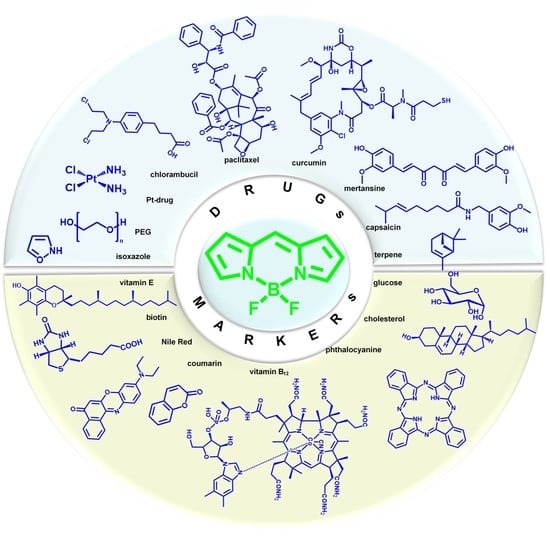
BODIPY Conjugates as Functional Compounds for Medical Diagnostics and Treatment
Abstract
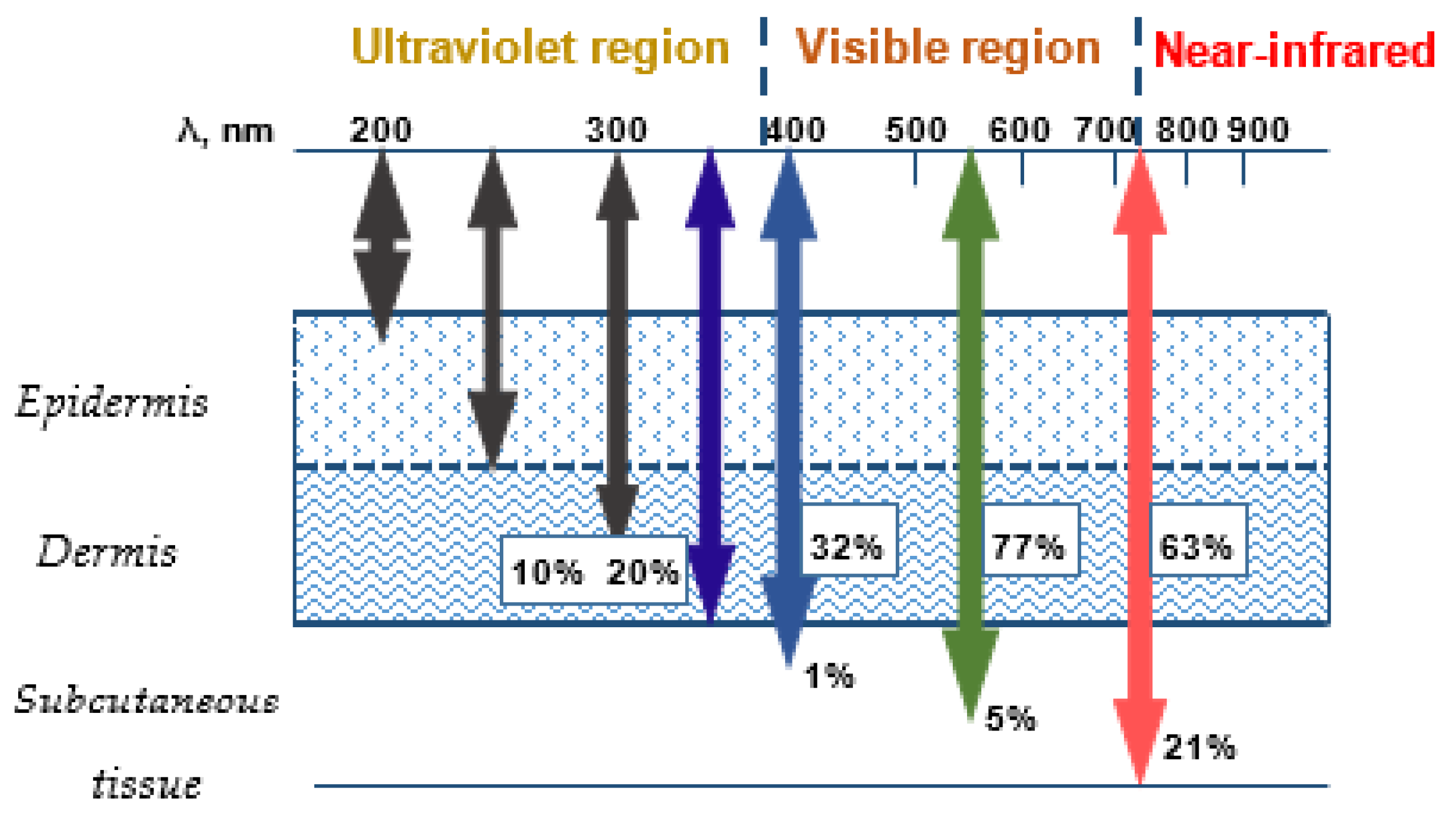
:1. Introduction
2. Multifunctional Bio-Conjugates BODIPY for Bioimaging and Therapy
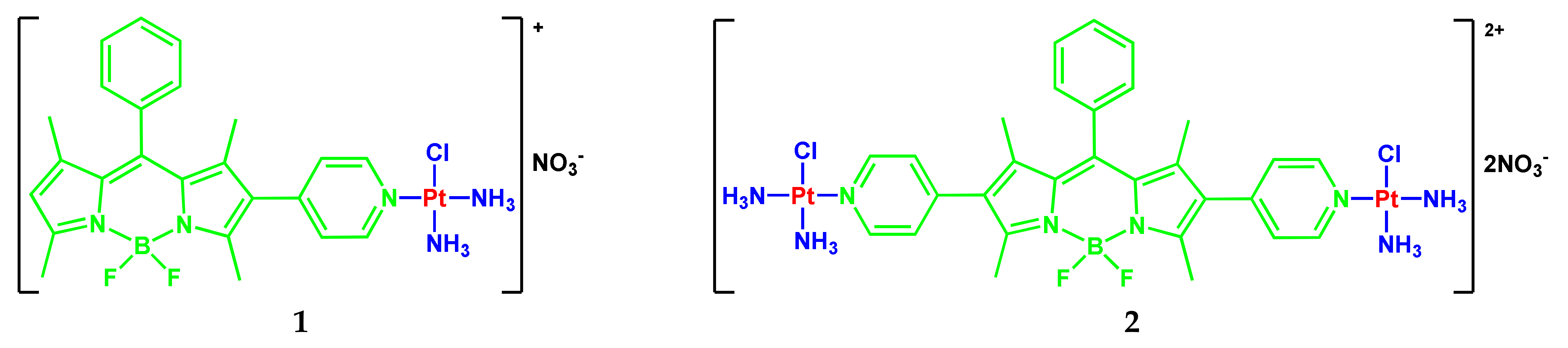
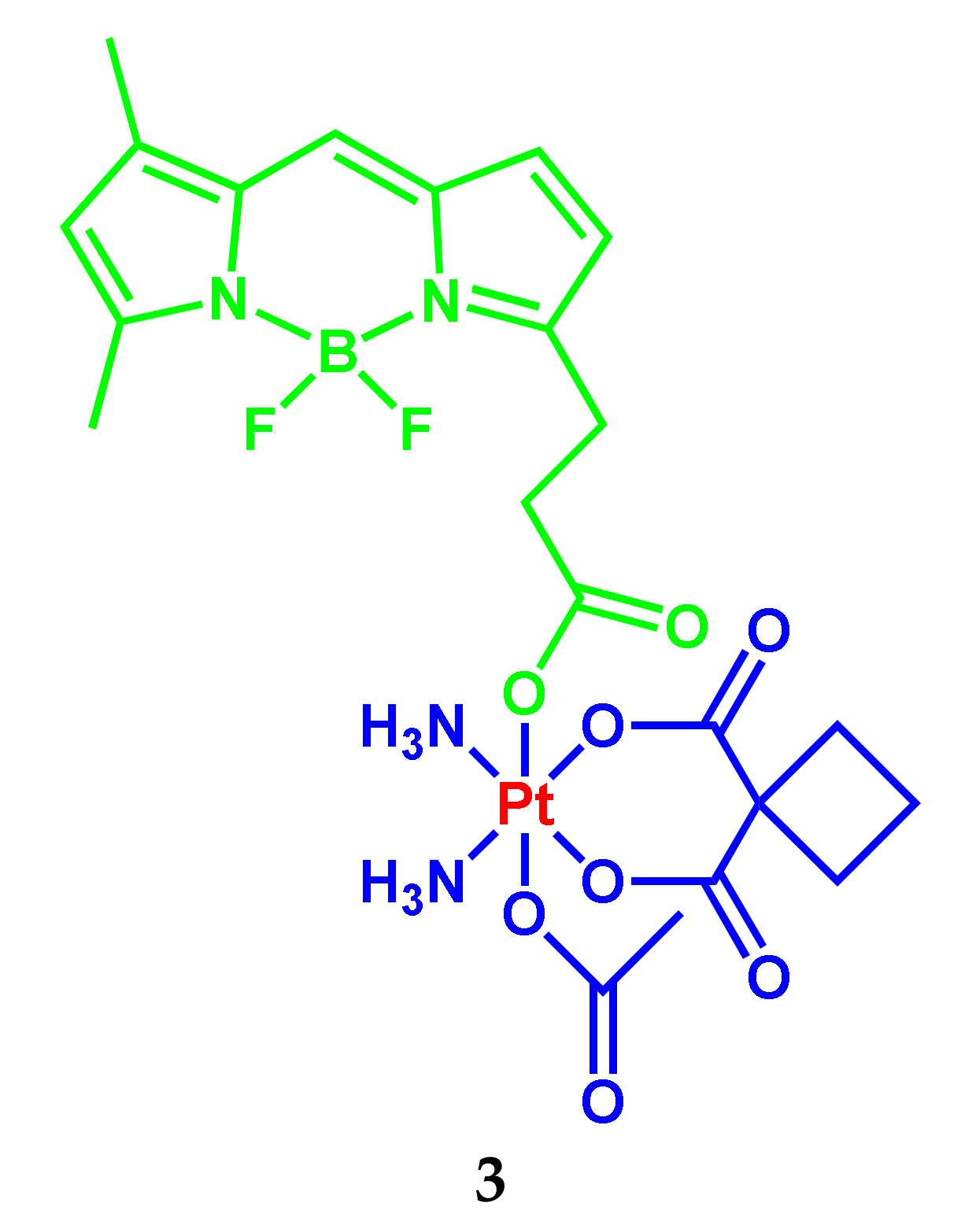
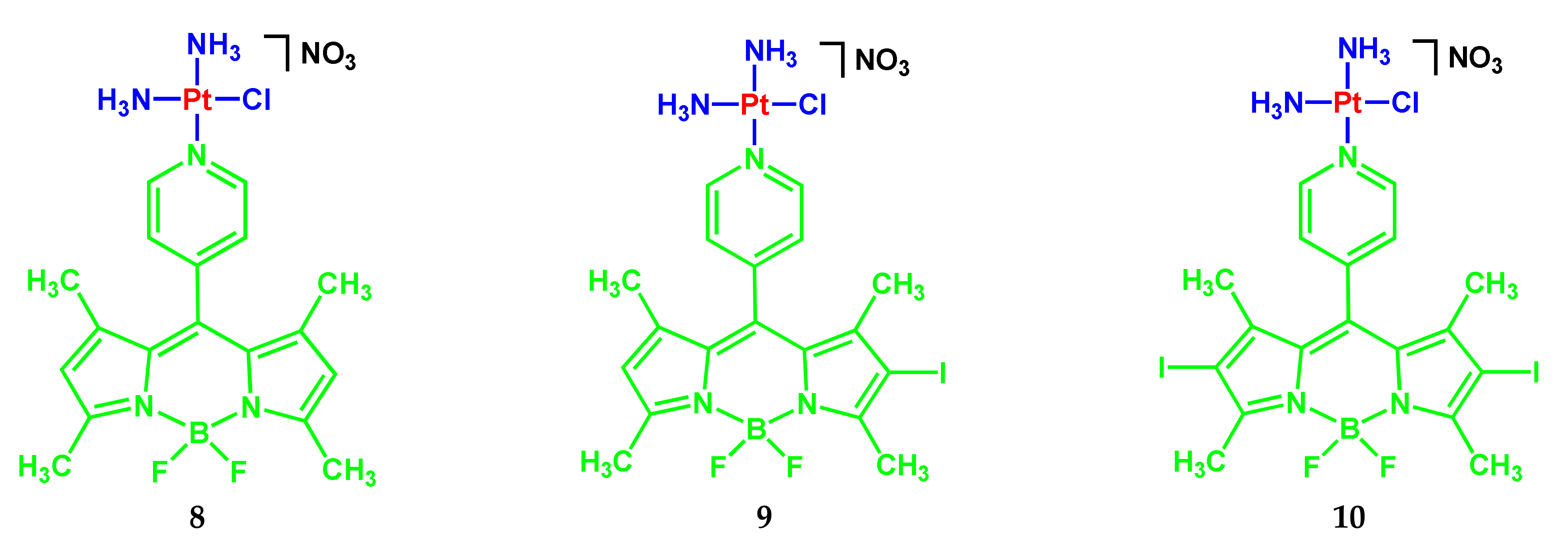
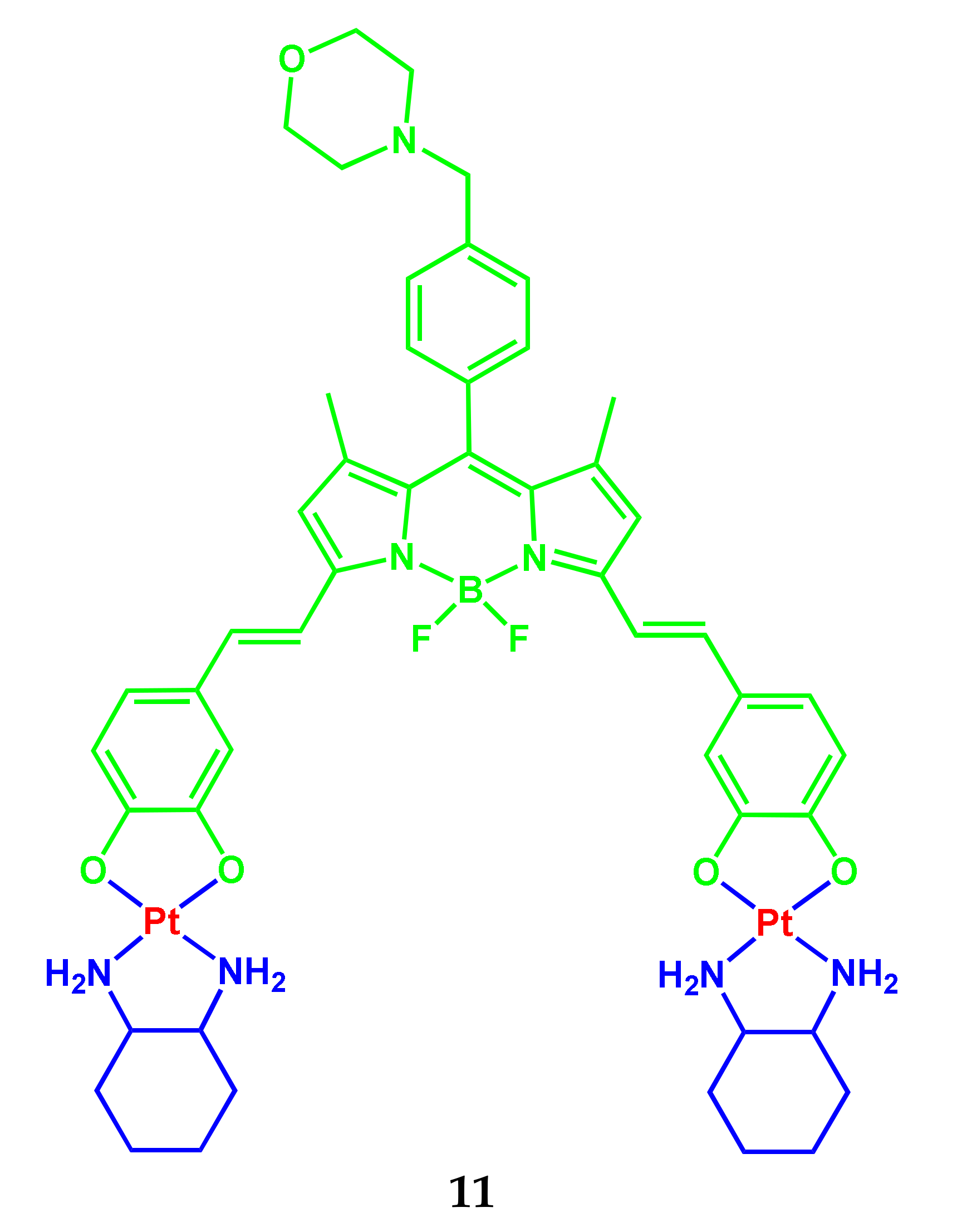
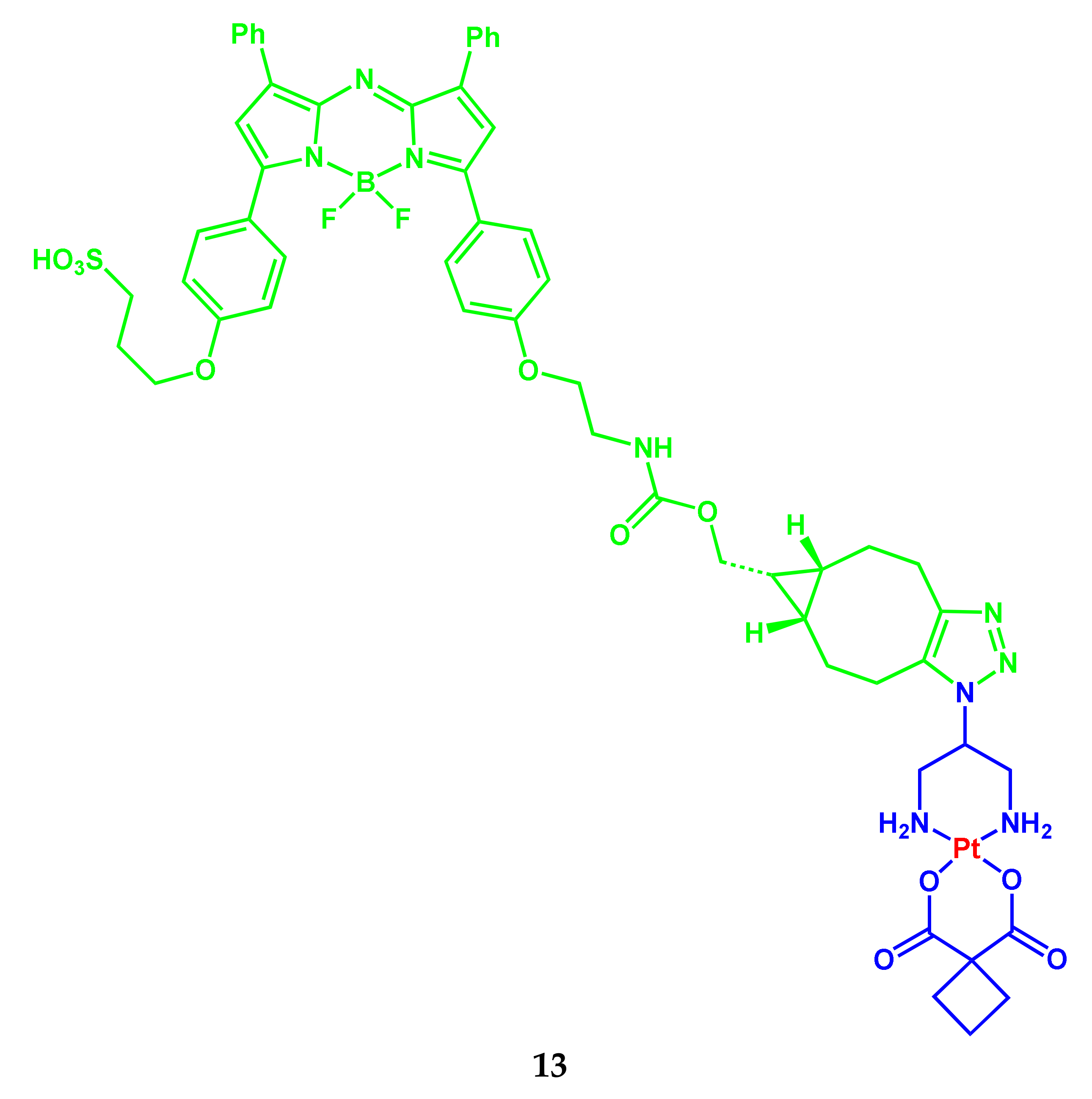
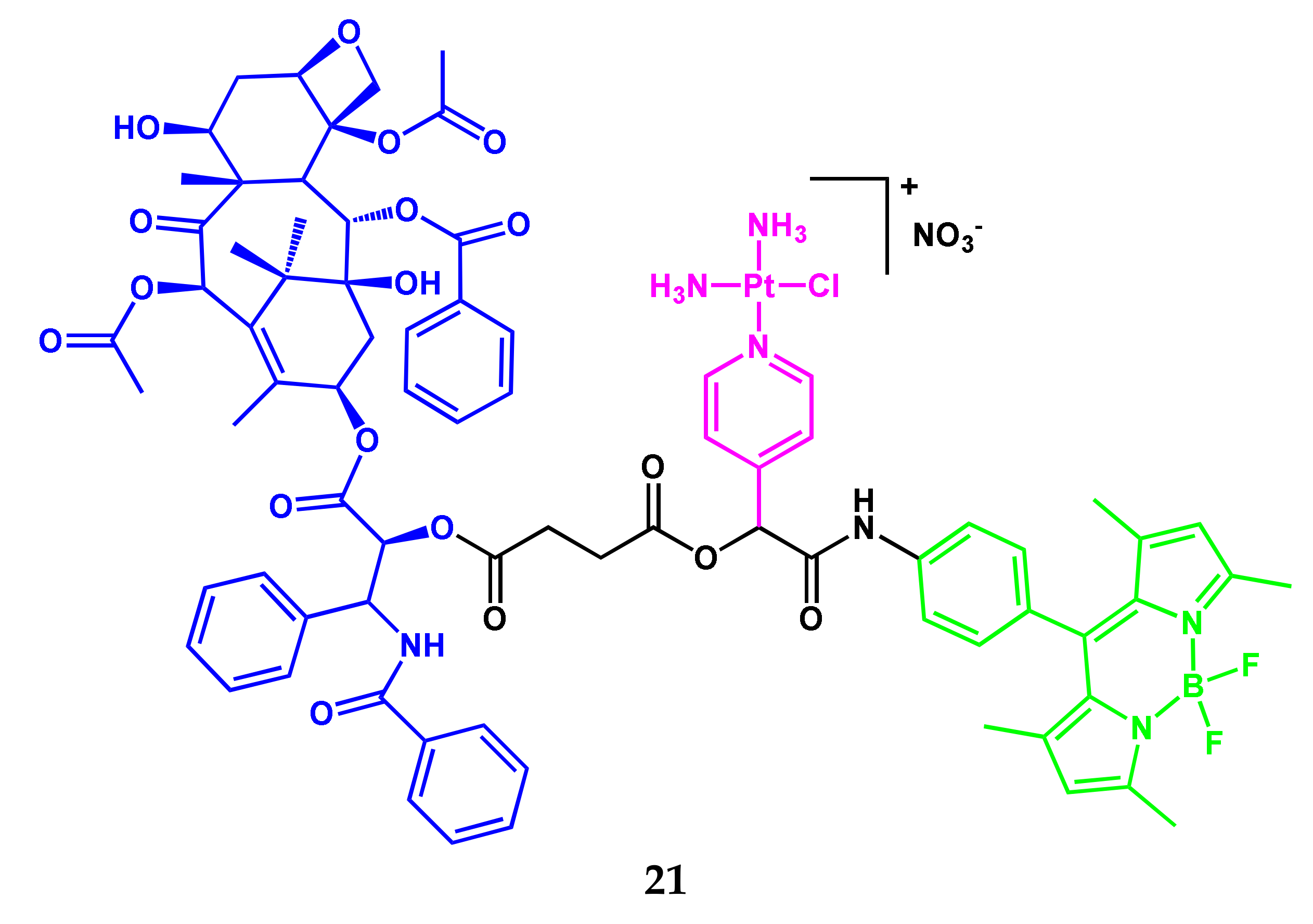
2.1. BODIPY Conjugates with Platinum Drugs
2.2. BODIPY-Conjugates with Curcumin
2.3. BODIPY-Conjugates with Paclitaxel
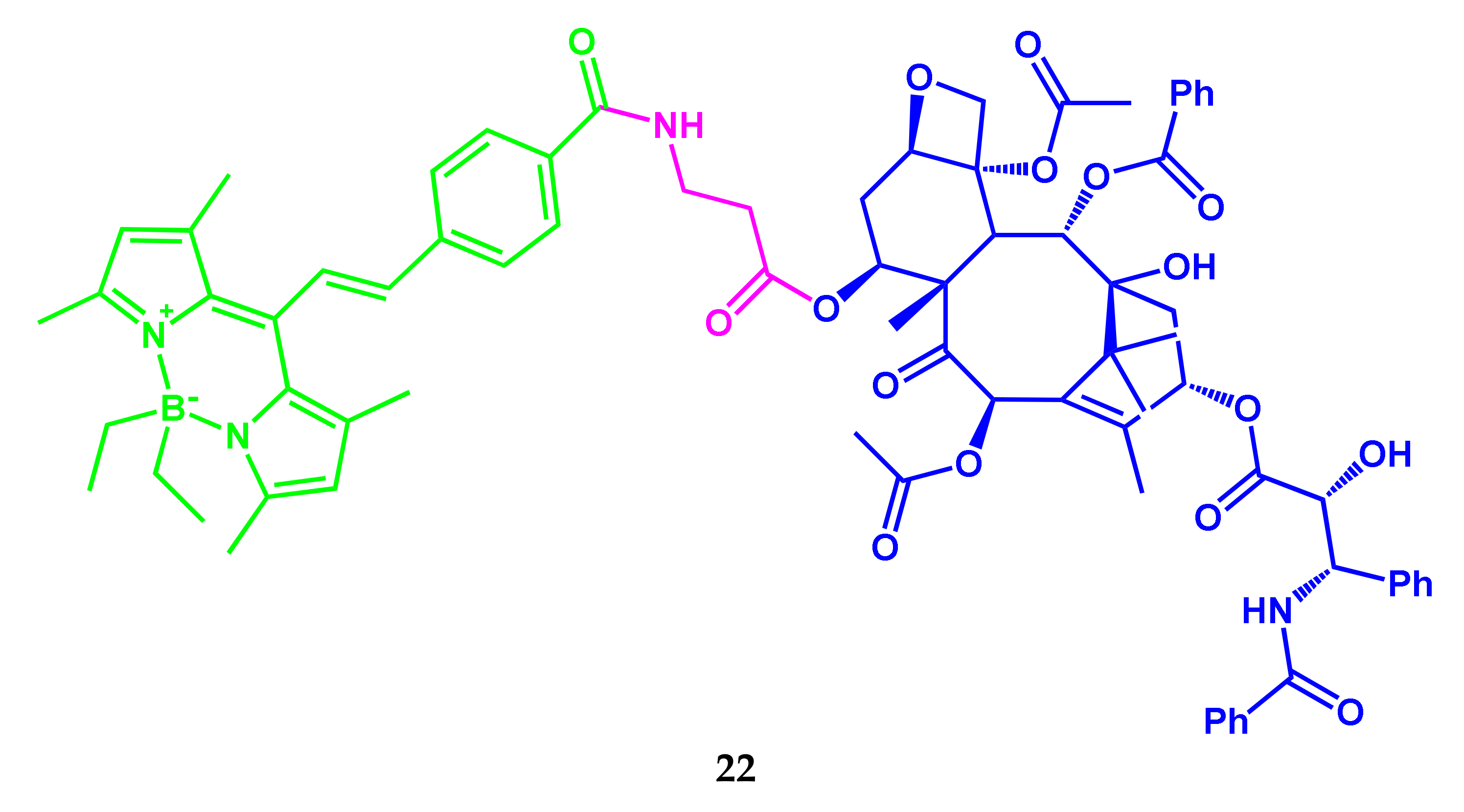
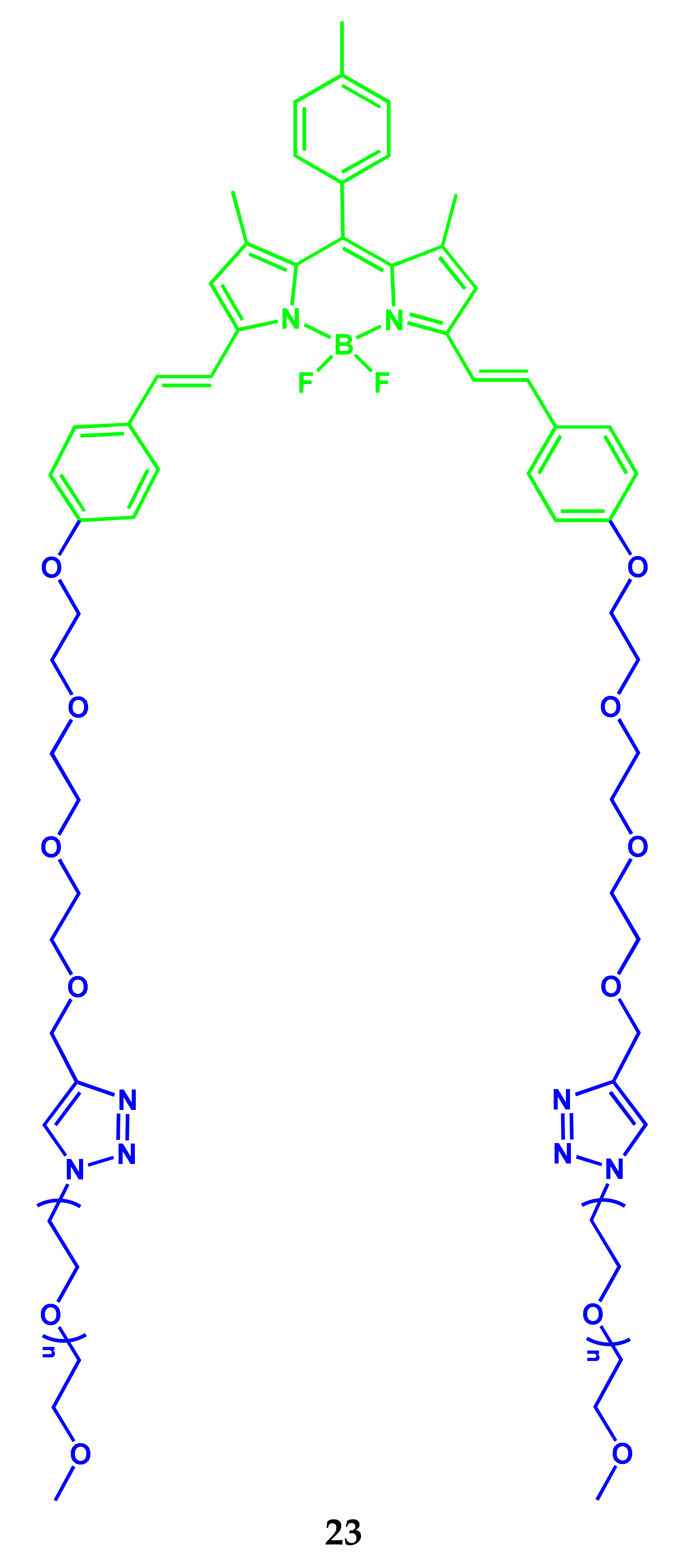
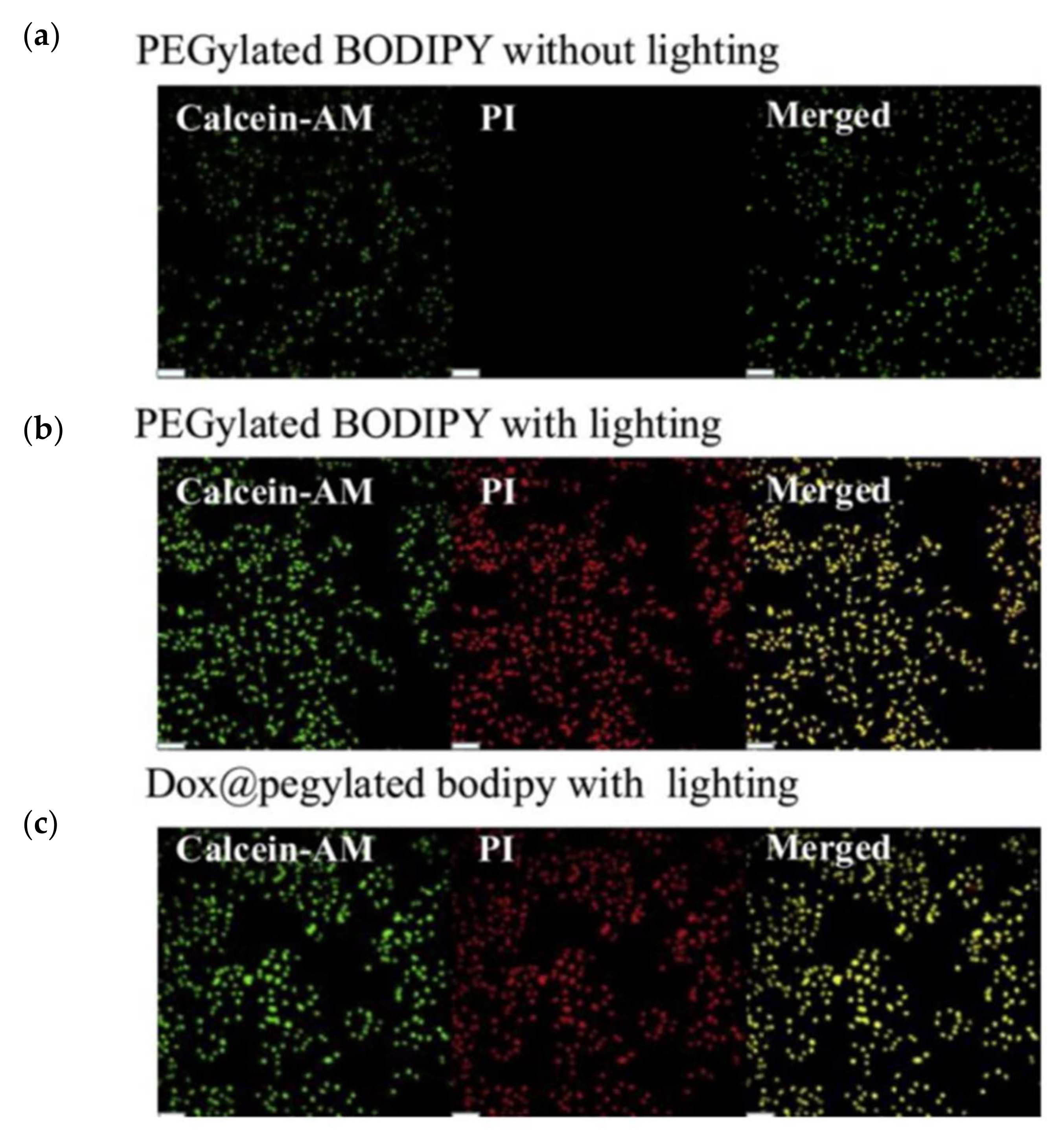
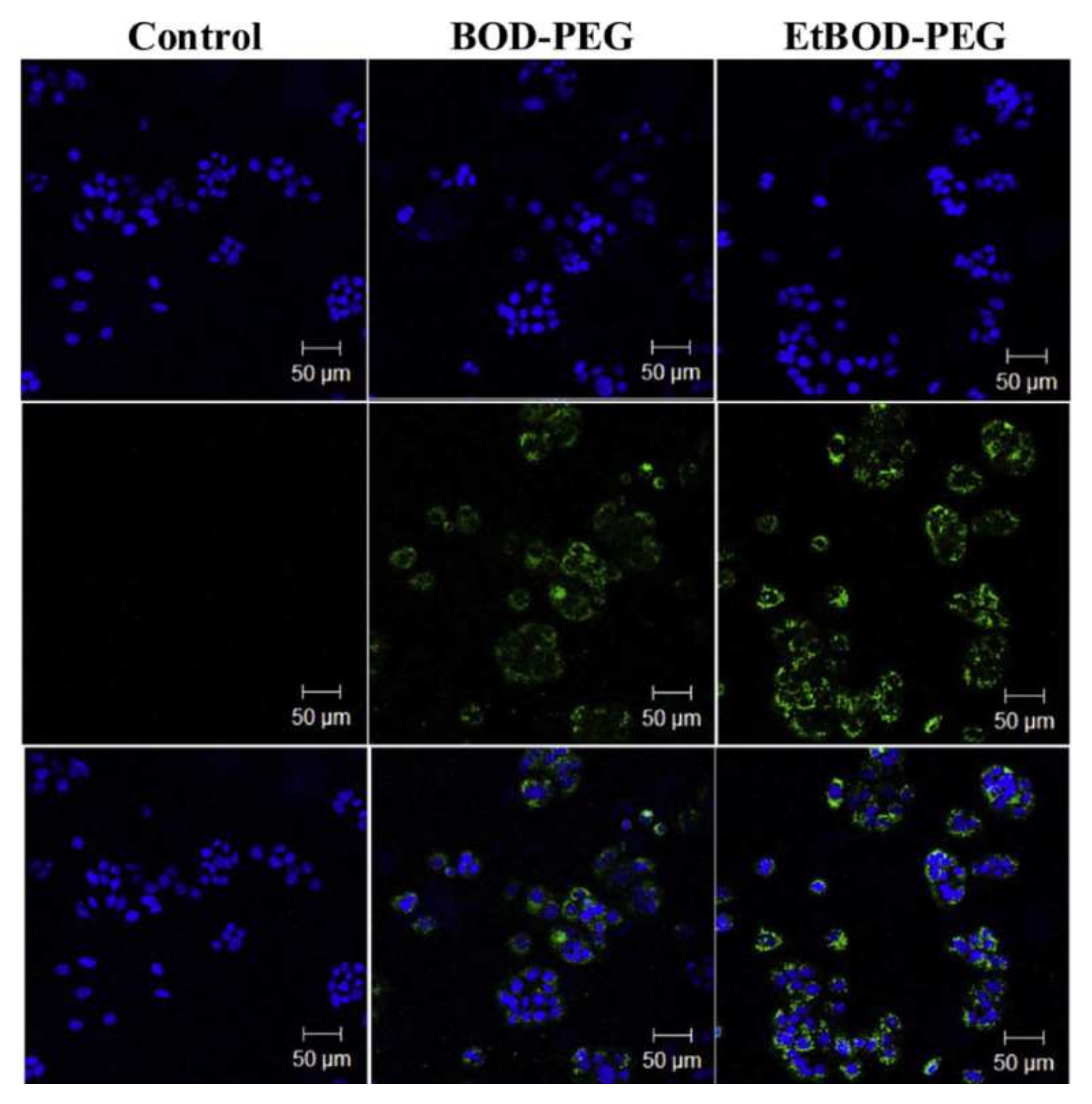
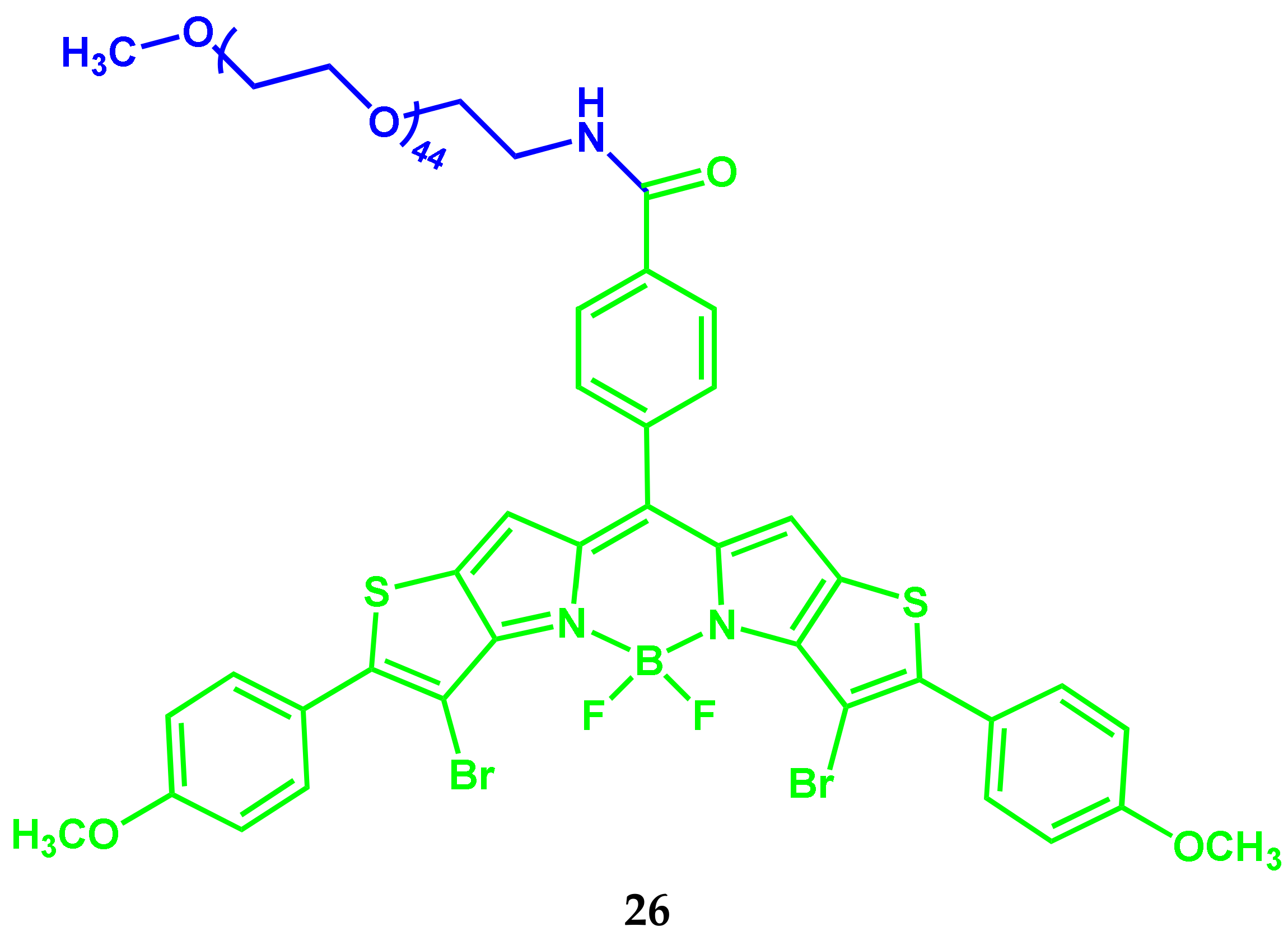
2.4. BODIPY-PEG Conjugates
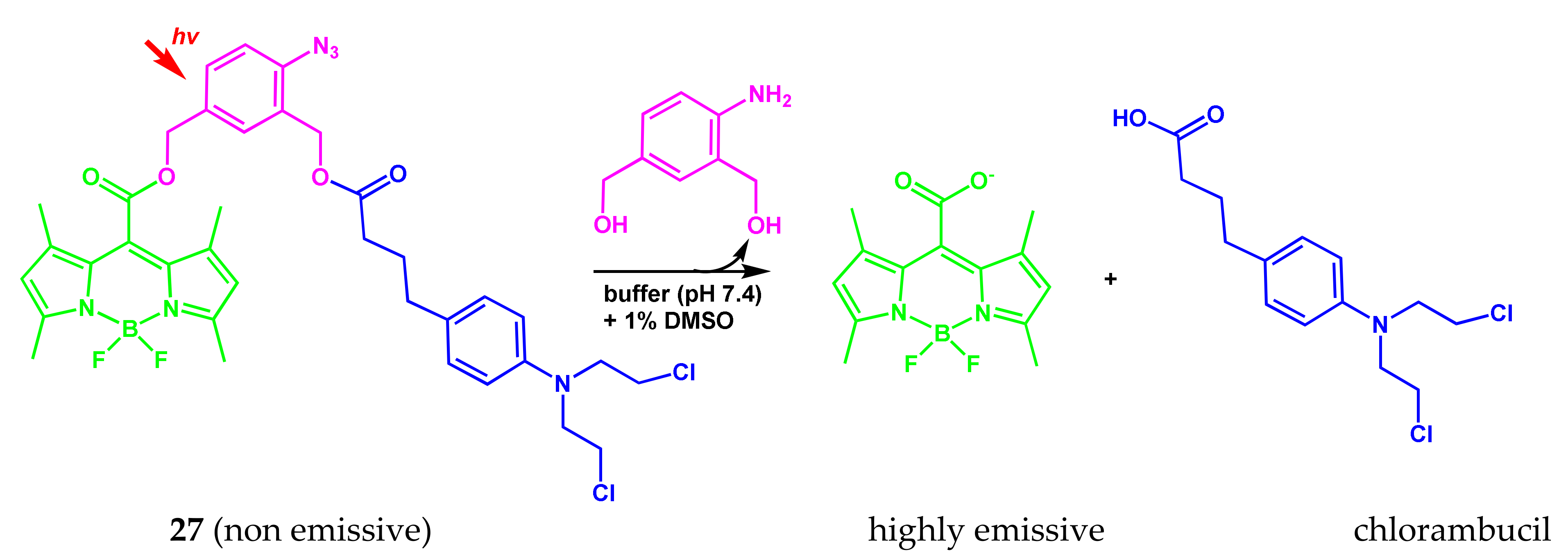
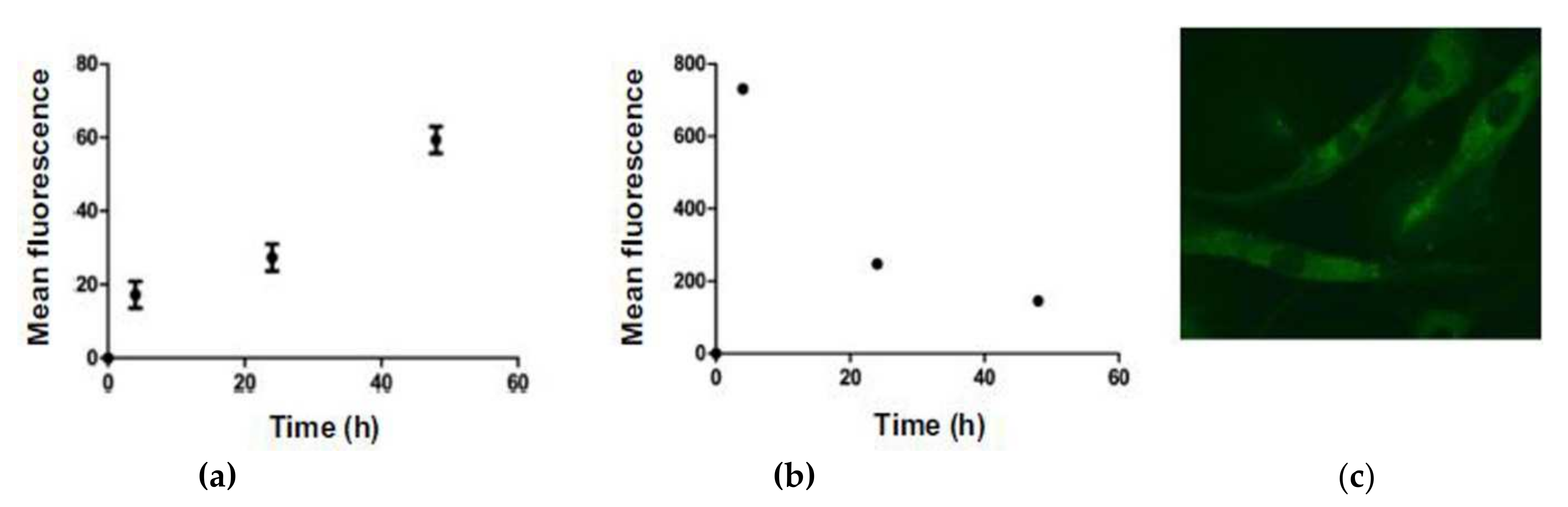
2.5. BODIPY Conjugate with Chlorambucil
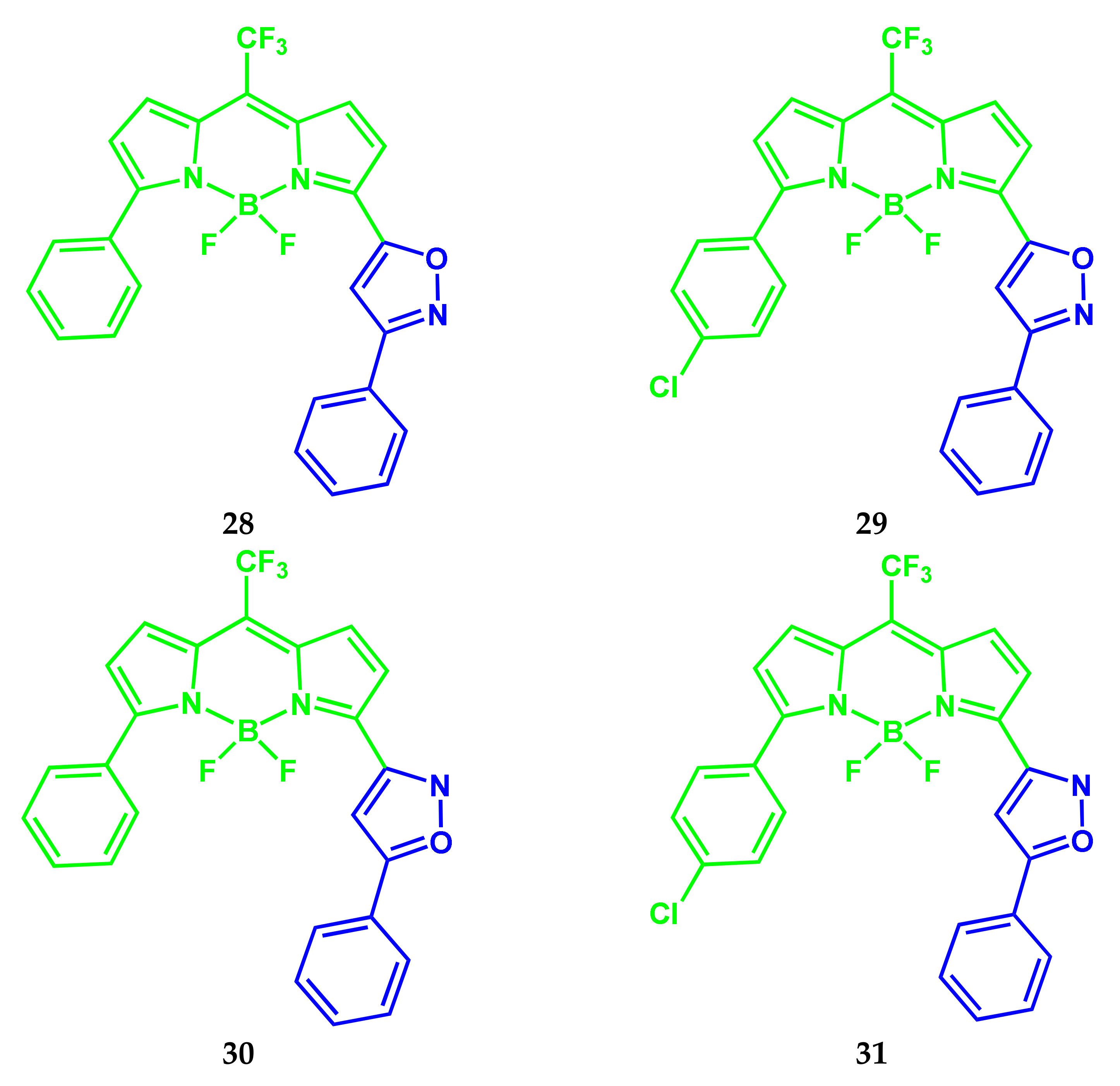
2.6. BODIPY Conjugates with Isoxazole
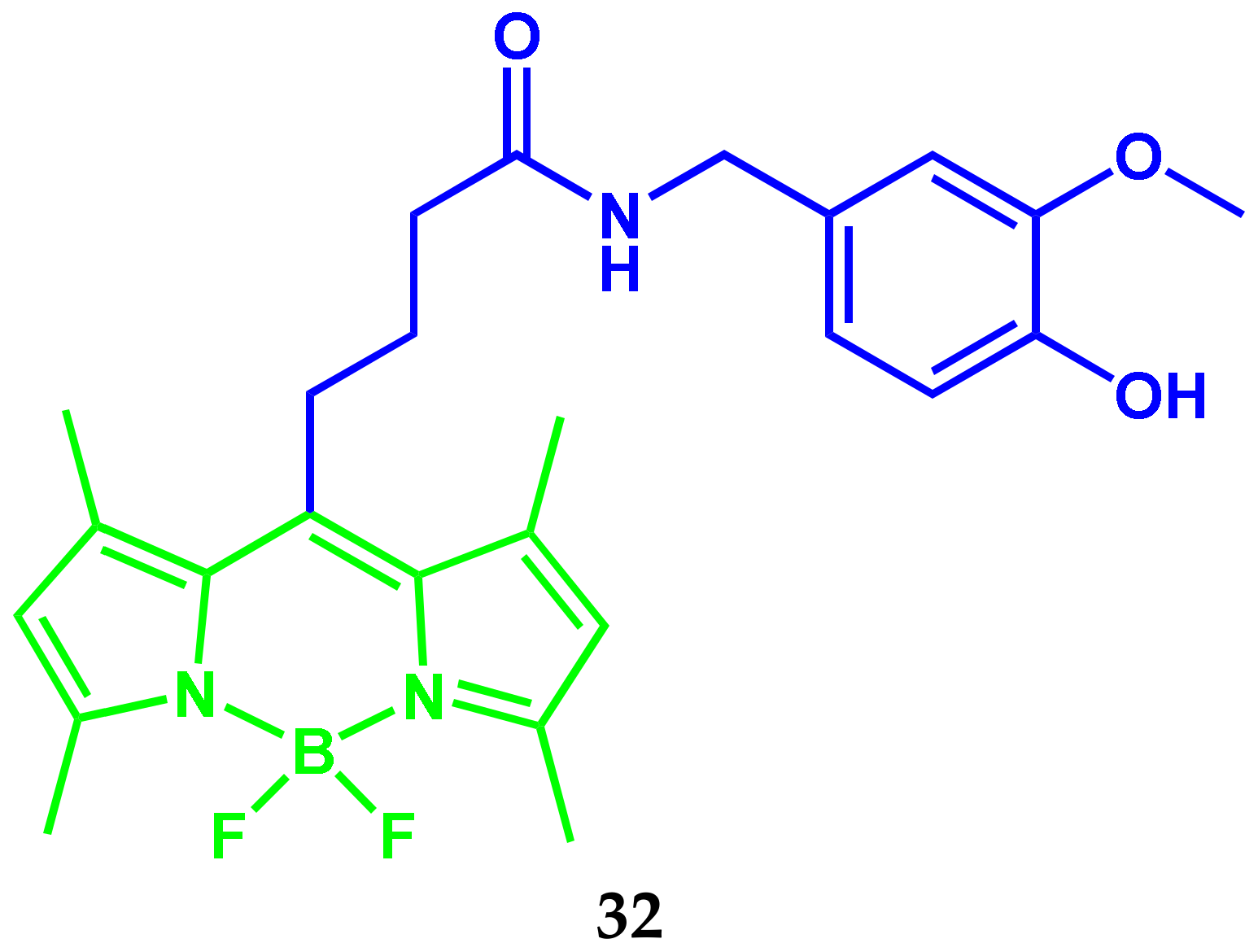
2.7. BODIPY Conjugate with Capsaicin
2.8. BODIPY Conjugates with Mertansine
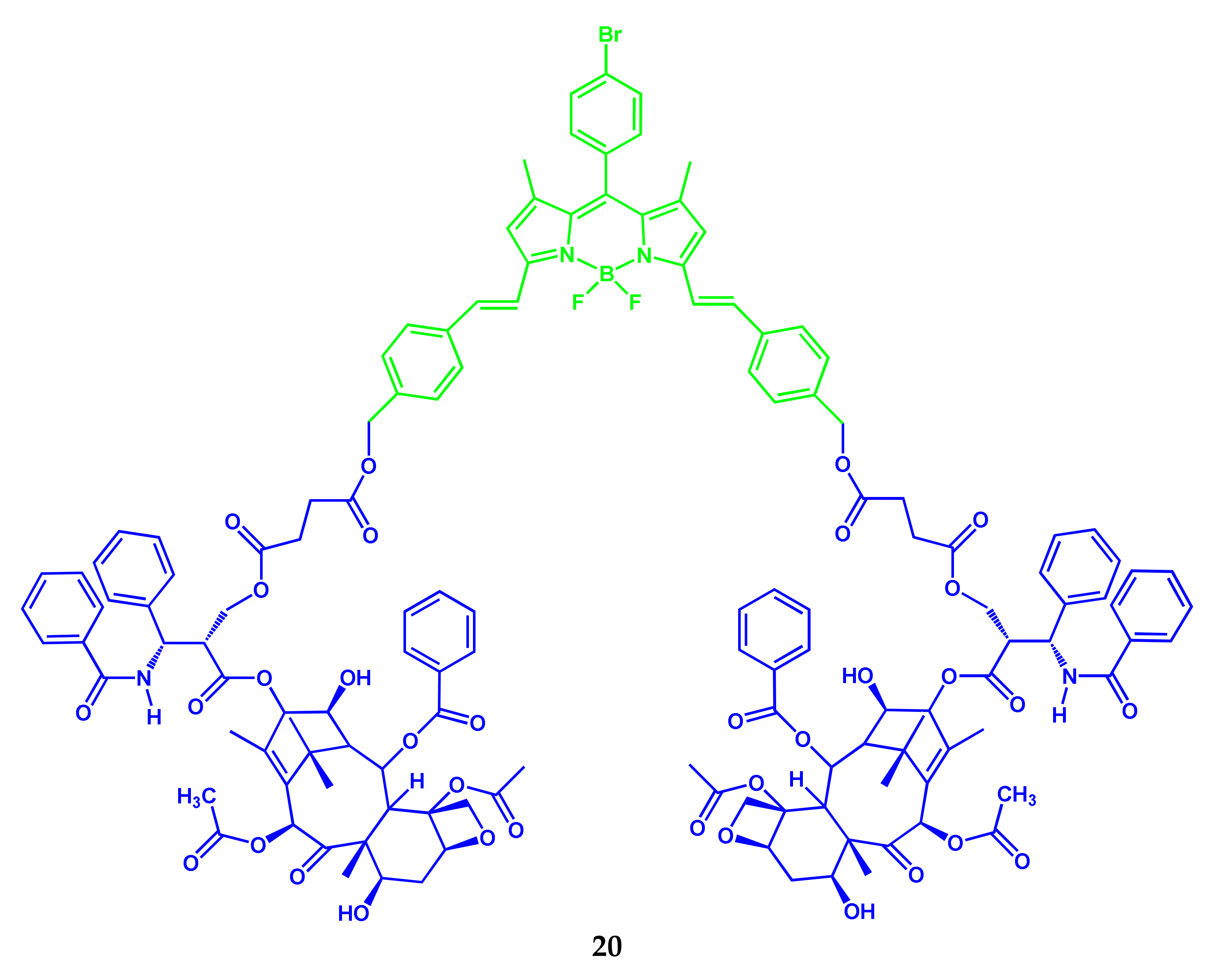
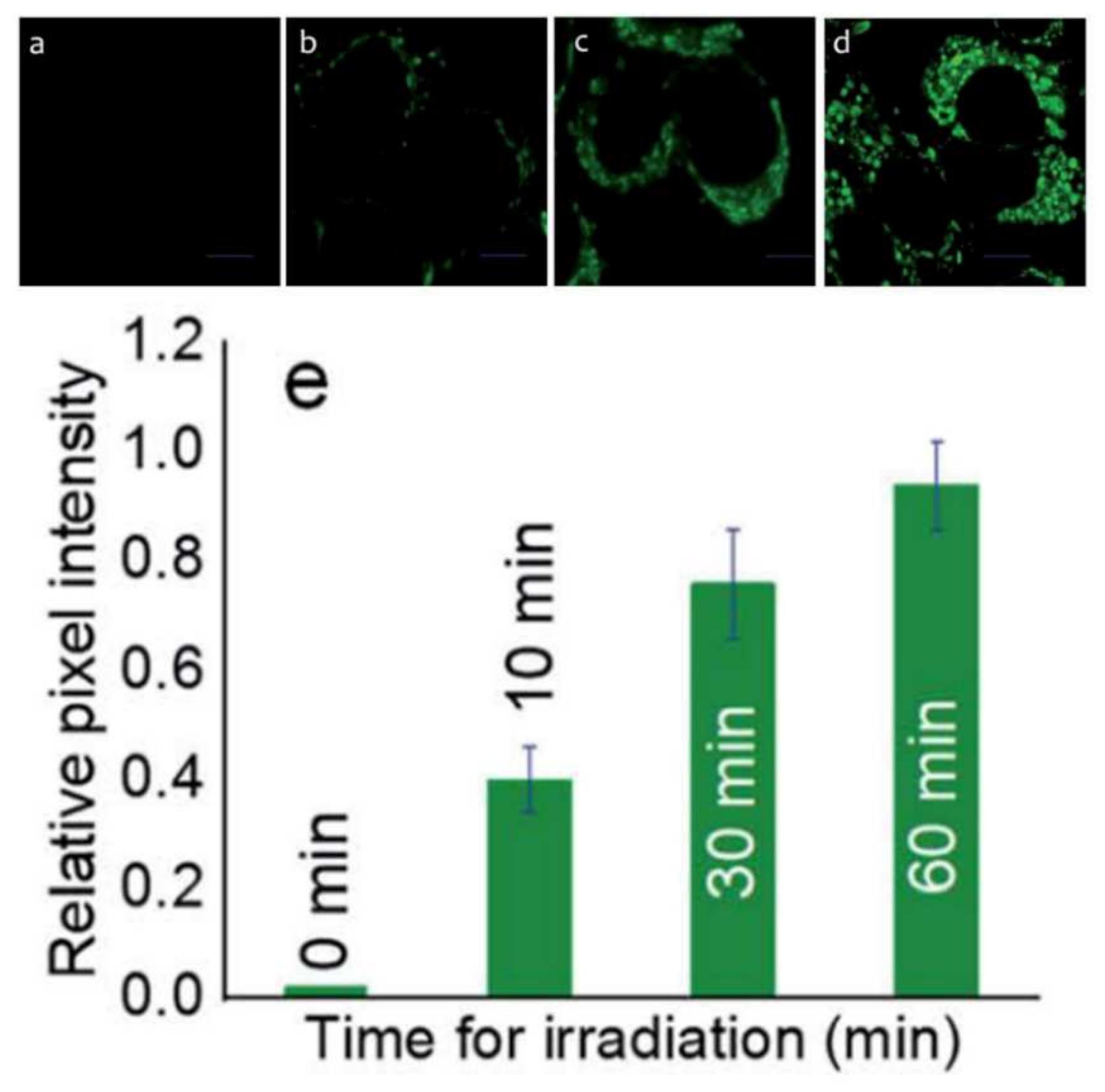
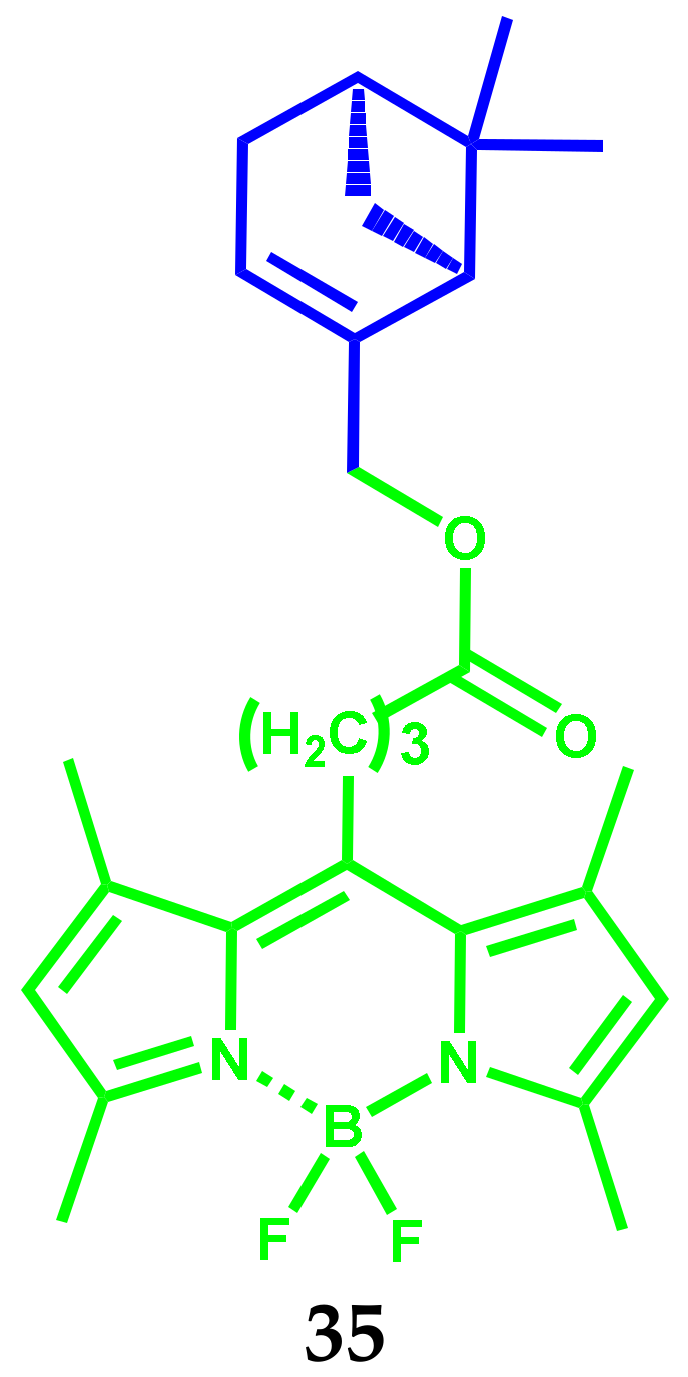
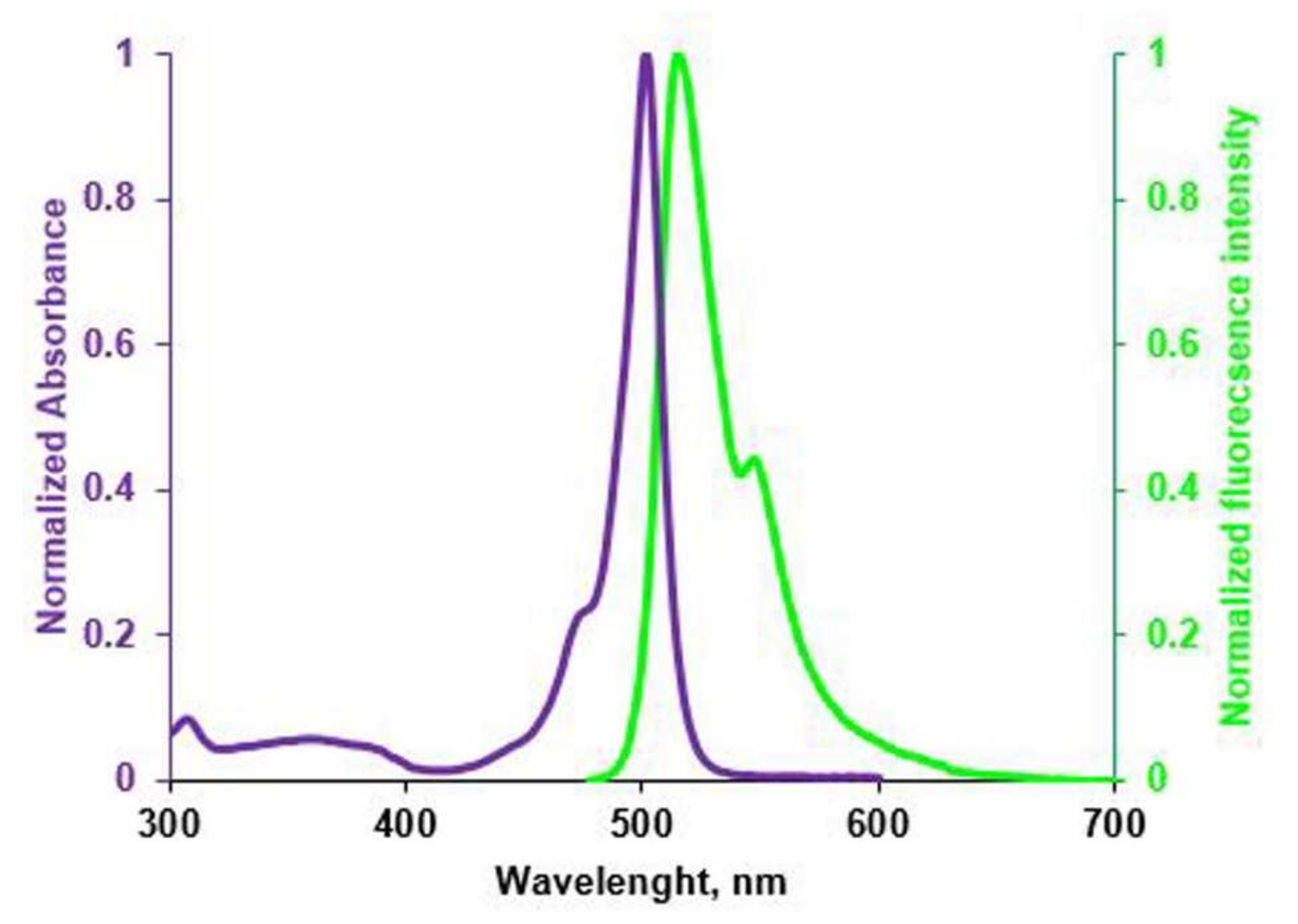
2.9. BODIPY Conjugates with Terpenes
3. BODIPY Conjugates for Bioimaging
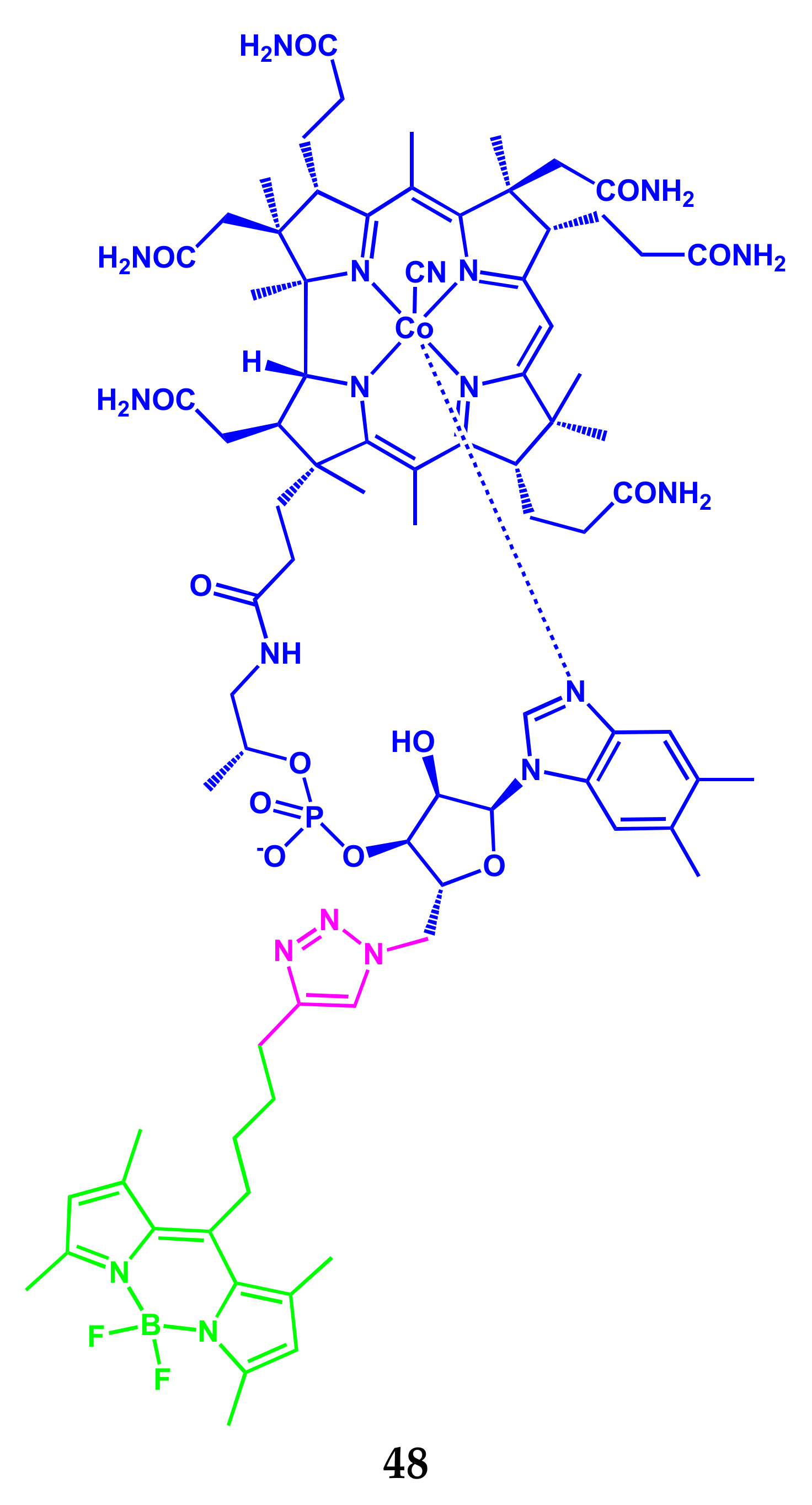
3.1. BODIPY-Conjugates with Vitamins
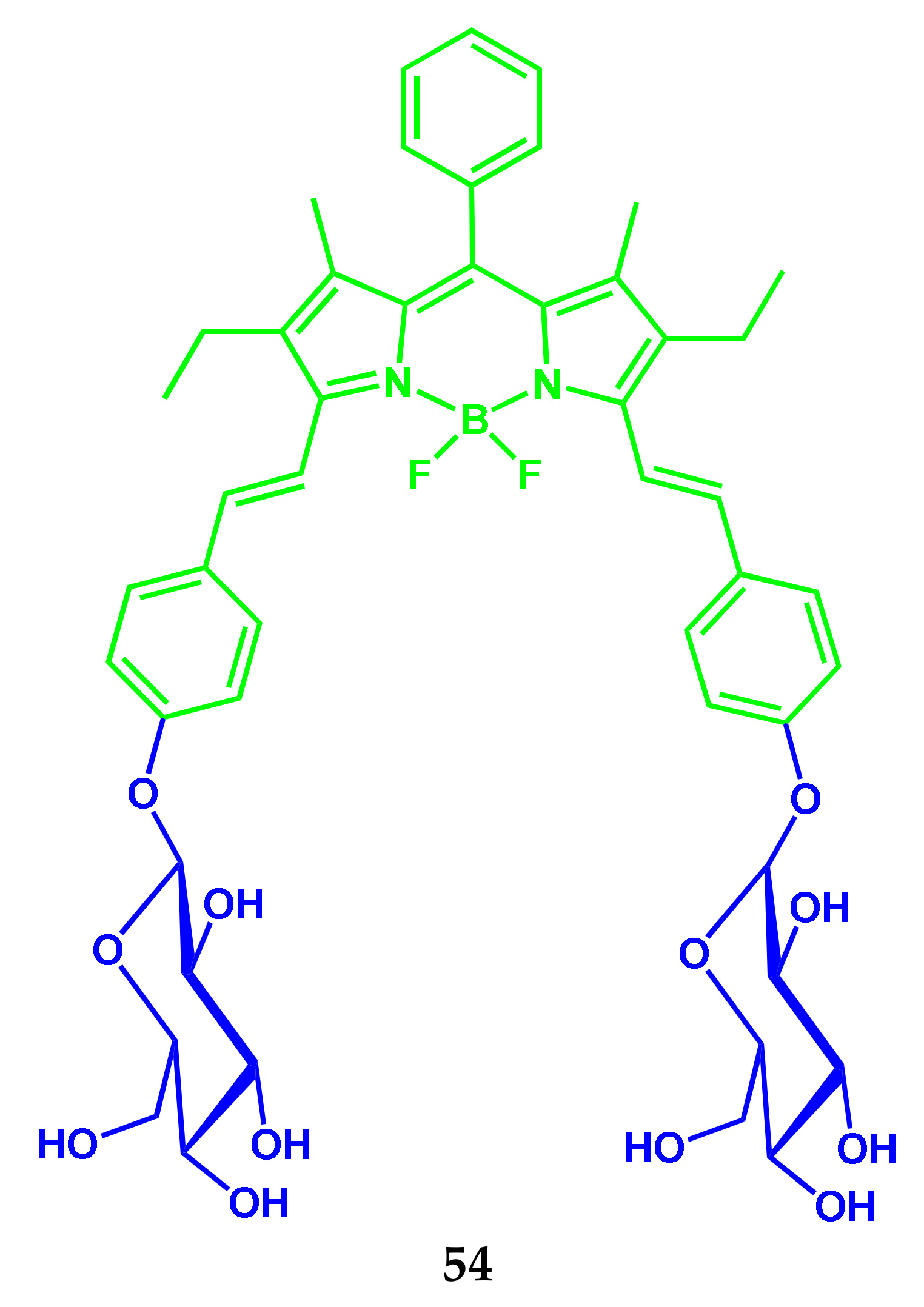
3.2. BODIPY Conjugates with Carbohydrates
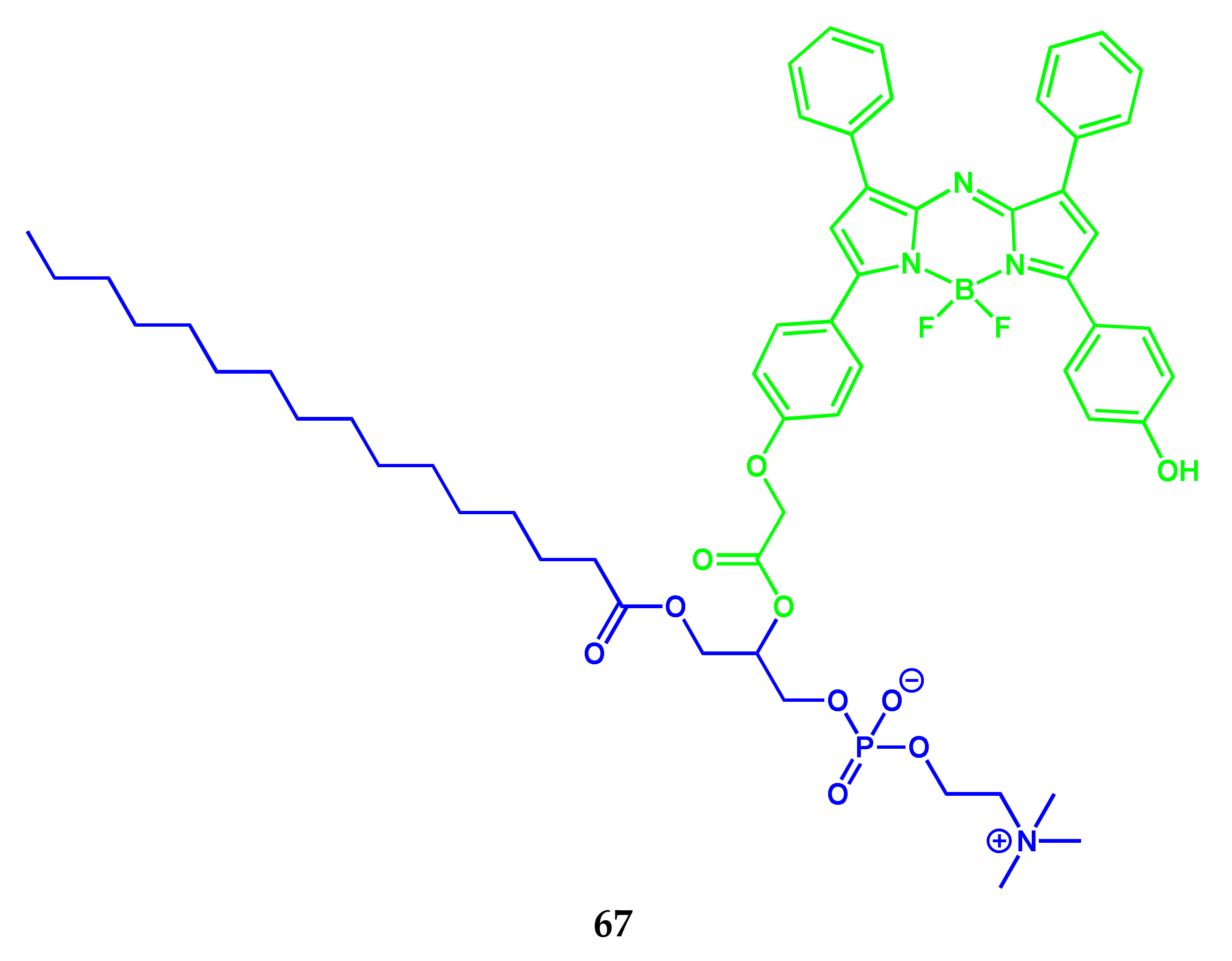
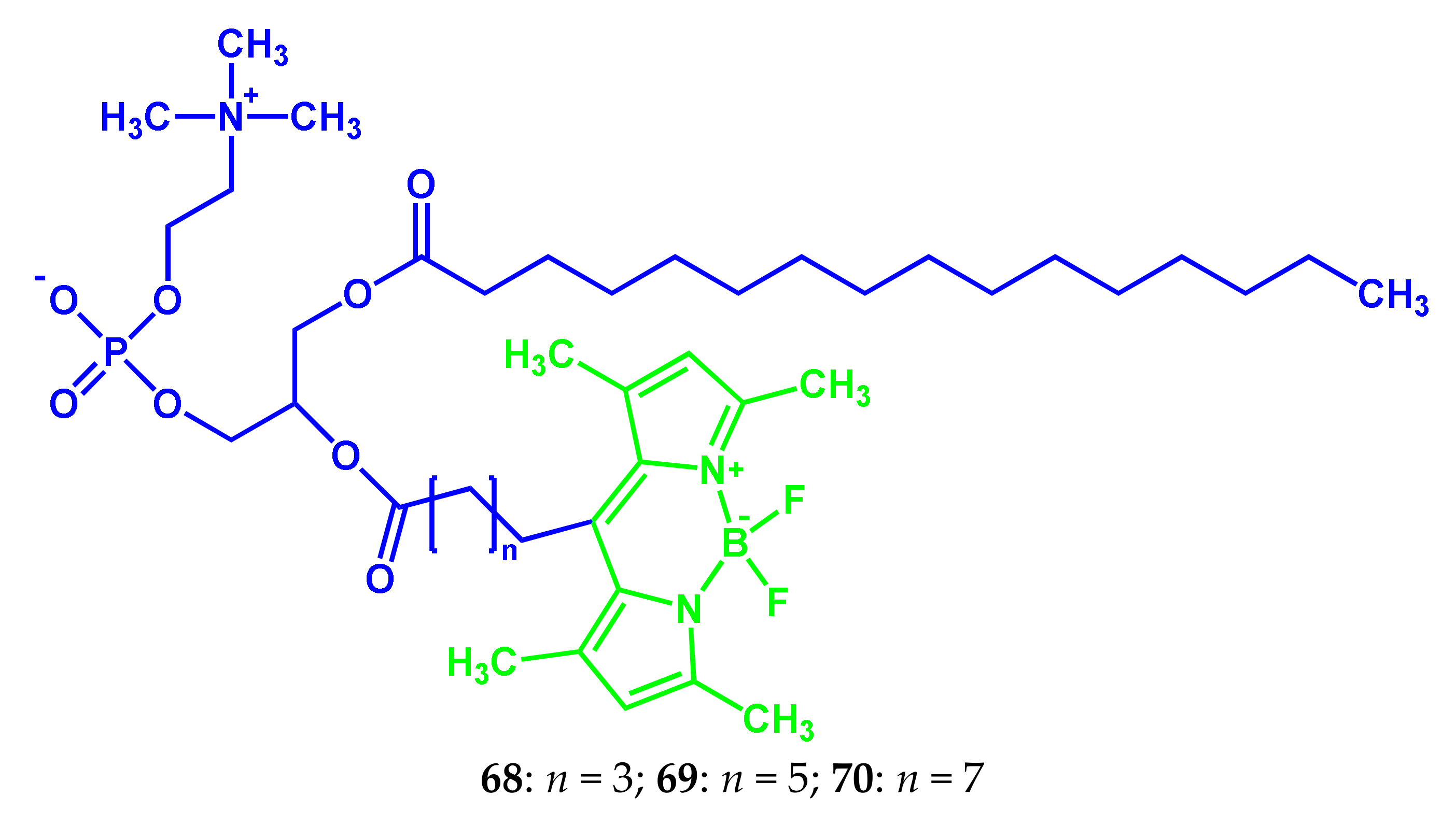
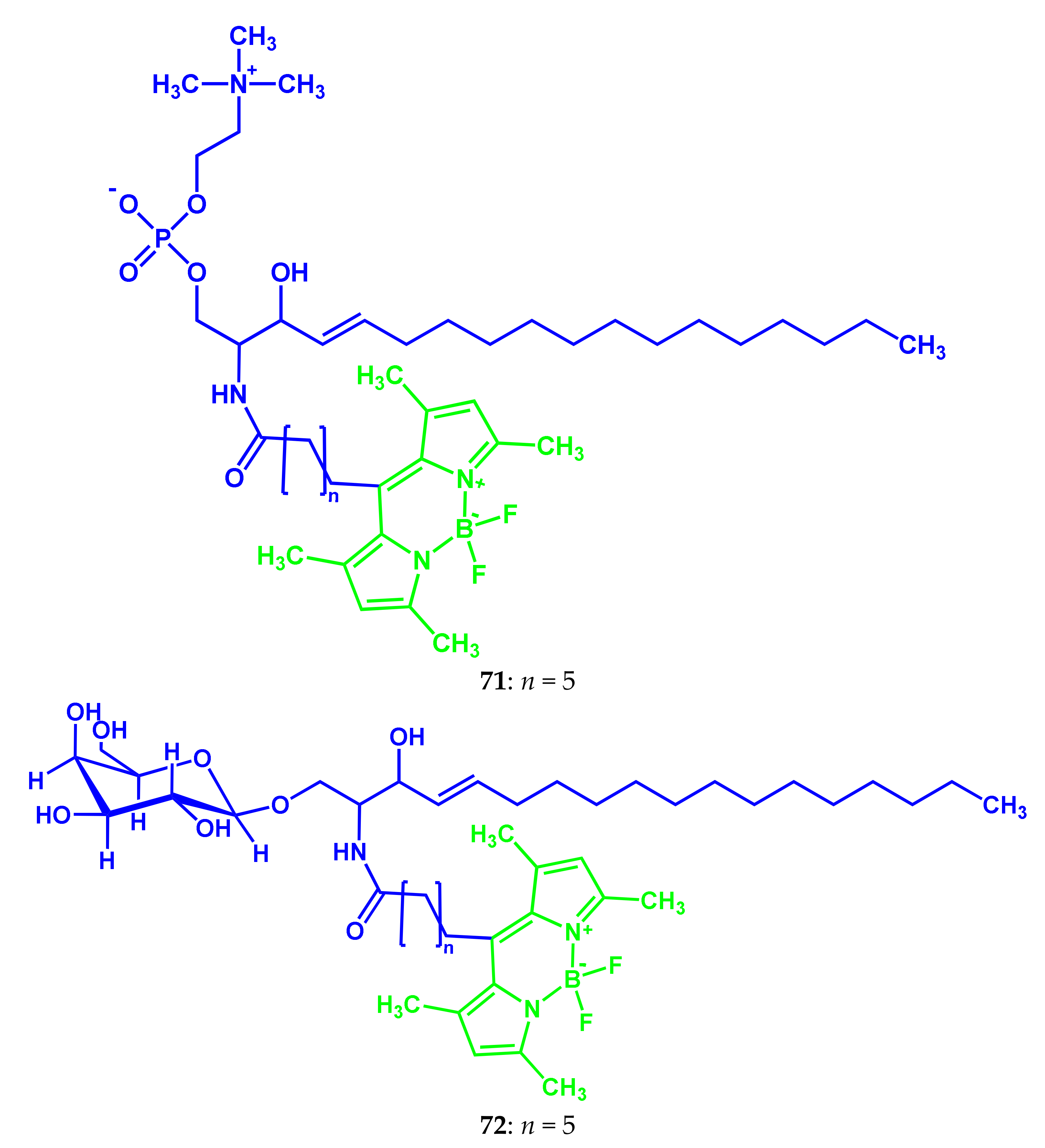
3.3. BODIPY Conjugates with Lipids
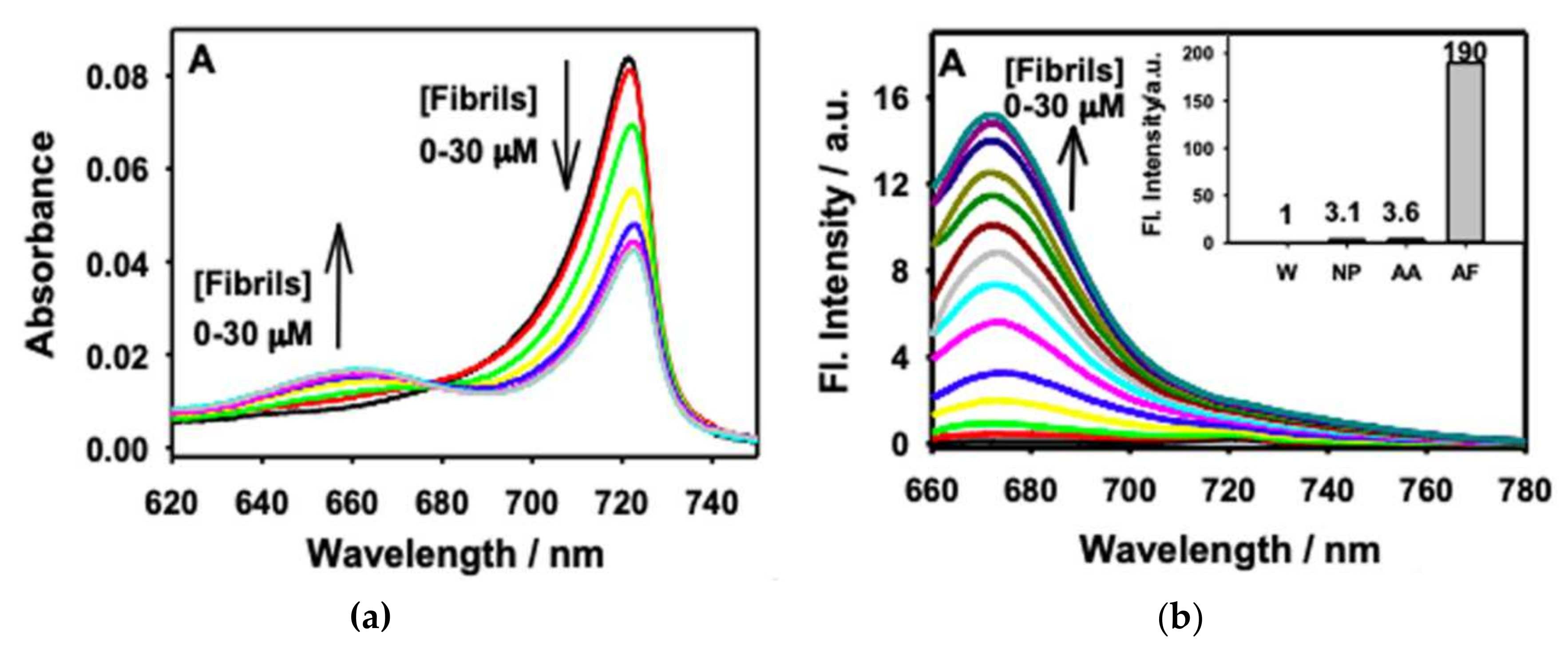
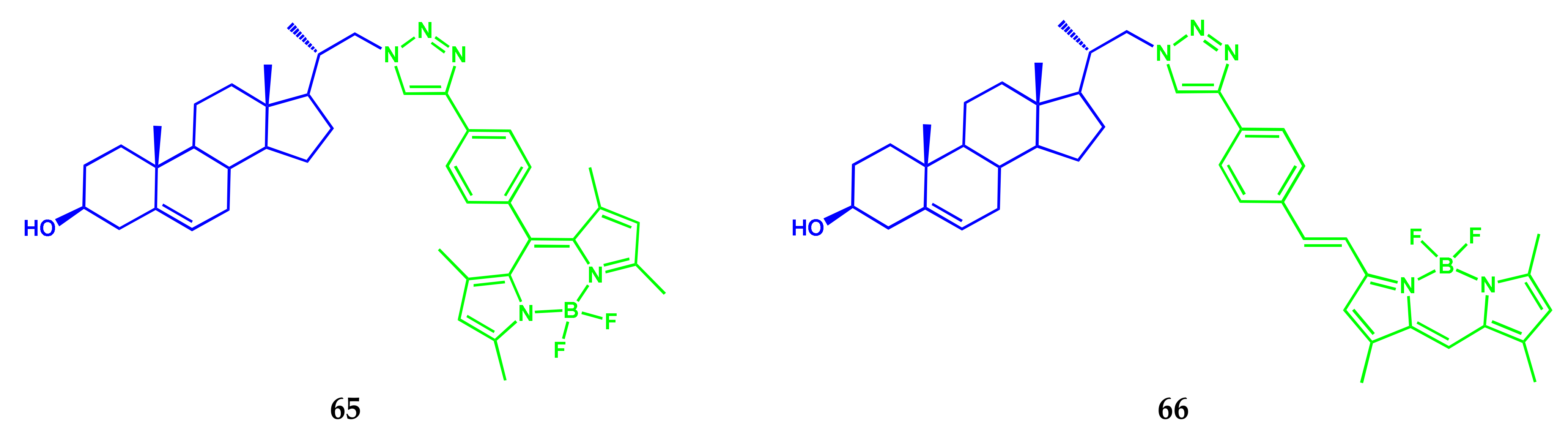
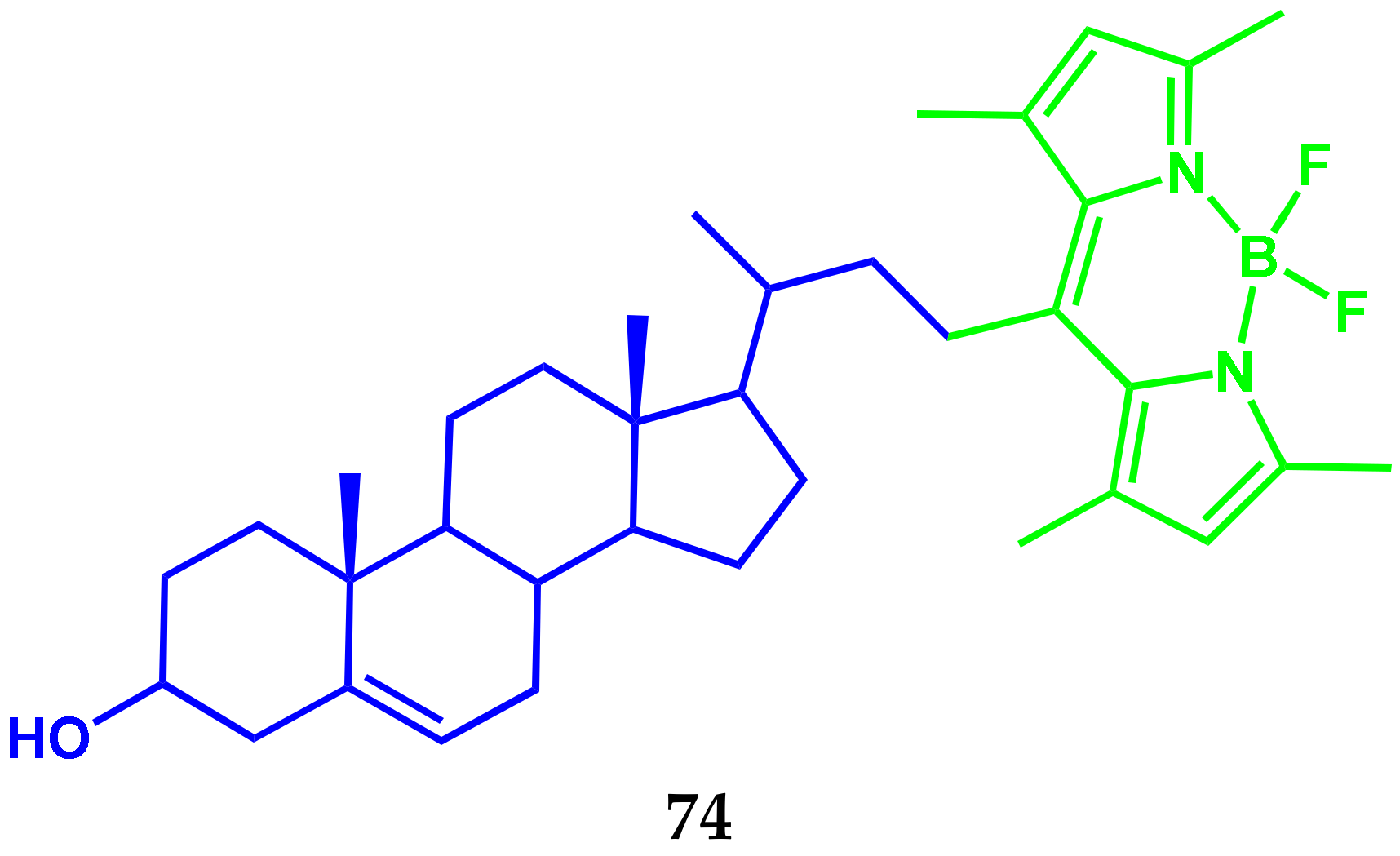
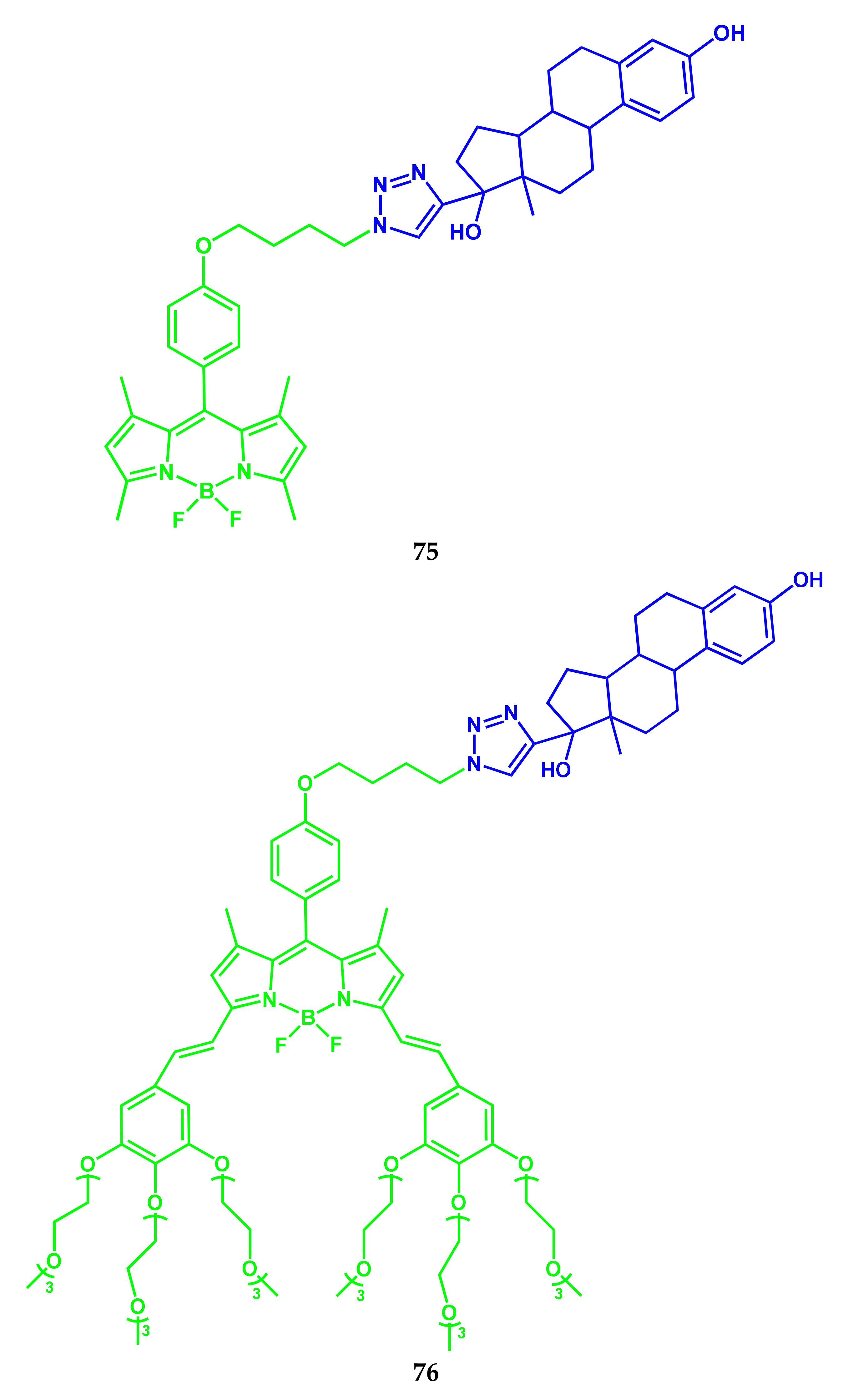
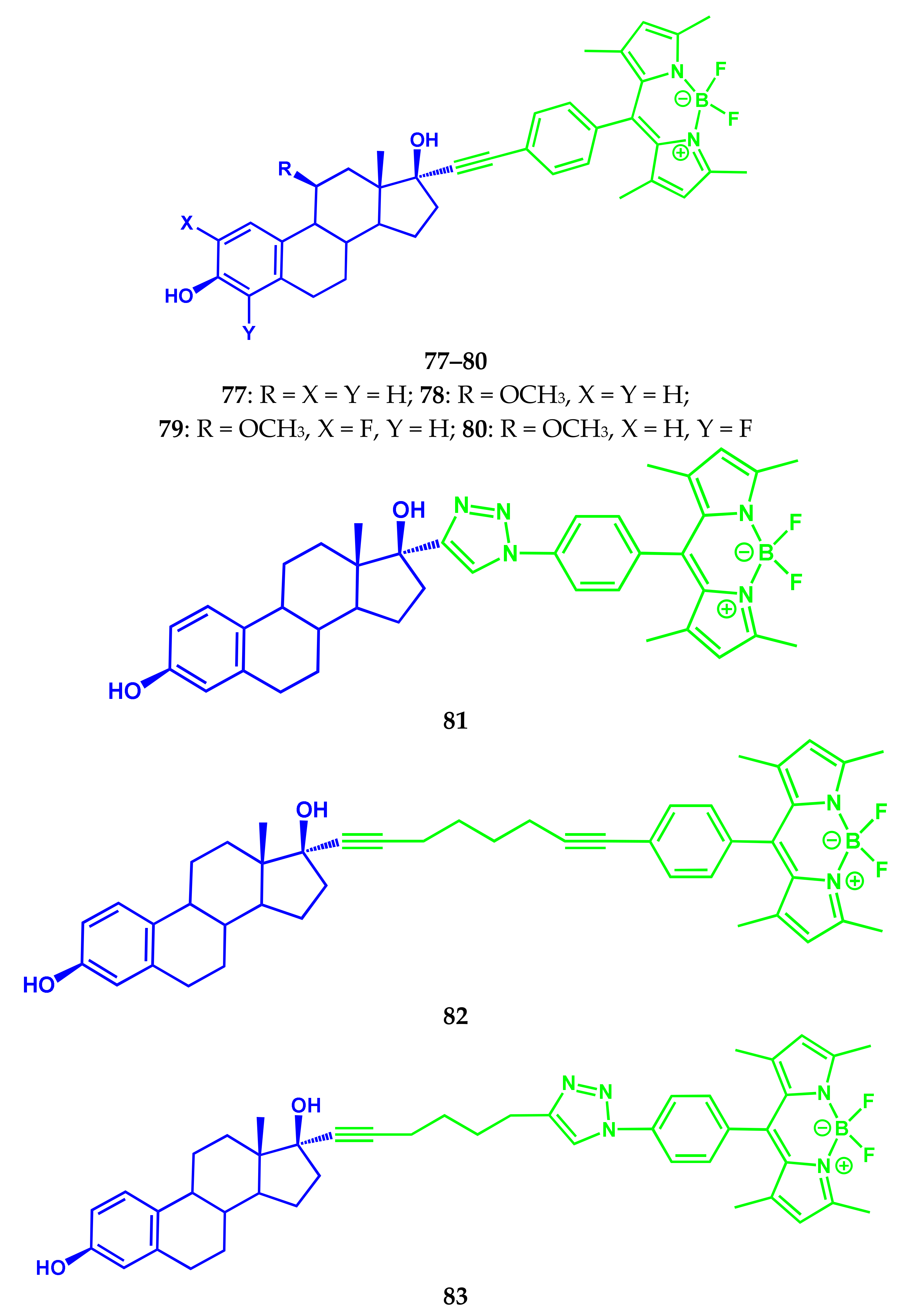
3.4. BODIPY Conjugates with Steroids
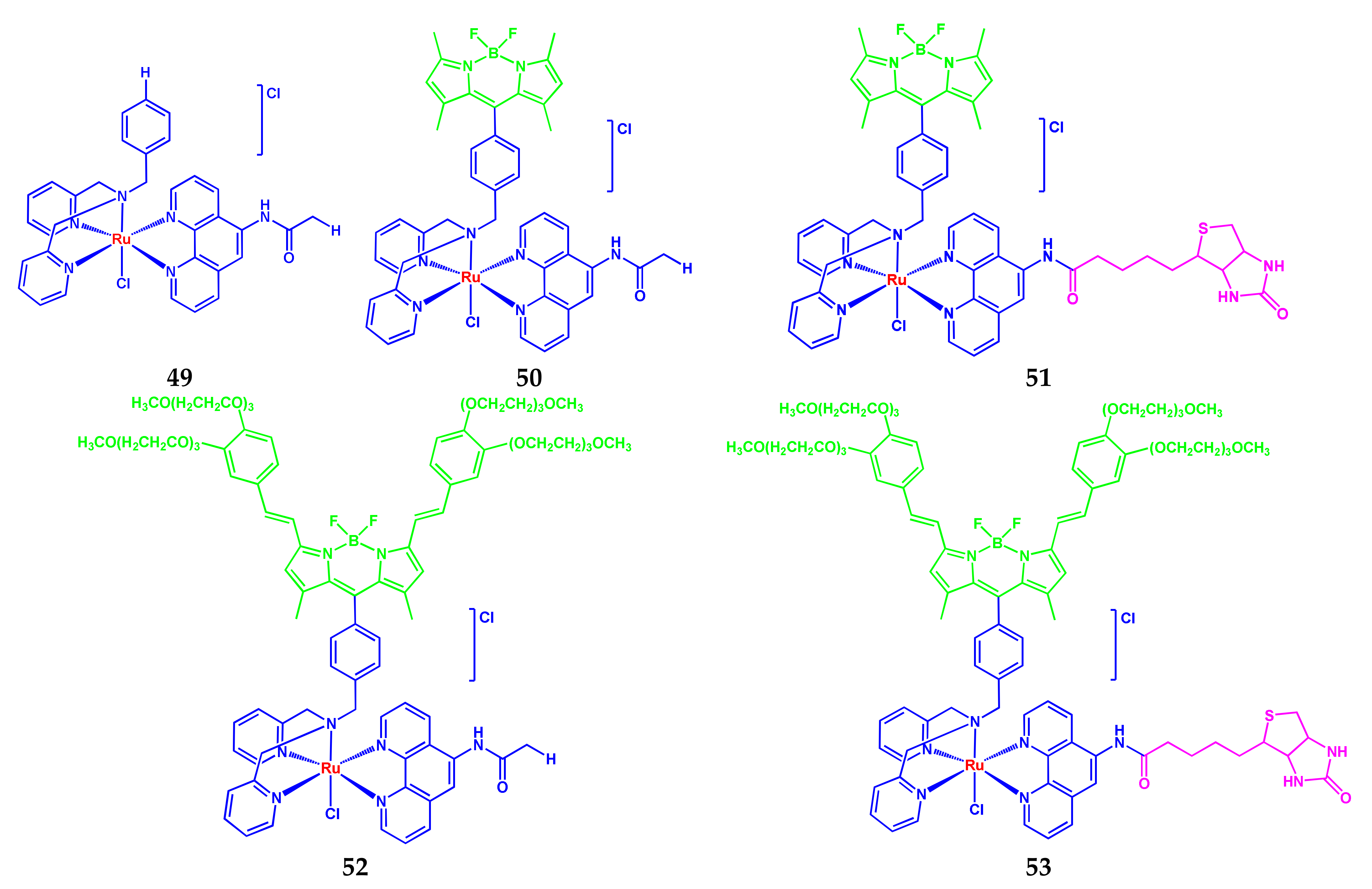
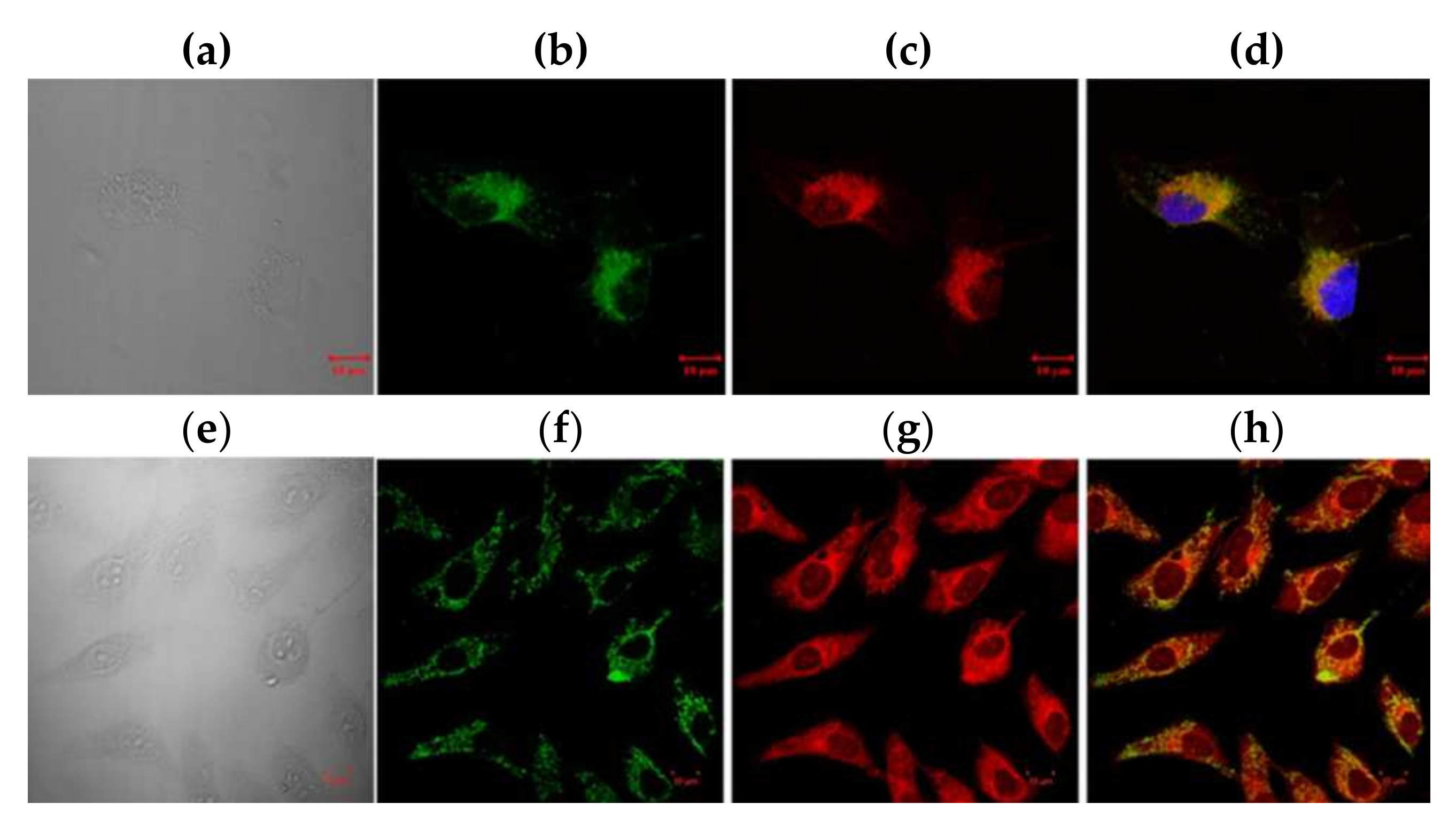
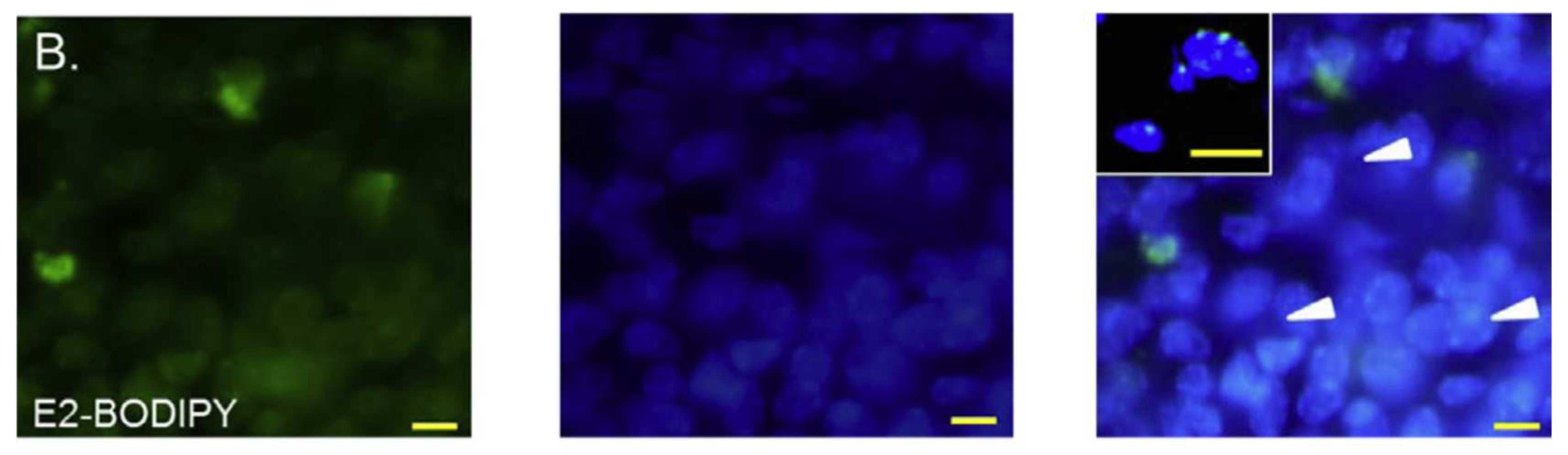
3.5. BODIPY Conjugate with Human Epidermal Growth Factor Receptor-2
4. BODIPY Conjugates with Chromo- and Fluorophores as Diagnostic and Treatment Agents
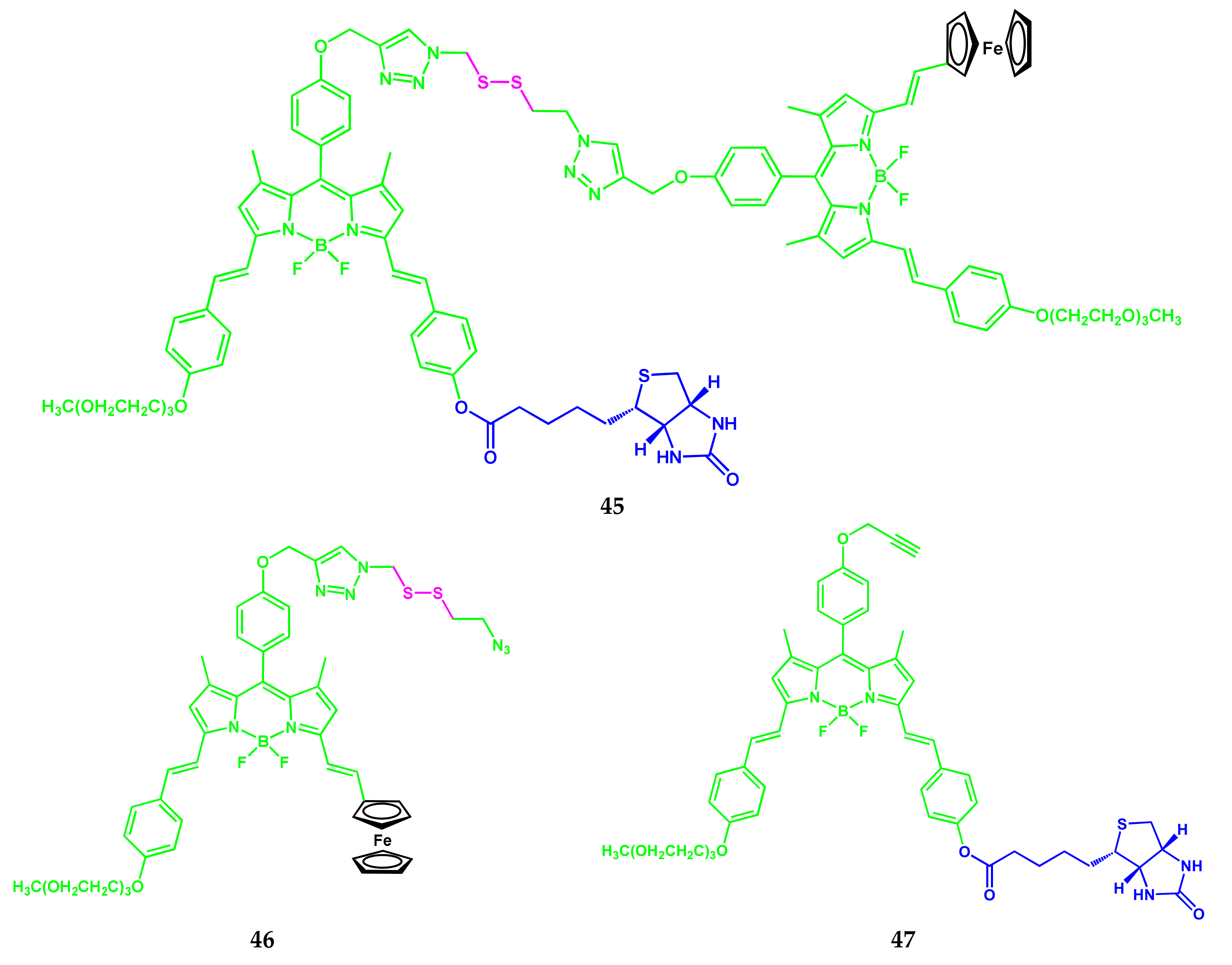
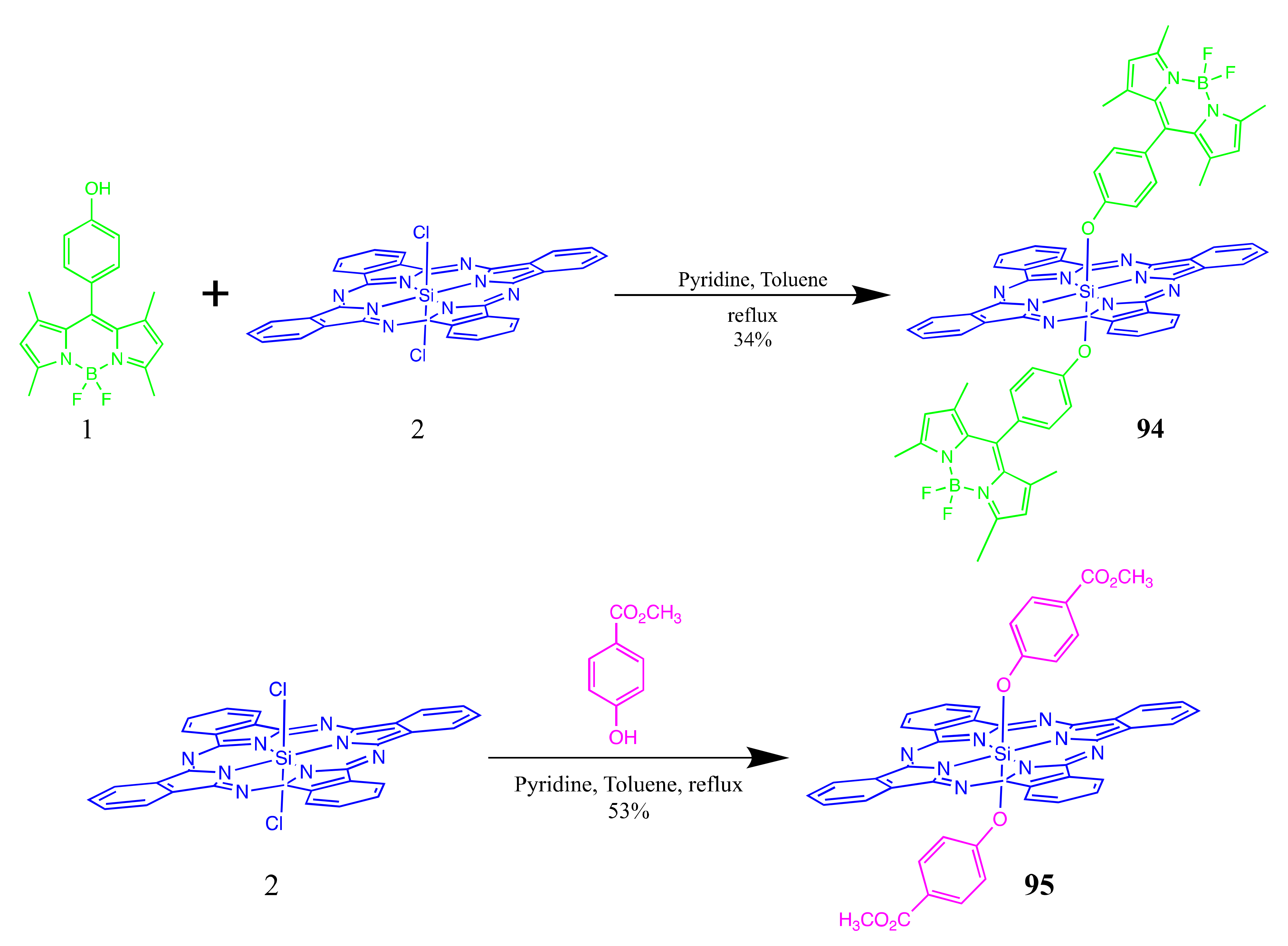
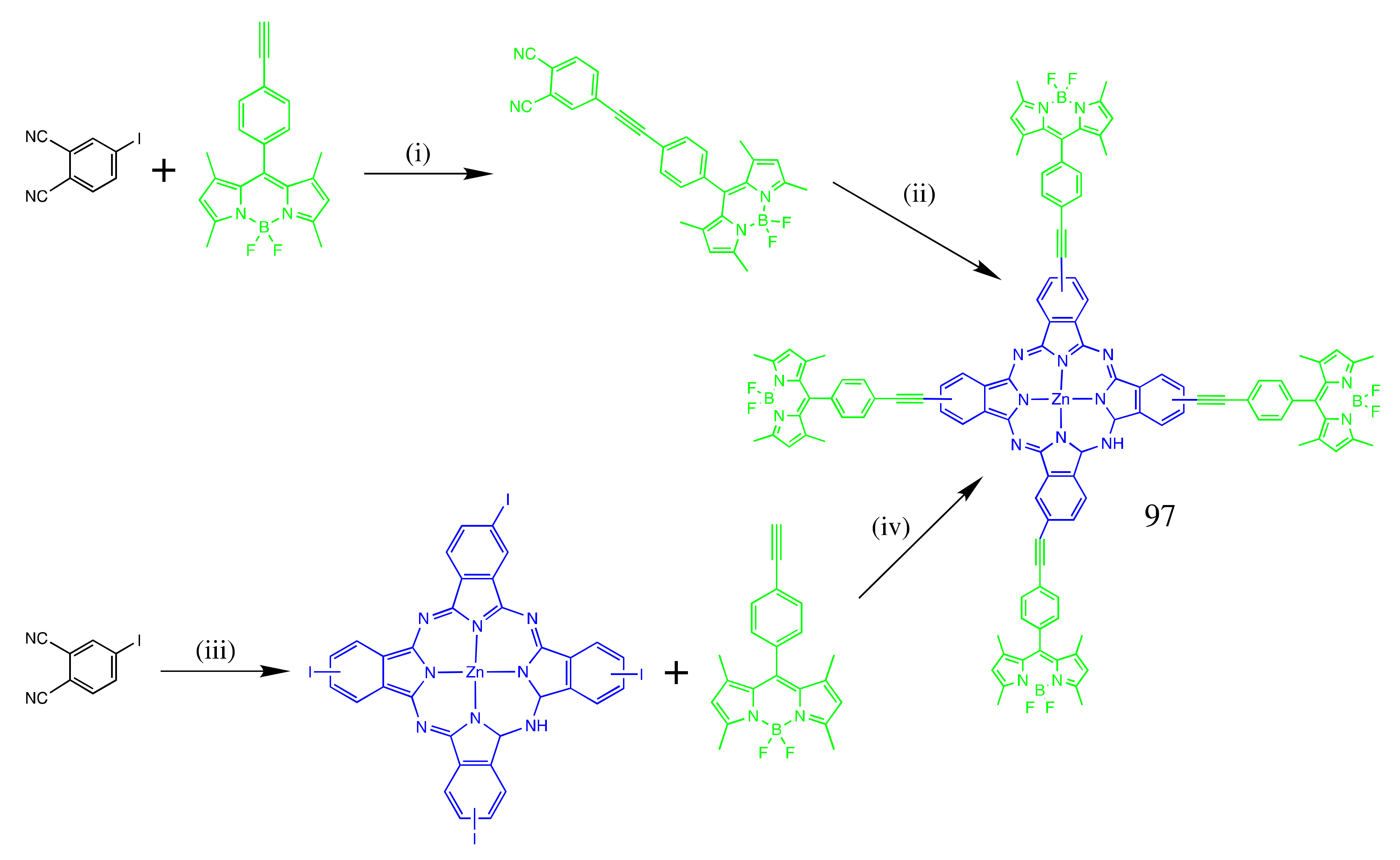
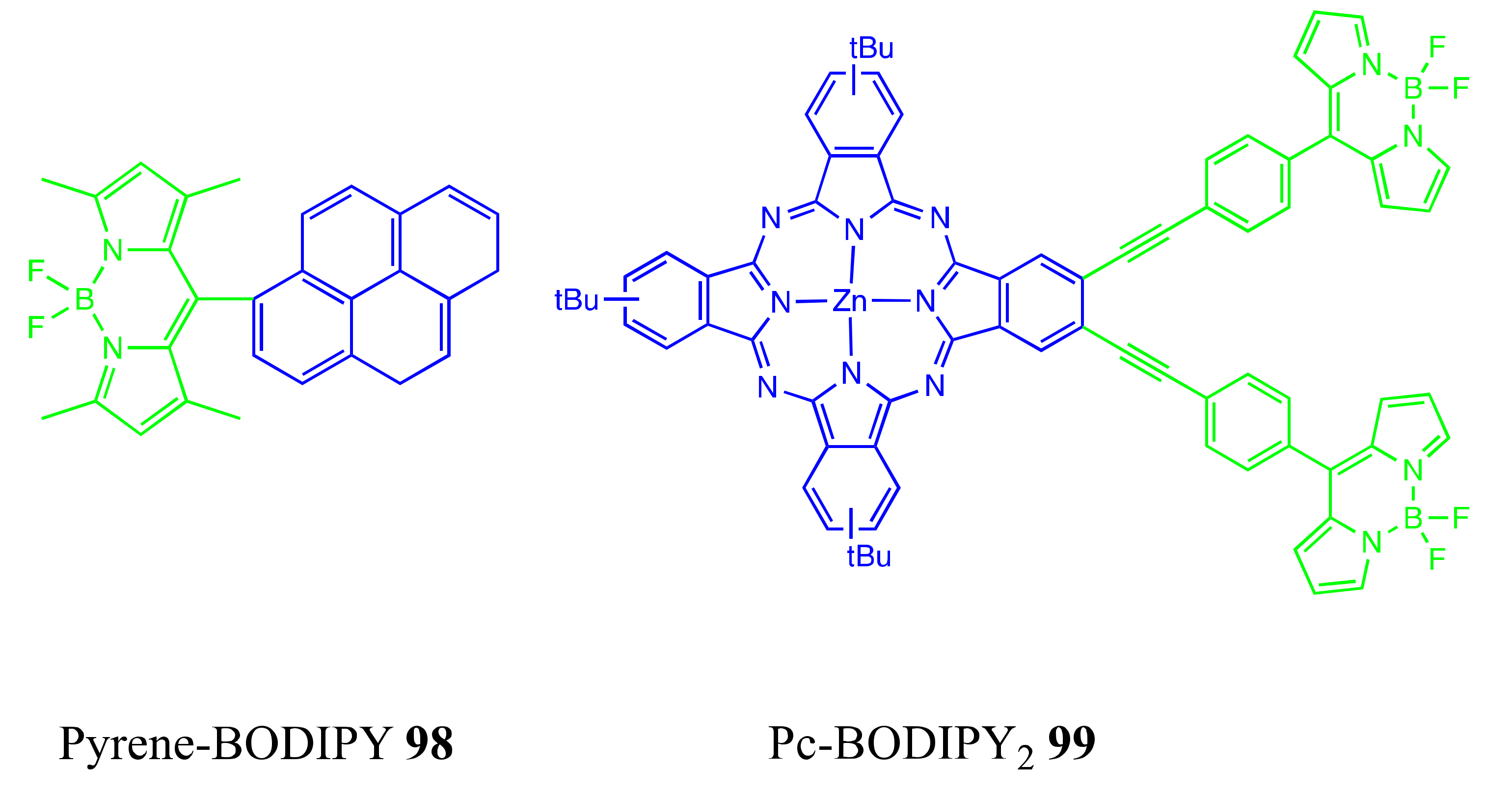
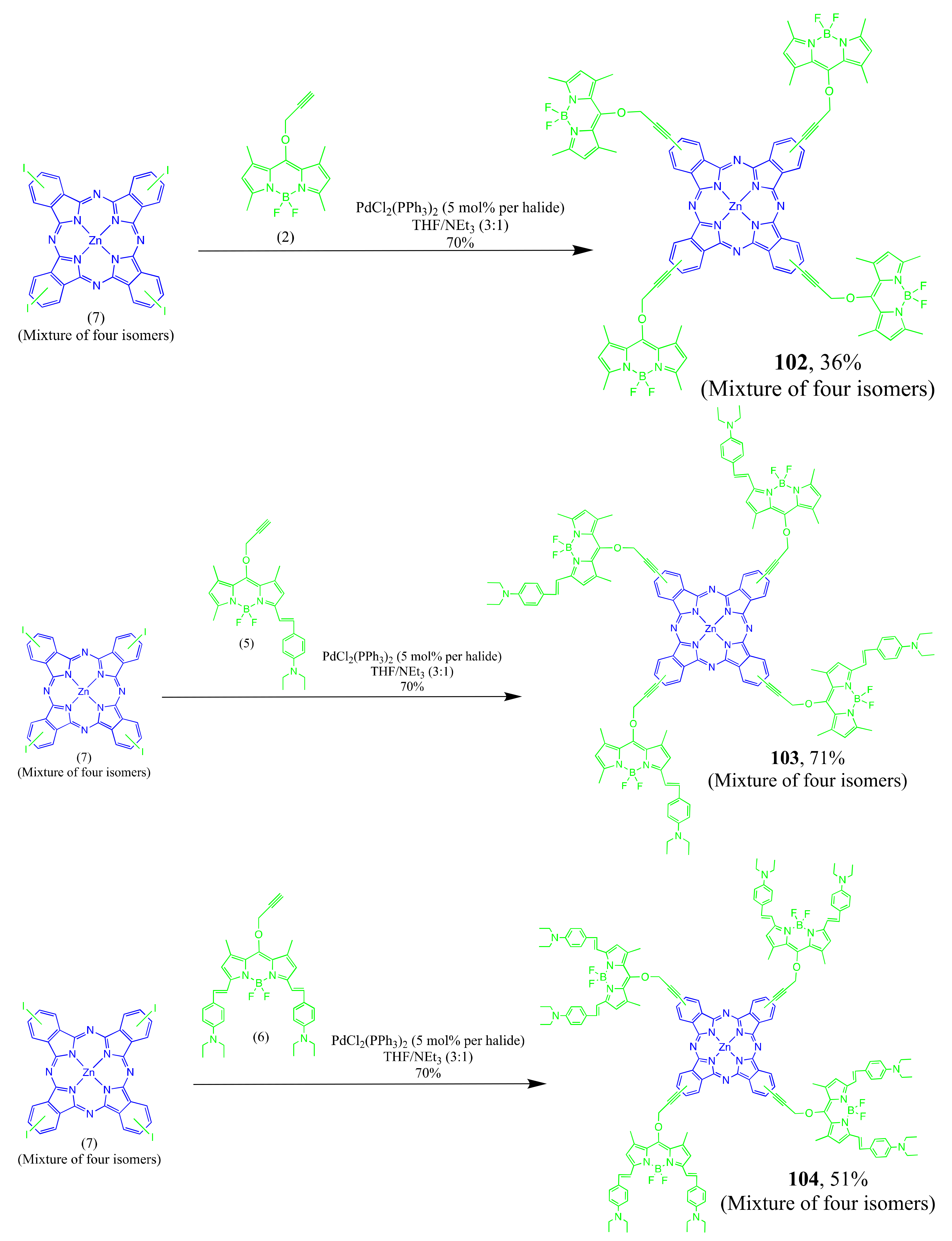
4.1. BODIPY Conjugates with Phthalocyanines
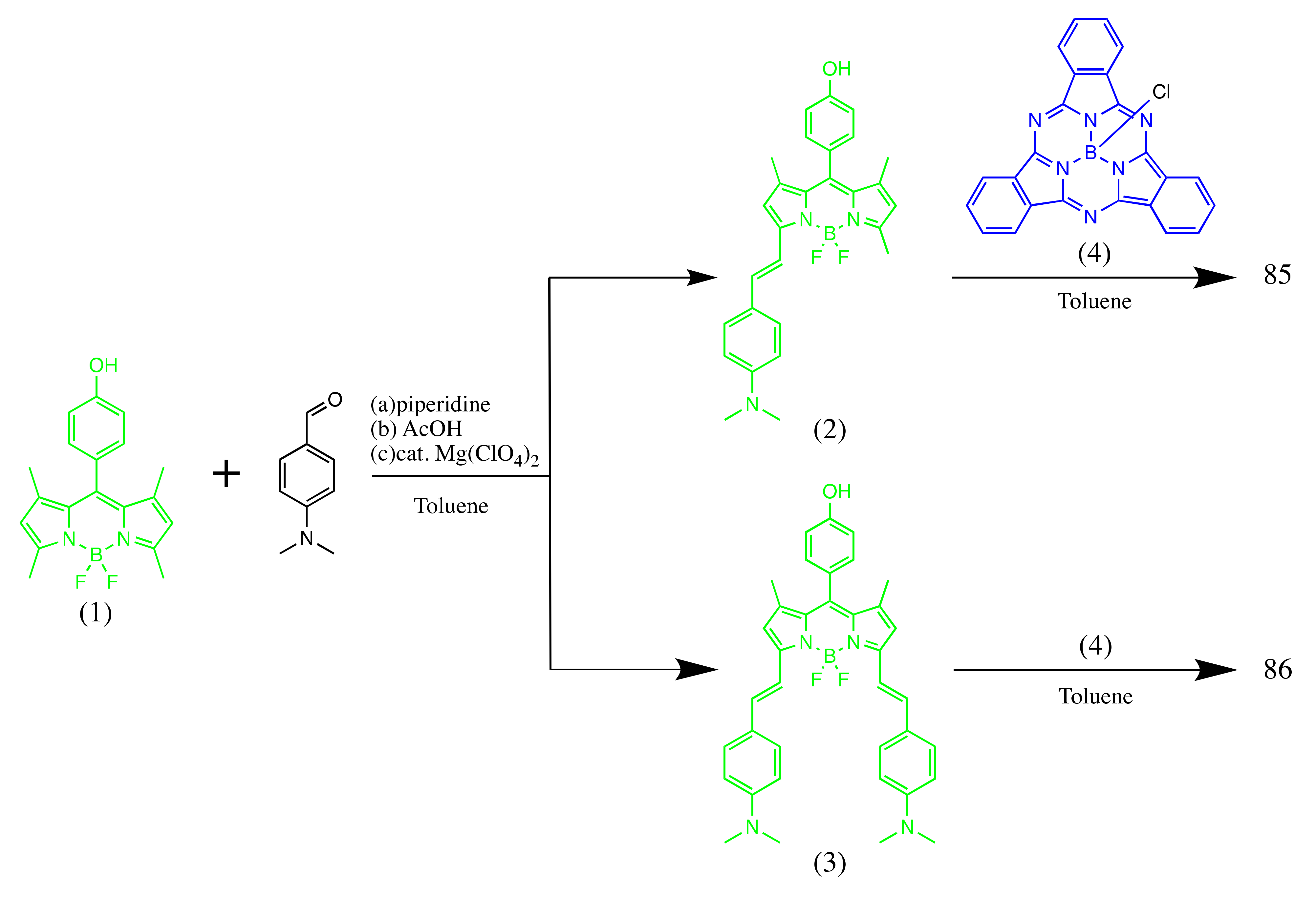
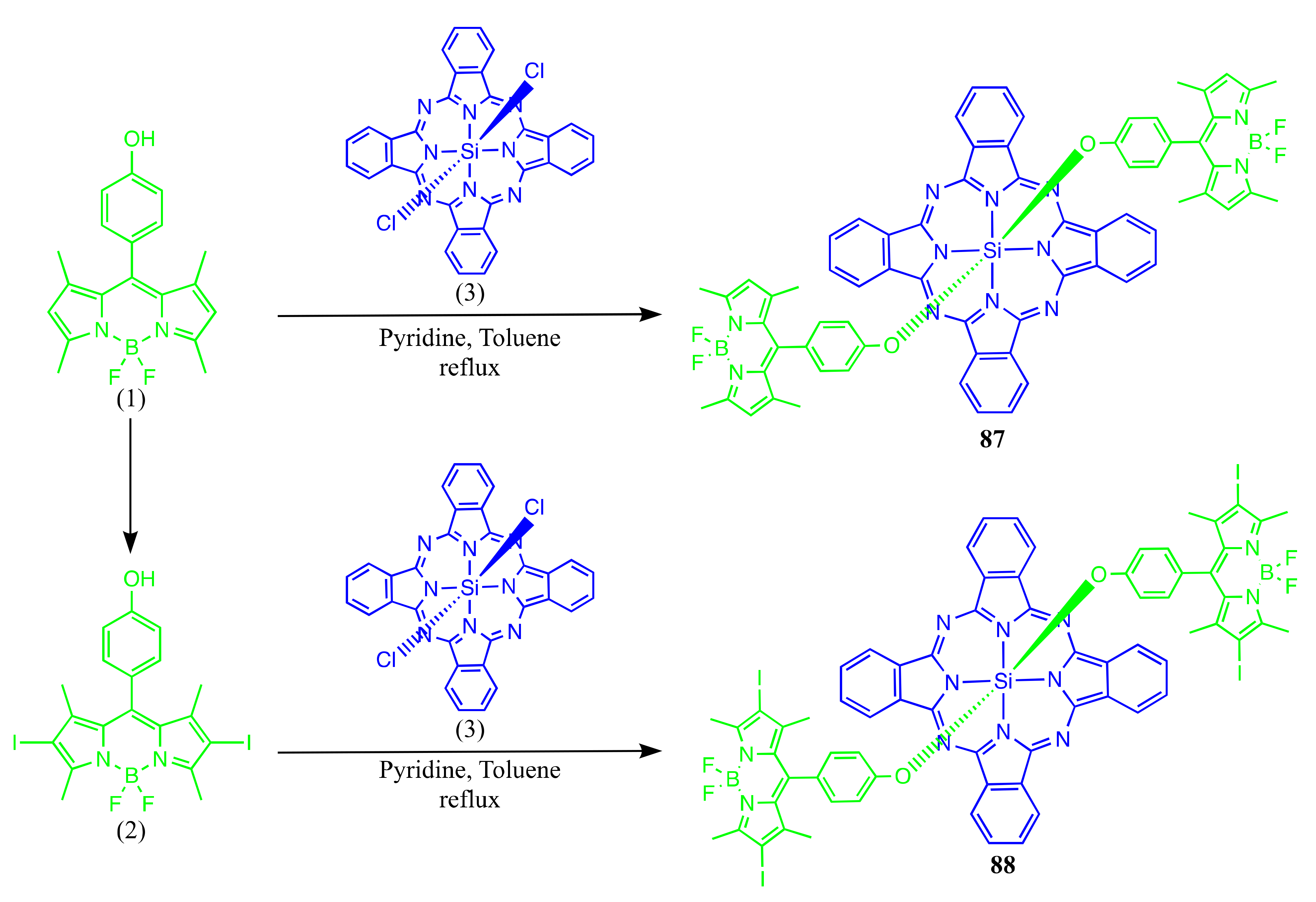
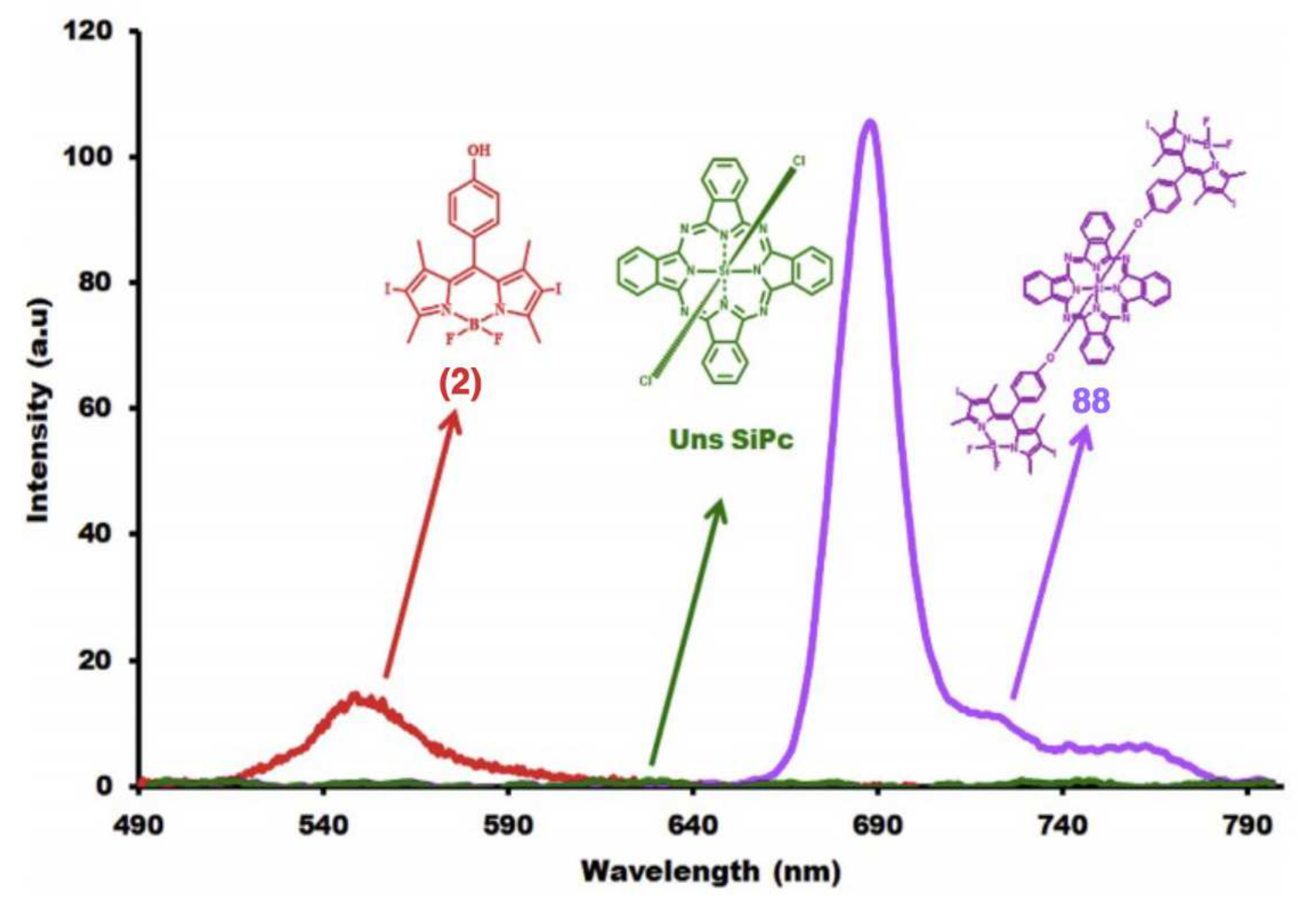
Axially Coordinated BODIPY-Pc Conjugates
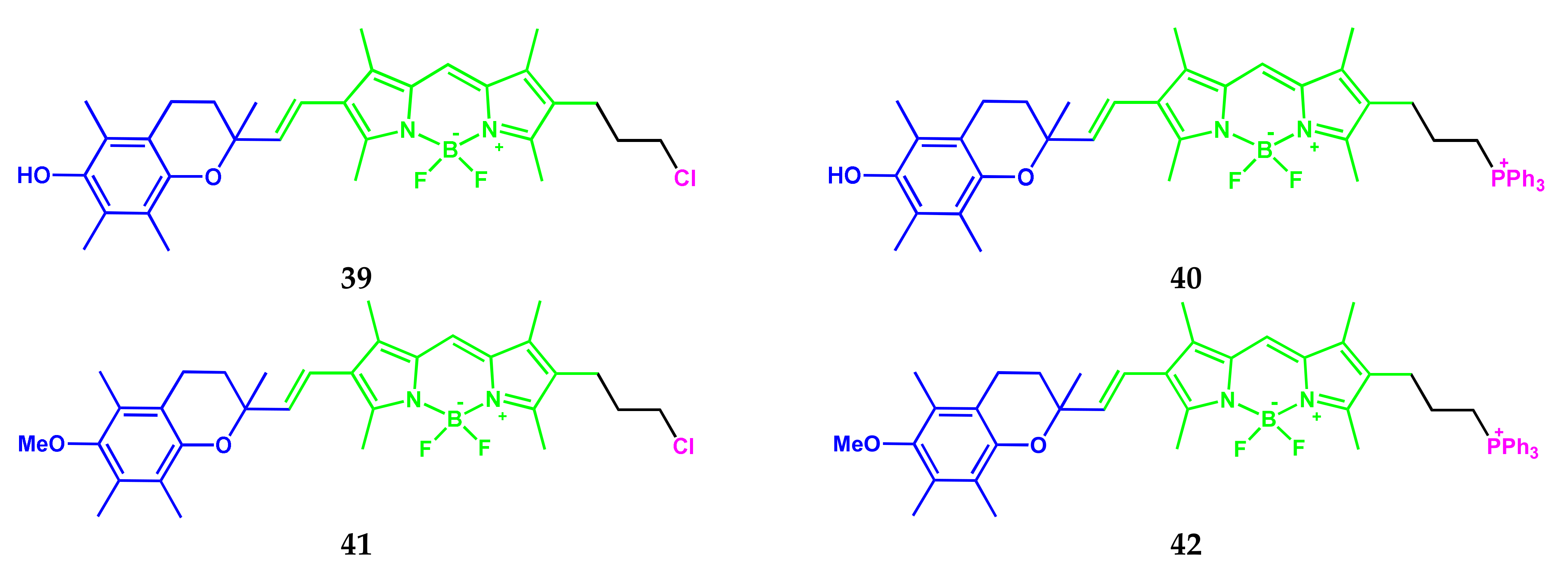
4.2. BODIPY Conjugates with Coumarin
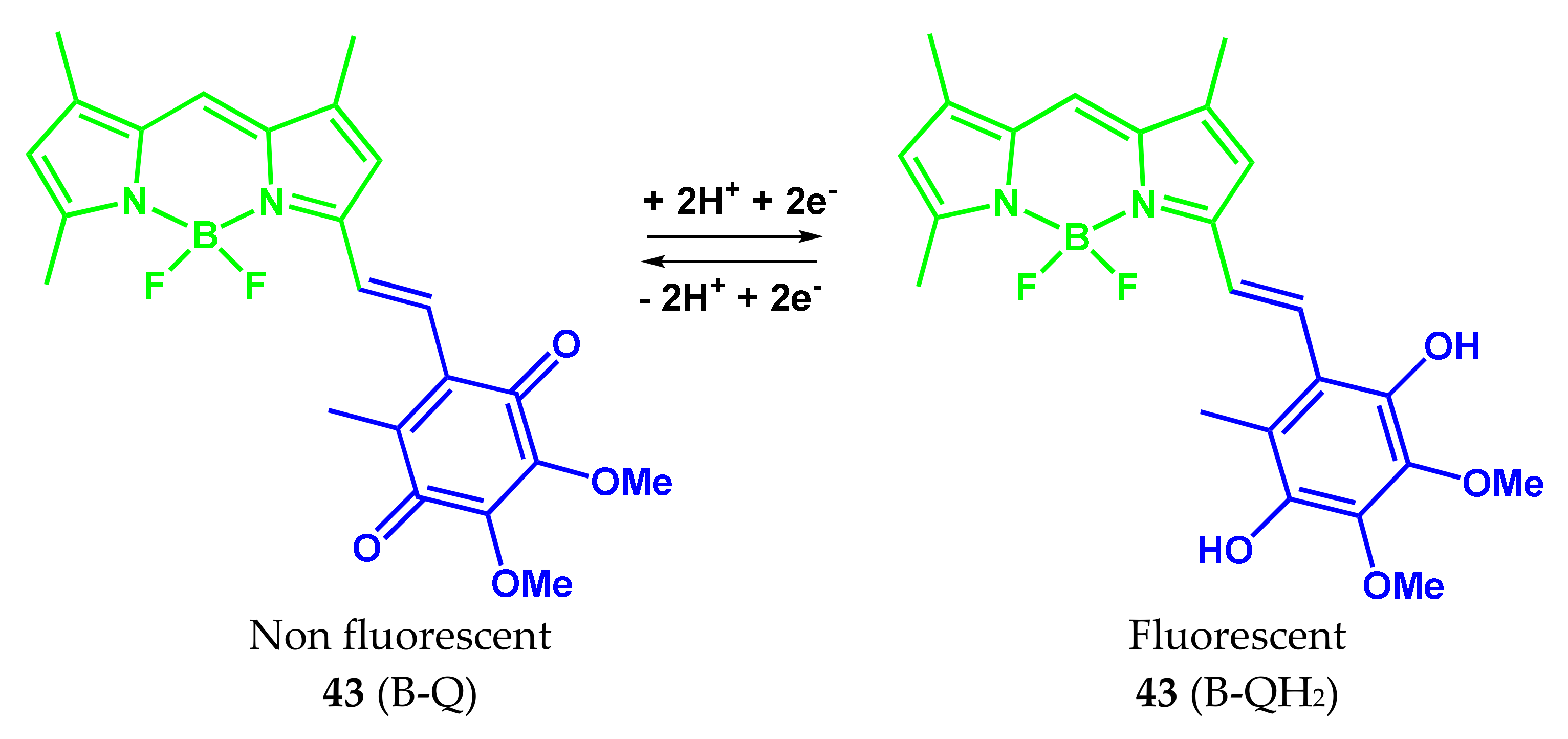
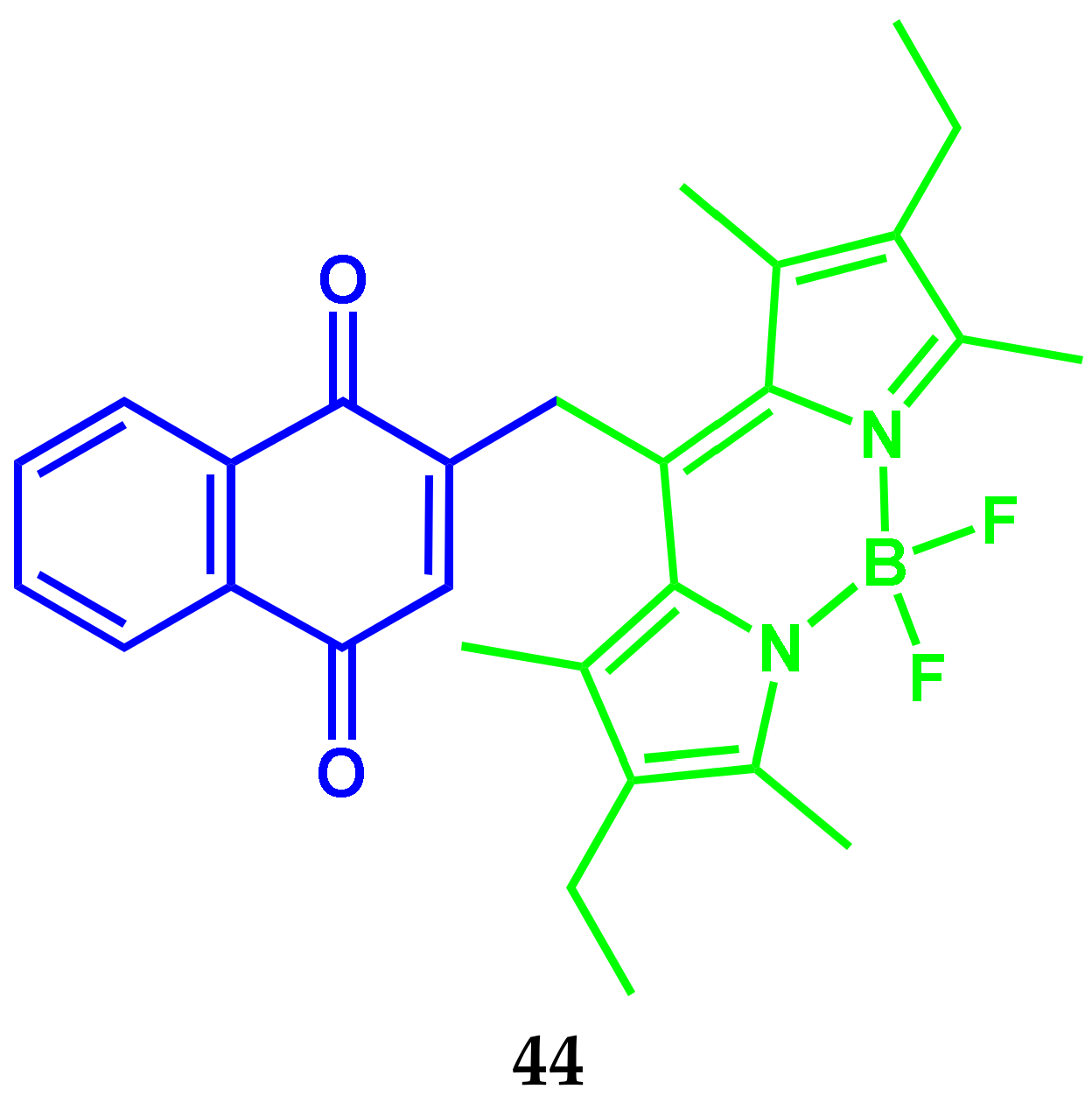
4.3. BODIPY Conjugates with Nile Red
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Deng, Z.; Wang, N.; Ai, F.; Wang, Z.; Zhu, G. Nanomaterial-mediated platinum drug-based combinatorial cancer therapy. View 2021, 2, 20200030. [Google Scholar] [CrossRef]
- Cheff, D.M.; Hall, M.D. A drug of such damned nature.1 challenges and opportunities in translational platinum drug research. View 2017, 60, 4517–4532. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted pt(II) agents, nanoparticle delivery, and pt(IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [Green Version]
- Farrell, N.P. Multi-platinum anti-cancer agents. Substitution-inert compounds for tumor selectivity and new targets. Chem. Soc. Rev. 2015, 44, 8773–8785. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Guo, Z. Functionalization of platinum complexes for biomedical applications. Acc. Chem. Res. 2015, 48, 2622–2631. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Yuan, H.; Chen, Y.; Guo, Y.; Zhang, S.; Liu, Z.; He, W.; Guo, Z. BODIPY-based monofunctional pt (II) complexes for specific photocytotoxicity against cancer cells. J. Inorg. Biochem. 2021, 218, 111394. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Chen, S.; Deng, Z.; Tse, M.-K.; Matsuda, Y.; Zhu, G. BODI-Pt, a green-light-activatable and carboplatin-based platinum(iv) anticancer prodrug with enhanced activation and cytotoxicity. Inorg. Chem. 2020, 59, 11823–11833. [Google Scholar] [CrossRef]
- Ramu, V.; Kundu, P.; Upadhyay, A.; Kondaiah, P.; Chakravarty, A.R. Lysosome specific platinum(II) catecholates with photoactive BODIPY for imaging and photodynamic therapy in near-ir light. Eur. J. Inorg. Chem. 2021, 2021, 831–839. [Google Scholar] [CrossRef]
- Raza, M.K.; Gautam, S.; Garai, A.; Mitra, K.; Kondaiah, P.; Chakravarty, A.R. Monofunctional BODIPY-appended imidazoplatin for cellular imaging and mitochondria-targeted photocytotoxicity. Inorg. Chem. 2017, 56, 11019–11029. [Google Scholar] [CrossRef]
- Raza, M.K.; Gautam, S.; Howlader, P.; Bhattacharyya, A.; Kondaiah, P.; Chakravarty, A.R. Pyriplatin-boron-dipyrromethene conjugates for imaging and mitochondria-targeted photodynamic therapy. Inorg. Chem. 2018, 57, 14374–14385. [Google Scholar] [CrossRef] [Green Version]
- Ramu, V.; Gautam, S.; Kondaiah, P.; Chakravarty, A.R. Diplatinum(II) catecholate of photoactive boron-dipyrromethene for lysosome-targeted photodynamic therapy in red light. Inorg. Chem. 2019, 58, 9067–9075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramu, V.; Kundu, P.; Kondaiah, P.; Chakravarty, A.R. Maloplatin-B, a cisplatin-based BODIPY-tagged mito-specific “chemo-PDT” agent active in red light. Inorg. Chem. 2021, 60, 6410–6420. [Google Scholar] [CrossRef] [PubMed]
- Kitteringham, E.; Wu, D.; Cheung, S.; Twamley, B.; O’Shea, D.F.; Griffith, D.M. Development of a novel carboplatin like cytoplasmic trackable near infrared fluorophore conjugate via strain-promoted azide alkyne cycloaddition. J. Inorg. Biochem. 2018, 182, 150–157. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Yu, G.; Crawley, M.R.; Fulong, C.R.P.; Friedman, A.E.; Sengupta, S.; Sun, J.; Li, Q.; Huang, F.; et al. Highly emissive self-assembled BODIPY-platinum supramolecular triangles. J. Am. Chem. Soc. 2018, 140, 7730–7736. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, U.; Kumar, B.; Garai, A.; Bhattacharyya, A.; Kumar, A.; Banerjee, S.; Kondaiah, P.; Chakravarty, A.R. Curcumin “drug” stabilized in oxidovanadium(IV)-BODIPY conjugates for mitochondria-targeted photocytotoxicity. Inorg. Chem. 2017, 56, 12457–12468. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Zheng, M.; Xie, Z. Near-infrared BODIPY-paclitaxel conjugates assembling organic nanoparticles for chemotherapy and bioimaging. J. Colloid Interface Sci. 2018, 514, 584–591. [Google Scholar] [CrossRef]
- Sun, T.; Lin, W.; Zhang, W.; Xie, Z. Self-assembly of amphiphilic drug-dye conjugates into nanoparticles for imaging and chemotherapy. Chem. Asian J. 2016, 11, 3174–3177. [Google Scholar] [CrossRef]
- Wijesooriya, C.S.; Peterson, J.A.; Shrestha, P.; Gehrmann, E.J.; Winter, A.H.; Smith, E.A. A Photoactivatable BODIPY probe for localization-based super-resolution cellular imaging. Angew. Chem. Int. Ed. Engl. 2018, 57, 12685–12689. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Li, J.; Ye, Z.; Li, J.; Wang, A.; Hu, J.; Bai, S.; Yin, J. Self-assembly of photosensitive and chemotherapeutic drugs for combined photodynamic-chemo cancer therapy with real-time tracing property. Colloids Surf. A Physicochem. Eng. Asp. 2019, 574, 44–51. [Google Scholar] [CrossRef]
- Badon, I.W.; Lee, J.; Pegarro Vales, T.; Cho, B.K.; Kim, H.-J. Synthesis and photophysical characterization of highly water-soluble PEGylated BODIPY derivatives for cellular imaging. J. Photochem. Photobiol. A Chem. 2019, 377, 214–219. [Google Scholar] [CrossRef]
- Ruan, Z.; Zhao, Y.; Yuan, P.; Liu, L.; Wang, Y.; Yan, L. PEG conjugated BODIPY-Br2 as macro-photosensitizer for efficient imaging-guided photodynamic therapy. J. Mater. Chem. B 2018, 6, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Kim, T.-I.; Kim, H.; Choi, Y.; Kim, Y. Photoactivatable BODIPY platform: Light-triggered anticancer drug release and fluorescence monitoring. ACS Appl. Bio. Mater. 2019, 2, 2567–2572. [Google Scholar] [CrossRef] [PubMed]
- Tomilin, D.N.; Sobenina, L.N.; Petrushenko, K.B.; Ushakov, I.A.; Trofimov, B.A. Design of novel meso-CF3-BODIPY dyes with isoxazole substituents. Dye. Pigment. 2018, 152, 14–18. [Google Scholar] [CrossRef]
- Sampedro, A.; Ramos-Torres, Á.; Schwöppe, C.; Mück-Lichtenfeld, C.; Helmers, I.; Bort, A.; Díaz-Laviada, I.; Fernández, G. Hierarchical Self-Assembly of BODIPY Dyes as a tool to improve the antitumor activity of capsaicin in prostate cancer. Angew. Chem. Int. Ed. Engl. 2018, 57, 17235–17239. [Google Scholar] [CrossRef]
- Tiwari, R.; Shinde, P.S.; Sreedharan, S.; Dey, A.K.; Vallis, K.A.; Mhaske, S.B.; Pramanik, S.K.; Das, A. Photoactivatable prodrug for simultaneous release of mertansine and CO along with a BODIPY derivative as a luminescent marker in mitochondria: A proof of concept for NIR image-guided cancer therapy. Chem. Sci. 2020, 12, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, H.; Sharma, Y.P.; Manhas, R.K.; Kumar, K. Ethnomedicinal plants used by the villagers of district Udhampur, J&K, India. J. Ethnopharmacol. 2014, 151, 1005–1018. [Google Scholar] [CrossRef]
- Cano-Ramírez, C.; Armendáriz-Toledano, F.; Macías-Sámano, J.E.; Sullivan, B.T.; Zúñiga, G. Electrophysiological and behavioral responses of the bark beetle Dendroctonus rhizophagus to volatiles from host pines and conspecifics. J. Chem. Ecol. 2012, 38, 512–524. [Google Scholar] [CrossRef]
- Lawal, O.A.; Oyedeji, A.O. Chemical composition of the essential oils of Cyperus rotundus L. from South Africa. Molecules 2009, 14, 2909–2917. [Google Scholar] [CrossRef]
- Gomes, B.S.; Neto, B.P.S.; Lopes, E.M.; Cunha, F.V.M.; Araújo, A.R.; Wanderley, C.W.S.; Wong, D.V.T.; Júnior, R.C.P.L.; Ribeiro, R.A.; Sousa, D.P.; et al. Anti-inflammatory effect of the monoterpene myrtenol is dependent on the direct modulation of neutrophil migration and oxidative stress. Chem. Biol. Interact. 2017, 273, 73–81. [Google Scholar] [CrossRef]
- Rivas da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [Green Version]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on monoterpenes as antimicrobial agents: A particular focus on p-cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- De Cássia da Silveira e Sá, R.; Andrade, L.N.; de Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef]
- Kozioł, A.; Stryjewska, A.; Librowski, T.; Sałat, K.; Gaweł, M.; Moniczewski, A.; Lochyński, S. An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini Rev. Med. Chem. 2014, 14, 1156–1168. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, S.V.; Nikitina, L.E.; Startseva, V.A.; Artemova, N.P.; Bodrov, A.V.; Boichuk, S.V.; Vorontsova, M.M.; Rakhmatullina, A.A.; Turaev, R.G.; Klochkov, V.V. Hemocoagulation activity of sulfur-containing pinane-type terpenoids. Pharm. Chem. J. 2017, 51, 343–347. [Google Scholar] [CrossRef]
- Nikitina, L.E.; Artemova, N.P.; Startseva, V.A.; Fedyunina, I.V.; Klochkov, V.V. Biological activity of s-containing monoterpenoids. Chem. Nat. Compd. 2017, 53, 811–819. [Google Scholar] [CrossRef]
- Nikitina, L.E.; Kiselev, S.V.; Startseva, V.A.; Lodochnikova, O.A.; Rakhmatullina, A.A.; Fedyunina, I.V.; Gilfanov, I.R. New aspects of using biologically active thioterpenoids of pinane series. Russ. Chem. Bull. 2019, 68, 1031–1035. [Google Scholar] [CrossRef]
- Nikitina, L.E.; Lisovskaya, S.A.; Startseva, V.A.; Pavelyev, R.S.; Gilfanov, I.R.; Fedyunina, I.V.; Ostolopovskaya, O.V.; Akhverdiev, R.F. Development of novel effective agents against candida albicans biofilms. BioNanoSci. 2019, 9, 539–544. [Google Scholar] [CrossRef]
- Brandes, B.; Hoenke, S.; Fischer, L.; Csuk, R. Design, synthesis and cytotoxicity of BODIPY FL labelled triterpenoids. Eur. J. Med. Chem. 2020, 185, 111858. [Google Scholar] [CrossRef]
- Krajcovicova, S.; Stankova, J.; Dzubak, P.; Hajduch, M.; Soural, M.; Urban, M. A synthetic approach for the rapid preparation of BODIPY conjugates and their use in imaging of cellular drug uptake and distribution. Chemistry 2018, 24, 4957–4966. [Google Scholar] [CrossRef]
- Guseva, G.B.; Antina, E.V.; Berezin, M.B.; Pavelyev, R.S.; Kayumov, A.R.; Ostolopovskaya, O.V.; Gilfanov, I.R.; Frolova, L.L.; Kutchin, A.V.; Akhverdiev, R.F.; et al. Design, spectral characteristics, and possibilities for practical application of BODIPY FL-labeled monoterpenoid. ACS Appl. Bio. Mater. 2021, 4, 6227–6235. [Google Scholar] [CrossRef]
- West, R.; Panagabko, C.; Atkinson, J. Synthesis and characterization of BODIPY-alpha-tocopherol: A fluorescent form of vitamin E. J. Org. Chem. 2010, 75, 2883–2892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panagabko, C.; Morley, S.; Hernandez, M.; Cassolato, P.; Gordon, H.; Parsons, R.; Manor, D.; Atkinson, J. Ligand specificity in the CRAL-TRIO protein family. Biochemistry 2003, 42, 6467–6474. [Google Scholar] [CrossRef] [PubMed]
- Krumova, K.; Greene, L.E.; Cosa, G. Fluorogenic α-tocopherol analogue for monitoring the antioxidant status within the inner mitochondrial membrane of live cells. J. Am. Chem. Soc. 2013, 135, 17135–17143. [Google Scholar] [CrossRef] [PubMed]
- Greene, L.E.; Godin, R.; Cosa, G. Fluorogenic ubiquinone analogue for monitoring chemical and biological redox processes. J. Am. Chem. Soc. 2016, 138, 11327–11334. [Google Scholar] [CrossRef]
- Belzile, M.-N.; Godin, R.; Durantini, A.M.; Cosa, G. Monitoring chemical and biological electron transfer reactions with a fluorogenic vitamin k analogue probe. J. Am. Chem. Soc. 2016, 138, 16388–16397. [Google Scholar] [CrossRef]
- Shi, W.-J.; Lo, P.-C.; Zhao, S.; Wong, R.C.H.; Wang, Q.; Fong, W.-P.; Ng, D.K.P. A biotin-conjugated glutathione-responsive FRET-based fluorescent probe with a ferrocenyl BODIPY as the dark quencher. Dalton Trans. 2016, 45, 17798–17806. [Google Scholar] [CrossRef]
- Haghdoost, M.M.; Sauvageau, E.; Oguadinma, P.; Tran, H.-V.; Lefrancois, S.; Castonguay, A. Cu-catalyzed click conjugation of cobalamin to a BODIPY-based fluorophore: A versatile tool to explore the cellular biology of vitamin B12. J. Inorg. Biochem. 2020, 210, 111105. [Google Scholar] [CrossRef]
- Paul, S.; Kundu, P.; Bhattacharyya, U.; Garai, A.; Maji, R.C.; Kondaiah, P.; Chakravarty, A.R. Ruthenium(II) Conjugates of boron-dipyrromethene and biotin for targeted photodynamic therapy in red light. Inorg. Chem. 2020, 59, 913–924. [Google Scholar] [CrossRef]
- Mora, A.K.; Murudkar, S.; Shivran, N.; Mula, S.; Chattopadhyay, S.; Nath, S. Monitoring the formation of insulin oligomers using a NIR emitting glucose-conjugated BODIPY dye. Int. J. Biol. Macromol. 2021, 166, 1121–1130. [Google Scholar] [CrossRef]
- Biagiotti, G.; Purić, E.; Urbančič, I.; Krišelj, A.; Weiss, M.; Mravljak, J.; Gellini, C.; Lay, L.; Chiodo, F.; Anderluh, M.; et al. Combining cross-coupling reaction and Knoevenagel condensation in the synthesis of glyco-BODIPY probes for DC-SIGN super-resolution bioimaging. Bioorg. Chem. 2021, 109, 104730. [Google Scholar] [CrossRef]
- Taki, S.; Ardestani, M.S. Novel nanosized AS1411-chitosan-BODIPY conjugate for molecular fluorescent imaging. Int. J. Nanomed. 2019, 14, 3543–3555. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, N.E.M.; Meng, Q.; Griffin, K.E.; Singh, S.S.; Dahal, A.; Zhou, Z.; Fronczek, F.R.; Mathis, J.M.; Jois, S.D.; Vicente, M.G.H. Synthesis, characterization, and evaluation of near-IR boron dipyrromethene bioconjugates for labeling of adenocarcinomas by selectively targeting the epidermal growth factor receptor. J. Med. Chem. 2019, 62, 3323–3335. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Byrne, A.; Keyes, T.E. Linker length in fluorophore–cholesterol conjugates directs phase selectivity and cellular localisation in GUVs and live cells. RSC Adv. 2019, 9, 22805–22816. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Bittman, R. Synthesis and spectral properties of cholesterol- and FTY720-containing boron dipyrromethene dyes. J. Org. Chem. 2007, 72, 8376–8382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, M.H.Y.; Harmatys, K.M.; Charron, D.M.; Chen, J.; Zheng, G. Stable J-Aggregation of an aza-BODIPY-lipid in a liposome for optical cancer imaging. Angew. Chem. Int. Ed. Engl. 2019, 58, 13394–13399. [Google Scholar] [CrossRef]
- Boldyrev, I.A.; Molotkovsky, J.G. A synthesis and properties of new 4,4-difluoro-3a,4a-diaza-s-indacene (BODIPY)-labeled lipids. Russ. J. Bioorg. Chem. 2006, 32, 78–83. [Google Scholar] [CrossRef]
- Okamoto, M.; Kobayashi, S.; Ikeuchi, H.; Yamada, S.; Yamanouchi, K.; Nagasawa, K.; Maekawa, S.; Kato, T.; Shimizu, I. Synthesis and bioassay of a boron-dipyrromethene derivative of estradiol for fluorescence imaging in vivo. Steroids 2012, 77, 845–849. [Google Scholar] [CrossRef]
- Faletrov, Y.; Brzostek, A.; Plocinska, R.; Dziadek, J.; Rudaya, E.; Edimecheva, I.; Shkumatov, V. Uptake and metabolism of fluorescent steroids by mycobacterial cells. Steroids 2017, 117, 29–37. [Google Scholar] [CrossRef]
- Kazan, H.H.; Özcan, E.; Eçik, E.T.; Çoşut, B. Novel 17α-Etinylestradiol-substituted BODIPY dyes: Synthesis, photophysical properties and fluorescence imaging studies in breast cancer cell lines. ChemistrySelect 2018, 3, 2962–2967. [Google Scholar] [CrossRef]
- Osati, S.; Ali, H.; Marques, F.; Paquette, M.; Beaudoin, S.; Guerin, B.; Leyton, J.V.; van Lier, J.E. BODIPY-17α-ethynylestradiol conjugates: Synthesis, fluorescence properties and receptor binding affinities. Bioorg. Med. Chem. Lett. 2017, 27, 443–446. [Google Scholar] [CrossRef]
- Cheng, M.H.Y.; Maruani, A.; Savoie, H.; Chudasama, V.; Boyle, R.W. Synthesis of a novel HER2 targeted aza-BODIPY-antibody conjugate: Synthesis, photophysical characterisation and in vitro evaluation. Org. Biomol. Chem. 2018, 16, 1144–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çetindere, S.; Çoşut, B.; Yeşilot, S.; Durmuş, M.; Kılıç, A. Synthesis and properties of axially BODIPY conjugated subphthalocyanine dyads. Dye. Pigment. 2014, 101, 234–239. [Google Scholar] [CrossRef]
- Kaya, E.N.; Köksoy, B.; Yeşilot, S.; Durmuş, M. Purple silicon(IV) phthalocyanine axially substituted with BODIPY groups. Dye. Pigment. 2020, 172, 107867. [Google Scholar] [CrossRef]
- Borges-Martínez, M.; Alvarez, D.; Montenegro-Pohlhammer, N.; Menéndez, M.I.; López, R.; Cárdenas-Jirón, G. Assessment of BODIPY–oxasmaragdyrin dyads for dye-sensitized solar cells: Aromaticity, photosensitization capability, and charge transport. J. Phys. Chem. C 2019, 123, 19362–19375. [Google Scholar] [CrossRef]
- Shi, W.-J.; Ng, D.K.P.; Zhao, S.; Lo, P.-C. A phthalocyanine-based glutathione-activated photosensitizer with a ferrocenyl boron dipyrromethene dark quencher for photodynamic therapy. ChemPhotoChem 2019, 3, 1004–1013. [Google Scholar] [CrossRef]
- Matshitse, R.; Ngoy, B.P.; Managa, M.; Mack, J.; Nyokong, T. Photophysical properties and photodynamic therapy activities of detonated nanodiamonds-BODIPY-phthalocyanines nanoassemblies. Photodiagnosis Photodyn. Ther. 2019, 26, 101–110. [Google Scholar] [CrossRef]
- Zhou, J.; Gai, L.; Zhou, Z.; Yang, W.; Mack, J.; Xu, K.; Zhao, J.; Zhao, Y.; Qiu, H.; Chan, K.S.; et al. Rational design of emissive NIR-absorbing chromophores: Rh(III) porphyrin-aza-BODIPY conjugates with orthogonal metal-carbon bonds. Chemistry 2016, 22, 13201–13209. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Ermilov, E.A.; Röder, B.; Ng, D.K.P. Switching the photo-induced energy and electron-transfer processes in BODIPY-phthalocyanine conjugates. Chem. Commun. 2009, 12, 1517–1519. [Google Scholar] [CrossRef]
- Göl, C.; Malkoç, M.; Yeşilot, S.; Durmuş, M. A first archetype of boron dipyrromethene-phthalocyanine pentad dye: Design, synthesis, and photophysical and photochemical properties. Dalton Trans. 2014, 43, 7561–7569. [Google Scholar] [CrossRef]
- Bizet, F.; Ipuy, M.; Bernhard, Y.; Lioret, V.; Winckler, P.; Goze, C.; Perrier-Cornet, J.-M.; Decréau, R.A. Cellular imaging using BODIPY-, pyrene- and phthalocyanine-based conjugates. Bioorg. Med. Chem. 2018, 26, 413–420. [Google Scholar] [CrossRef]
- Yanık, H.; Göksel, M.; Yeşilot, S.; Durmuş, M. Novel phthalocyanine–BODIPY conjugates and their photophysical and photochemical properties. Tetrahedron Lett. 2016, 57, 2922–2926. [Google Scholar] [CrossRef]
- Zheng, K.; Chen, H.; Fang, S.; Wang, Y. A ratiometric fluorescent probe based on a Bodipy-Coumarin conjugate for sensing of nitroxyl in living cells. Sens. Actuators B Chem. 2016, 233, 193–198. [Google Scholar] [CrossRef]
- Yang, Z.; He, Y.; Lee, J.H.; Chae, W.-S.; Ren, W.X.; Lee, J.H.; Kang, C.; Kim, J.S. A Nile Red/BODIPY-based bimodal probe sensitive to changes in the micropolarity and microviscosity of the endoplasmic reticulum. Chem. Commun. 2014, 50, 11672–11675. [Google Scholar] [CrossRef] [PubMed] [Green Version]




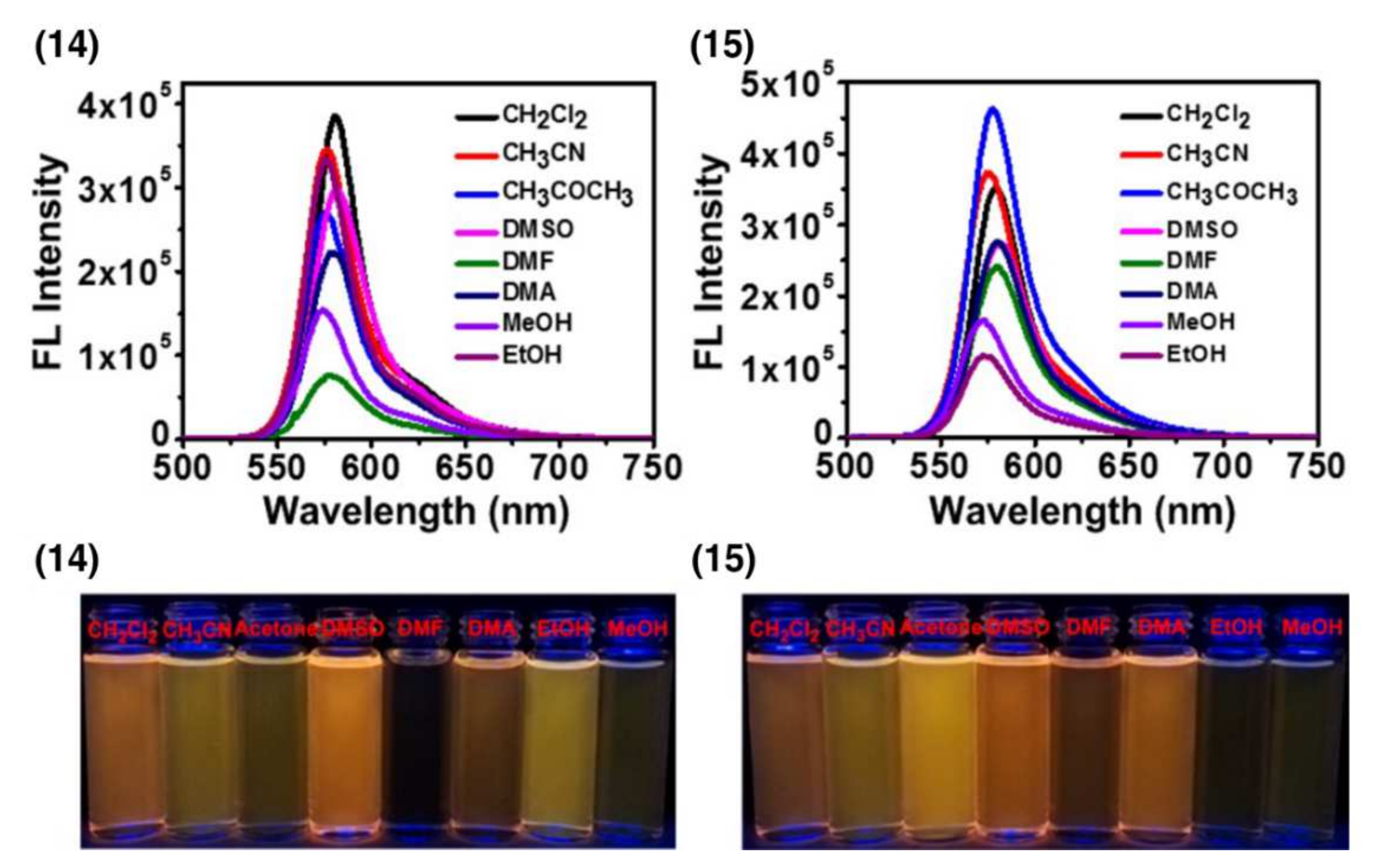

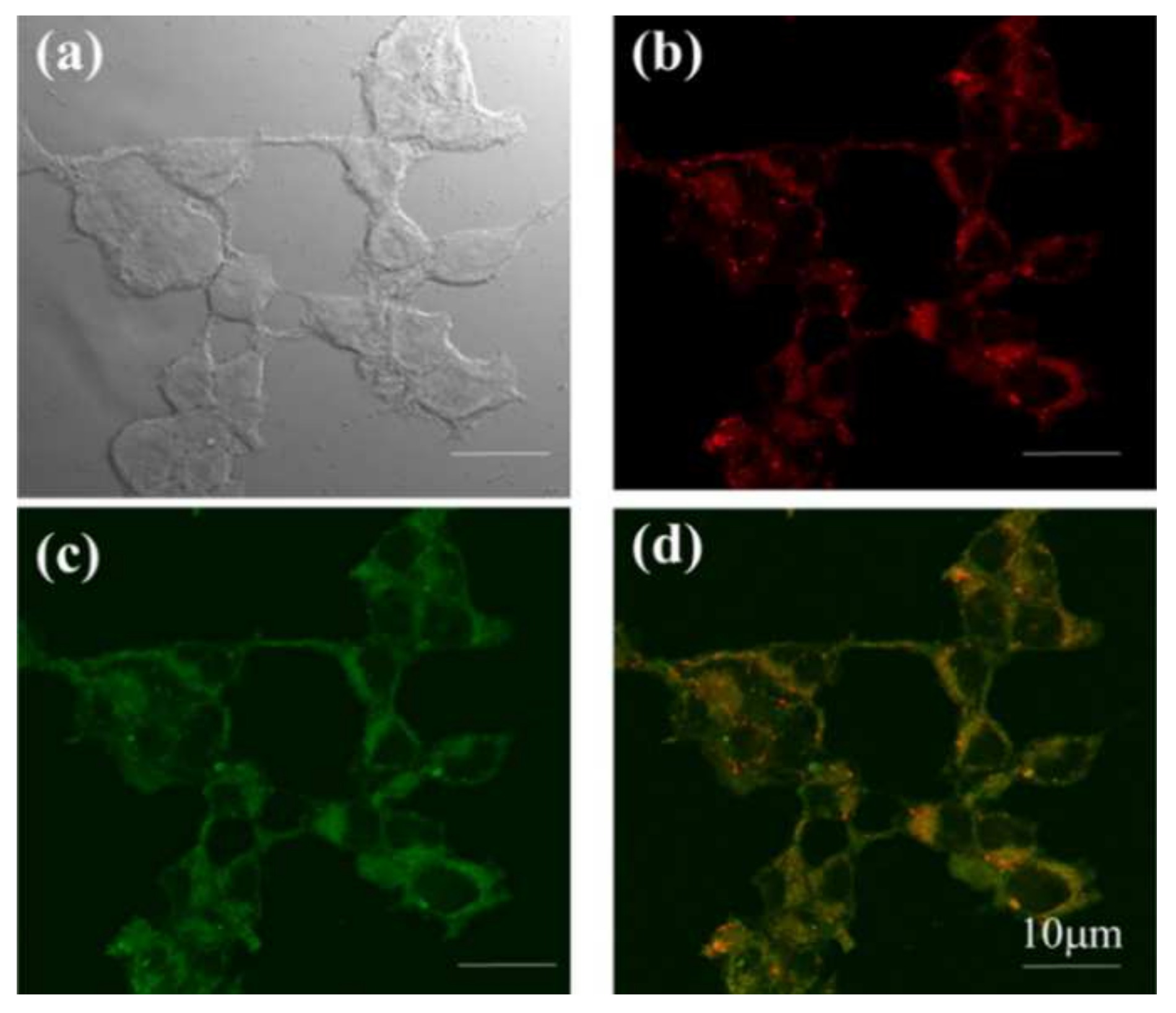
















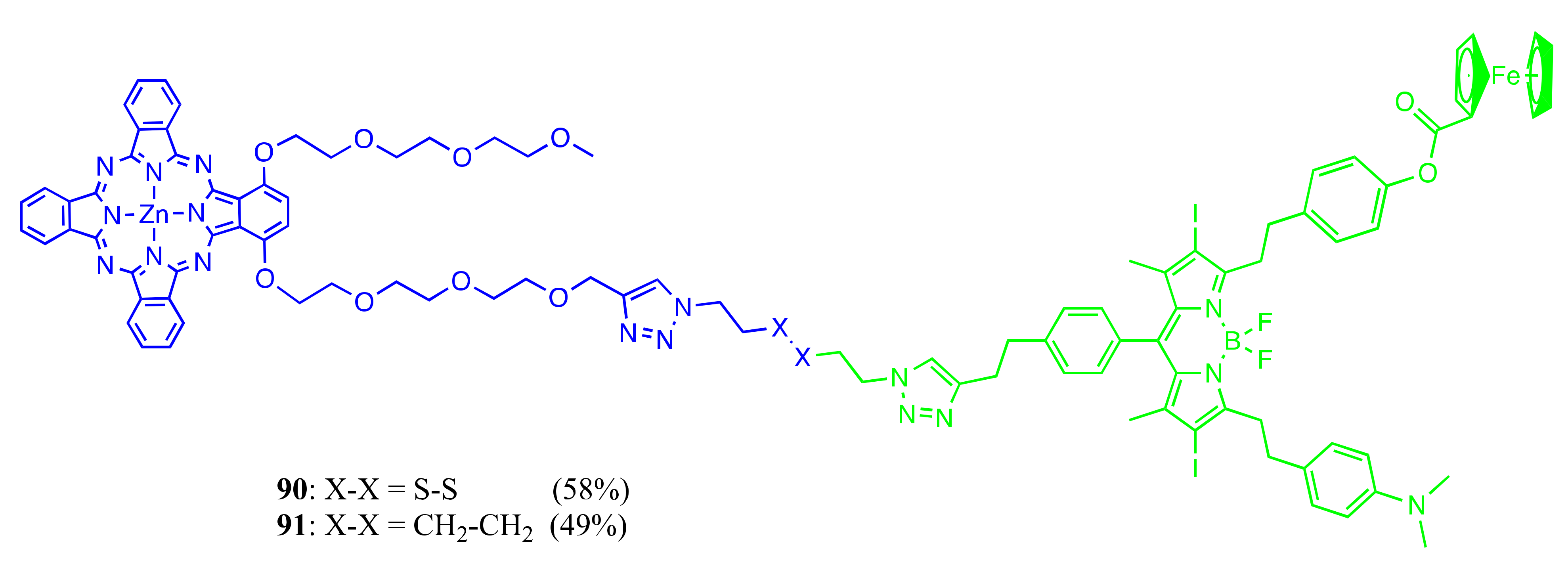
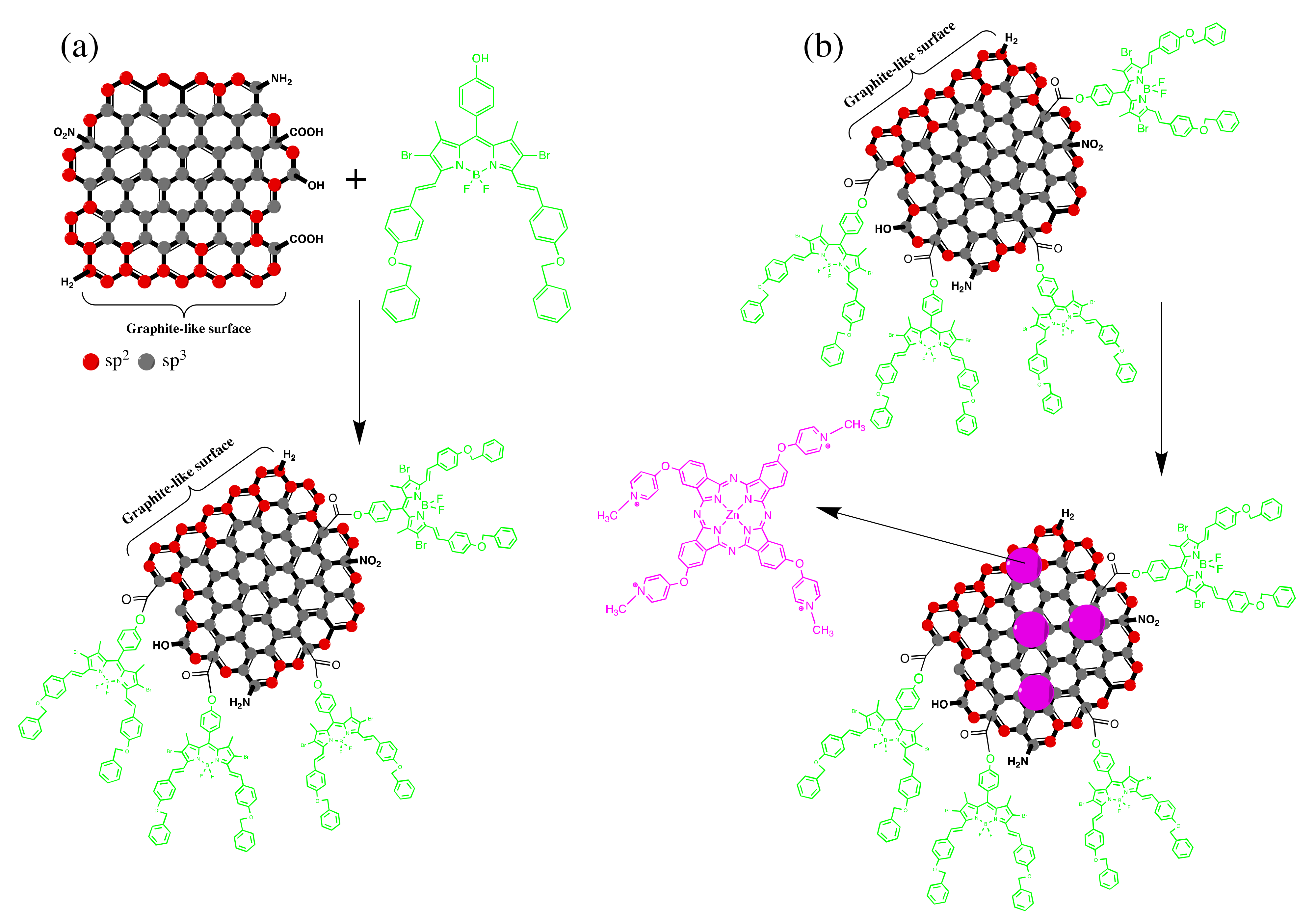
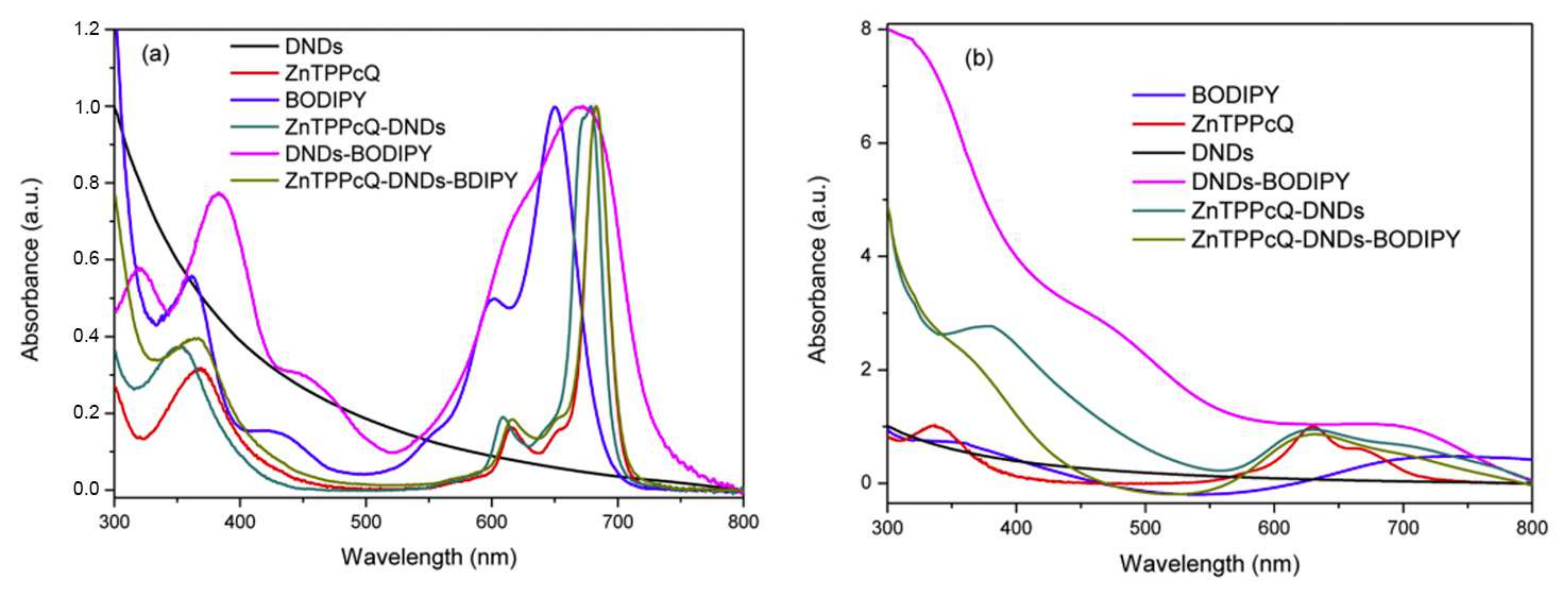
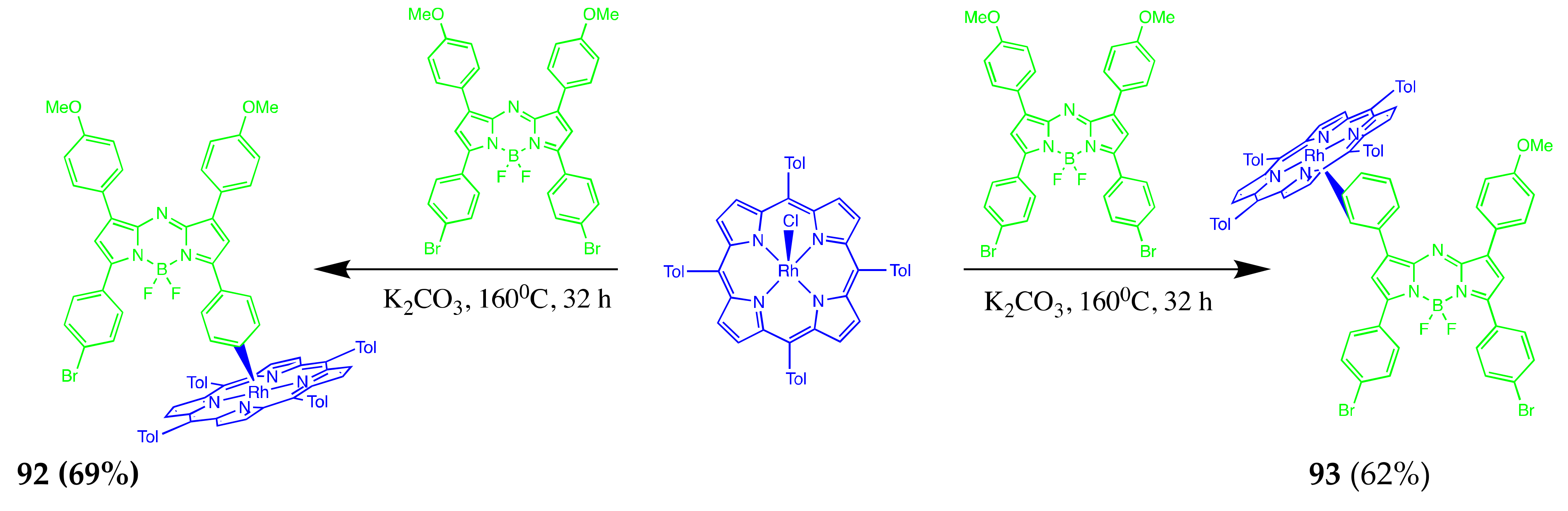


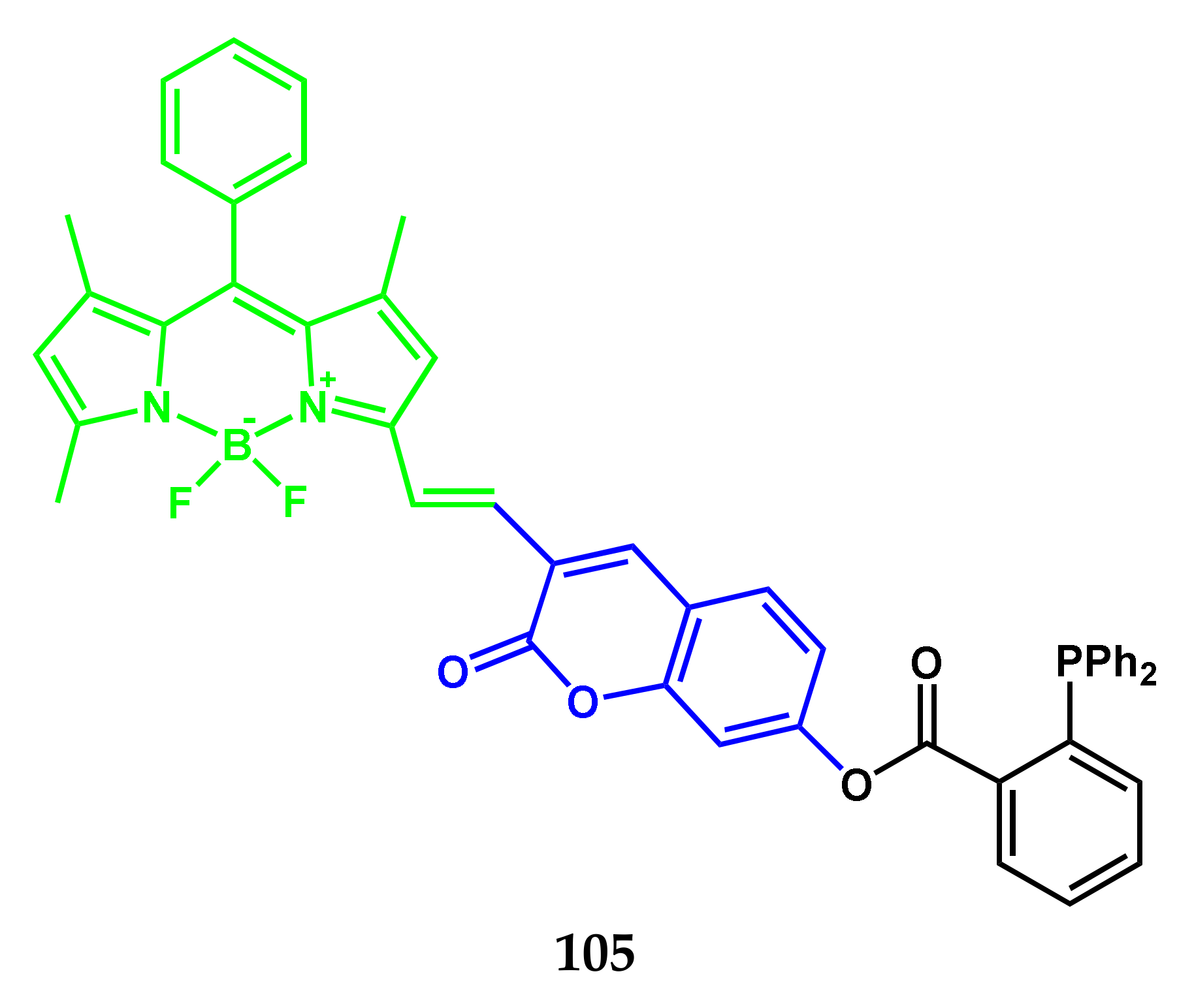
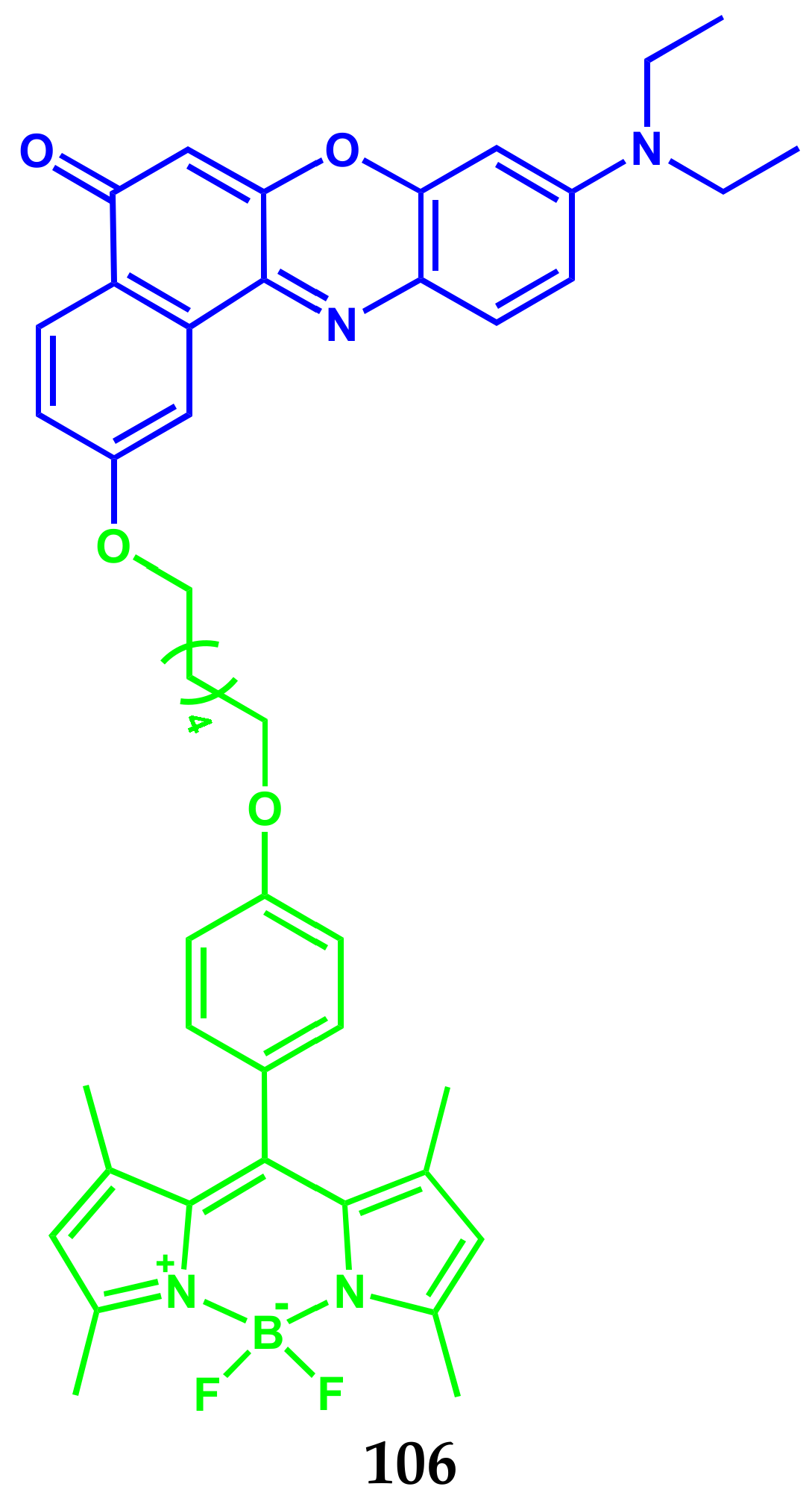
| Conjugate | λabs, nm (ε, M−1 cm−1) | λfl, nm | ΦF | ΦΔ | Comments | IC50, μM | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 506 (33,300) | 519 | 0.27 absolute | 0.19 | 10 μM in PBS containing 5% DMSO (V/V) | 13.1 μM (HeLa); 7.6 μM (MCF-7) | [6] |
| 2 | 519 (26,300) | 533 | 0.14 absolute | 0.14 | 10 μM in PBS containing 5% DMSO (V/V) | 28.6 μM (HeLa); 18.9 μM (MCF-7) | [6] |
| 3 | 504 (68,630) | 513 | 0.03 | - | PBS buffer (pH 7.4) | 15.7 μM (MCF-7); 19.1 μM (MDA-MB-231); 22.4 μM (SKOV3); 31.0 μM (A2780); 37.0 μM (HeLa); 60.0 μM (A549) | [7] |
| 4 | 596 | 615 | 0.04 | - | DMSO (10%)-DME; λex 580 nm | 6.1 μM (HeLa); 4.1 μM (A549) | [8] |
| 5 | 710 | 718 | - | - | DMSO (10%)-DME; λex 685 nm | 0.85 μM (HeLa); 1.1 μM (A549) | [8] |
| 6 | 530 (18,000); 500 (27,000) | 510 | 0.09 | 1% DMSO/DMEM | 0.15 μM (HaCaT); 6.7 μM (MCF-7) | [9] | |
| 7 | 540 (14,000); 510 (19,000) | - | - | - | 1% DMSO/DMEM | 0.10 μM (HaCaT); 2.6 μM (MCF-7) | [9] |
| 8 | 505 (41,000); 475 (11,000) | 530 | 0.07 | 0.26 | 1% DMSO/DMEM; singlet oxygen quantum yield in air-saturated DMSO/DMF | 1.70 μM (A549); 0.4 μM (MCF-7); 1.0 μM (HaCaT) | [10] |
| 9 | 525 (20,100); 490 (7000sh) | 540 | 0.02 | 0.48 | 1% DMSO/DMEM; singlet oxygen quantum yield in air-saturated DMSO/DMF | 0.09 μM (A549); 0.13 μM (MCF-7); 0.09 μM (HaCaT) | [10] |
| 10 | 555 (11,000) | - | - | 0.65 | 1% DMSO/DMEM; singlet oxygen quantum yield in air-saturated DMSO/DMF | 0.05 μM (A549); 0.07 μM (MCF-7); 0.06 μM (HaCaT) | [10] |
| 11 | 650–660 | 668 | 0.02 | - | λex 650 nm; 10% DMSO−DMEM of pH 7.2 | 0.6 μM (HeLa) 0.7 μM (A549) 3.4 μM (MDA-MB-231) | [11] |
| 12 | 617 (29,000) | 720 | 0.032 | 0.48 | 10% DMSO/DMEM buffer | 1.6 μM (A549) 2.4 μM (HeLa) | [12] |
| 13 | 695 (64,000) | 720 | 0.11 | - | H2O/1% SDS | ~200 μM (A2780P and A2780cisR) | [13] |
| 14 | ~556 | 582 | 0.595 | - | DMSO | 6.41 μM (HeLa) | [14] |
| 15 | ~556 | 581 | 0.719 | - | DMSO | 2.11 μM (HeLa) | [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antina, E.; Bumagina, N.; Marfin, Y.; Guseva, G.; Nikitina, L.; Sbytov, D.; Telegin, F. BODIPY Conjugates as Functional Compounds for Medical Diagnostics and Treatment. Molecules 2022, 27, 1396. https://doi.org/10.3390/molecules27041396
Antina E, Bumagina N, Marfin Y, Guseva G, Nikitina L, Sbytov D, Telegin F. BODIPY Conjugates as Functional Compounds for Medical Diagnostics and Treatment. Molecules. 2022; 27(4):1396. https://doi.org/10.3390/molecules27041396
Chicago/Turabian StyleAntina, Elena, Natalia Bumagina, Yuriy Marfin, Galina Guseva, Liliya Nikitina, Dmitry Sbytov, and Felix Telegin. 2022. "BODIPY Conjugates as Functional Compounds for Medical Diagnostics and Treatment" Molecules 27, no. 4: 1396. https://doi.org/10.3390/molecules27041396
APA StyleAntina, E., Bumagina, N., Marfin, Y., Guseva, G., Nikitina, L., Sbytov, D., & Telegin, F. (2022). BODIPY Conjugates as Functional Compounds for Medical Diagnostics and Treatment. Molecules, 27(4), 1396. https://doi.org/10.3390/molecules27041396