Drug Repurposing and De Novo Drug Discovery of Protein Kinase Inhibitors as New Drugs against Schistosomiasis
Abstract
1. Introduction
2. Discovery of Natural Compounds as Source for Anthelmintic Drugs
3. Protein Kinases as Central Targets for Drug Repurposing
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization Schistosomiasis. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 20 December 2021).
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nat. Rev. Dis. Prim. 2018, 4, 13. [Google Scholar] [CrossRef]
- Vale, N.; Gouveia, M.J.; Rinaldi, G.; Brindley, P.J.; Gärtner, F.; Da Costa, J.M.C. Praziquantel for schistosomiasis: Single-drug metabolism revisited, mode of action, and resistance. Antimicrob. Agents Chemother. 2017, 61, e02582-16. [Google Scholar] [CrossRef] [PubMed]
- Pica-Mattoccia, L.; Cioli, D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int. J. Parasitol. 2004, 34, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Sabah, A.A.; Fletcher, C.; Webbe, G.; Doenhoff, M.J. Schistosoma mansoni: Chemotherapy of infections of different ages. Exp. Parasitol. 1986, 61, 294–303. [Google Scholar] [CrossRef]
- Bergquist, R.; Utzinger, J.; Keiser, J. Controlling schistosomiasis with praziquantel: How much longer without a viable alternative? Infect. Dis. Poverty 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Molehin, A.J. Schistosomiasis vaccine development: Update on human clinical trials. J. Biomed. Sci. 2020, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Blume-Jensen, P.; Hunter, T. Oncogenic kinase signalling. Nature 2001, 411, 355–365. [Google Scholar] [CrossRef]
- Roskoski, R. Properties of FDA-approved small molecule protein kinase inhibitors: A 2020 update. Pharmacol. Res. 2020, 152, 104609. [Google Scholar] [CrossRef]
- Bournez, C.; Carles, F.; Peyrat, G.; Aci-Sèche, S.; Bourg, S.; Meyer, C.; Bonnet, P. Comparative assessment of protein kinase inhibitors in public databases and in PKIDB. Molecules 2020, 25, 3226. [Google Scholar] [CrossRef]
- Carles, F.; Bourg, S.; Meyer, C.; Bonnet, P. PKIDB: A curated, annotated and updated database of protein kinase inhibitors in clinical trials. Molecules 2018, 23, 908. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.D.; Fox, A.W.; Stonier, P.D. Principles and Practice of Pharmaceutical Medicine, 3rd ed.; Academic Press: Washington, DC, USA, 2010. [Google Scholar]
- Trouiller, P.; Olliaro, P.; Torreele, E.; Orbinski, J.; Laing, R.; Ford, N. Drug development for neglected diseases: A deficient market and a public-health policy failure. Lancet 2002, 359, 2188–2194. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Geary, T.G.; Sakanari, J.A.; Caffrey, C.R. Anthelmintic drug discovery: Into the future. J. Parasitol. 2015, 101, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Neves, B.J.; Andrade, C.H.; Cravo, P.V.L. Natural products as leads in schistosome drug discovery. Molecules 2015, 20, 1872–1903. [Google Scholar] [CrossRef]
- Ndjonka, D.; Rapado, L.N.; Silber, A.M.; Liebau, E.; Wrenger, C. Natural products as a source for treating neglected parasitic diseases. Int. J. Mol. Sci. 2013, 14, 3395–3439. [Google Scholar] [CrossRef]
- Abou El Dahab, M.M.; Shahat, S.M.; Mahmoud, S.S.M.; Mahana, N.A. In vitro effect of curcumin on Schistosoma species viability, tegument ultrastructure and egg hatchability. Exp. Parasitol. 2019, 199, 1–8. [Google Scholar] [CrossRef]
- Magalhães, L.G.; Machado, C.B.; Morais, E.R.; Bueno De Carvalho Moreira, É.; Soares, C.S.; Da Silva, S.H.; Da Silva Filho, A.A.; Rodrigues, V. In vitro schistosomicidal activity of curcumin against Schistosoma mansoni adult worms. Parasitol. Res. 2009, 104, 1197–1201. [Google Scholar] [CrossRef]
- Golonko, A.; Lewandowska, H.; Świsłocka, R.; Jasińska, U.T.; Priebe, W.; Lewandowski, W. Curcumin as tyrosine kinase inhibitor in cancer treatment. Eur. J. Med. Chem. 2019, 181, 111512. [Google Scholar] [CrossRef]
- Banerjee, S.; Ji, C.; Mayfield, J.E.; Goel, A.; Xiao, J.; Dixon, J.E.; Guo, X. Ancient drug curcumin impedes 26S proteasome activity by direct inhibition of dual-specificity tyrosine-regulated kinase 2. Proc. Natl. Acad. Sci. USA 2018, 115, 8155–8160. [Google Scholar] [CrossRef]
- Cunha, N.L.; Uchôa, C.J.D.M.; Cintra, L.S.; De Souza, H.C.; Peixoto, J.A.; Silva, C.P.; Magalhães, L.G.; Gimenez, V.M.M.; Groppo, M.; Rodrigues, V.; et al. In vitro schistosomicidal activity of some brazilian cerrado species and their isolated compounds. Evid. -Based Complement. Altern. Med. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Baier, A.; Szyszka, R. Compounds from natural sources as protein kinase inhibitors. Biomolecules 2020, 10, 1546. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Ishida, J.; Nakagawa, S.; Ogawara, H.; Watanabe, S.; Itoh, N.; Shibuya, M.; Fukami, Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987, 262, 5592–5595. [Google Scholar] [CrossRef]
- Tandon, V.; Das, B. Genistein: Is the multifarious botanical a natural anthelmintic too? J. Parasit. Dis. 2018, 42, 151–161. [Google Scholar] [CrossRef]
- Roy, B.; Tandon, V. Effect of root-tuber extract of Flemingia vestita, a leguminous plant, on Artyfechinostomum sufrartyfex and Fasciolopsis buski: A scanning electron microscopy study. Parasitol. Res. 1996, 82, 248–252. [Google Scholar] [CrossRef][Green Version]
- Kamdem, S.D.; Moyou-Somo, R.; Brombacher, F.; Nono, J.K. Host regulators of liver fibrosis during human schistosomiasis. Front. Immunol. 2018, 9, 2781. [Google Scholar] [CrossRef]
- Sobhy, M.M.K.; Mahmoud, S.S.; El-Sayed, S.H.; Rizk, E.M.A.; Raafat, A.; Negm, M.S.I. Impact of treatment with a Protein Tyrosine Kinase Inhibitor (Genistein) on acute and chronic experimental Schistosoma mansoni infection. Exp. Parasitol. 2018, 185, 115–123. [Google Scholar] [CrossRef]
- Chae, H.S.; Xu, R.; Won, J.Y.; Chin, Y.W.; Yim, H. Molecular targets of genistein and its related flavonoids to exert anticancer effects. Int. J. Mol. Sci. 2019, 20, 2420. [Google Scholar] [CrossRef]
- Li, T.; Wang, N.; Zhang, T.; Zhang, B.; Sajeevan, T.P.; Joseph, V.; Armstrong, L.; He, S.; Yan, X.; Benjamin Naman, C. A systematic review of recently reported marine derived natural product kinase inhibitors. Mar. Drugs 2019, 17, 493. [Google Scholar] [CrossRef]
- Mbengue, A.; Bhattacharjee, S.; Pandharkar, T.; Liu, H.; Estiu, G.; Stahelin, R.V.; Rizk, S.S.; Njimoh, D.L.; Ryan, Y.; Chotivanich, K.; et al. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature 2015, 520, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Jisaka, M.; Kawanaka, M.; Michael, M.; Takegawa, K.; Ohigash, H.; Koshimizu, K.; Sugiyama, H.; Huffman, H. Antischistosomal Activities of Sesquiterpene Lactones and Steroid Glucosides from Vernonia amygdalina, Possibly Used by Wild Chimpanzees against Parasite-related Diseases. Biosci. Biotechnol. Biochem. 1992, 56, 845–846. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, N.; Pandurangan, A.K.; Mustafa, M.R.; Anandasadagopan, S.K.; Alagumuthu, T. Vernodalin induces apoptosis through the activation of ROS/JNK pathway in human colon cancer cells. J. Biochem. Mol. Toxicol. 2020, 34. [Google Scholar] [CrossRef]
- Mengarda, A.C.; Mendonça, P.S.; Morais, C.S.; Cogo, R.M.; Mazloum, S.F.; Salvadori, M.C.; Teixeira, F.S.; Morais, T.R.; Antar, G.M.; Lago, J.H.G.; et al. Antiparasitic activity of piplartine (piperlongumine) in a mouse model of schistosomiasis. Acta Trop. 2020, 205, 105350. [Google Scholar] [CrossRef]
- De Moraes, J.; Nascimento, C.; Lopes, P.O.M.V.; Nakano, E.; Yamaguchi, L.F.; Kato, M.J.; Kawano, T. Schistosoma mansoni: In vitro schistosomicidal activity of piplartine. Exp. Parasitol. 2011, 127, 357–364. [Google Scholar] [CrossRef]
- De Moraes, J.; Nascimento, C.; Yamaguchi, L.F.; Kato, M.J.; Nakano, E. Schistosoma mansoni: In vitro schistosomicidal activity and tegumental alterations induced by piplartine on schistosomula. Exp. Parasitol. 2012, 132, 222–227. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Pessoa, C.; De Moraes, M.O.; Saker-Neto, N.; Silveira, E.R.; Costa-Lotufo, L.V. Overview of the therapeutic potential of piplartine (piperlongumine). Eur. J. Pharm. Sci. 2013, 48, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Vasilevich, N.I.; Kombarov, R.V.; Genis, D.V.; Kirpichenok, M.A. Lessons from natural products chemistry can offer novel approaches for synthetic chemistry in drug discovery miniperspective. J. Med. Chem. 2012, 55, 7003–7009. [Google Scholar] [CrossRef] [PubMed]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Peter Ventura, A.M.; Haeberlein, S.; Lange-Grünweller, K.; Grünweller, A.; Hartmann, R.K.; Grevelding, C.G.; Schlitzer, M. Development of Biarylalkyl Carboxylic Acid Amides with Improved Anti-schistosomal Activity. ChemMedChem 2019, 14, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Peter Ventura, A.M.; Haeberlein, S.; Konopka, L.; Obermann, W.; Grünweller, A.; Grevelding, C.G.; Schlitzer, M. Synthesis and antischistosomal activity of linker- and thiophene-modified biaryl alkyl carboxylic acid derivatives. Arch. Pharm. 2021, 354, e2100259. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.H.; Keiser, J.; Chollet, J.; Utzinger, J.; Dong, Y.; Endriss, Y.; Vennerstrom, J.L.; Tanner, M. In vitro and in vivo activities of synthetic trioxolanes against major human schistosome species. Antimicrob. Agents Chemother. 2007, 51, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Cowan, N.; Yaremenko, I.A.; Krylov, I.B.; Terent’ev, A.O.; Keiser, J. Elucidation of the in vitro and in vivo activities of bridged 1,2,4-trioxolanes, bridged 1,2,4,5-tetraoxanes, tricyclic monoperoxides, silyl peroxides, and hydroxylamine derivatives against Schistosoma mansoni. Bioorg. Med. Chem. 2015, 23, 5175–5181. [Google Scholar] [CrossRef]
- Mossallam, S.F.; Amer, E.I.; El-Faham, M.H. Efficacy of SynriamTM, a new antimalarial combination of OZ277 and piperaquine, against different developmental stages of Schistosoma mansoni. Acta Trop. 2015, 143, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Ingram, K.; Vargas, M.; Chollet, J.; Wang, X.; Dong, Y.; Vennerstrom, J.L. In vivo activity of aryl ozonides against Schistosoma species. Antimicrob. Agents Chemother. 2012, 56, 1090–1092. [Google Scholar] [CrossRef]
- Khan, M.O.F.; Keiser, J.; Amoyaw, P.N.A.; Hossain, M.F.; Vargas, M.; Le, J.G.; Simpson, N.C.; Roewe, K.D.; Freeman, T.N.C.; Hasley, T.R.; et al. Discovery of antischistosomal drug leads based on tetraazamacrocyclic derivatives and their metal complexes. Antimicrob. Agents Chemother. 2016, 60, 5331–5336. [Google Scholar] [CrossRef]
- Meleti, V.R.; Esperandim, V.R.; Flauzino, L.G.B.; Prizantelli, A.H.; Paula, L.A.d.L.; Magalhães, L.G.; Cunha, W.R.; Laurentiz, R.d.S.; Pissurno, A.P.d.R.; Nanayakkara, N.P.D.; et al. (±)-Licarin A and its semi-synthetic derivatives: In vitro and in silico evaluation of trypanocidal and schistosomicidal activities. Acta Trop. 2020, 202, 105248. [Google Scholar] [CrossRef]
- Krieg, R.; Jortzik, E.; Goetz, A.A.; Blandin, S.; Wittlin, S.; Elhabiri, M.; Rahbari, M.; Nuryyeva, S.; Voigt, K.; Dahse, H.M.; et al. Arylmethylamino steroids as antiparasitic agents. Nat. Commun. 2017, 8, 14478. [Google Scholar] [CrossRef]
- Padalino, G.; El-Sakkary, N.; Liu, L.J.; Liu, C.; Harte, D.S.G.; Barnes, R.E.; Sayers, E.; Forde-Thomas, J.; Whiteland, H.; Bassetto, M.; et al. Anti-schistosomal activities of quinoxaline-containing compounds: From hit identification to lead optimisation. Eur. J. Med. Chem. 2021, 226, 113823. [Google Scholar] [CrossRef]
- Yao, H.; Liu, F.; Chen, J.; Li, Y.; Cui, J.; Qiao, C. Antischistosomal activity of N,N′-arylurea analogs against Schistosoma japonicum. Bioorg. Med. Chem. Lett. 2016, 26, 1386–1390. [Google Scholar] [CrossRef]
- Monti, L.; Cornec, A.S.; Oukoloff, K.; Kovalevich, J.; Prijs, K.; Alle, T.; Brunden, K.R.; Smith, A.B.; El-Sakkary, N.; Liu, L.J.; et al. Congeners Derived from Microtubule-Active Phenylpyrimidines Produce a Potent and Long-Lasting Paralysis of Schistosoma mansoni in vitro. ACS Infect. Dis. 2021, 7, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.D.S.; Rezende, C.O.; Fernandes, T.S.; Da Silveira, L.S.; Rezende, C.A.M.; De Almeida, M.V.; De Paula, R.G.; Rodrigues, V.; Da Silva Filho, A.A.; Couri, M.R.C. Anthelmintic effects of alkylated diamines and amino alcohols against Schistosoma mansoni. Biomed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Silva Torres, D.; Alves de Oliveira, B.; Souza d Silveira, L.; Paulo da Silva, M.; Rodrigues Durães Pereira, V.; Moraes, J.; Rúbia Costa Couri, M.; Fortini Grenfell e Queiroz, R.; Martins Parreiras, P.; Roberto Silva, M.; et al. Synthetic Aurones: New Features for Schistosoma mansoni Therapy. Chem. Biodivers. 2021, 18, e2100439. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, M.; Kumagai, T.; Shimogawara, R.; Blay, E.A.; Hino, A.; Ichimura, K.; Sato, A.; Kim, H.S.; Ohta, N. Novel synthetic compounds with endoperoxide structure damage juvenile stage of Schistosoma mansoni by targeting lysosome-like organelles. Parasitol. Int. 2017, 66, 917–924. [Google Scholar] [CrossRef]
- Laufkötter, O.; Laufer, S.; Bajorath, J. Kinase inhibitor data set for systematic analysis of representative kinases across the human kinome. Data Brief 2020, 32, 106189. [Google Scholar] [CrossRef]
- Veale, C.G.L. Unpacking the Pathogen Box—An Open Source Tool for Fighting Neglected Tropical Disease. ChemMedChem 2019, 14, 386–453. [Google Scholar] [CrossRef]
- Maccesi, M.; Aguiar, P.H.N.; Pasche, V.; Padilla, M.; Suzuki, B.M.; Montefusco, S.; Abagyan, R.; Keiser, J.; Mourão, M.M.; Caffrey, C.R. Multi-center screening of the Pathogen Box collection for schistosomiasis drug discovery. Parasites Vectors 2019, 12, 1–10. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Cowan, N.; Keiser, J. Repurposing of anticancer drugs: In vitro and in vivo activities against Schistosoma mansoni. Parasites Vectors 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Gouveia, M.J.; Brindley, P.J.; Gärtner, F.; Correia Da Costa, J.M.; Vale, N. Drug repurposing for schistosomiasis: Combinations of drugs or biomolecules. Pharmaceuticals 2018, 11, 15. [Google Scholar] [CrossRef]
- Siqueira, L.d.P.; Fontes, D.A.F.; Aguilera, C.S.B.; Timóteo, T.R.R.; Ângelos, M.A.; Silva, L.C.P.B.B.; de Melo, C.G.; Rolim, L.A.; da Silva, R.M.F.; Neto, P.J.R. Schistosomiasis: Drugs used and treatment strategies. Acta Trop. 2017, 176, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Cioli, D.; Pica-Mattoccia, L.; Basso, A.; Guidi, A. Schistosomiasis control: Praziquantel forever? Mol. Biochem. Parasitol. 2014, 195, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Mäder, P.; Rennar, G.A.; Ventura, A.M.P.; Grevelding, C.G.; Schlitzer, M. Chemotherapy for Fighting Schistosomiasis: Past, Present and Future. ChemMedChem 2018, 13, 2374–2389. [Google Scholar] [CrossRef] [PubMed]
- You, H.; McManus, D.P.; Hu, W.; Smout, M.J.; Brindley, P.J.; Gobert, G.N. Transcriptional Responses of In vivo Praziquantel Exposure in Schistosomes Identifies a Functional Role for Calcium Signalling Pathway Member CamKII. PLoS Pathog. 2013, 9, e1003254. [Google Scholar] [CrossRef]
- Nawaratna, S.S.K.; McManus, D.P.; Gasser, R.B.; Brindley, P.J.; Boyle, G.M.; Rivera, V.; Ranasinghe, S.L.; Jones, M.K.; You, H.; Gobert, G.N. Use of kinase inhibitors against schistosomes to improve and broaden praziquantel efficacy. Parasitology 2020, 147, 1488–1498. [Google Scholar] [CrossRef]
- Meggio, F.; Deana, A.D.; Ruzzene, M.; Brunati, A.M.; Cesaro, L.; Guerra, B.; Meyer, T.; Mett, H.; Fabbro, D.; Furet, P.; et al. Different Susceptibility of Protein Kinases to Staurosporine Inhibition: Kinetic Studies and Molecular Bases for the Resistance of Protein Kinase CK2. Eur. J. Biochem. 1995, 234, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Tamaoki, T.; Nomoto, H.; Takahashi, I.; Kato, Y.; Morimoto, M.; Tomita, F. Staurosporine, a potent inhibitor of phospholipid Ca++dependent protein kinase. Biochem. Biophys. Res. Commun. 1986, 135, 397–402. [Google Scholar] [CrossRef]
- Long, T.; Cailliau, K.; Beckmann, S.; Browaeys, E.; Trolet, J.; Grevelding, C.G.; Dissous, C. Schistosoma mansoni Polo-like kinase 1: A mitotic kinase with key functions in parasite reproduction. Int. J. Parasitol. 2010, 40, 1075–1086. [Google Scholar] [CrossRef]
- Long, T.; Neitz, R.J.; Beasley, R.; Kalyanaraman, C.; Suzuki, B.M.; Jacobson, M.P.; Dissous, C.; McKerrow, J.H.; Drewry, D.H.; Zuercher, W.J.; et al. Structure-Bioactivity Relationship for Benzimidazole Thiophene Inhibitors of Polo-Like Kinase 1 (PLK1), a Potential Drug Target in Schistosoma mansoni. PLoS Negl. Trop. Dis. 2016, 10, e0004356. [Google Scholar] [CrossRef]
- Andrade, L.F.; Nahum, L.A.; Avelar, L.G.A.; Silva, L.L.; Zerlotini, A.; Ruiz, J.C.; Oliveira, G. Eukaryotic Protein Kinases (ePKs) of the Helminth Parasite Schistosoma mansoni. BMC Genom. 2011, 12, 215. [Google Scholar] [CrossRef]
- Stroehlein, A.J.; Young, N.D.; Jex, A.R.; Sternberg, P.W.; Tan, P.; Boag, P.R.; Hofmann, A.; Gasser, R.B. Defining the Schistosoma haematobium kinome enables the prediction of essential kinases as anti-schistosome drug targets. Sci. Rep. 2015, 5, 17759. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, S.; Silva, A.C.; Borba, J.V.V.B.; Ramos, P.I.P.; Paveley, R.A.; Muratov, E.N.; Andrade, C.H.; Furnham, N. Computationally-guided drug repurposing enables the discovery of kinase targets and inhibitors as new schistosomicidal agents. PLOS Comput. Biol. 2018, 14, e1006515. [Google Scholar] [CrossRef] [PubMed]
- Grevelding, C.G.; Langner, S.; Dissous, C. Kinases: Molecular Stage Directors for Schistosome Development and Differentiation. Trends Parasitol. 2018, 34, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Levitzki, A.; Mishani, E. Tyrphostins and other tyrosine kinase inhibitors. Annu. Rev. Biochem. 2006, 75, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Vicogne, J.; Pin, J.P.; Lardans, V.; Capron, M.; Noël, C.; Dissous, C. An unusual receptor tyrosine kinase of Schistosoma mansoni contains a Venus Flytrap module. Mol. Biochem. Parasitol. 2003, 126, 51–62. [Google Scholar] [CrossRef]
- Ahier, A.; Khayath, N.; Vicogne, J.; Dissous, C. Insulin receptors and glucose uptake in the human parasite Schistosoma mansoni. Parasite 2008, 15, 573–579. [Google Scholar] [CrossRef]
- Buro, C.; Oliveira, K.C.; Lu, Z.; Leutner, S.; Beckmann, S.; Dissous, C.; Cailliau, K.; Verjovski-Almeida, S.; Grevelding, C.G. Transcriptome Analyses of Inhibitor-treated Schistosome Females Provide Evidence for Cooperating Src-kinase and TGFβ Receptor Pathways Controlling Mitosis and Eggshell Formation. PLoS Pathog. 2013, 9, e1003448. [Google Scholar] [CrossRef]
- Beckmann, S.; Buro, C.; Dissous, C.; Hirzmann, J.; Grevelding, C.G. The Syk kinase SmTK4 of Schistosoma mansoni is involved in the regulation of spermatogenesis and oogenesis. PLoS Pathog. 2010, 6, e1000769. [Google Scholar] [CrossRef]
- You, H.; Zhang, W.; Jones, M.K.; Gobert, G.N.; Mulvenna, J.; Rees, G.; Spanevello, M.; Blair, D.; Duke, M.; Brehm, K.; et al. Cloning and characterisation of Schistosoma japonicum insulin receptors. PLoS ONE 2010, 5, e9868. [Google Scholar] [CrossRef]
- Gelmedin, V.; Dissous, C.; Grevelding, C.G. Re-positioning protein-kinase inhibitors against schistosomiasis. Future Med. Chem. 2015, 7, 737–752. [Google Scholar] [CrossRef]
- Morel, M.; Vanderstraete, M.; Hahnel, S.; Grevelding, C.G.; Dissous, C. Receptor tyrosine kinases and schistosome reproduction: New targets for chemotherapy. Front. Genet. 2014, 5, 238. [Google Scholar] [CrossRef][Green Version]
- Knobloch, J.; Kunz, W.; Grevelding, C.G. Herbimycin A suppresses mitotic activity and egg production of female Schistosoma mansoni. Int. J. Parasitol. 2006, 36, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, S.; Grevelding, C.G. Imatinib has a fatal impact on morphology, pairing stability and survival of adult Schistosoma mansoni in vitro. Int. J. Parasitol. 2010, 40, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Haeberlein, S.; Zhao, L.; Mughal, M.N.; Zhu, T.; Liu, L.; Fang, R.; Zhou, Y.; Zhao, J.; Grevelding, C.G.; et al. The ABL kinase inhibitor imatinib causes phenotypic changes and lethality in adult Schistosoma japonicum. Parasitol. Res. 2019, 118, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Hemer, S.; Brehm, K. In vitro efficacy of the anticancer drug imatinib on Echinococcus multilocularis larvae. Int. J. Antimicrob. Agents 2012, 40, 458–462. [Google Scholar] [CrossRef]
- Morawietz, C.M.; Houhou, H.; Puckelwaldt, O.; Hehr, L.; Dreisbach, D.; Mokosch, A.; Roeb, E.; Roderfeld, M.; Spengler, B.; Haeberlein, S. Targeting Kinases in Fasciola hepatica: Anthelminthic Effects and Tissue Distribution of Selected Kinase Inhibitors. Front. Vet. Sci. 2020, 7, 1270. [Google Scholar] [CrossRef]
- O’Connell, E.M.; Kamenyeva, O.; Lustigman, S.; Bell, A.; Nutman, T.B. Defining the target and the effect of imatinib on the filarial c-Abl homologue. PLoS Negl. Trop. Dis. 2017, 11, e0005690. [Google Scholar] [CrossRef]
- Njouendou, A.J.; Fombad, F.F.; O’Neill, M.; Zofou, D.; Nutting, C.; Ndongmo, P.C.; Kengne-Ouafo, A.J.; Geary, T.G.; MacKenzie, C.D.; Wanji, S. Heterogeneity in the in vitro susceptibility of Loa loa microfilariae to drugs commonly used in parasitological infections. Parasites Vectors 2018, 11, 223. [Google Scholar] [CrossRef]
- Moslehi, M.; Namdar, F.; Esmaeilifallah, M.; Hejazi, S.; Sokhanvari, F.; Siadat, A.; Hosseini, S.; Iraji, F. Evaluation of different concentrations of imatinib on the viability of Leishmania major: An In vitro study. Adv. Biomed. Res. 2019, 8, 61. [Google Scholar] [CrossRef]
- Juárez-Saldivar, A.; Barbosa-Cabrera, E.; Lara-Ramírez, E.E.; Paz-González, A.D.; Martínez-Vázquez, A.V.; Bocanegra-García, V.; Palos, I.; Campillo, N.E.; Rivera, G. Virtual screening of fda-approved drugs against triose phosphate isomerase from entamoeba histolytica and giardia lamblia identifies inhibitors of their trophozoite growth phase. Int. J. Mol. Sci. 2021, 22, 5943. [Google Scholar] [CrossRef]
- Kesely, K.R.; Pantaleo, A.; Turrini, F.M.; Olupot-Olupot, P.; Low, P.S. Inhibition of an erythrocyte tyrosine kinase with imatinib prevents Plasmodium falciparum egress and terminates parasitemia. PLoS ONE 2016, 11, e0164895. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, E.M.; Nutman, T.B. Reduction of Loa loa Microfilaremia with Imatinib—A Case Report. N. Engl. J. Med. 2017, 377, 2095–2096. [Google Scholar] [CrossRef] [PubMed]
- Mokosch, A.S.; Gerbig, S.; Grevelding, C.G.; Haeberlein, S.; Spengler, B. High-resolution AP-SMALDI MSI as a tool for drug imaging in Schistosoma mansoni. Anal. Bioanal. Chem. 2021, 413, 2755–2766. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, S.; Leutner, S.; Gouignard, N.; Dissous, C.; Grevelding, C.G. Protein Kinases as Potential Targets for Novel Anti-Schistosomal Strategies. Curr. Pharm. Des. 2012, 18, 3579–3594. [Google Scholar] [CrossRef]
- Avelar, L.d.G.A.; Gava, S.G.; Neves, R.H.; Soares Silva, M.C.; Araújo, N.; Tavares, N.C.; El Khal, A.; Alves Mattos, A.C.; Machado-Silva, J.R.; Oliveira, G.; et al. Smp38 MAP kinase regulation in Schistosoma mansoni: Roles in survival, oviposition, and protection against oxidative stress. Front. Immunol. 2019, 10, 21. [Google Scholar] [CrossRef]
- de Andrade, L.F.; de Mourão, M.M.; Geraldo, J.A.; Coelho, F.S.; Silva, L.L.; Neves, R.H.; Volpini, A.; Machado-Silva, J.R.; Araujo, N.; Nacif-Pimenta, R.; et al. Regulation of Schistosoma mansoni Development and Reproduction by the Mitogen-Activated Protein Kinase Signaling Pathway. PLoS Negl. Trop. Dis. 2014, 8, e3081. [Google Scholar] [CrossRef]
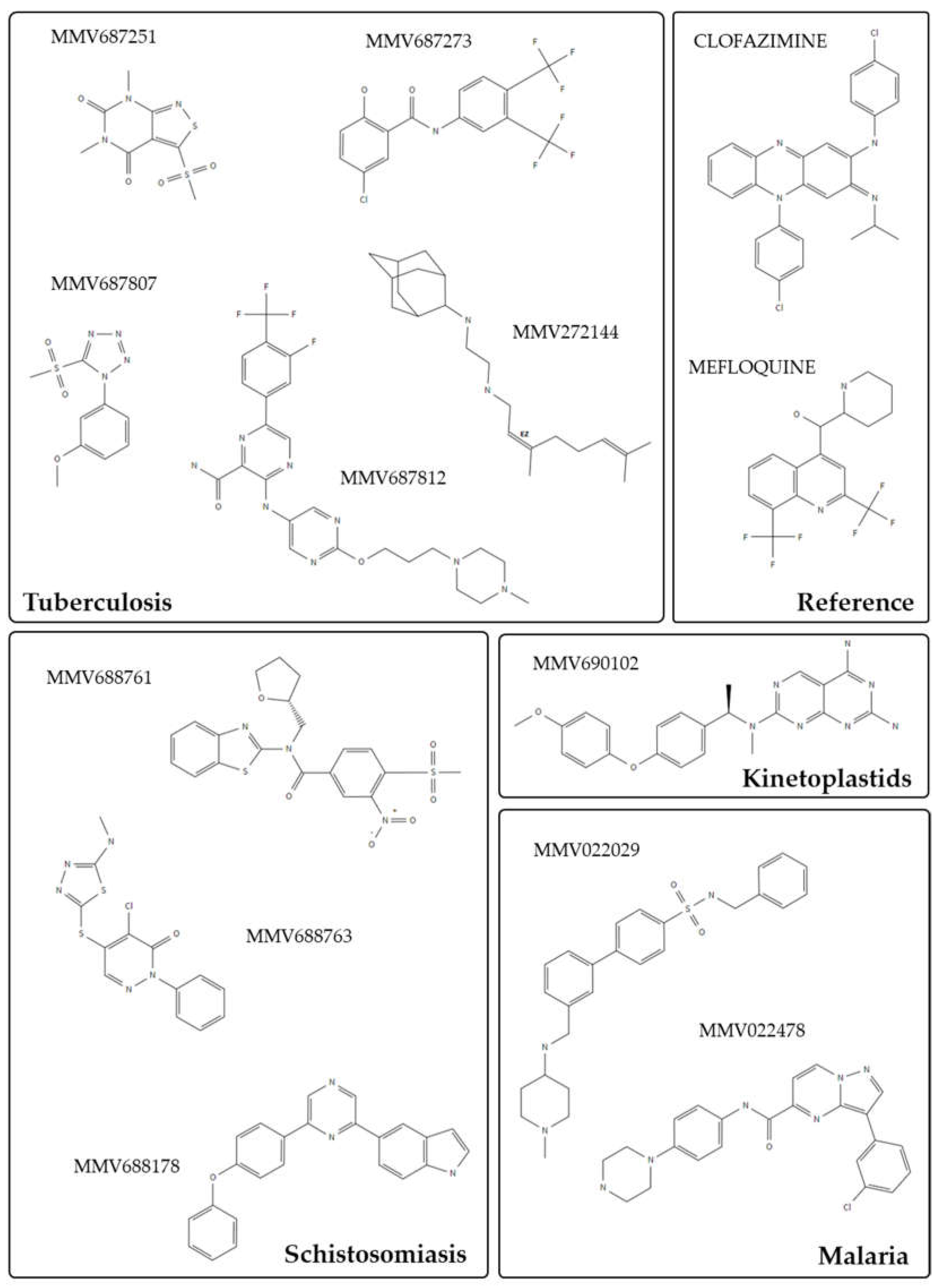

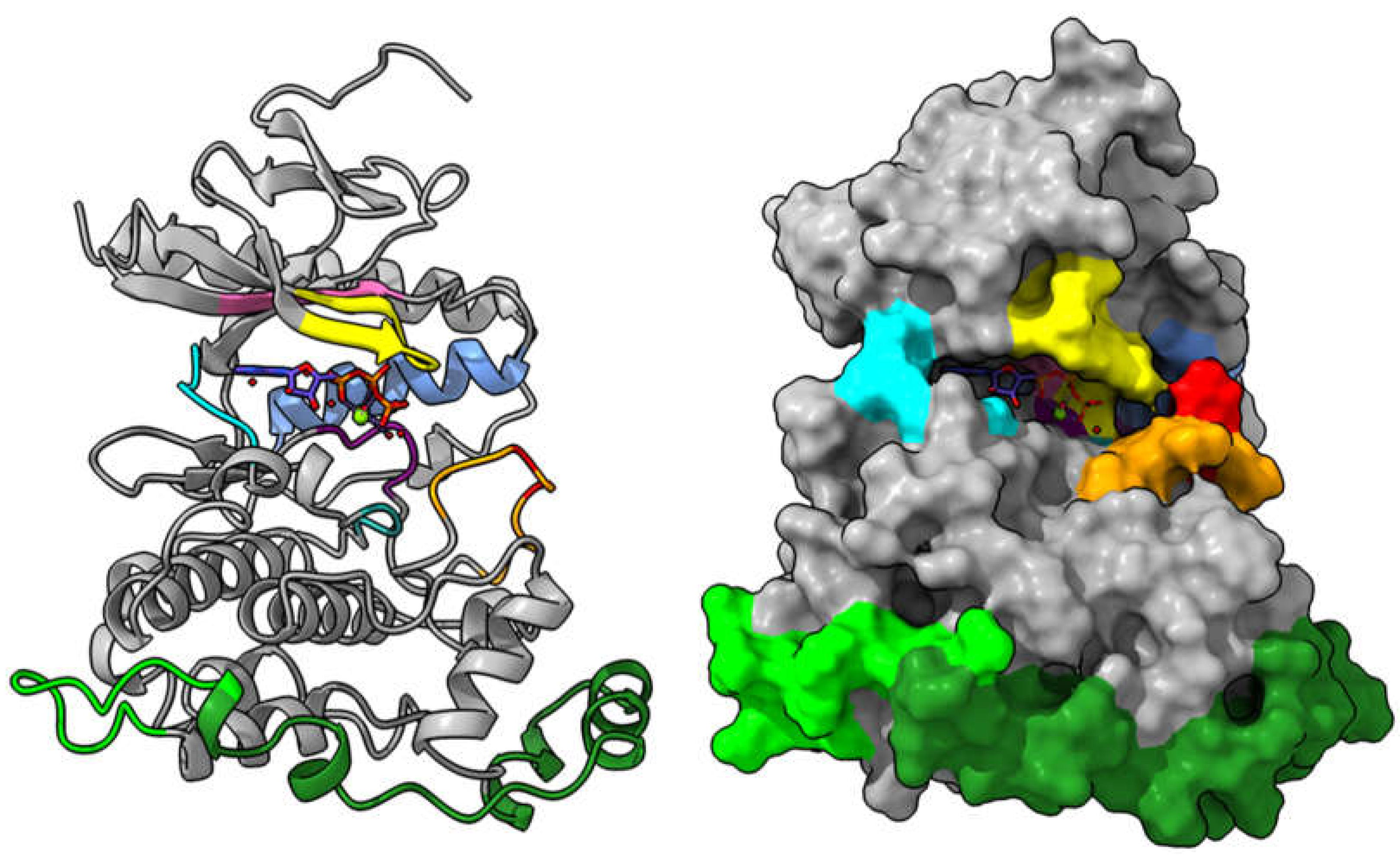
| De Novo Drug Discovery and Development | Drug Repurposing |
|---|---|
| Higher investment cost and financial return | Lower research, development (R&D) costs and more favorable financial return on investment |
| Long development time for new drugs | Reduces the drug development timeline (might not require Phase 1 clinical trials) |
| Ever-expanding toolbox of small-molecule compounds | Potential for reuse of compounds (despite evidence of adverse effects and failed efficacy in some indications) |
| Higher risk of failure | Higher probability of success |
| Focused on the discovery of drugs to treat chronic diseases and complex syndromes | Development of drugs for emerging and re-emerging infectious diseases |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira Moreira, B.; Weber, M.H.W.; Haeberlein, S.; Mokosch, A.S.; Spengler, B.; Grevelding, C.G.; Falcone, F.H. Drug Repurposing and De Novo Drug Discovery of Protein Kinase Inhibitors as New Drugs against Schistosomiasis. Molecules 2022, 27, 1414. https://doi.org/10.3390/molecules27041414
Pereira Moreira B, Weber MHW, Haeberlein S, Mokosch AS, Spengler B, Grevelding CG, Falcone FH. Drug Repurposing and De Novo Drug Discovery of Protein Kinase Inhibitors as New Drugs against Schistosomiasis. Molecules. 2022; 27(4):1414. https://doi.org/10.3390/molecules27041414
Chicago/Turabian StylePereira Moreira, Bernardo, Michael H. W. Weber, Simone Haeberlein, Annika S. Mokosch, Bernhard Spengler, Christoph G. Grevelding, and Franco H. Falcone. 2022. "Drug Repurposing and De Novo Drug Discovery of Protein Kinase Inhibitors as New Drugs against Schistosomiasis" Molecules 27, no. 4: 1414. https://doi.org/10.3390/molecules27041414
APA StylePereira Moreira, B., Weber, M. H. W., Haeberlein, S., Mokosch, A. S., Spengler, B., Grevelding, C. G., & Falcone, F. H. (2022). Drug Repurposing and De Novo Drug Discovery of Protein Kinase Inhibitors as New Drugs against Schistosomiasis. Molecules, 27(4), 1414. https://doi.org/10.3390/molecules27041414







