Molecular Mechanisms of Action of Selected Substances Involved in the Reduction of Benzo[a]pyrene-Induced Oxidative Stress
Abstract
1. Introduction
2. The Effect of BaP on Oxidative Stress In Vitro and In Vivo
3. Protective Properties of Selected Substances against Oxidative Effects of BaP
3.1. Vitamin E
3.2. Polyphenols
3.2.1. Curcumin
3.2.2. Resveratrol and Other Polyphenols
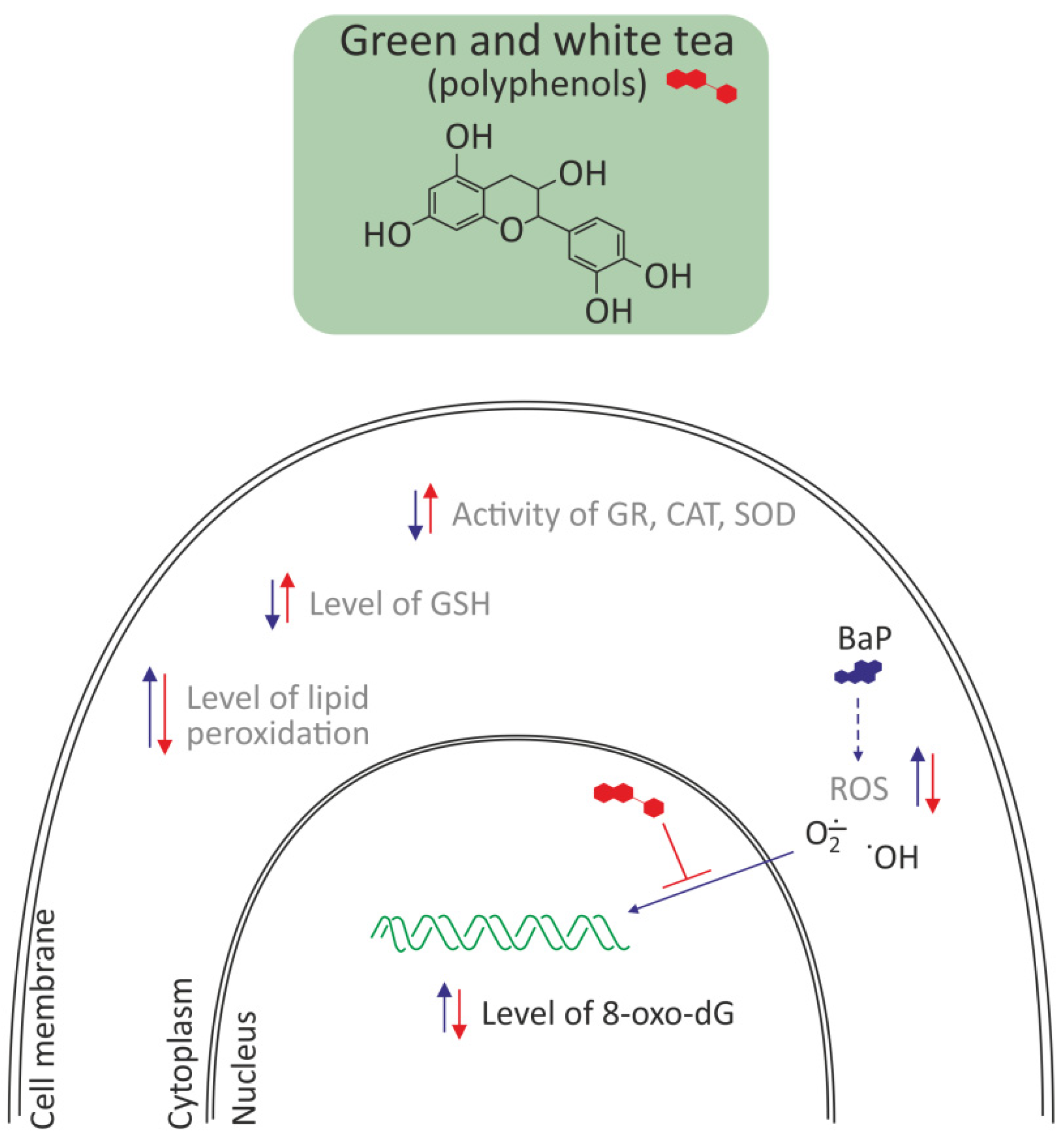
3.2.3. Polyphenols from Green and White Tea
3.2.4. Flawonoids
Baicalein
Myricetin
Catechin Hydrate
Hesperetin
3.3. Rhaponticin
3.4. Taurine
3.5. Atorvastatin
3.6. Organosulfur Compounds of Garlic
4. Summary
5. Conclusions
- Decrease of AhR translocation (e.g., resveratrol, curcumin)
- Downregulation of Cyp 450 level and other proteins involved in BaP metabolism (e.g., resveratrol, curcumin)
- Decrease of BPDA-DNA adducts and other DNA damage formation (e.g., curcumin; polyphenols from white and green tea)
- Decrease of expression of genes related to oxidative stress and inflammation (e.g., hesperetin, rhaponticin, curcumin, taurine, atorvastatin)
- Increase of expression and activity on antioxidative enzymes and antioxidants (e.g., vitamin E, diallyl sulfide, hesperetin)
- Decrease of fatty acid synthase (FASN), and in consequence decrease of fatty acid level in the liver (resveratrol, DAS)
- Decrease of ROS level by binding of hydrogen ions and scavenging free radicals (e.g., polyphenols from white and green tea, curcumin, resveratrol)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Siddique, R.; Zahoor, A.F.; Ahmad, H.; Zahid, F.M.; Karrar, E. Impact of different cooking methods on polycyclic aromatic hydrocarbons in rabbit meat. Food Sci. Nutr. 2021, 9, 3219–3227. [Google Scholar] [CrossRef] [PubMed]
- Susanto, A.; Yusril, N.; Zaini, J.; Nuwidya, F. Comparison of Serum Benzo(a)pyrene Diol Epoxide—Protein Adducts Level between Kretek Cigarette Smokers and Nonsmokers and the Related Factors. J. Nat. Sci. Biol. Med. 2021, 12, 52. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Guizellini, G.M.; Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A.F.S. Polycyclic Aromatic Hydrocarbons in Foods: Biological Effects, Legislation, Occurrence, Analytical Methods, and Strategies to Reduce Their Formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, C.; He, W.; Xu, F. Nationwide health risk assessment of juvenile exposure to polycyclic aromatic hydrocarbons (PAHs) in the water body of Chinese lakes. Sci. Total Environ. 2020, 723, 138099. [Google Scholar] [CrossRef]
- Air Quality in Europe—2020 Report. 2020. Available online: https://www.eea.europa.eu/publications/air-quality-in-europe-2020-report (accessed on 1 January 2022).
- IARC Working Groups Benzo[a]pyrene. 2018. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono100F-14.pdf (accessed on 1 January 2022).
- Birkett, N.; Al-Zoughool, M.; Bird, M.; Baan, R.A.; Zielinski, J.; Krewski, D. Overview of biological mechanisms of human carcinogens. J. Toxicol. Environ. Health Part B 2019, 22, 288–359. [Google Scholar] [CrossRef]
- Chakravarti, D.; Venugopal, D.; Mailander, P.C.; Meza, J.L.; Higginbotham, S.; Cavalieri, E.L.; Rogan, E.G. The role of polycyclic aromatic hydrocarbon–DNA adducts in inducing mutations in mouse skin. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2008, 649, 161–178. [Google Scholar] [CrossRef]
- Mangal, D.; Vudathala, D.; Park, J.-H.; Lee, S.H.; Penning, T.M.; Blair, I.A. Analysis of 7,8-Dihydro-8-oxo-2′-deoxyguanosine in Cellular DNA during Oxidative Stress. Chem. Res. Toxicol. 2009, 22, 788–797. [Google Scholar] [CrossRef]
- Barnes, J.L.; Zubair, M.; John, K.; Poirier, M.C.; Martin, F.L. Carcinogens and DNA damage. Biochem. Soc. Trans. 2018, 46, 1213–1224. [Google Scholar] [CrossRef]
- Briedé, J.J.; Godschalk, R.W.; Emans, M.T.; de Kok, T.M.; van Agen, E.; van Maanen, J.M.; van Schooten, F.-J.; Kleinjans, J.C. In Vitro and In Vivo Studies on Oxygen Free Radical and DNA Adduct Formation in Rat Lung and Liver during Benzo[a]pyrene Metabolism. Free Radic. Res. 2004, 38, 995–1002. [Google Scholar] [CrossRef]
- Park, J.-H.; Mangal, D.; Tacka, K.A.; Quinn, A.M.; Harvey, R.G.; Blair, I.A.; Penning, T.M. Evidence for the aldo-keto reductase pathway of polycyclic aromatic trans-dihydrodiol activation in human lung A549 cells. Proc. Natl. Acad. Sci. USA 2008, 105, 6846–6851. [Google Scholar] [CrossRef]
- Harvey, R.G.; Dai, Q.; Ran, C.; Lim, K.; Blair, I.; Penning, T.M. Syntheses of adducts of active metabolites of carcinogenic polycyclic aromatic hydrocarbons with 2′-deoxyribonucleosides. Polycycl. Aromat. Compd. 2005, 25, 371–391. [Google Scholar] [CrossRef]
- Møller, P.; Jacobsen, N.R.; Folkmann, J.K.; Danielsen, P.H.; Mikkelsen, L.; Hemmingsen, J.G.; Vesterdal, L.K.; Forchhammer, L.; Wallin, H.; Loft, S. Role of oxidative damage in toxicity of particulates. Free Radic. Res. 2010, 44, 1–46. [Google Scholar] [CrossRef]
- Rendic, S.P.; Guengerich, F.P. Human Family 1-4 cytochrome P450 enzymes involved in the metabolic activation of xenobiotic and physiological chemicals: An update. Arch. Toxicol. 2021, 95, 395–472. [Google Scholar] [CrossRef]
- Rendic, S.P.; Guengerich, F.P. Development and Uses of Offline and Web-Searchable Metabolism Databases—The Case of Benzoapyrene. Curr. Drug Metab. 2018, 19, 3–46. [Google Scholar] [CrossRef]
- Penning, T.M. Human aldo-keto reductases and the metabolic activation of polycyclic aromatic hydrocarbons. Chem. Res. Toxicol. 2014, 27, 1901–1917. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Tatli, P.; Yilmaz, S.; Seven, I.; Tuna, G. The Effects of Propolis in Animals Exposed Oxidative Stress. In Oxidative Stress—Environmental Induction and Dietary Antioxidants; Lushchak, V., Ed.; InTech: Vienna, Austria, 2012; ISBN 978-953-51-0553-4. [Google Scholar]
- McNeill, J.M.; Wills, E.D. The formation of mutagenic derivatives of benzoapyrene by peroxidising fatty acids. Chem. Biol. Interact. 1985, 53, 197–207. [Google Scholar] [CrossRef]
- Uno, S.; Makishima, M. Benzo[a]pyrene Toxicity and Inflammatory Disease. Curr. Rheumatol. Rev. 2009, 5, 266–271. [Google Scholar] [CrossRef]
- Gao, D.; Wu, M.; Wang, C.; Wang, Y.; Zuo, Z. Chronic exposure to low benzo[a]pyrene level causes neurodegenerative disease-like syndromes in zebrafish (Danio rerio). Aquat. Toxicol. 2015, 167, 200–208. [Google Scholar] [CrossRef]
- Ueng, T.-H.; Chang, Y.-L.; Tsai, Y.-Y.; Su, J.-L.; Chan, P.-K.; Shih, J.-Y.; Lee, Y.-C.; Ma, Y.-C.; Kuo, M.-L. Potential roles of fibroblast growth factor-9 in the benzo(a)pyrene-induced invasion in vitro and the metastasis of human lung adenocarcinoma. Arch. Toxicol. 2010, 84, 651–660. [Google Scholar] [CrossRef]
- Labib, S.; Guo, C.H.; Williams, A.; Yauk, C.L.; White, P.A.; Halappanavar, S. Toxicogenomic outcomes predictive of forestomach carcinogenesis following exposure to benzo(a)pyrene: Relevance to human cancer risk. Toxicol. Appl. Pharmacol. 2013, 273, 269–280. [Google Scholar] [CrossRef][Green Version]
- Ba, Q.; Li, J.; Huang, C.; Qiu, H.; Li, J.; Chu, R.; Zhang, W.; Xie, D.; Wu, Y.; Wang, H. Effects of Benzo[ a ]pyrene Exposure on Human Hepatocellular Carcinoma Cell Angiogenesis, Metastasis, and NF- κ B Signaling. Environ. Health Perspect. 2015, 123, 246–254. [Google Scholar] [CrossRef]
- White, A.J.; Bradshaw, P.T.; Herring, A.H.; Teitelbaum, S.L.; Beyea, J.; Stellman, S.D.; Steck, S.E.; Mordukhovich, I.; Eng, S.M.; Engel, L.S.; et al. Exposure to multiple sources of polycyclic aromatic hydrocarbons and breast cancer incidence. Environ. Int. 2016, 89–90, 185–192. [Google Scholar] [CrossRef]
- Petit, P.; Maître, A.; Persoons, R.; Bicout, D.J. Lung cancer risk assessment for workers exposed to polycyclic aromatic hydrocarbons in various industries. Environ. Int. 2019, 124, 109–120. [Google Scholar] [CrossRef]
- He, J.; Pang, Q.; Huang, C.; Xie, J.; Hu, J.; Wang, L.; Wang, C.; Meng, L.; Fan, R. Environmental dose of 16 priority-controlled PAHs mixture induce damages of vascular endothelial cells involved in oxidative stress and inflammation. Toxicol. Vitr. 2022, 79, 105296. [Google Scholar] [CrossRef]
- Ji, K.; Xing, C.; Jiang, F.; Wang, X.; Guo, H.; Nan, J.; Qian, L.U.; Yang, P.; Lin, J.; Li, M.; et al. Benzo[a]pyrene induces oxidative stress and endothelial progenitor cell dysfunction via the activation of the NF-κB pathway. Int. J. Mol. Med. 2013, 31, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Wu, C.-Y.; Hu, C.-H.; Pai, T.-W.; Chen, Y.-R.; Wang, W.-D. Integrated Hypoxia Signaling and Oxidative Stress in Developmental Neurotoxicity of Benzo[a]Pyrene in Zebrafish Embryos. Antioxidants 2020, 9, 731. [Google Scholar] [CrossRef]
- Aparna, S.; Patri, M. Benzo[a]pyrene exposure and overcrowding stress impacts anxiety-like behavior and impairs learning and memory in adult zebrafish, Danio rerio. Environ. Toxicol. 2021, 36, 352–361. [Google Scholar] [CrossRef]
- González, A.; Espinoza, D.; Vidal, C.; Moenne, A. Benzopyrene induces oxidative stress and increases expression and activities of antioxidant enzymes, and CYP450 and GST metabolizing enzymes in Ulva lactuca (Chlorophyta). Planta 2020, 252, 107. [Google Scholar] [CrossRef]
- Gao, M.; Zheng, A.; Chen, L.; Dang, F.; Liu, X.; Gao, J. Benzo(a)pyrene affects proliferation with reference to metabolic genes and ROS/HIF-1α/HO-1 signaling in A549 and MCF-7 cancer cells. Drug Chem. Toxicol. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Cromie, M.M.; Cai, Q.; Lv, T.; Singh, K.; Gao, W. Curcumin and Vitamin E Protect against Adverse Effects of Benzo[a]pyrene in Lung Epithelial Cells. PLoS ONE 2014, 9, e92992. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, S.; Sinha, N.; Kodidela, S.; Kumar, S. Benzo(a)pyrene in Cigarette Smoke Enhances HIV-1 Replication through NF-κB Activation via CYP-Mediated Oxidative Stress Pathway. Sci. Rep. 2018, 8, 10394. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ren, A.; Wang, L.; Huang, Y.; Wang, Y.; Wang, C.; Greene, N.D. Oxidative Stress and Apoptosis in Benzo[a]pyrene-Induced Neural Tube Defects. Free Radic. Biol. Med. 2018, 116, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-C.; Jee, S.-C.; Kim, M.; Kim, S.; Shin, M.K.; Kim, Y.; Sung, J.-S. Curcumin Suppresses the Lipid Accumulation and Oxidative Stress Induced by Benzo[a]pyrene Toxicity in HepG2 Cells. Antioxidants 2021, 10, 1314. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ito, T.; Tsuji, G.; Furue, M. Baicalein Inhibits Benzo[a]pyrene-Induced Toxic Response by Downregulating Src Phosphorylation and by Upregulating NRF2-HMOX1 System. Antioxidants 2020, 9, 507. [Google Scholar] [CrossRef]
- Bodduluru, L.N.; Kasala, E.R.; Barua, C.C.; Karnam, K.C.; Dahiya, V.; Ellutla, M. Antiproliferative and antioxidant potential of hesperetin against benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Chem. Biol. Interact. 2015, 242, 345–352. [Google Scholar] [CrossRef]
- Jee, S.-C.; Kim, M.; Kim, K.S.; Kim, H.-S.; Sung, J.-S. Protective Effects of Myricetin on Benzoapyrene-Induced 8-Hydroxy-2’-Deoxyguanosine and BPDE-DNA Adduct. Antioxidants 2020, 9, 446. [Google Scholar] [CrossRef]
- Gao, H.; Ye, G.; Lin, Y.; Chi, Y.; Dong, S. Benzo[a]pyrene at human blood equivalent level induces human lung epithelial cell invasion and migration via aryl hydrocarbon receptor signaling. J. Appl. Toxicol. 2020, 40, 1087–1098. [Google Scholar] [CrossRef]
- Wang, X.; Priya Veeraraghavan, V.; Krishna Mohan, S.; Lv, F. Anticancer and immunomodulatory effect of rhaponticin on Benzo(a)Pyrene-induced lung carcinogenesis and induction of apoptosis in A549 cells. Saudi J. Biol. Sci. 2021, 28, 4522–4531. [Google Scholar] [CrossRef]
- Owumi, S.E.; Popoola, O.; Da Otunla, M.T.; Okuu, U.A.; Najophe, E.S. Benzo-a-pyrene-induced reproductive toxicity was abated in rats co-treated with taurine. Toxin Rev. 2021, 1–14. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, V.L.; Sehgal, A.; Jain, M. Protective Effects of Green and White Tea Against Benzo(a)pyrene Induced Oxidative Stress and DNA Damage in Murine Model. Nutr. Cancer 2012, 64, 300–306. [Google Scholar] [CrossRef]
- Du, X.; Li, D.; Wang, G.; Fan, Y.; Li, N.; Chai, L.; Li, G.; Li, J. Chemoprotective effect of atorvastatin against benzo(a)pyrene-induced lung cancer via the inhibition of oxidative stress and inflammatory parameters. Ann. Transl. Med. 2021, 9, 355. [Google Scholar] [CrossRef]
- Khan, A.; Alhumaydhi, F.A.; Alwashmi, A.S.S.; Allemailem, K.S.; Alsahli, M.A.; Alrumaihi, F.A.; Almatroudi, A.; Mobark, M.A.; Mousa, A.; Khan, M.A. Diallyl Sulfide-Mediated Modulation of the Fatty Acid Synthase (FASN) Leads to Cancer Cell Death in BaP-Induced Lung Carcinogenesis in Swiss Mice. J. Inflamm. Res. 2020, 13, 1075–1087. [Google Scholar] [CrossRef]
- Yamauchi, R. Vitamin E: Mechanism of Its Antioxidant Activity. FSTI 1997, 3, 301–309. [Google Scholar] [CrossRef]
- Rimbach, G.; Moehring, J.; Huebbe, P.; Lodge, J.K. Gene-regulatory activity of alpha-tocopherol. Molecules 2010, 15, 1746–1761. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Liczbiński, P.; Michałowicz, J.; Bukowska, B. Molecular mechanism of curcumin action in signaling pathways: Review of the latest research. Phytother. Res. 2020, 34, 1992–2005. [Google Scholar] [CrossRef]
- Dutta, B. Study of secondary metabolite constituents and curcumin contents of six different species of genus curcuma. J. Med. Plants Stud. 2015, 3, 116–119. [Google Scholar]
- Perdew, G.H.; Murray, I.A.; Peters, J.M. Xenobiotic Receptor-Mediated Toxicity. Compr. Toxicol. 2018, 1, 202–228. [Google Scholar] [CrossRef]
- Omidian, K.; Rafiei, H.; Bandy, B. Polyphenol inhibition of benzo[a]pyrene-induced oxidative stress and neoplastic transformation in an in vitro model of carcinogenesis. Food Chem. Toxicol. 2017, 106, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Olas, B.; Wachowicz, B. Resveratrol, a phenolic antioxidant with effects on blood platelet functions. Platelets 2005, 16, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Gao, H.; Zhang, X.; Liu, X.; Chen, J.; Liao, X.; Zhang, H.; Huang, Q. Aryl hydrocarbon receptor mediates benzo[a]pyrene-induced metabolic reprogramming in human lung epithelial BEAS-2B cells. Sci. Total Environ. 2021, 756, 144130. [Google Scholar] [CrossRef] [PubMed]
- Çelik, S.; Baysal, B.; Şen, S. Resveratrol Attenuates Benzo(a)pyrene-Induced Dysfunctions, Oxidative Stress and Apoptosis in Pancreatic Beta-Cells. Adv. Biosci. Biotechnol. 2019, 10, 389–404. [Google Scholar] [CrossRef]
- Liczbiński, P.; Bukowska, B. Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations. Ind. Crops Prod. 2022, 175, 114265. [Google Scholar] [CrossRef]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement. Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef]
- Khattab, S.A.; Hussien, W.F.; Raafat, N.; Ahmed Alaa El-Din, E. Effects of catechin hydrate in benzo[a]pyrene-induced lung toxicity: Roles of oxidative stress, apoptosis, and DNA damage. Toxicol. Mech. Methods 2021, 31, 467–475. [Google Scholar] [CrossRef]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and Anti-Inflammatory Effects of Citrus Flavonoid Hesperetin: Special Focus on Neurological Disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef]
- Kim, H.W.; Woo, H.J.; Yang, J.Y.; Kim, J.-B.; Kim, S.-H. Hesperetin Inhibits Expression of Virulence Factors and Growth of Helicobacter pylori. Int. J. Mol. Sci. 2021, 22, 10035. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Czepas, J. Rhaponticin as an anti-inflammatory component of rhubarb: A minireview of the current state of the art and prospects for future research. Phytochem. Rev. 2019, 18, 1375–1386. [Google Scholar] [CrossRef]
- Reddy, K.P.; Girish, B.P.; Reddy, P.S. Reproductive and paternal mediated developmental toxicity of benzo(a)pyrene in adult male Wistar rats. Toxicol. Res. 2015, 4, 223–232. [Google Scholar] [CrossRef]
- Jorge, B.C.; Reis, A.C.C.; Stein, J.; Da Balin, P.S.; Sterde, É.T.; Barbosa, M.G.; de Aquino, A.M.; Kassuya, C.A.L.; Arena, A.C. Parental exposure to benzo(a)pyrene in the peripubertal period impacts reproductive aspects of the F1 generation in rats. Reprod. Toxicol. 2021, 100, 126–136. [Google Scholar] [CrossRef]
- Orita, H.; Coulter, J.; Tully, E.; Kuhajda, F.P.; Gabrielson, E. Inhibiting Fatty Acid Synthase for Chemoprevention of Chemically Induced Lung Tumors. Clin. Cancer Res. 2008, 14, 2458–2464. [Google Scholar] [CrossRef]
- Rahayu Lestari, S.; Lukiati, B.; Nur Arifah, S.; Rofiqotun Nurul Alimah, A.; Gofur, A. Medicinal Uses of Single Garlic in Hyperlipidemia by Fatty Acid Synthase Enzyme Inhibitory: Molecular Docking. IOP Conf. Ser. Earth Environ. Sci. 2019, 276, 12008. [Google Scholar] [CrossRef]
- Yeh, Y.-Y.; Liu, L. Cholesterol-Lowering Effect of Garlic Extracts and Organosulfur Compounds: Human and Animal Studies. J. Nutr. 2001, 131, 989S–993S. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, N.; He, H.; Tang, X. Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J. Control. Release 2019, 316, 359–380. [Google Scholar] [CrossRef]
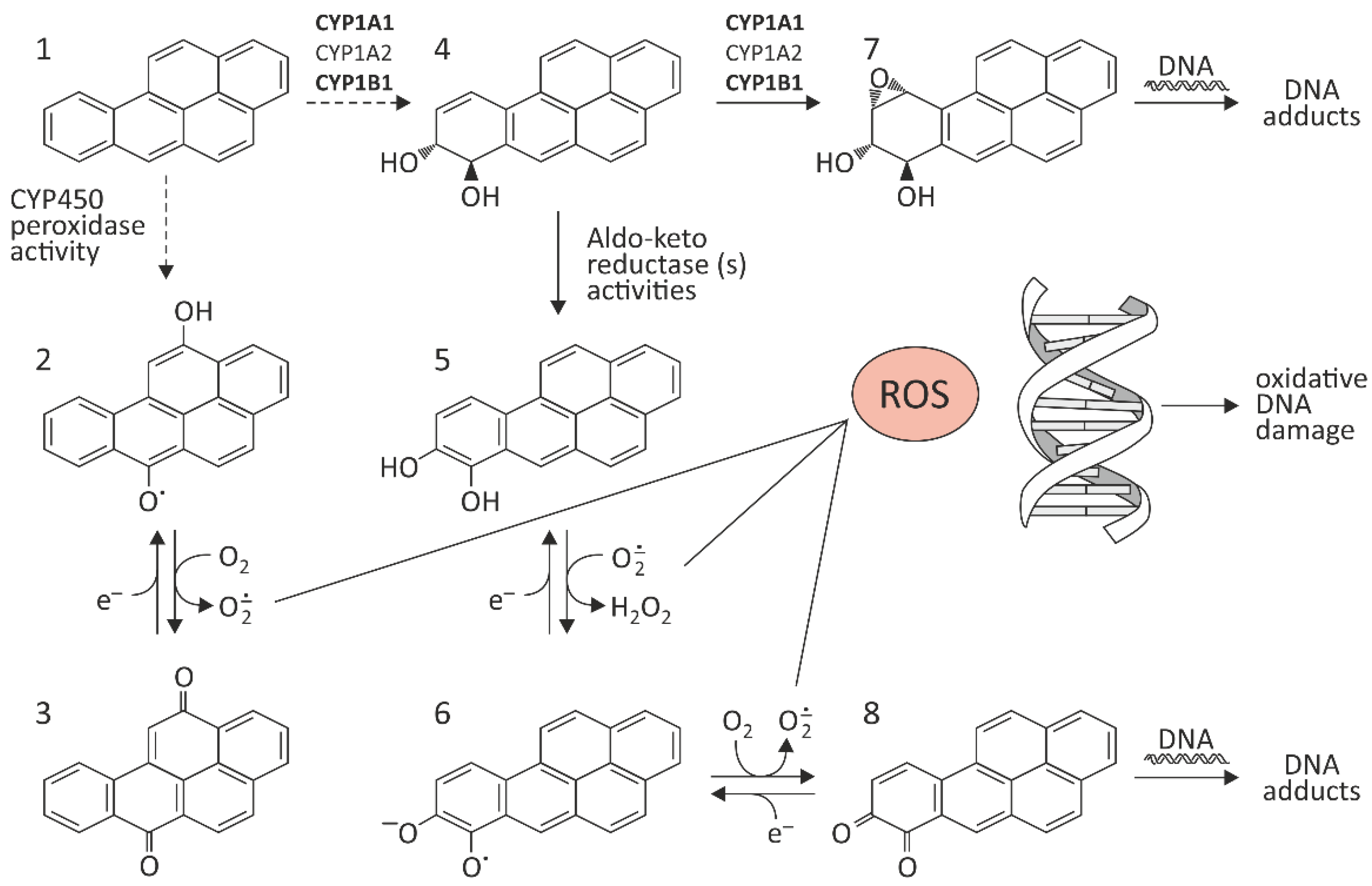
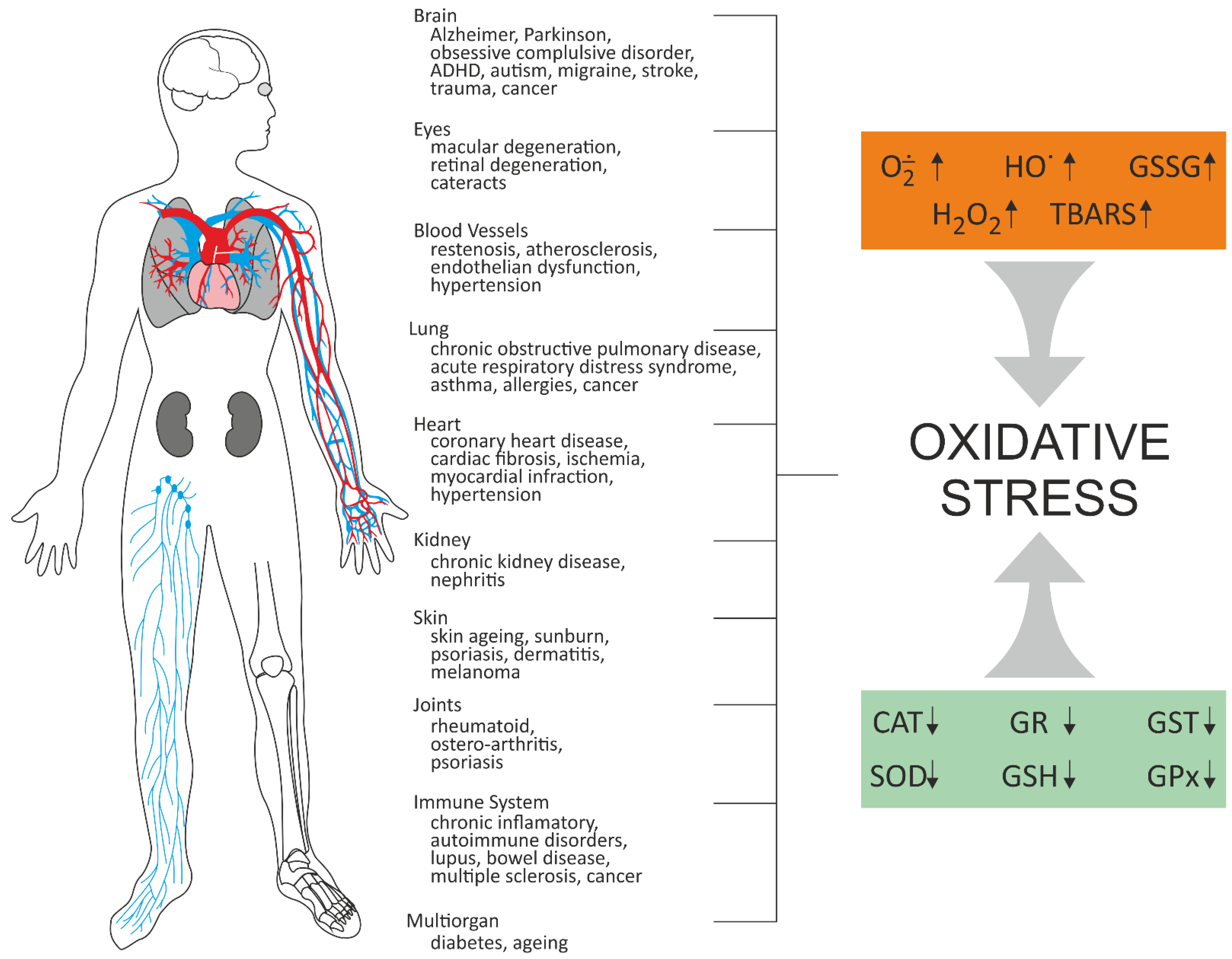
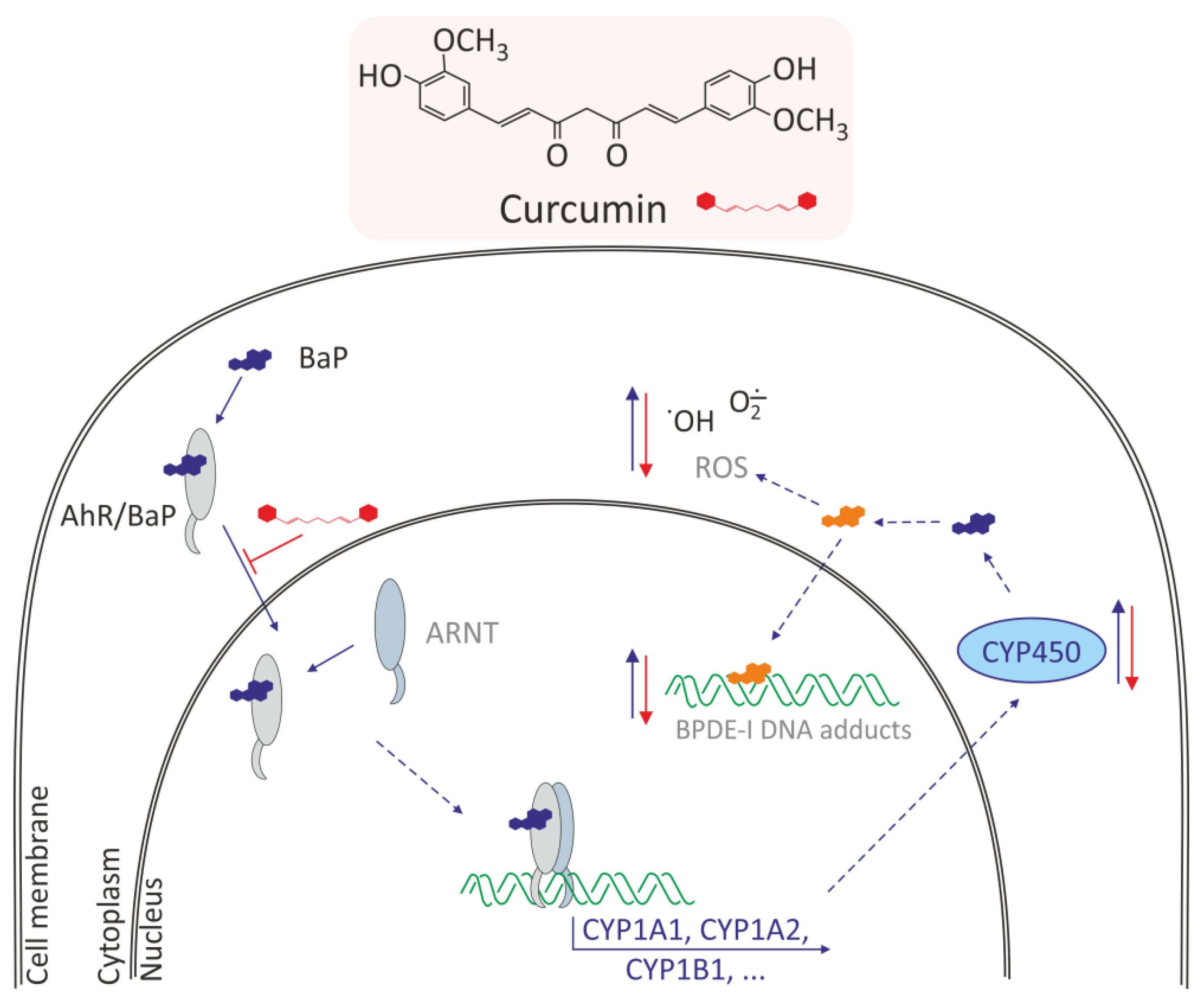
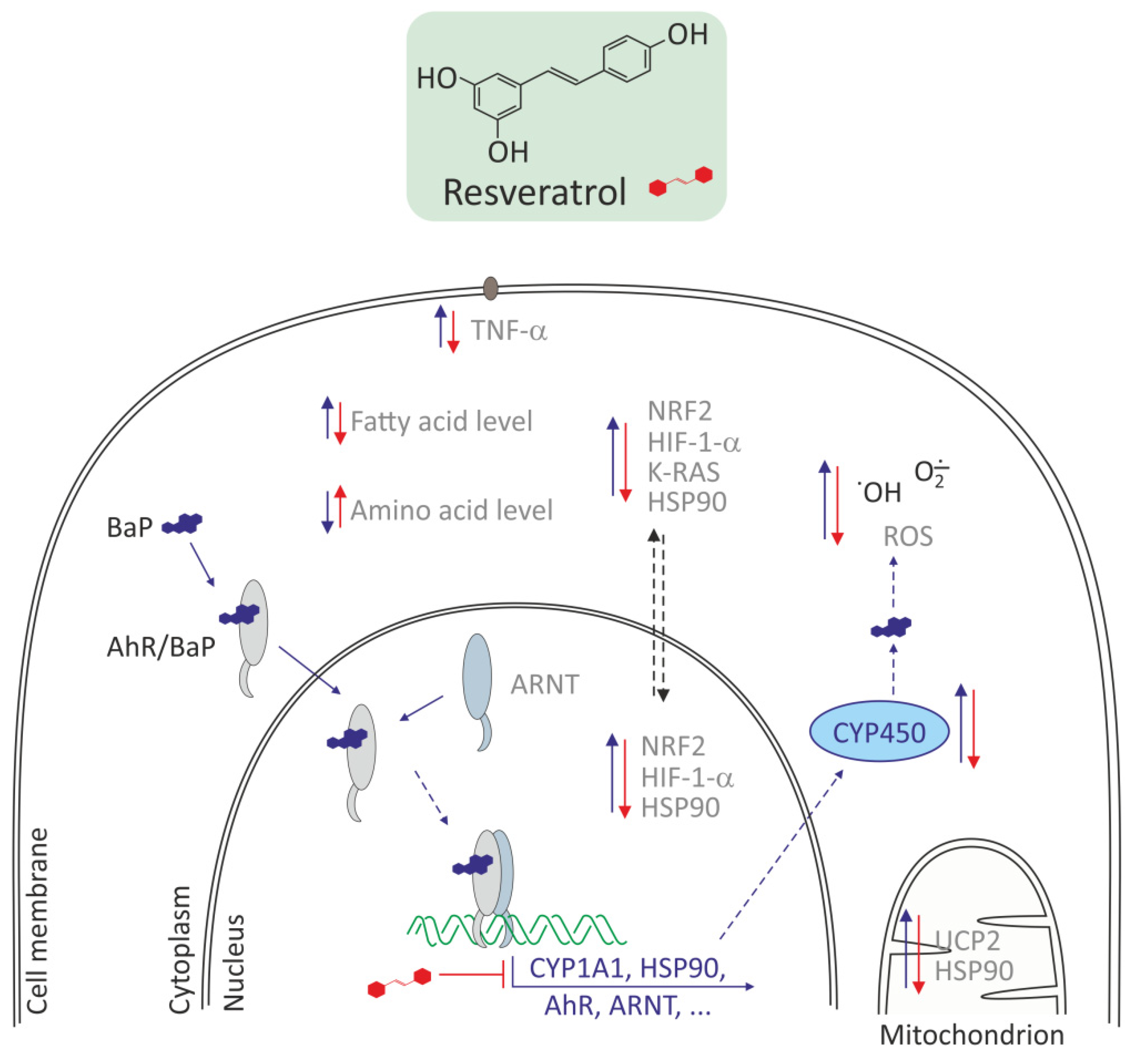

| BaP Concentrations | Cells/ Organisms | Oxidative Effects Induced by BaP | References |
|---|---|---|---|
| 10; 20; 50 µM | Human endothelial progenitor cells (EPCs) | Release of IL-1β and TNF-α from these cells | [30] |
| 1; 5 µM | A549 and MCF-7 cancer cells | Formation of ROS by influencing ROS/HIF-1α/HO-1 signaling pathway | [34] |
| 5 µM | Human lung epithelial cells (BEAS-2B) | ROS production and DNA damage | [35] |
| 100 nM; 1 µM | Human monocytes and U937 cells | ROS production, increase in the expression of CYP1A1 gene, and induction of NF-κB pathway | [36] |
| 250 mg kg–1 | Mice–maternal exposure to BaP | Increased expression of CYP1A1, SOD1, and SOD2 gene, and repressed GPX1 gene | [37] |
| 10; 20 µM | Zebrafish embryos | Decrease of CAT and SOD1 gene expression, increase H2O2 and MDA level | [31] |
| 0.2 mg L−1 | Adult zebrafish | Increase of lipid peroxidation and protein carbonyl formation, decrease CAT activity and reduce GSH level | [32] |
| 5 µM | Green algae (Ulva lactuca) | Changes in the level of ROS, changes in SOD, CAT, AP, GR, GPX activities | [33] |
| BaP Concentrations | Cells/Organisms | Oxidative Effects Induced by BaP | Compounds That Reduce the Oxidative Effect of BaP | References |
|---|---|---|---|---|
| 250 mg kg–1 | Female mice | Increased expression of CYP1A1, SOD1, and SOD2; decreased GPX1 | Vitamin E 0.125%, w/w | [37] |
| 0–80 µM; 5 µM | Human lung epithelial cells (BEAS-2B) | ROS production | Vitamin E and curumine 0–80 µM | [35] |
| 10 µM | HepG2 cells | Lipid accumulation and oxidative stress | Curcumin 1; 5; 10; 20; 40 µM | [38] |
| 4 µM | Bhas 42 cells | Increased expression of NRF2, UCP2, TNF-α | Polyphenols 5 µM | [54] |
| 1.28; 6.4; 32; 160; 800; 4000; 20000 μg/L | BEAS-2B lung cells | Changes in expression levels of AhR mRNA and genes involved in BaP metabolism | Resveratrol 0.1; 0.2; 0.4; 0.8; 1; 3.2; 6.4 μg/mL | [42] |
| 5 µg/L | BEAS-2B lung cells | Changes in 30 metabolic pathways | Resveratrol 0.1 mg/L | [56] |
| 20 μM | INS-1 (832/13) insulinoma cells | Decrease of GSH level and antioxidant status | Resveratrol 80 μM | [57] |
| 1 μM | NHEKs epidermal keratinocytes and HaCaT keratinocytes | Increase in CYP1A1 expression, ROS production and expression of pro-inflammatory cytokines | Baicalein 10 µM for NHEKs and 25 µM for HaCaT cells | [39] |
| 125 mg/kg b.w. orally | Balb/c mice | Increased LPO level and decrease in antioxidants level | Green tea and white tea−2% | [45] |
| 1; 2.5; 5; 10 μM 2 mg/kg | HepG2 cells rats | Oxidative damage to DNA and increase of CYP1A1 expression | Myricetin 15 mg/kg | [41] |
| 50 mg/kg 1/20 of LD50 | Adult albino rats | Decrease in the activity of SOD, CAT, and an increase in MDA level | Catechin hydrate 20 mg/kg body weight | [58] |
| 50 mg/kg p.o. | Swiss albino mice | Increase in LPO and decrease in SOD, CAT, GPx, GR, and GST activities and decrease in GSH, Vit C, and Vit E levels | Hesperetin 50 mg/kg body weight | [40] |
| 50 mg/kg body weight | Mice | Decrease of CAT and SOD activities and increase in LPO level | Rhaponticin 30 mg/kg body weight | [43] |
| 10 mg/kg | Rats | Oxidative stress and high level of inflammatory biomarkers | Taurine 100, 200 mg/kg | [44] |
| 50 mg/kg, body weight | Swiss Wistar rats | Decreased GPx, SOD, GST activities, and GSH level, increased nitrate and LPO levels | Atorvastatin 5, 10, 20 mg/kg body weight | [46] |
| 50 mg/kg of BaP twice a week for 4 weeks | Mice | Increased activity of enzymatic tumor markers: ADA, AHH, γ-GT, LDH, and decreased activity of antioxidant enzymes: SOD and CAT. High level of fatty acid synthase | Diallyl sulfide 100 mg/kg body weight | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukowska, B.; Duchnowicz, P. Molecular Mechanisms of Action of Selected Substances Involved in the Reduction of Benzo[a]pyrene-Induced Oxidative Stress. Molecules 2022, 27, 1379. https://doi.org/10.3390/molecules27041379
Bukowska B, Duchnowicz P. Molecular Mechanisms of Action of Selected Substances Involved in the Reduction of Benzo[a]pyrene-Induced Oxidative Stress. Molecules. 2022; 27(4):1379. https://doi.org/10.3390/molecules27041379
Chicago/Turabian StyleBukowska, Bożena, and Piotr Duchnowicz. 2022. "Molecular Mechanisms of Action of Selected Substances Involved in the Reduction of Benzo[a]pyrene-Induced Oxidative Stress" Molecules 27, no. 4: 1379. https://doi.org/10.3390/molecules27041379
APA StyleBukowska, B., & Duchnowicz, P. (2022). Molecular Mechanisms of Action of Selected Substances Involved in the Reduction of Benzo[a]pyrene-Induced Oxidative Stress. Molecules, 27(4), 1379. https://doi.org/10.3390/molecules27041379







