | Kelch-like protein 22 | Klhl22 | A0A0G2KA06 | 0.57a | 0.75 | 0.43a |
| 69 | Protein FAM162A | Fam162a | Q4QQV3 | 0.57a | 1.06 | 0.61a |
| 70 | SCY1-like pseudokinase 2 | Scyl2 | D4A1Y0 | 0.58a | 1.05 | 0.61a |
| 71 | Ras GTPase-activating protein 2 | Rasa2 | Q63713 | 0.61a | 0.69 | 0.42a |
| 72 | Chromatin-modifying protein 4B-like 1 | Chmp4bl1 | D4A9Z8 | 0.62a | 0.81 | 0.50a |
| 73 | Prostamide/prostaglandin F synthase | Fam213b | D3ZVR7 | 0.64a | 0.94 | 0.61a |
| 74 | Isochorismatase domain-containing protein 1 | LOC103694869 | F2Z3T7 | 0.65a | 1.02 | 0.66a |
| 75 | RCG43947 | Txndc5 | D3ZZC1 | 0.66a | 0.79 | 0.52a |
| 76 | Calpain-5 | Capn5 | G3V7U6 | 0.66a | 0.99 | 0.65a |
| 77 | Phosphatidic acid phosphatase type 2B | Plpp3 | Q6IMX4 | 0.66a | 0.90 | 0.60a |
| 78 | Similar to RIKEN cDNA 1110063G11 (Predicted) | Tmcc2 | D3ZE26 | 0.66a | 0.87 | 0.57a |
| 79 | Capping protein regulator and myosin 1 linker 2 | Carmil2 | D3ZC15 | 1.68a | 0.93 | 1.57a |
| 80 | Proton myo-inositol cotransporter | Slc2a13 | Q921A2 | 1.69a | 1.24 | 2.10a |
| 81 | Retinoid-inducible serine carboxypeptidase | Scpep1 | Q920A6 | 1.72a | 0.90 | 1.56a |
| 82 | ELKS/Rab6-interacting/CAST family member 1 | Erc1 | A0A0G2JYT1 | 1.74a | 0.96 | 1.68a |
| 83 | ASPSCR1 tether for SLC2A4, UBX domain-containing | Aspscr1 | F1LR71 | 1.78a | 1.13 | 2.00a |
| 84 | Hemoglobin subunit beta-1 | Hbb | P02091 | 1.89a | 0.81 | 1.53a |
| 85 | Hemoglobin subunit beta-2 | ENSRNOG00000031230 | P11517 | 1.92a | 0.84 | 1.61a |
| 86 | Nuclear cap-binding protein subunit 1 | Ncbp1 | Q56A27 | 2.06a | 1.06 | 2.17a |
| 87 | Acid sphingomyelinase-like phosphodiesterase | Smpdl3b | Q4V7D9 | 2.23a | 0.73 | 1.62a |
| 88 | SH3 and multiple ankyrin repeat domains protein 1 | Shank1 | Q9WV48 | 2.33a | 0.74 | 1.73a |
| 89 | Proteasome inhibitor PI31 subunit | Psmf1 | F1M7S2 | 2.45a | 0.89 | 2.17a |
| 90 | YTH N(6)-methyladenosine RNA-binding protein 1 | Ythdf1 | Q4V8J6 | 2.50a | 1.14 | 2.87a |
| 91 | Spermine synthase | Sms | Q3MIE9 | 3.36a | 0.92 | 3.07a |
| 92 | Serine/threonine-protein kinase PRP4 homolog | Prpf4b | Q5RKH1 | 4.79a | 0.64 | 3.07a |
| 93 | Fam81a | Fam81a | D4A7T8 | 0.83 | 0.60a | 0.50a |
| 94 | 40S ribosomal protein S15 | Rps15 | P62845 | 1.03 | 0.60a | 0.58a |
| 95 | Ras suppressor protein 1 | Rsu1 | D4A8F2 | 0.84 | 1.82a | 1.52a |
| 96 | Target of rapamycin complex 2 subunit MAPKAP1 | Mapkap1 | Q6AYF1 | 1.08 | 1.56a | 1.68a |
| 97 | CaM kinase-like vesicle-associated protein | Camkv | A0A0G2K1R5 | 0.93 | 2.81a | 2.66a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Hu, T.; Wang, Y.; Zhang, X.; Zhang, H.; Lin, J.; Tang, X.; Liu, X.; Chen, M.; Khan, N.U.; et al. Investigating the Neurotoxic Impacts of Arsenic and the Neuroprotective Effects of Dictyophora Polysaccharide Using SWATH-MS-Based Proteomics. Molecules 2022, 27, 1495. https://doi.org/10.3390/molecules27051495
Zhang J, Hu T, Wang Y, Zhang X, Zhang H, Lin J, Tang X, Liu X, Chen M, Khan NU, et al. Investigating the Neurotoxic Impacts of Arsenic and the Neuroprotective Effects of Dictyophora Polysaccharide Using SWATH-MS-Based Proteomics. Molecules. 2022; 27(5):1495. https://doi.org/10.3390/molecules27051495
Chicago/Turabian StyleZhang, Jun, Ting Hu, Yi Wang, Xinglai Zhang, Huajie Zhang, Jing Lin, Xiaoxiao Tang, Xukun Liu, Margy Chen, Naseer Ullah Khan, and et al. 2022. "Investigating the Neurotoxic Impacts of Arsenic and the Neuroprotective Effects of Dictyophora Polysaccharide Using SWATH-MS-Based Proteomics" Molecules 27, no. 5: 1495. https://doi.org/10.3390/molecules27051495
APA StyleZhang, J., Hu, T., Wang, Y., Zhang, X., Zhang, H., Lin, J., Tang, X., Liu, X., Chen, M., Khan, N. U., Shen, L., & Luo, P. (2022). Investigating the Neurotoxic Impacts of Arsenic and the Neuroprotective Effects of Dictyophora Polysaccharide Using SWATH-MS-Based Proteomics. Molecules, 27(5), 1495. https://doi.org/10.3390/molecules27051495






