Microwave-Assisted Extraction of Anticancer Flavonoid, 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethyl Chalcone (DMC), Rich Extract from Syzygium nervosum Fruits
Abstract
1. Introduction
2. Results and Discussion
2.1. Method Validation of HPLC Determination
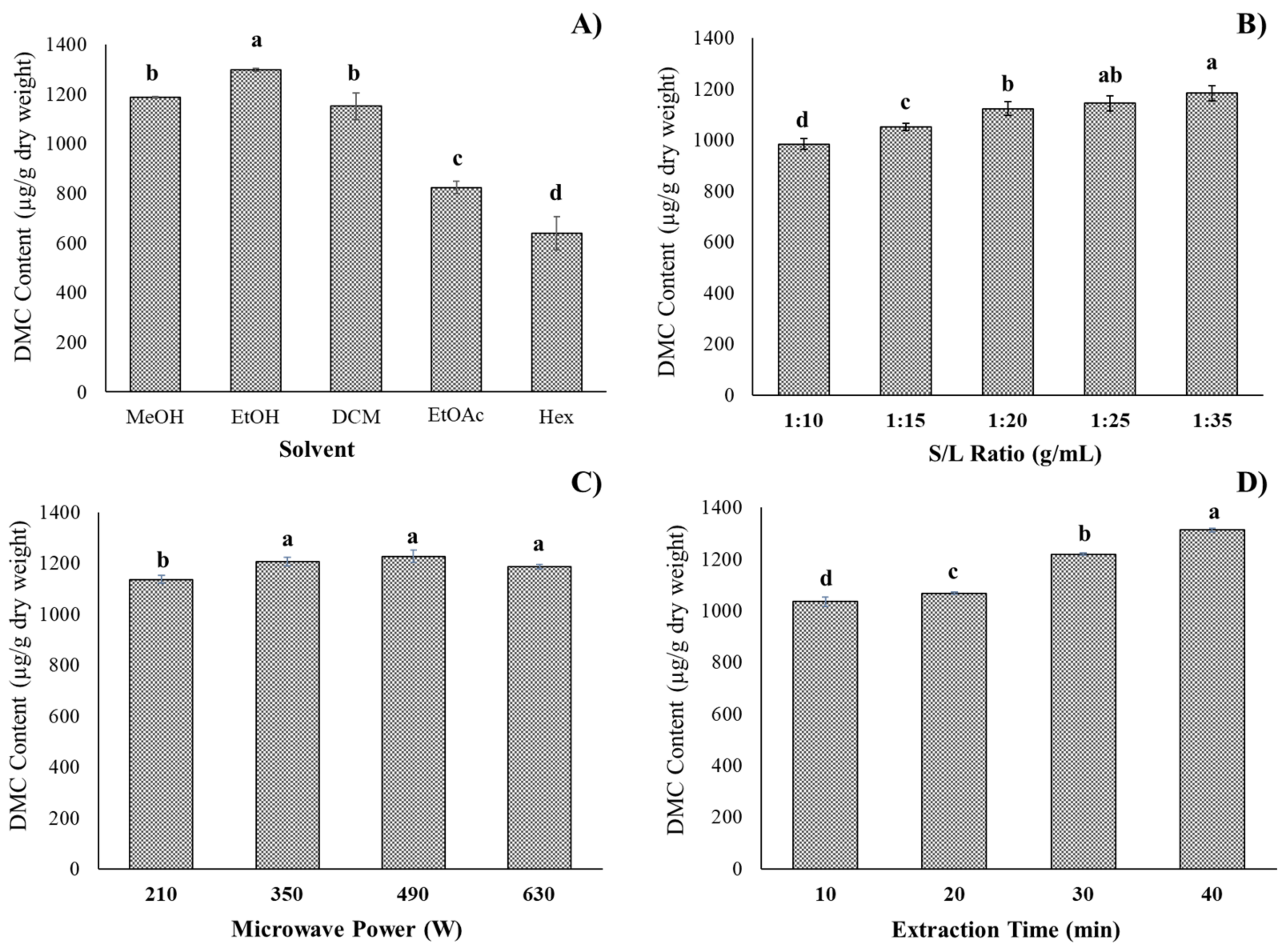
2.2. Single Variables Experiment of DMC Microwave-Assisted Extraction
2.3. Fitting the Model
- Y is replaced by a DMC content (µg/g dry sample);
- X1 is replaced by a solid-to-liquid ratio (g/mL);
- X2 is replaced by a microwave power (W);
- X3 is replaced by a microwave time (min).
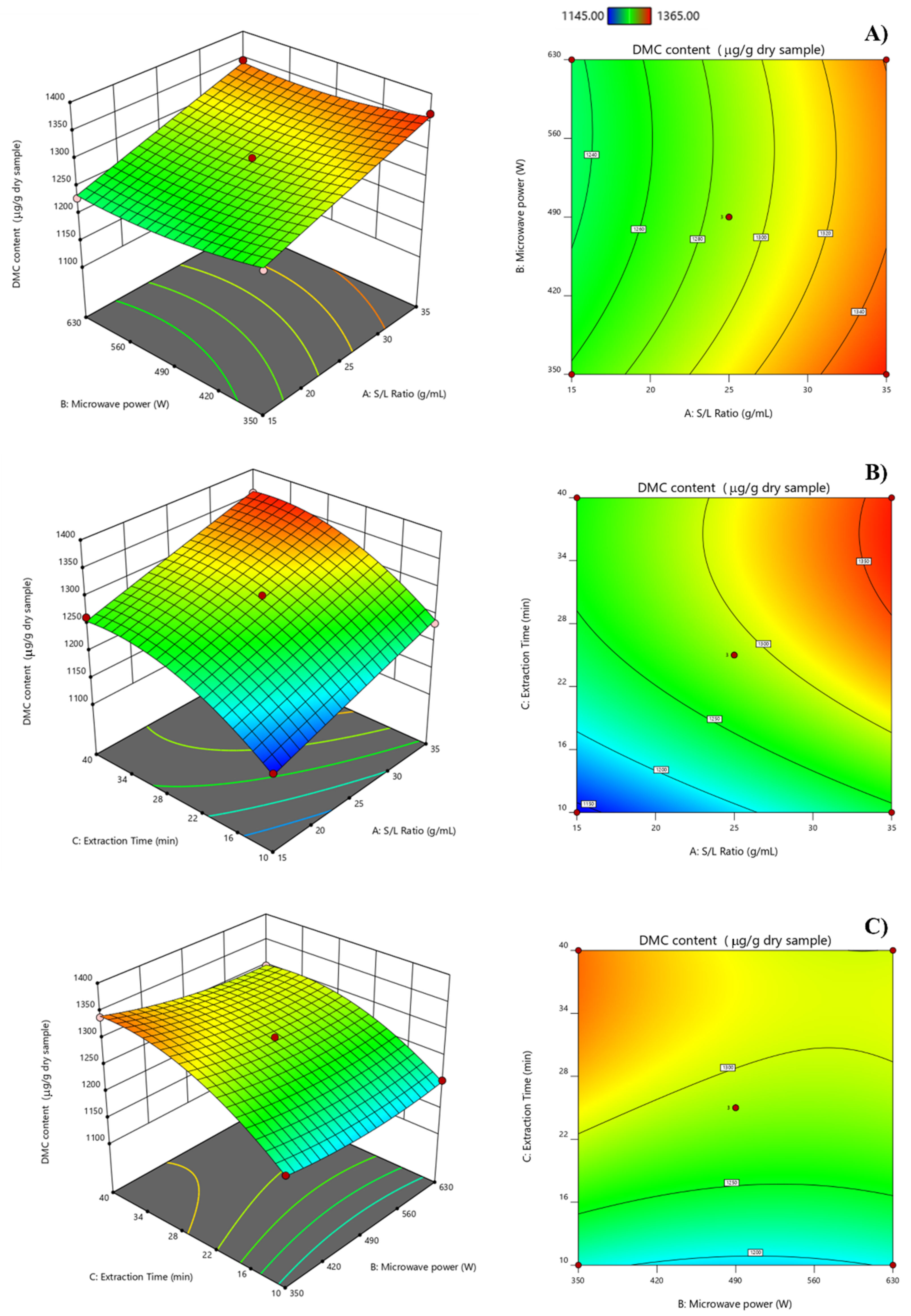
2.4. Analysis of Response Surface and Optimization of MAE Process by RSM
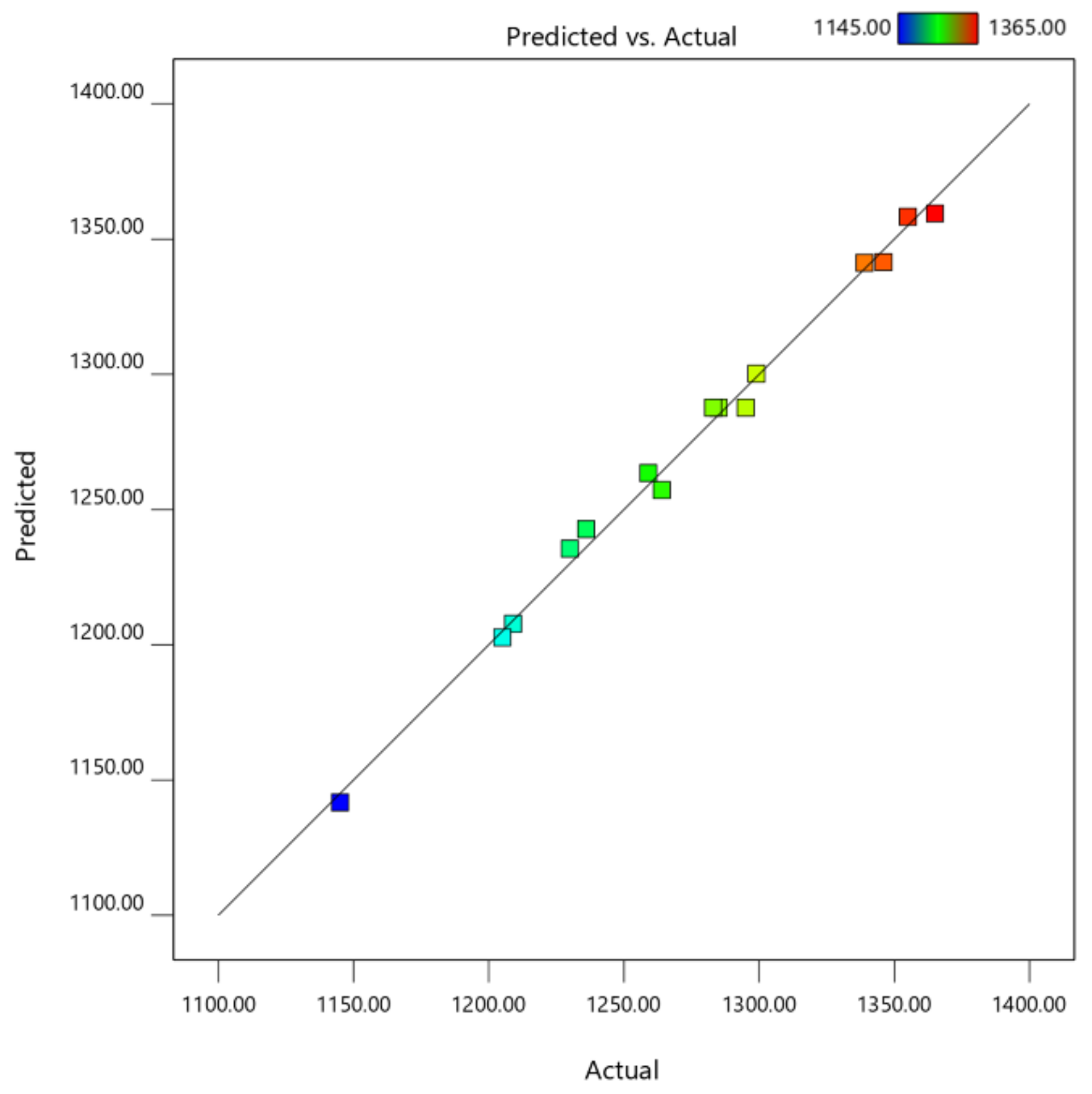
2.5. Verification of the DMC Predictive Model and Comparison of MAE with Conventional Methods
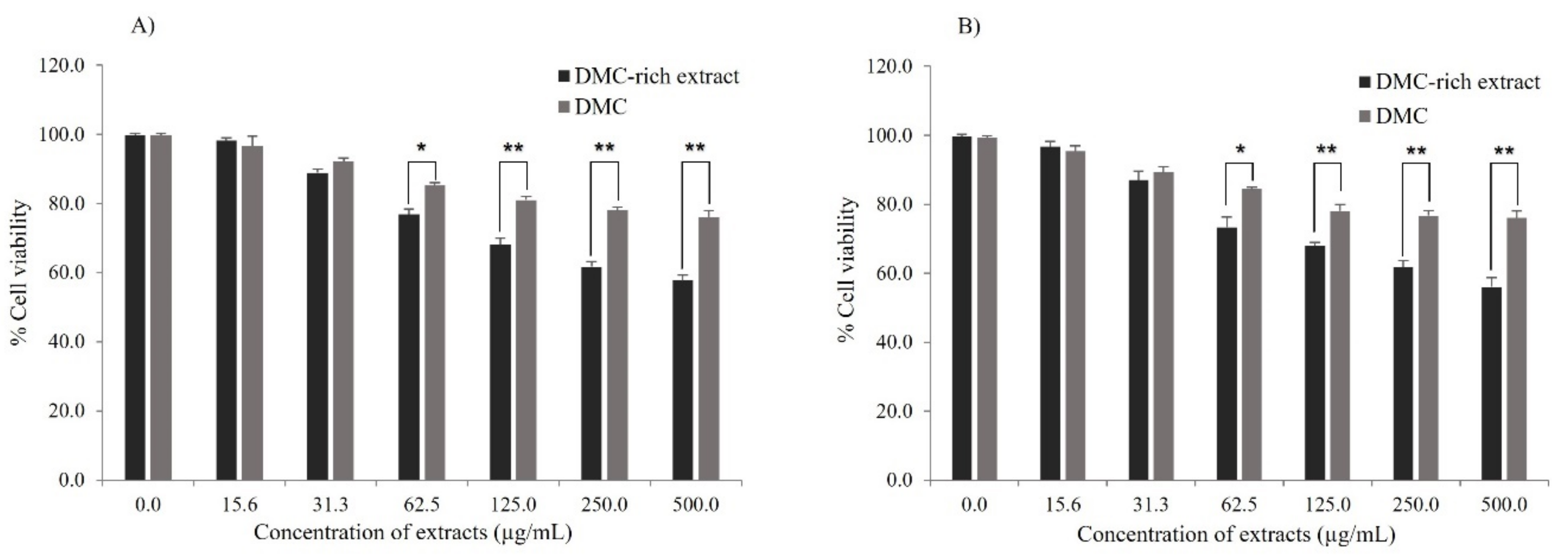
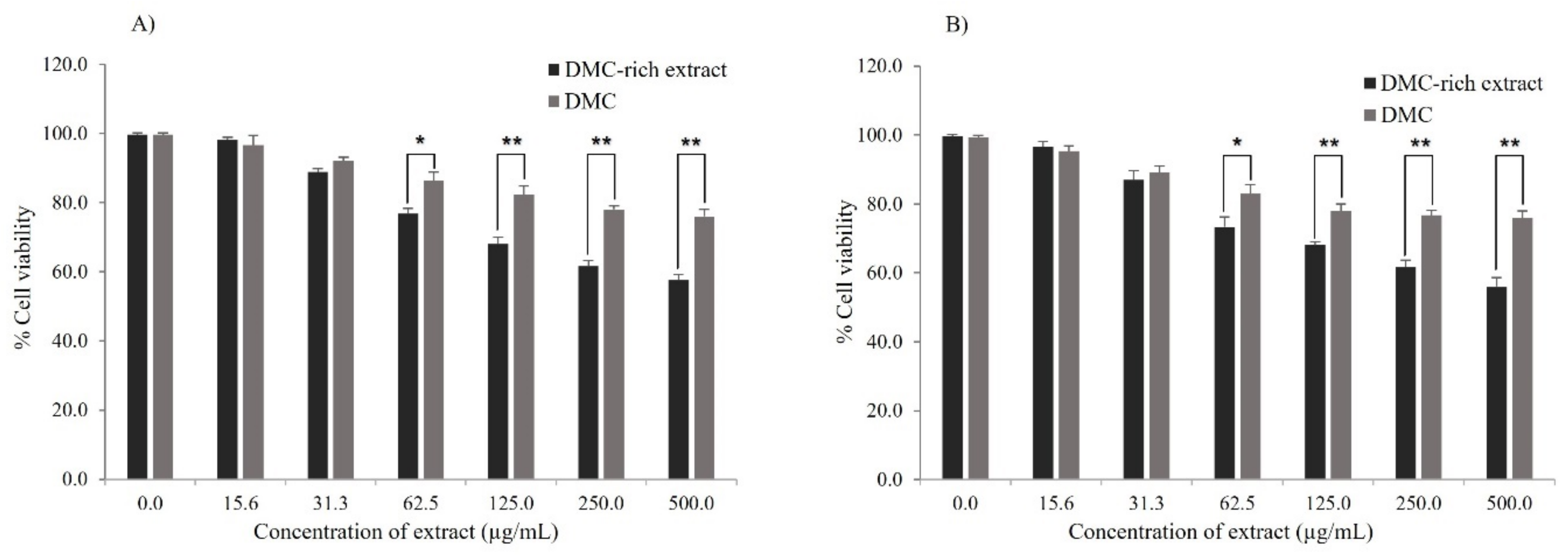
2.6. Cell Viability
3. Materials and Methods
3.1. Materials and Apparatuses
3.2. Isolation and Characterization of Standard DMC Compound
3.3. HPLC Conditions and Method Validation for Quantification of DMC
3.4. Microwave-Assisted Extraction (MAE) Approach of DMC-Rich Extract
3.5. Single Variable Investigation
3.6. Experimental Design
3.7. Verification of the DMC Predictive Model
3.8. Conventional Maceration and Heat Reflux Extraction (HRE)
3.9. Statistical Analysis
3.10. Cell Lines and MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Craven, L.A.; Biffin, E. An infrageneric classification of Syzygium (Myrtaceae). Blumea-Biodivers. Evol. Biogeogr. Plants 2010, 55, 94–99. [Google Scholar] [CrossRef]
- Aisha, A.F.; Abu-Salah, K.M.; Alrokayan, S.A.; Siddiqui, M.J.; Ismail, Z.; Majid, A.M.S.A. Syzygium aromaticum extracts as good source of betulinic acid and potential anti-breast cancer. Rev. Bras. Farmacogn. 2012, 22, 335–343. [Google Scholar] [CrossRef]
- Samy, M.N.; Sugimoto, S.; Matsunami, K.; Otsuka, H.; Kamel, M.S. One new flavonoid xyloside and one new natural triterpene rhamnoside from the leaves of Syzygium grande. Phytochem. Lett. 2014, 10, 86–90. [Google Scholar] [CrossRef]
- Miyazawa, M.; Hisama, M. Antimutagenic activity of phenylpropanoids from clove (Syzygium aromaticum). J. Agric. Food Chem. 2003, 51, 6413–6422. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Shaw, A.K.; Kulshreshtha, D.K. Triterpenoids and chalcone from Syzygium samarangense. Phytochemistry 1995, 38, 687–689. [Google Scholar] [CrossRef]
- Gordon, A.; Jungfer, E.; da Silva, B.A.; Maia, J.G.S.; Marx, F. Phenolic constituents and antioxidant capacity of four underutilized fruits from the Amazon region. J. Agric. Food Chem. 2011, 59, 7688–7699. [Google Scholar] [CrossRef]
- Tanaka, T.; Orii, Y.; Nonaka, G.-I.; Nishioka, I.; Kouno, I. Syzyginins A and B, two ellagitannins from Syzygium aromaticum. Phytochemistry 1996, 43, 1345–1348. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, Z.; Cao, D.; Rong, F.; Ding, H.; Zhang, D. Antitermitic and antifungal activities of eugenol and its congeners from the flower buds of Syzgium aromaticum (clove). Ind. Crops Prod. 2015, 77, 780–786. [Google Scholar] [CrossRef]
- Djoukeng, J.; Abou-Mansour, E.; Tabacchi, R.; Tapondjou, A.; Bouda, H.; Lontsi, D. Antibacterial triterpenes from Syzygium guineense (Myrtaceae). J. Ethnopharmacol. 2005, 101, 283–286. [Google Scholar] [CrossRef]
- Nomi, Y.; Shimizu, S.; Sone, Y.; Tuyet, M.T.; Gia, T.P.; Kamiyama, M.; Shibamoto, T.; Shindo, K.; Otsuka, Y. Isolation and antioxidant activity of zeylaniin A, a new macrocyclic ellagitannin from Syzygium zeylanicum leaves. J. Agric. Food Chem. 2012, 60, 10263–10269. [Google Scholar] [CrossRef]
- Memon, A.H.; Ismail, Z.; Aisha, A.F.; Al-Suede, F.S.R.; Hamil, M.S.R.; Hashim, S.; Saeed, M.A.A.; Laghari, M.; Abdul Majid, A.M.S. Isolation, characterization, crystal structure elucidation, and anticancer study of dimethyl cardamonin, isolated from Syzygium campanulatum Korth. Evid.-Based Complement. Altern. Med. 2014, 2014, 470179. [Google Scholar] [CrossRef]
- Annadurai, G.; Masilla, B.; Jothiramshekar, S.; Palanisami, E.; Puthiyapurayil, S.; Parida, A. Antimicrobial, antioxidant, anticancer activities of Syzygium caryophyllatum (L.) Alston. Int. J. Green Pharm. 2012, 6, 285. [Google Scholar]
- Setchell, K.D.; Cassidy, A. Dietary isoflavones: Biological effects and relevance to human health. J. Nutr. 1999, 129, 758S–767S. [Google Scholar] [CrossRef] [PubMed]
- Chailungka, A.; Junpirom, T.; Pompimon, W.; Nuntasaen, N.; Meepowpan, P. Two flavonoids first isolated from the seed of Syzygium nervosum and preliminary study of their anti-cancer and anti-HIV-1 reverse transcriptase activities. Maejo Int. J. Sci. Technol. 2017, 11, 58–67. [Google Scholar]
- Gafner, S.; Wolfender, J.-L.; Mavi, S.; Hostettmann, K. Antifungal and antibacterial chalcones from Myrica serrata. Planta Med. 1996, 62, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Ghayur, M.; Gilani, A.; Khan, A.; Amor, E.; Villaseñor, I.; Choudhary, M. Presence of calcium antagonist activity explains the use of Syzygium samarangense in diarrhoea. Phytother. Res. 2006, 20, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Pinela, J.; Prieto, M.; Carvalho, A.M.; Barreiro, M.F.; Oliveira, M.B.P.; Barros, L.; Ferreira, I.C. Microwave-assisted extraction of phenolic acids and flavonoids and production of antioxidant ingredients from tomato: A nutraceutical-oriented optimization study. Sep. Purif. Technol. 2016, 164, 114–124. [Google Scholar] [CrossRef]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Hamid, A.; Zaidul, I. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process. 2011, 89, 67–72. [Google Scholar] [CrossRef]
- Bhadoriya, U.; Tiwari, S.; Mourya, M.; Ghule, S. Microwave-assisted extraction of flavonoids from Zanthoxylum budrunga W. optimization of extraction process. Asian J. Pharm. Life Sci. 2011, 1, 81–86. [Google Scholar]
- Chang, C.-W.; Yen, C.-C.; Wu, M.-T.; Hsu, M.-C.; Wu, Y.-T. Microwave-assisted extraction of cannabinoids in hemp nut using response surface methodology: Optimization and comparative study. Molecules 2017, 22, 1894. [Google Scholar] [CrossRef]
- Narkprasom, N.; Narkprasom, K.; Upara, U. Optimization of total phenolic from Cleistocalyx nervosum by microwave-assisted extraction. Am. J. Eng. Appl. Sci. 2015, 8, 302. [Google Scholar] [CrossRef][Green Version]
- Jin, C.; Wei, X.; Yang, S.; Yao, L.; Gong, G. Microwave-assisted extraction and antioxidant activity of flavonoids from sedum aizoon leaves. Food Sci. Technol. Res. 2017, 23, 111–118. [Google Scholar] [CrossRef]
- Imam, M.U.; Ismail, M.; Ooi, D.J.; Azmi, N.H.; Sarega, N.; Chan, K.W.; Bhanger, M.I. Are bioactive-rich fractions functionally richer? Crit. Rev. Biotechnol. 2016, 36, 585–593. [Google Scholar] [CrossRef]
- Ghafari, S.; Aziz, H.A.; Isa, M.H.; Zinatizadeh, A.A. Application of response surface methodology (RSM) to optimize coagulation–flocculation treatment of leachate using poly-aluminum chloride (PAC) and alum. J. Hazard. Mater. 2009, 163, 650–656. [Google Scholar] [CrossRef]
- Yu, M.; Wang, B.; Qi, Z.; Xin, G.; Li, W. Response surface method was used to optimize the ultrasonic assisted extraction of flavonoids from Crinum asiaticum. Saudi J. Biol. Sci. 2019, 26, 2079–2084. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, Y.; Zhang, P.; Zhang, A.; Wu, H. Optimisation of microwave-assisted extraction of flavonoids and phenolics from celery (Apium graveolens L.) leaves by response surface methodology. Czech J. Food Sci. 2016, 34, 341–349. [Google Scholar] [CrossRef]
- Huber, L. Validation and Qualification in Analytical Laboratories; Informa Healthcare: New York, NY, USA, 2007. [Google Scholar]
- Feldsine, P.; Abeyta, C.; Andrews, W.H. AOAC International methods committee guidelines for validation of qualitative and quantitative food microbiological official methods of analysis. J. AOAC Int. 2002, 85, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Dirar, A.; Alsaadi, D.; Wada, M.; Mohamed, M.; Watanabe, T.; Devkota, H. Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. S. Afr. J. Bot. 2019, 120, 261–267. [Google Scholar] [CrossRef]
- Chaves, J.O.; De Souza, M.C.; Da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; da Fonseca Machado, A.P.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F. Extraction of flavonoids from natural sources using modern techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Ibrahim, N.; Zaini, M.A.A. Solvent selection in microwave assisted extraction of castor oil. Chem. Eng. Trans. 2017, 56, 865–870. [Google Scholar]
- Shang, A.; Luo, M.; Gan, R.-Y.; Xu, X.-Y.; Xia, Y.; Guo, H.; Liu, Y.; Li, H.-B. Effects of microwave-assisted extraction conditions on antioxidant capacity of sweet tea (Lithocarpus polystachyus Rehd.). Antioxidants 2020, 9, 678. [Google Scholar] [CrossRef] [PubMed]
- Le, X.D.; Nguyen, M.C.; Vu, D.H.; Pham, M.Q.; Pham, Q.L.; Nguyen, Q.T.; Nguyen, T.A.; Pham, V.T.; Bach, L.G.; Nguyen, T.V. Optimization of microwave-assisted extraction of total phenolic and total flavonoid contents from fruits of Docynia indica (Wall.) Decne. using response surface methodology. Processes 2019, 7, 485. [Google Scholar] [CrossRef]
- Xu, J.; Wu, J.; Qi, J.; Li, J.; Liu, Y.; Miao, Z.; Qiu, G.; Jia, W. Microwave-assisted extraction of flavonoids from phyllostachys heterocycla leaves: Optimization, mechanism, and antioxidant activity in vitro. BioResources 2021, 16, 8060–8081. [Google Scholar] [CrossRef]
- Xu, M.; Shao, Q.; Ye, S.; Li, S.; Wu, M.; Ding, M.; Li, Y. Simultaneous extraction and identification of phenolic compounds in anoectochilus roxburghii using microwave-assisted extraction combined with UPLC-Q-TOF-MS/MS and their antioxidant activities. Front. Plant Sci. 2017, 8, 1474. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.B.; Yousfi, M.; Viaene, J.; Dejaegher, B.; Demeyer, K.; Mangelings, D.; Vander Heyden, Y. Determination of optimal extraction conditions for phenolic compounds from Pistacia atlantica leaves using the response surface methodology. Anal. Methods 2016, 8, 6107–6114. [Google Scholar] [CrossRef]
- Feki, F.; Klisurova, D.; Masmoudi, M.A.; Choura, S.; Denev, P.; Trendafilova, A.; Chamkha, M.; Sayadi, S. Optimization of microwave assisted extraction of simmondsins and polyphenols from Jojoba (Simmondsia chinensis) seed cake using Box-Behnken statistical design. Food Chem. 2021, 356, 129670. [Google Scholar] [CrossRef]
- Xiao, W.; Han, L.; Shi, B. Optimization of microwave-assisted extraction of flavonoid from Radix astragali using response surface methodology. Sep. Sci. Technol. 2008, 43, 671–681. [Google Scholar] [CrossRef]
- Chemat, F.; Cravotto, G. Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Food Engineering Series; Chemat, F., Cravotto, G., Eds.; Springer: New York, NY, USA, 2013; Chapter 2; pp. 15–43. ISBN 978-1-4614-4829-7. [Google Scholar]
- Ling, Y.Y.; Fun, P.S.; Yeop, A.; Yusoff, M.M.; Gimbun, J. Assessment of maceration, ultrasonic and microwave assisted extraction for total phenolic content, total flavonoid content and kaempferol yield from Cassia alata via microstructures analysis. Mater. Today Proc. 2019, 19, 1273–1279. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Zhang, J.-J.; Li, Y.; Meng, X.; Li, H.-B. Microwave-assisted extraction of phenolic compounds from Melastoma sanguineum fruit: Optimization and identification. Molecules 2018, 23, 2498. [Google Scholar] [CrossRef]
- Wang, C.; Wu, P.; Shen, X.-L.; Wei, X.-Y.; Jiang, Z.-H. Synthesis, cytotoxic activity and drug combination study of tertiary amine derivatives of 2′, 4′-dihydroxyl-6′-methoxyl-3′, 5′-dimethylchalcone. RSC Adv. 2017, 7, 48031–48038. [Google Scholar] [CrossRef]
- Li, F.; Zhao, C.; Wang, L. Molecular-targeted agents combination therapy for cancer: Developments and potentials. Int. J. Cancer 2014, 134, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10, S4. [Google Scholar] [CrossRef] [PubMed]
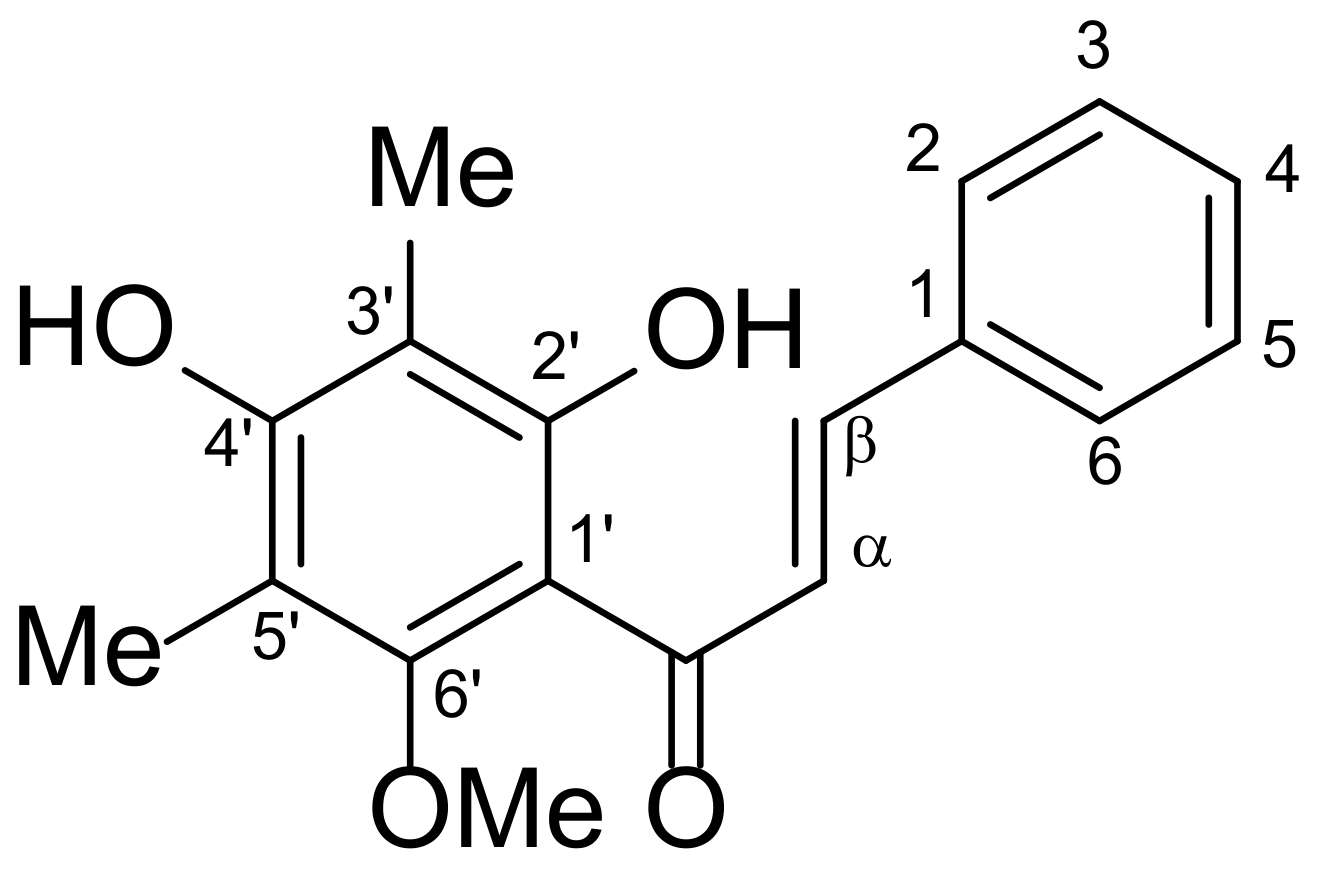
| Concentration of DMC (µg/mL) | %RSD | %Recovery (µg/mL ± SD) | |
|---|---|---|---|
| Intra-Day | Inter-Day | ||
| 0.25 | 5.75 | 7.39 | 85.3 ± 1.5 |
| 50 | 1.36 | 1.87 | 98.5 ± 2.5 |
| 100 | 0.67 | 1.22 | 97.3 ± 3.8 |
| Standard Order | Extraction Variables | DMC Content (µg/g Dry Sample) | ||
|---|---|---|---|---|
| X1: Solid-to-Liquid Ratio (g/mL) | X2: Microwave Power (W) | X3: Microwave Time (min) | ||
| 2 | 1:35 | 350 | 25 | 1365 |
| 10 | 1:25 | 630 | 10 | 1205 |
| 5 | 1:15 | 490 | 10 | 1145 |
| 7 | 1:15 | 490 | 40 | 1264 |
| 13 | 1:25 | 490 | 25 | 1295 |
| 8 | 1:35 | 490 | 40 | 1355 |
| 4 | 1:35 | 630 | 25 | 1346 |
| 9 | 1:25 | 350 | 10 | 1209 |
| 11 | 1:25 | 350 | 40 | 1339 |
| 15 | 1:25 | 490 | 25 | 1283 |
| 1 | 1:15 | 350 | 25 | 1259 |
| 14 | 1:25 | 490 | 25 | 1285 |
| 6 | 1:35 | 490 | 10 | 1236 |
| 3 | 1:15 | 630 | 25 | 1230 |
| 12 | 1:25 | 630 | 40 | 1299 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | Remark |
|---|---|---|---|---|---|---|
| Model | 54,540.17 | 9 | 6060.02 | 98.01 | <0.0001 | significant |
| X1-S-to-L Ratio | 20,402.00 | 1 | 20,402.00 | 329.95 | <0.0001 | significant |
| X2-Microwave Power | 1058.00 | 1 | 1058.00 | 17.11 | 0.0090 | significant |
| X3-Microwave Time | 26,680.50 | 1 | 26,680.50 | 431.49 | <0.0001 | significant |
| X1X2 | 25.00 | 1 | 25.00 | 0.4043 | 0.5528 | |
| X1X3 | 0.00 | 1 | 0.00 | 0.0000 | 1.0000 | |
| X2X3 | 324.00 | 1 | 324.00 | 5.24 | 0.0707 | |
| X1² | 0.4103 | 1 | 0.4103 | 0.0066 | 0.9382 | |
| X2² | 592.41 | 1 | 592.41 | 9.58 | 0.0270 | significant |
| X3² | 5146.26 | 1 | 5146.26 | 85.23 | 0.0003 | significant |
| Residual | 309.17 | 5 | 61.83 | |||
| Lack of Fit | 226.50 | 3 | 75.50 | 1.83 | 0.3729 | not significant |
| Pure Error | 82.67 | 2 | 41.33 | |||
| Cor Total | 54,849.33 | 14 | ||||
| R2 | 0.9944 | |||||
| R2 (adj) | 0.9842 | |||||
| R2(pred) | 0.9305 | |||||
| CV (%) | 0.6171 | |||||
| Standard Deviation | 7.86 |
| Extraction Method | Optimal MAE Conditions | Maximum Value (µg/g Dry Sample) | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Predicted Value | Experimental Value | |
| MAE | 1:35 g/mL | 350 W | 38 min | 1389 | 1409 ± 24 a |
| HRE | 1:35 g/mL | - | 2 h | - | 1337 ± 37 a |
| Maceration | 1:35 g/mL | - | 20 h | - | 1225 ± 81 b |
| Independent Variable | Variable Symbol | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Sample–Solvent ratio (g/mL) | X1 | 1:15 | 1:25 | 1:35 |
| Microwave Power (W) | X2 | 350 | 490 | 630 |
| Microwave Time (min) | X3 | 10 | 25 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choommongkol, V.; Punturee, K.; Klumphu, P.; Rattanaburi, P.; Meepowpan, P.; Suttiarporn, P. Microwave-Assisted Extraction of Anticancer Flavonoid, 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethyl Chalcone (DMC), Rich Extract from Syzygium nervosum Fruits. Molecules 2022, 27, 1397. https://doi.org/10.3390/molecules27041397
Choommongkol V, Punturee K, Klumphu P, Rattanaburi P, Meepowpan P, Suttiarporn P. Microwave-Assisted Extraction of Anticancer Flavonoid, 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethyl Chalcone (DMC), Rich Extract from Syzygium nervosum Fruits. Molecules. 2022; 27(4):1397. https://doi.org/10.3390/molecules27041397
Chicago/Turabian StyleChoommongkol, Vachira, Khanittha Punturee, Piyatida Klumphu, Parintip Rattanaburi, Puttinan Meepowpan, and Panawan Suttiarporn. 2022. "Microwave-Assisted Extraction of Anticancer Flavonoid, 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethyl Chalcone (DMC), Rich Extract from Syzygium nervosum Fruits" Molecules 27, no. 4: 1397. https://doi.org/10.3390/molecules27041397
APA StyleChoommongkol, V., Punturee, K., Klumphu, P., Rattanaburi, P., Meepowpan, P., & Suttiarporn, P. (2022). Microwave-Assisted Extraction of Anticancer Flavonoid, 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethyl Chalcone (DMC), Rich Extract from Syzygium nervosum Fruits. Molecules, 27(4), 1397. https://doi.org/10.3390/molecules27041397





