Revisiting the Physicochemical Properties and Applications of Deep Eutectic Solvents
Abstract
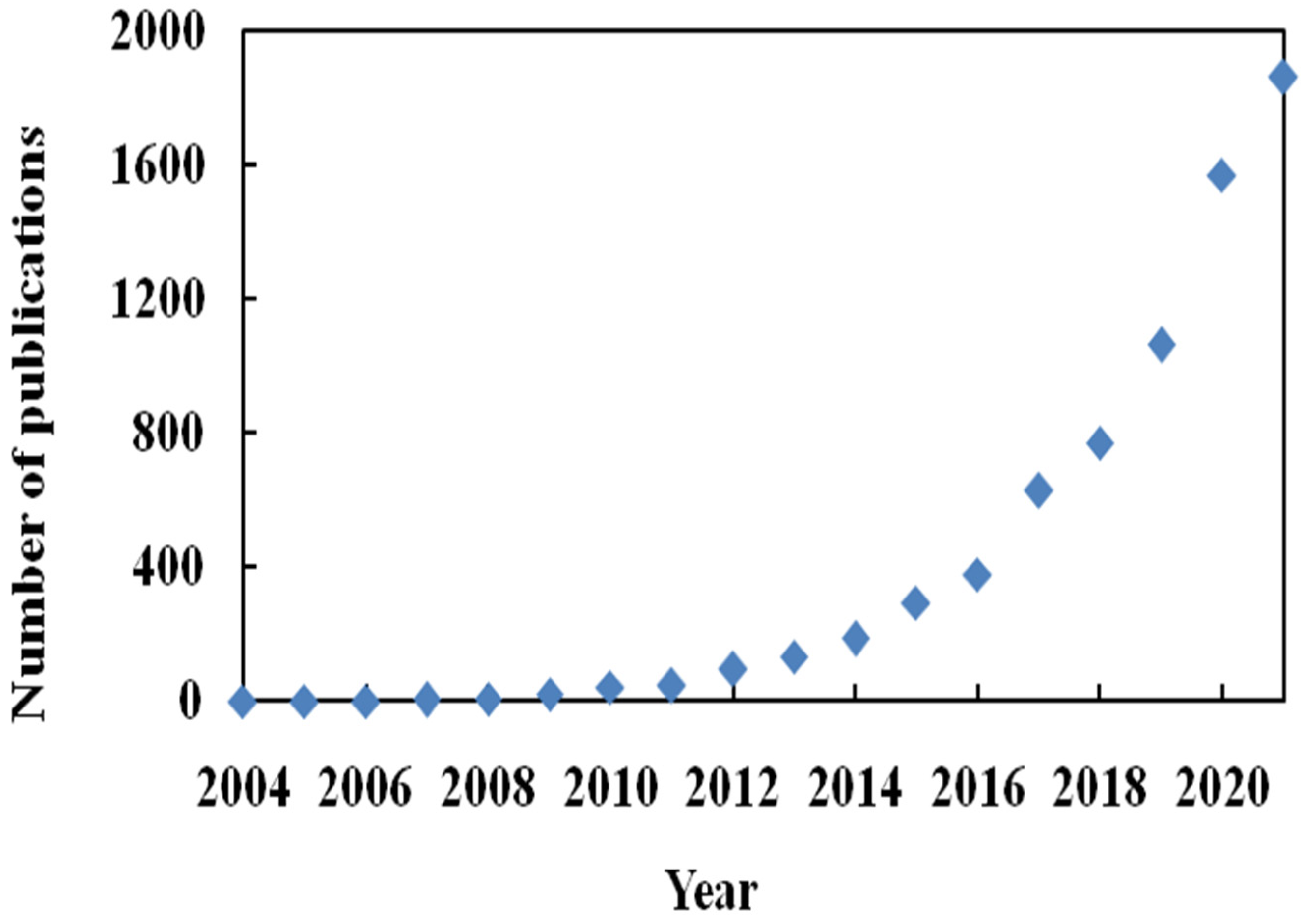
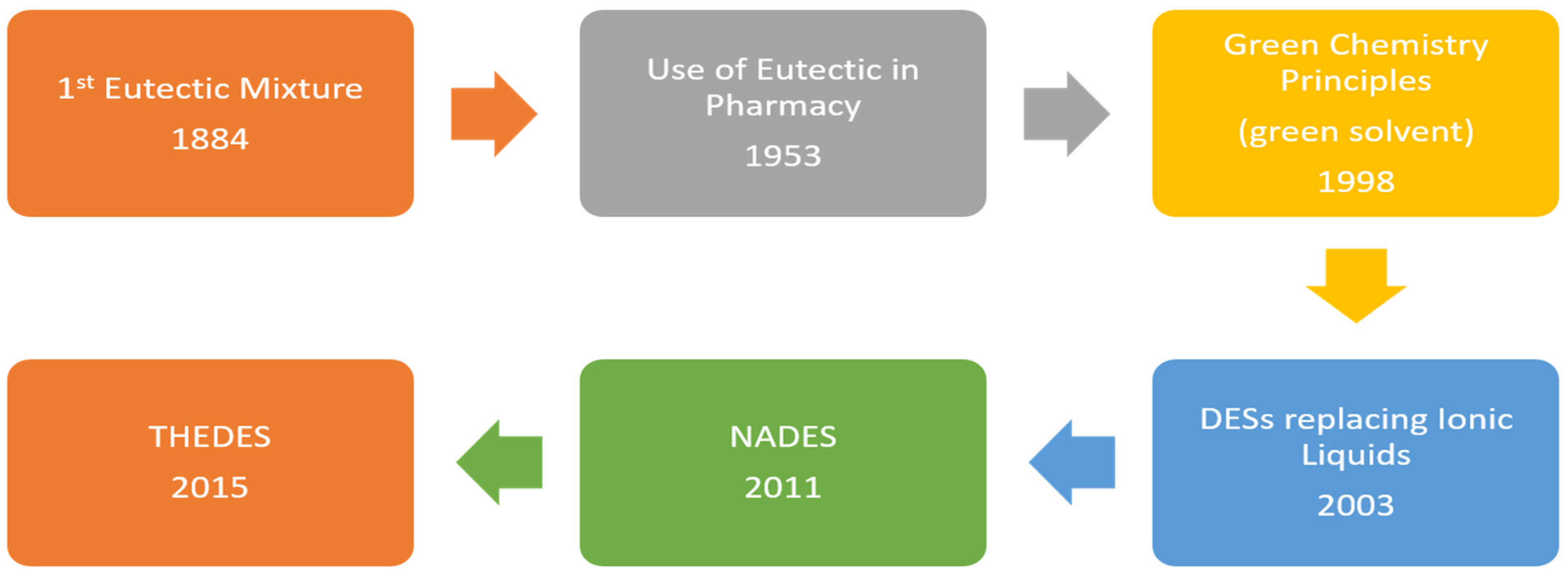
:1. Introduction
2. Deep Eutectic Solvents
2.1. Definition
2.2. Method of Preparation
2.3. Types of DES
| Type of DES | General Formula | Terms | Example |
|---|---|---|---|
| I | R+ A− + cMClx | M = In, Zn, Fe, Al, Sn | ChCl + SnCl2 |
| II | R+A− + cMClx.cH2O | M = Ni, Cr, Fe, Cu | ChCl + FeCl3·6H2O |
| III | R+ A− + cRW | W = OH, CONH2, COOH | ChCl + Urea |
| IV | MClx + cRW | M = Al, Zn and W = CONH2, OH | ZnCl2 + Urea |
| V | HBD + HBA | HBD = hydrogen bond donor HBA = hydrogen bond acceptor | Thymol + Menthol |
3. Physicochemical Properties of DESs
3.1. Density
| HBA | HBD | Molar Ratio | Density (ρ) (g cm3) | References |
|---|---|---|---|---|
| ChCl | Urea | 1:2 | 1.25 | [42] |
| ChAc | Urea | 1:2 | 1.206 | [42] |
| ChCl | 1-(trifluoromethyl)urea | 1:1.5 | 1.324 | [42] |
| ChCl | Glycerol | 1:1 | 1.16 | [43] |
| ChCl | Glycerol | 1:2 | 1.18 | [44,45] |
| ChCl | Ethylene glycol | 1:2 | 1.12 | [43,45] |
| ChCl | Ethylene glycol | 1:3 | 1.12 | [43,45] |
| ChCl | Oxalic acid | 1:1 | 1.259 | [25] |
| ChCl | Glycolic acid | 1:1 | 1.195 | [25] |
| ChCl | Malonic acid | 1:1 | 1.231 | [25] |
| ChCl | Glutaric acid | 1:1 | 1.188 | [25] |
| ChCl | Levulinic acid | 1:2 | 1.138 | [25] |
| ChCl | o-Cresol | 1:3 | 1.07 | [46] |
| ChCl | p-Cresol | 1:2 | 1.0681 | [47] |
| ChCl | Phenol | 1: 3 | 1.092 | [46] |
| ChCl | p-Chlorophenol | 1:2 | 1.1988 | [47] |
| ChCl | Glucose | 2:1 | 1.2423 | [41] |
| ChCl | D-Glucose | 1:1 | 1.273 | [38] |
| ChCl | D-Mannose | 1:1 | 1.278 | [38] |
| ChCl | D-Ribose | 1:1 | 1.267 | [38] |
| ChCl | D-Xylose | 1:1 | 1.257 | [38] |
| ChCl | D-Fructose | 1:1 | 1.272 | [38] |
| AcChCl | D-Glucose | 1:1 | 1.252 | [38] |
| AcChCl | D-Mannose | 1:1 | 1.260 | [38] |
| AcChCl | D-Ribose | 1:1 | 1.243 | [38] |
| AcChCl | D-Xylose | 1:1 | 1.224 | [38] |
| AcChCl | D-Fructose | 1:1 | 1.239 | [38] |
| BzChCl | D-Glucose | 1:1 | 1.263 | [38] |
| BzChCl | D-Mannose | 1:1 | 1.272 | [38] |
| BzChCl | D-Ribose | 1:1 | 1.255 | [38] |
| BzChCl | D-Xylose | 1:1 | 1.254 | [38] |
| BzChCl | D-Fructose | 1:1 | 1.263 | [38] |
| BTMACl | Ethylene glycol | 1:3 | 1.1009 | [37] |
| BTMACl | Diethylene glycol | 1:3 | 1.1106 | [37] |
| BTMACl | Triethylene glycol | 1:3 | 1.1173 | [37] |
| EACl | Urea | 1:1.5 | 1.140 | [42] |
| EACl | 1-(trifluoromethyl) urea | 1:1.5 | 1.273 | [42] |
| EACl | Acetamide | 1:1.5 | 1.041 | [42] |
| DEACl | Glycerol | 1:2 | 1.17 | [43] |
| DEACl | Glycerol | 1:3 | 1.21 | [43] |
| DEACl | Glycerol | 1:4 | 1.22 | [43] |
| DEACl | Ethylene glycol | 1:2 | 1.10 | [43] |
| DEACl | Ethylene glycol | 1:3 | 1.10 | [43] |
| DEACl | Ethylene glycol | 1:4 | 1.10 | [43] |
| MTPhPBr | Glycerol | 1:2 | 1.31 | [43] |
| MTPhPBr | Glycerol | 1:3 | 1.30 | [43] |
| MTPhPBr | Glycerol | 1:4 | 1.30 | [43] |
| MTPhPBr | Ethylene glycol | 1:3 | 1.25 | [43] |
| MTPhPBr | Ethylene glycol | 1:4 | 1.23 | [43] |
| ZnCl2 | Acetamide | 1:4 | 1.36 | [29] |
| ZnCl2 | Ethylene glycol | 1:4 | 1.45 | [29] |
| ZnCl2 | Hexanediol | 1:3 | 1.38 | [29] |
| TEACl | Levulinic acid | 1:2 | 1.0939 | [48] |
| TEABr | Levulinic acid | 1:2 | 1.1736 | [48] |
| TBACl | Levulinic acid | 1:2 | 1.0310 | [48] |
| TBABr | Levulinic acid | 1:2 | 1.0972 | [48] |
| TEACl | Levulinic acid | 1:4 | 1.1020 | [48] |
| TEABr | Ethylene glycol | 1:4 | 1.1596 | [49] |
| TEABr | Triethylene glycol | 1:4 | 1.1468 | [49] |
| TEABr | Levulinic acid | 1:4 | 1.1669 | [49] |
| TPACl | Levulinic acid | 1:4 | 1.0759 | [49] |
| TPABr | Triethylene glycol | 1:4 | 1.1204 | [49] |
| TPABr | Ethylene glycol | 1:4 | 1.1314 | [49] |
| TPACl | Levulinic acid | 1:4 | 1.0484 | [49] |
| TBABr | Ethylene glycol | 1:4 | 1.0762 | [49] |
| TBABr | Triethylene glycol | 1:4 | 1.0976 | [49] |
| TBABr | Levulinic acid | 1:4 | 1.1061 | [49] |
| TBABr | Ethylene glycol | 1:4 | 1.1339 | [50] |
| TBABr | Glycerol | 1:3 | 1.1924 | [50] |
| TBABr | Triethylene glycol | 1:3 | 1.1426 | [50] |
| TBACl | Glycerol | 1:5 | 1.1417 | [51] |
| TBACl | Ethylene glycol | 1:3 | 1.0263 | [51] |
| TBACl | Trietylene glycol | 1:2 | 1.0043 | [51] |
| TBACl | Ethylene glycol | 1:2 | 0.9890 | [52] |
| TBACl | PEG 400 | 1:2 | 1.0771 | [52] |
| TBACl | Propanoic acid | 1:2 | 1.1183 | [52] |
| TBACl | Phenylacetic acid | 1:2 | 1.0401 | [52] |
| TBACl | Glycerol | 1:4 | 1.1714 | [53] |
| TBACl | Glycerol | 1:4 | 1.1748 | [53] |
| TBACl | Decanoic acid | 1:2 | 0.9168 | [59] |
| TPABr | Decanoic acid | 1:2 | 0.8907 | [39] |
| TOACl | Decanoic acid | 1:2 | 0.8889 | [39] |
| TPABr | Decanoic acid | 1:2 | 0.9298 | [39] |
| TBACl | Glutamic acid | 10:1 | 0.9630 | [54] |
| TBACl | Aspartic acid | 9:1 | 0.9582 | [54] |
| TBACl | Arginine | 6:1 | 1.0042 | [54] |
| TBACl | Serine | 8:1 | 0.9906 | [55] |
| TBACl | Threonine | 9:1 | 0.9393 | [55] |
| TBACl | Methionine | 11:1 | 0.9393 | [55] |
| TBABr | Ethanolamine | 1:4 | 1.0547 | [56] |
| TBABr | Ethyleneglycol | 1:2 | 1.0045 | [56] |
| TBABr | Glycerol | 1:2 | 1.0426 | [57] |
| BTACl | p-toulenesulfonic acid | 1:1 | 1.1904 | [58] |
| BTACl | Oxalic acid | 1:1 | 1.1940 | [58] |
| MTPPhBr | Ethylene glycol | 1:4 | 1.393 | [59] |
| MTPPhBr | Glycerol | 1:1.75 | 1.233 | [59] |
| MTPPhBr | Trifluroacetamide | 1:8 | 1.123 | [59] |
| BTPPhCl | Glycerol | 1:16 | 1.2407 | [60] |
| BTPPhCl | Triethylene glycol | 1:8 | 1.140 | [61] |
| BTPPhCl | Glycerol | 1:16 | 1.2337 | [62] |
| ATPPhBr | Glycerol | 1:14 | 1.2630 | [62] |
| ATPPhBr | Diethylene glycol | 1:10 | 1.1563 | [63] |
| ATPPhBr | Triethylene glycol | 1:10 | 1.1555 | [63] |
| FeCl3 6H2O | Ethylene Glycol | 2:1 | 1.605 | [64] |
| FeCl3 6H2O | Glycerol | 3:1 | 1.637 | [64] |
| FeCl3 6H2O | Malonic acid | 2:1 | 1.619 | [64] |
| FeCl3 6H2O | Xylitol | 2:1 | 1.630 | [64] |
| FeCl3 6H2O | Serine | 2:1 | 1.670 | [64] |
| FeCl3 6H2O | Alanine | 2:1 | 1.628 | [64] |
| FeCl3 6H2O | Glycine | 2:1 | 1.677 | [64] |
| DESs | 298.15 K | 303.15 K | 308.15 K | 313.15 K | 318.15 K | 323.15 K | Ref. |
|---|---|---|---|---|---|---|---|
| ChCl:TEG | - | 1.1254 | - | 1.1189 | - | 1.1125 | [41] |
| ChCl:Glucose | - | 1.2397 | - | 1.2346 | - | 1.2294 | [41] |
| TBABr:PEG 200 | 1.0976 | 1.0942 | 1.0906 | 1.0872 | 1.0837 | 1.0802 | [33] |
| DEAC:Glycerol | 1.1766 | 1.1735 | 1.1703 | 1.1672 | 1.1642 | 1.1582 | [65] |
| DEAC:Ethylene glycol | 1.0999 | 1.0968 | 1.0938 | 1.0909 | 1.0879 | 1.0848 | [65] |
| ChCl:Ethylene glycol | - | - | 1.1109 | 1.1081 | 1.1053 | 1.1025 | [66] |
| ChCl:Glycerol | - | 1.1941 | - | 1.1913 | - | 1.1884 | [67] |
| ChCl:Glycerol | 1.1921 | 1.1892 | 1.1862 | 1.1834 | 1.1805 | 1.1777 | [68] |
| ChCl:Urea | - | 1.1942 | - | 1.1886 | - | 1.1832 | [69] |
| ChCl:Ethylene glycol | 1.1171 | - | 1.1114 | - | 1.1057 | 1.1001 | [70] |
| ChCl:Ethylene glycol | - | 1.1139 | - | 1.1081 | - | 1.1025 | [71] |
| ChCl:Levulinic acid | - | 1.1352 | - | 1.1285 | - | 1.1219 | [71] |
| ChCl:Phenol | - | 1.0934 | - | 1.0874 | - | 1.0815 | [71] |
| ATPPhBr:TEG(1:4) | 1.1871 | 1.1834 | 1.1797 | 1.1761 | 1.1724 | 1.1687 | [72] |
| ATPPhBr:TEG(1:10) | 1.1555 | 1.1517 | 1.1480 | 1.1442 | 1.1405 | 1.1367 | [72] |
| ATPPhBr:TEG(1:16) | 1.1425 | 1.1388 | 1.1350 | 1.1313 | 1.1275 | 1.1237 | [72] |
| ChCl:Phenol(1:2) | 1.0967 | 1.0930 | 1.0901 | 1.0873 | 1.0843 | - | [45] |
| ChCl:Phenol(1:3) | 1.0921 | 1.0890 | 1.0858 | 1.0829 | 1.0795 | - | [45] |
| ChCl:Phenol(1:4) | 1.0893 | 1.0860 | 1.0819 | 1.0803 | 1.0782 | - | [45] |
| ChCl:Phenol(1:5) | 1.0870 | 1.0838 | 1.0803 | 1.0761 | 1.0736 | - | [45] |
| ChCl:Phenol(1:6) | 1.0852 | 1.0818 | 1.0782 | 1.0745 | 1.0717 | - | [45] |
3.2. Viscosity
| HBA | HBD | Molar Ratio | Viscosity (cP) | Temp (K) | Ref. |
|---|---|---|---|---|---|
| ChCl | Urea | 1:2 | 750 | 298.15 | [82] |
| ChCl | Ethylene glycol | 1:2 | 36 | 293.15 | [82] |
| ChCl | Ethylene glycol | 1:2 | 37 | 298.15 | [82] |
| ChCl | Ethylene glycol | 1:3 | 19 | 293.15 | [45] |
| ChCl | Ethylene glycol | 1:4 | 19 | 293.15 | [45] |
| ChCl | 1,4-butanediol | 1:3 | 140 | 293.15 | [45] |
| ChCl | 1,4-butanediol | 1:4 | 88 | 293.15 | [45] |
| ChCl | Glycolic acid | 1:1 | 394.8 | 303.15 | [25] |
| ChCl | Levulinic acid | 1:2 | 164.5 | 303.15 | [25] |
| ChCl | Malonic acid | 1:2 | 1124 | 303.15 | [25] |
| ChCl | o-Cresol | 1:3 | 77.65 | 303.15 | [46] |
| ChCl | Phenol | 1:2 | 64.41 | 303.15 | [46] |
| ChCl | Phenol | 1:3 | 35.17 | 303.15 | [46] |
| ChCl | Phenol | 1:4 | 25.20 | 303.15 | [46] |
| ChCl | Phenol | 1:5 | 19.75 | 303.15 | [46] |
| ChCl | Phenol | 1:6 | 16.82 | 303.15 | [46] |
| ChCl | D-Sorbitol | 1:1 | 12730 | 303.15 | [81] |
| ChCl | Xylitol | 1:1 | 5230 | 303.15 | [81] |
| ChCl | ZnCl2 | 1:2 | 8500 | 298.15 | [27] |
| ChCl | Urea | 1:2 | 750 | 298.15 | [82] |
| ChCl | Urea | 1:2 | 449 | 303.15 | [42] |
| ChCl | Urea | 1:2 | 169 | 313.15 | [42] |
| ChCl | Glycerol | 1:2 | 376 | 293.15 | [45] |
| ChCl | Glycerol | 1:2 | 259 | 298.15 | [82] |
| ChCl | Glycerol | 1:2 | 246.79 | 303.15 | [45] |
| ChCl | Glycerol | 1:3 | 450 | 293.15 | [45] |
| ChCl | Glycerol | 1:4 | 503 | 293.15 | [45] |
| TBACl | Ethylene glycol | 1:3 | 56.9 | 303.15 | [51] |
| TBACl | Glycerol | 1:4 | 476.1 | 303.15 | [51] |
| TPABr | Ethylene glycol | 1:3 | 58.2 | 303.15 | [50] |
| TPABr | Triethylene glycol | 1:3 | 71.9 | 303.15 | [50] |
| TPABr | Ethylene glycol | 1:3 | 77 | 303.15 | [83] |
| TPABr | Glycerol | 1:3 | 467.2 | 303.15 | [83] |
| TPABr | 1,3-propanediol | 1:3 | 135 | 303.15 | [83] |
| TPABr | 1,5-propanediol | 1:3 | 183 | 303.15 | [83] |
| TBABr | Glycerol | 1:3 | 467.2 | 303.15 | [51] |
| TBABr | Glycerol | 1:4 | 476.1 | 303.15 | [51] |
| TBABr | PEG 200 | 1:3 | 115.82 | 298.15 | [33] |
| TBABr | PEG 400 | 1:3 | 157.14 | 298.15 | [33] |
| TBABr | PEG 600 | 1:3 | 182.32 | 298.15 | [33] |
| MTPBr | Ethylene glycol | 1:4 | 109.8 | 298.15 | [59] |
| DEACl | Glycerol | 1:2 | 351 | 303.15 | [65] |
| BTMACl | Glycerol | 1:5 | 553.7 | 328.15 | [59] |
| EtNH3Cl | Acetamide | 1:1.5 | 64 | 313.15 | [42] |
| EtNH3Cl | CF3CONH2 | 1:1.5 | 256 | 313.15 | [42] |
| EtNH3Cl | Urea | 1:1.5 | 128 | 313.15 | [42] |
4. Applications of DES
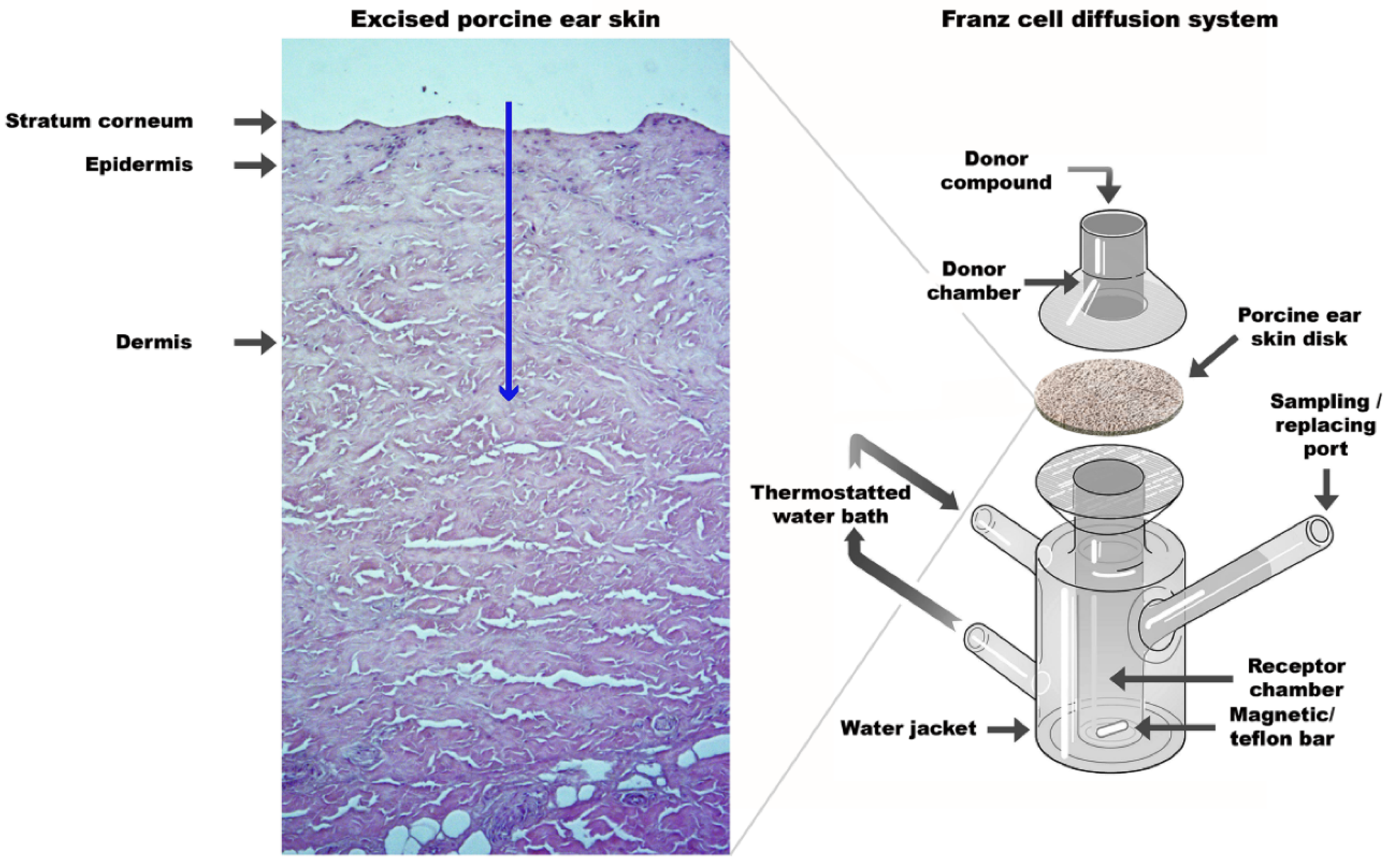
4.1. Drug Delivery/Solubilization
4.2. Therapeutic Deep Eutectic Solvents (THEDES)
| HBD | HBA | Molar Ratio | Ref. |
|---|---|---|---|
| DL-menthol | Ibuprofen | 2.5:7.5 | [97] |
| L-menthol | Ibuprofen | 3:7 | [97] |
| Methyl nicotinate | Ibuprofen | 1:1 | [97] |
| 1,8-Cineole | Ibuprofen | 2:3 | [97] |
| Lidocaine | Ibuprofen | 1:1 | [97] |
| Decanoic acid | Propranolol | 3.5:6.5 | [100] |
| Phenol | Itraconazole | 2.4:7.6 | [101] |
| Glycerol | Ranitidine | 2:1 | [102] |
| Aspirin | ChCl | 1:1 | [103] |
| Phenylacetic acid | ChCl | 3:2 | [103] |
| Ascorbic acid | ChCl | 2:1 | [104] |
| Salicylic acid | ChCl | 1:1 | [105] |
| Paracetamol | ChCl | 1:1 | [105] |
| Thymol | ChCl | 1:2 | [105] |
4.3. Extraction of Biomolecules
| Natural Source | Bioactive Compounds | Ref. |
|---|---|---|
| Chinese rhubarb (Rheum palmatum L.) | Chrysophanol, physcion, rhein, emodin, aloe-emodin | [137] |
| Red Sage (Salvia miltiorrhiza) | Tanshonene IIA, cryptotanshinone, salvianolic acid B | [138] |
| Grape pomace (Vitis vinifera) | Anthocyanin | [139] |
| Mulberry leaves (Morus alba L.) | Gallic acid, vanillic acid, benzoic acid, gentisic acid | [140] |
| Mulberry leaves (Morus alba L.) | Quercetin, Astragalin, rutin, syringic acid, chlorogenic acid, catechinic acid | [141] |
| Marjoram (Origanum majorana), Mint (Mentha spicata), Sage (Salvia officinalis), Fennel (Foeniculum vulgare), Dittany(Origanum Dictamnus) | Total polyphenol | [140] |
| Olive pomace (Olea europaea) | Total phenol | [141] |
| Grape skin (Vitis vinifera) | Anthocyanin, total phenol | [143] |
| Cinnamon bark (Cinnamomum burmanniiis) | Coumarin, trans-cinnamaldehyde | [144] |
| Corn mint (Mentha arvensis) | Total flavonoid, total phenol | [145] |
| Rosemary (Rosmarinus officinalis L.), Chinese hickory peels (Carya cathayensis Sarg.), Mudou leaves (Cajanus cajan), French lavender (Lavandula pedunculata) | Total phenol | [146,147,148,149] |
| Roselle (Hibiscus sabdariffa L.), Alkanet root (Alkanna tinctoria), Chickpea (Cicer arietinum L.) sprouts | Total phenol, total flavonoid | [150,151,152] |
4.3.1. Flavonoids
4.3.2. Polysaccharides
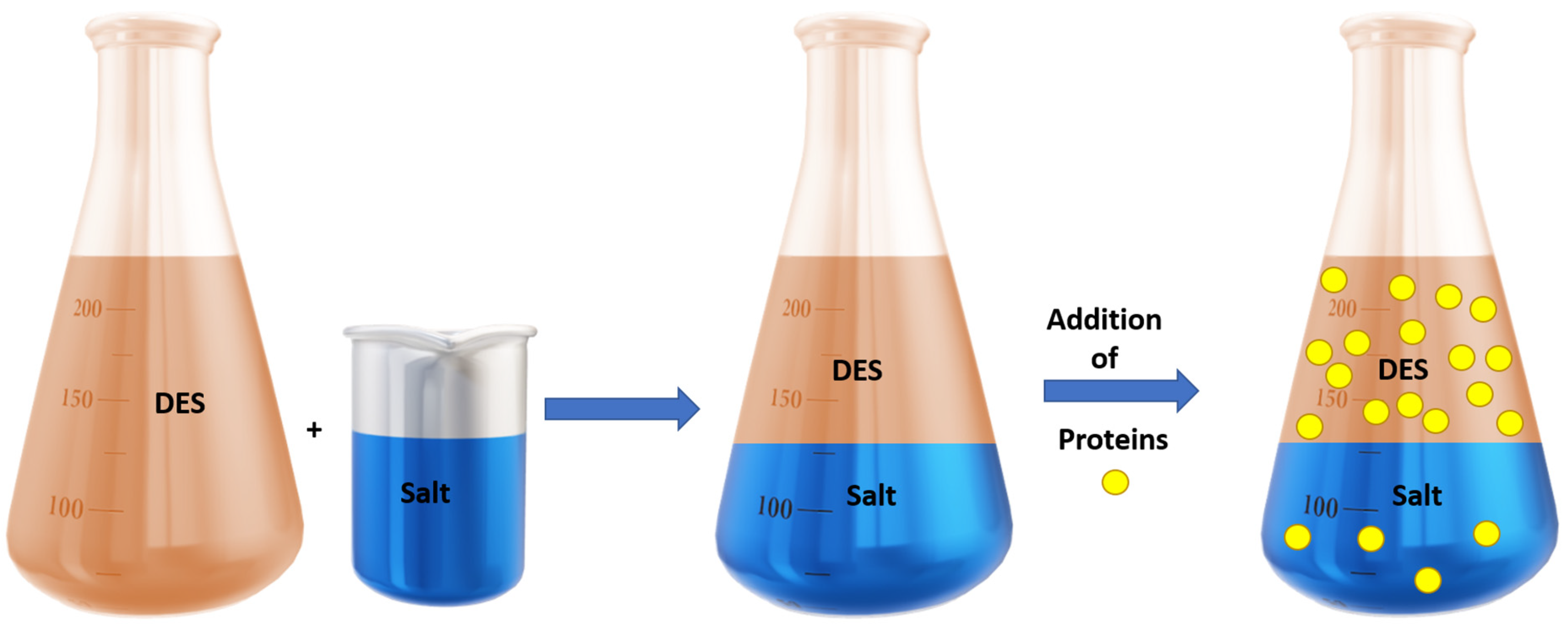
4.3.3. Proteins
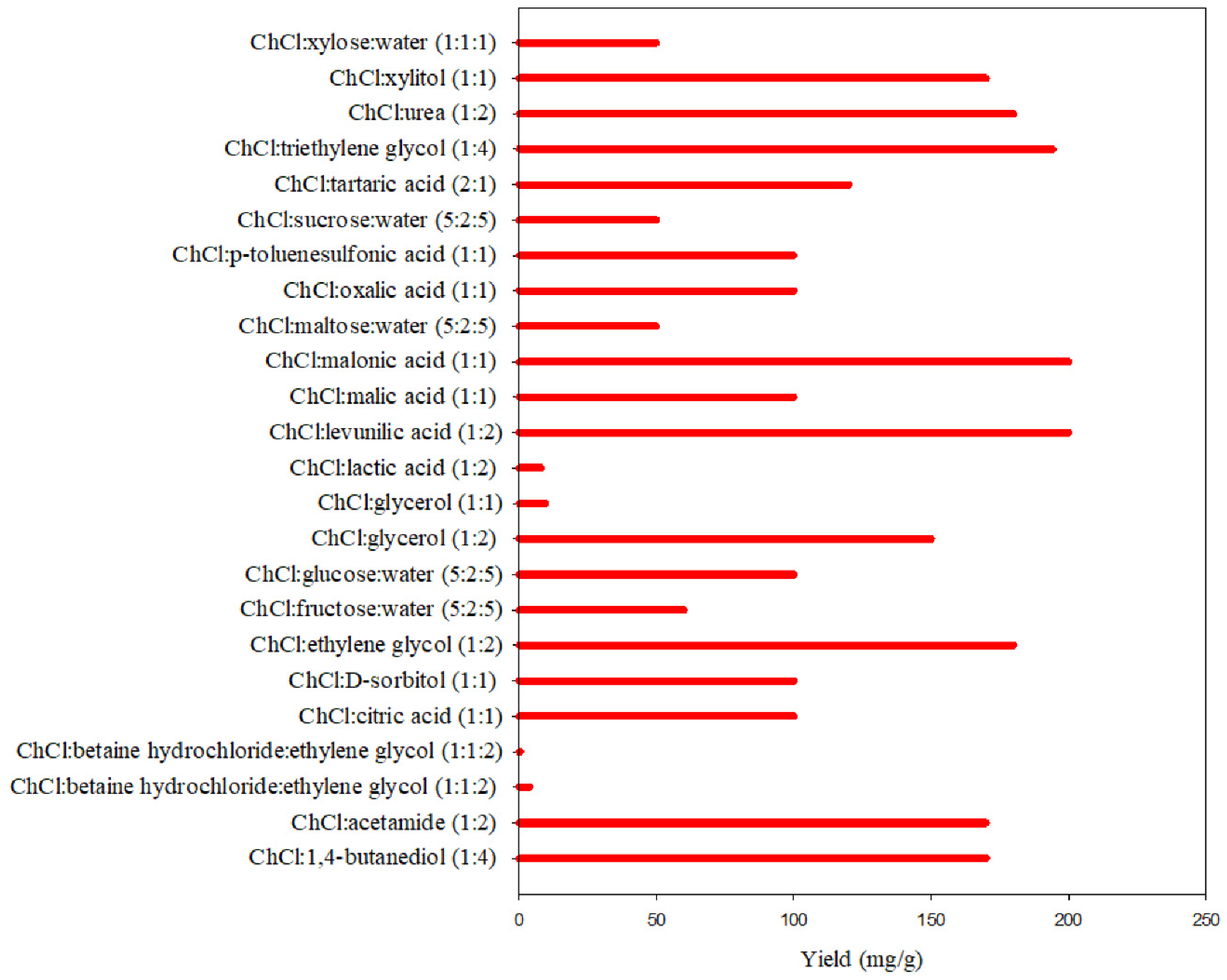
| DES | Flavonoids | Proteins | Polysaccharide Source | Ref. |
|---|---|---|---|---|
| ChCl:betaine hydrochloride:ethylene glycol | Genkwanin | - | - | [165] |
| ChCl:betaine hydrochloride:ethylene glycol | Apigenin | - | - | |
| ChCl:betaine hydrochloride:ethylene glycol | Quercetin-3-O-β-d-glucopyranoside | - | - | |
| ChCl:betaine hydrochloride:ethylene glycol | Kaempferol-3-O-β-d-glucopyranoside-7-O-β-d-glucopyranoside | - | - | |
| ChCl:betaine hydrochloride:ethylene glycol | Luteolin | - | - | |
| ChCl:betaine hydrochloride:ethylene glycol | Luteolin-7-O-β-d-glucopyranoside | - | - | |
| ChCl:betaine hydrochloride:ethylene glycol | Genkwanin-5-O-β-d-glucopyranoside | - | - | |
| ChCl:betaine hydrochloride:ethylene glycol | Apigenin-5-O-β-d-glucopyranoside | - | - | |
| ChCl:citric acid | Rutin | - | - | [164] |
| ChCl:glycerol | - | - | ||
| ChCl:glucose:water | - | - | ||
| ChCl:fructose:water | - | - | ||
| ChCl:lactic acid | Wogononin | - | [166] | |
| ChCl:lactic acid | Baicalein | - | ||
| ChCl:lactic acid | Wogonoside | - | ||
| ChCl:Sorbitol:1,3-butanediol | - | - | Poria cocos polysaccharides | [173] |
| Lactic acid:glucose:water | - | Bovine Serum Albumin | - | [174] |
| Betaine:urea:water | Bovine Serum Albumin | - | [174] | |
| ChCl:ethylene glycol | - | - | Lilium lancifolium Thunb. | [184] |
| ChCl:ethylene glycol | - | - | Lotus leaves | [171] |
| ChCl:1,3-butanediol:D-sorbitol | - | - | Poria cocos (Schw.)wolf | [173] |
| ChCl:glycerol | - | Proteins from Rapeseed Cake and Primrose Cake | - | [179] |
| ChCl:ethylene glycol ChCl:glycerol ChCl:Sorbitol | - | Bovine Serum Albumin and Trypsin | - | [183] |
| Betaine–urea | - | Bovineserumalbumin, Ovalbumin, Trypsin | - | [180] |
| Sodium acetate:urea Potassium formate:urea | - | Brewer’s Spent Grain Proteins | - | [177] |
5. Conclusions and Future Perspective
Funding
Conflicts of Interest
References
- Coby, J.; Clarke, W.T.; Oliver, L.; Andreas, B.; Jason, H.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-Transition-Temperature Mixtures (LTTMs): A New Generation of Designer Solvents Angew. Chem. Int. Ed. 2013, 52, 3074–3075. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef] [Green Version]
- Marsh, K.N.; Deev, A.; Wu, A.C.T.; Tran, E.; Klamt, A. Korean Room Temperature Ionic Liquids as Replacements for Conventional Solvents—A Review. J. Chem. Eng. 2002, 19, 357–362. [Google Scholar]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Pârvulescu, V.I.; Hardacre, C. Catalysis in Ionic Liquids. Chem. Rev. 2007, 107, 2615–2665. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yan, F.; Texter, J. Advanced Applications of Ionic Liquids in Polymer Science. Prog. Polym. Sci. 2009, 34, 431–448. [Google Scholar] [CrossRef]
- Martínez-Palou, R.; Luque, R. Applications of ionic liquids in the removal of contaminants from refinery feedstocks: An industrial perspective. Energy Environ. Sci. 2014, 7, 2414–2447. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, V.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [Green Version]
- KaterynaYavir, K.; Marcinkowski, L.; Marcinkowska, R.; Namieśnik, J.; Kloskowski, A. Analytical applications and physicochemical properties of ionic liquid-based hybrid materials: A review. Anal. Chim. Acta 2019, 1054, 1–16. [Google Scholar]
- Lei, Z.; Chen, B.; Koo, Y.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.; Cho, C.; Yun, Y. Environmental fate and toxicity of ionic liquids: A review. Water Res. 2010, 44, 352–372. [Google Scholar] [CrossRef]
- Kunz, W.; Häckl, K. The hype with ionic liquids as solvents. Chem. Phys. Lett. 2016, 661, 6–12. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Carriazo, D.; Serrano, M.C.; Gutierrez, M.C.; Ferrer, M.L.; Monte, F. Deep-eutectic solvents playing multiple roles in the synthesis of polymers and related materials. Chem. Soc. Rev. 2012, 41, 4996–5014. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Namiesnik, J. Ionic Liquids and Deep Eutectic Mixtures: Sustainable Solvents for Extraction Processes. ChemSusChem 2015, 7, 1–18. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar]
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep Eutectic Solvents: Physicochemical Properties and Gas Separation Applications. Energy Fuel. 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- Oke, E.; Ijardar, S.P. Advances in the application of deep eutectic solvents based aqueous biphasic systems: An up-to-date review. Biochem. Eng. J. 2021, 176, 108211. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, L.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the synthesis and properties of deep eutectic solvents based on cholinium chloride and carboxylic acids, ACS Sustain. Chem. Eng. 2014, 2, 2416–2425. [Google Scholar]
- Gutiérrez, M.C.; Ferrer, M.L.; Mateo, C.R.; Monte, F. Freeze-drying of aqueous solutions of deep eutectic solvents: A suitable approach to deep eutectic suspensions of self-assembled structures. Langmuir 2009, 25, 5509–5515. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K. Ionic liquids based upon metal halide/substituted quaternary ammonium salt mixtures. Inorg. Chem. 2004, 43, 3447. [Google Scholar] [CrossRef]
- Xu, W.G.; Lu, X.M.; Zhang, Q.G.; Gui, J.S.; Yang, J.Z. study on thermodynamic properties of ionic liquids BMIMGaCl2. Chin. J. Chem. 2006, 24, 331. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K. Ionic Liquid Analogues Formed from Hydrated Metal Salts. Chem. Eur. J. 2004, 10, 3769. [Google Scholar] [CrossRef]
- Gambino, M.; Bros, J.P. Heat capacity of urea and of a group of eutectic mixtures based on urea between 30 and 140 °C. Thermochim. Acta 1988, 127, 223–236. [Google Scholar] [CrossRef]
- Abranches, D.O.; Martins, M.A.; Silva, L.P.; Schaeffer, N.; Pinho, S.P.; Coutinho, J.A.P. Phenolic Hydrogen Bond Donors in the Formation of Non-ionic Deep Eutectic Solvents: The Quest for Type V DES. Chem. Commun. 2019, 55, 10253–10256. [Google Scholar] [CrossRef] [Green Version]
- Assael, M.J. The Importance of Thermophysical Properties in Optimum Design and Energy Saving. In Energy and Environment; Springer: Tokyo, Japan, 2001. [Google Scholar]
- Ijardar, S.P. Deep eutectic solvents composed of tetrabutylammonium bromide and PEG: Density, speed of sound and viscosity as a function of temperature. J. Chem. Thermodyn. 2020, 140, 105897. [Google Scholar] [CrossRef]
- Mjalli, F.S. Mass connectivity index-based density prediction of deep eutectic solvents. Fluid Phase Equilib. 2016, 409, 312–317. [Google Scholar] [CrossRef]
- Cui, Y.; Li, C.; Yin, J.; Li, V.; Jia, Y.; Bao, M. Design, synthesis and properties of acidic deep eutectic solvents based on choline chloride. J. Mol. Liq. 2017, 236, 338–343. [Google Scholar] [CrossRef]
- Abbott, A.P. Application of Hole Theory to the Viscosity of Ionic and Molecular Liquids. Chem. Phys. Chem. 2004, 5, 1242–1246. [Google Scholar] [CrossRef]
- Basaiahgari, A.; Panda, S.; Gardas, R.L. Effect of Ethylene, Diethylene, and Triethylene Glycols and Glycerol on the Physicochemical Properties and Phase Behavior of Benzyltrimethyl and Benzyltributylammonium Chloride Based Deep Eutectic Solvents at 283.15−343.15 K. J. Chem. Eng. Data 2018, 63, 2613–2627. [Google Scholar] [CrossRef]
- Florindo, C.; Oliveira, M.M.; Branco, L.C.; Marrucho, I.M. Carbohydrates-based deep eutectic solvents: Thermophysical properties and rice straw dissolution. J. Mol. Liq. 2017, 247, 441–447. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Zubeir, L.F.; Avd, B.; Rocha, M.A.A.; Kroon, M.C. Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem. 2015, 17, 4518–4521. [Google Scholar] [CrossRef] [Green Version]
- Shafie, M.H.; Yusof, R.; Gan, C.Y. Synthesis of Citric Acid Monohydrate-Choline Chloride Based Deep Eutectic Solvents (DES)and Characterization of their Physicochemical Properties. J. Mol. Liq. 2019, 288, 111081. [Google Scholar] [CrossRef]
- Mjalli, F.S.; Ahmad, O. Density of aqueous choline chloride-based ionic liquid analogies. Thermochim. Acta 2017, 647, 8–14. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Gray, S. Design of Improved Deep Eutectic Solvents Using Hole Theory. Chem. Phys. Chem. 2006, 7, 803–806. [Google Scholar] [CrossRef]
- Shahbaz, K.; Baroutian, S.; Mjalli, F.S.; Hashim, M.A.; AlNashef, I.M. Densities of ammonium and phosphonium based deep eutectic solvents: Prediction using artificial intelligence and group contribution techniques. Thermochim. Acta 2012, 52, 59–66. [Google Scholar] [CrossRef]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S.; D’Agostino, C.; Gladden, L.F.; Mantle, M.D. Glycerol eutectics as sustainable solvent systems. Green Chem. 2011, 13, 82–90. [Google Scholar] [CrossRef]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S. Application of Hole Theory to Define Ionic Liquids by their Transport Properties. J. Phys. Chem. B 2007, 111, 4910–4913. [Google Scholar] [CrossRef]
- Guo, W.; Hou, Y.; Ren, S.; Tian, S.; Wu, W. Formation of Deep Eutectic Solvents by Phenols and Choline Chloride and Their Physical Properties. J. Chem. Eng. Data 2013, 58, 866–872. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, K.Y.; Ye, F.; Song, N.; Xu, Y. Physicochemical properties of deep eutectic solvents formed by choline chloride and phenolic compounds at T = (293.15 to 333.15) K: The influence of electronic effect of substitution group. J. Mol. Liq. 2017, 232, 182–187. [Google Scholar] [CrossRef]
- Deng, D.; Jiang, Y.; Liu, X.; Zhang, Z.; Ai, N. Investigation of solubilities of carbon dioxide in five levulinic acid-based deep eutectic solvents and their thermodynamic properties. J. Chem. Thermodyn. 2016, 103, 212–217. [Google Scholar] [CrossRef]
- Wang, Y.; Hiu, Y.; Wu, W.; Liu, D.; Ji, Y.; Ren, S. Roles of hydrogen bond donor and hydrogen bond acceptor in the extraction of toluene from n-heptane using deep eutectic solvents. Green Chem. 2016, 18, 3089–3097. [Google Scholar] [CrossRef]
- Jibril, B.; Mjalli, F.; Naser, J.; Gano, Z. New tetrapropylammonium bromide-based deep eutectic solvents: Synthesis and characterizations. J. Mol. Liq. 2014, 199, 462–469. [Google Scholar] [CrossRef]
- Mjalli, F.S.; Naser, J.; Jibril, B.; Alizadeh, V.; Gano, Z. Tetra butylammonium chloride based liquid analogues and their physical properties. J. Chem. Eng. Data 2014, 59, 2242–2251. [Google Scholar] [CrossRef]
- Hong-Zhen, S.; Jing-Mei, Y.N.; Qing-Shan, L.; Chang-Ping, L. Properties of four deep eutectic solvents: Density, electrical conductivity, dynamic viscosity and refractive index. Acta Phys. Chim. Sin. 2015, 31, 1468–1473. [Google Scholar]
- AlOmar, M.K.; Hayyan, M.; Alsaadi, M.A.; Akib, S.; Hayyan, A.; Hashim, M.A. Glycerol-based deep eutectic solvents: Physical properties. J. Mol. Liq. 2016, 215, 98–103. [Google Scholar] [CrossRef]
- Mjalli, F.S. Novel amino acid based ionic liquids analogues: Acidic and basic amino acids. J. Taiwan Inst. Chen. Eng. 2016, 61, 64–74. [Google Scholar] [CrossRef]
- Mjalli, F.S.; AlHajri, R.; Al-Muhtaseb, A.; Ahmed, O.; Nagaraju, M. Novel amino acid-based ionic liquid analogues: Neutral hydroxylic and sulfur-containing amino acids. Asia-Pacific J. Chem. Eng. 2016, 116, 83–694. [Google Scholar] [CrossRef]
- Mjalli, F.S.; Murshid, G.; Al-Zakwami, S.; Hayyan, A. Monoethanolamine-based deep eutectic solvents, their synthesis and characterization. Fluid Phase Equilib. 2017, 448, 30–40. [Google Scholar] [CrossRef]
- Rodriguez, N.R.; Requejo, P.F.; Kroon, M.C. Aliphatic-aromatic separation using deep eutectic solvents as extracting agents. Ind. Eng. Chem. Res. 2015, 54, 11404–11412. [Google Scholar] [CrossRef]
- Taysun, M.B.; Sert, E.; Atalay, F.S. Physical properties of benzyl triphenyl ammonium chloride based deep eutectic solvents and employment as catalyst. J. Mol. Liq. 2016, 223, 845–852. [Google Scholar] [CrossRef]
- Kareem, M.A.; Mjalli, F.S.; Hashim, M.A.; AlNashef, I.M. Phosphonium-based ionic liquids analogues and their physical properties. J. Chem. Eng. Data 2010, 55, 4632–4637. [Google Scholar] [CrossRef]
- Sun, S.; Niu, Y.; Xu, Q.; Sun, Z.; Wei, X. Efficient SO2 absorptions by four kinds of deep eutectic solvents based on choline chloride. Ind. Eng. Chem. Res. 2015, 54, 8019–8124. [Google Scholar] [CrossRef]
- Ali, E.; Hadj-Kali, M.K.; Mulyono, S.; Alnashef, I.; Fakeeha, A.; Mjalli, F.; Hayyan, A. Solubility of CO2 in deep eutectic solvents: Experiments and modelling using the Peng-Robinson equation of state. Chem. Eng. Res. Des. 2014, 92, 1898–1906. [Google Scholar] [CrossRef]
- Naser, J.; Mjalli, F.S.; Gano, Z. Molar heat capacity of type III deep eutectic solvents. J. Chem. Eng. Data 2016, 61, 1608–1615. [Google Scholar] [CrossRef]
- Ghaedi, H.; Ayoub, M.; Sufian, S.; Lal, B.; Shariff, A.M. Measurement and correlation of physicochemical properties of phosphonium-based deep eutectic solvents at several temperatures (293.15 K–343.15 K) for CO2 capture. J. Chem. Thermodyn. 2017, 113, 41–51. [Google Scholar] [CrossRef]
- Liu, F.; Xue, Z.; Zhao, X.; Mou, H.; He, J.; Mu, T. Catalytic deep eutectic solvents for highly efficient conversion of cellulose to gluconic acid with gluconic acid self-precipitation separation. Chem. Commun. 2018, 54, 6140–6143. [Google Scholar] [CrossRef] [PubMed]
- Siongco, K.R.; Leron, R.B.; Li, M.H. Densities, refractive indices, and viscosities of N,N-diethylethanol ammonium chloride–glycerol or –ethylene glycol deep eutectic solvents and their aqueous solutions. J. Chem. Thermodyn. 2013, 65, 65–72. [Google Scholar] [CrossRef]
- Harifi-Mood, A.R.; Buchner, R. Density, viscosity, and conductivity of choline chloride+ethylene glycol as a deep eutectic solvent and its binary mixtures with dimethyl sulfoxide. J. Mol. Liq. 2017, 225, 689–695. [Google Scholar] [CrossRef]
- Kim, K.; Park, B.H. Volumetric properties of solutions of choline chloride + glycerol deep eutectic solvent with water, methanol, ethanol, or iso-propanol. J. Mol. Liq. 2018, 254, 272–279. [Google Scholar] [CrossRef]
- Leron, B.R.; Wong, D.S.H.; Meng-Hui, l. Densities of a deep eutectic solvent based on choline chloride and glycerol and its aqueous mixtures at elevated pressures. Fluid Phase Equilib. 2012, 335, 32–38. [Google Scholar] [CrossRef]
- Haghbakhsh, R.; Raeissi, S. Investigation of solutions of ethyl alcohol and the deep eutectic solvent of Reline for their volumetric properties. Fluid Phase Equilib. 2018, 472, 39–47. [Google Scholar] [CrossRef]
- Yadav, A.; Kar, J.R.; Verma, M.; Naqvi, S.; Pandey, S. Densities of aqueous mixtures of (choline chloride+ethylene glycol) and (choline chloride+malonic acid) deep eutectic solvents in temperature range 283.15–363.15K. Thermochim. Acta 2015, 600, 95–101. [Google Scholar] [CrossRef]
- Gajardo-Parra, N.F.; Lubben, J.M.; Winnert, M.J.; Leiva, Á.; Brennecke, J.F.; Canales, R.I. Physicochemical properties of choline chloride-based deep eutectic solvents and excess properties of their pseudo-binary mixtures with 1-butanol. J. Chem. Thermodyn. 2019, 133, 272–284. [Google Scholar] [CrossRef]
- Ghaedi, H.; Ayoub, M.; Sufian, S.; Shariff, A.M.; Murshid, G.; Hailegiorgis, S.M.; Khan, S.N. Density, excess and limiting properties of (water and deep eutectic solvent) systems at temperatures from 293.15 K to 343.15 K. J. Mol. Liq. 2017, 248, 378–390. [Google Scholar] [CrossRef]
- Shah, D.; Mjalli, F.S. Effect of water on the thermo-physical properties of Reline: An experimental and molecular simulation based approach. Phys. Chem. Chem. Phys. 2014, 16, 23900–23907. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Pandey, S. Densities and Viscosities of (Choline Chloride + Urea) Deep Eutectic Solvent and Its Aqueous Mixtures in the Temperature Range 293.15 K to 363.15 K. J. Chem. Eng. Data 2014, 59, 2221–2229. [Google Scholar] [CrossRef]
- Lapeña, D.; Bergua, F.; Lomba, L.; Giner, B.; Lafuente, C. A comprehensive study of the thermophysical properties of reline and hydrated reline. J. Mol. Liq. 2020, 303, 112679. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Haghbakhsh, R.; Duarte, A.R.C.; Raeissi, S. Volumetric investigation of aqueous mixtures of the {choline chloride + phenol (1:4)} deep eutectic solvent. J. Chem. Thermodyn. 2021, 158, 106440. [Google Scholar] [CrossRef]
- Shekaari, H.; Zafarani-Moattar, M.T.; Mohammadi, B. Thermophysical characterization of aqueous deep eutectic solvent (choline chloride/urea) solutions in full ranges of concentration at T = (293.15–323.15) K. J. Mol. Liq. 2017, 243, 451–461. [Google Scholar] [CrossRef]
- Kuddushi, M.; Nangala, G.; Rajput, S.; Ijardar, S.P.; Malek, N.I. Understanding the peculiar effect of water on the physicochemical properties of choline chloride based deep eutectic solvents theoretically and experimentally. J. Mol. Liq. 2019, 278, 607–615. [Google Scholar] [CrossRef]
- Ghatee, M.H.; Bahrami, M.; Khanjari, N. Measurement and study of density, surface tension, and viscosity of quaternary ammonium-based ionic liquids ([N222(n)]Tf2N). J. Chem. Thermodyn. 2013, 65, 42–52. [Google Scholar] [CrossRef]
- Maugeri, Z.; Domınguez, P. Novel choline-chloride-based deep-eutectic-solvents with renewable hydrogen bond donors: Levulinic acid and sugar-based polyols. RSC Adv. 2012, 2, 421–425. [Google Scholar] [CrossRef]
- Agostino, C.; Harris, R.C.; Abbott, A.P.; Gladden, L.F.; Mantle, M.D. Molecular motion and ion diffusion in choline chloride based deep eutectic solvents studied by 1H pulsed field gradient NMR spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 21383–21391. [Google Scholar] [CrossRef] [PubMed]
- Yusof, R.; Abdulmalek, E.; Sirat, K.; Basyaruddin, M.; Rahman, M.A. Tetrabutylammonium bromide (TBABr)-based deep eutectic solvents (DESs) and their physical properties. Molecules 2014, 19, 8011–8026. [Google Scholar] [CrossRef] [PubMed]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedro, S.N.; Freire, M.G.; Freire, C.S.R.; Silvestre, A.J.D. Deep eutectic solvents comprising active pharmaceutical ingredients in the development of drug delivery systems. Expert Opin. Drug Del. 2019, 16, 497–506. [Google Scholar] [CrossRef]
- Indoria, S.; Singh, V.; Hsieh, M.F. Recent advances in theranostic polymeric nanoparticles for cancer treatment: A review. Int. J. Pharm. 2020, 582, 119314. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Obi, N. Studies on Absorption of Eutectic Mixture. I. A Comparison of the Behavior of Eutectic Mixture of Sulfathiazole and that of Ordinary Sulfathiazole in Man. Chem. Pharm. Bull. 1961, 9, 866–872. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, A.; Aparicio, S.; Atilhan, M. Design of arginine-based therapeutic deep eutectic solvents as drug solubilization vehicles for active pharmaceutical ingredients. Phys. Chem. Chem. Phys. 2019, 21, 10621–10634. [Google Scholar] [CrossRef]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef] [Green Version]
- Palmelund, H.; Eriksen, J.B.; Bauer-Brandl, A.; Rantanen, J.; Lobmann, K. Enabling formulations of aprepitant: In vitro and in vivo comparison of nanocrystalline, amorphous and deep eutectic solvent-based formulations. Intern. J. Pharma. X 2021, 3, 10083. [Google Scholar] [CrossRef]
- Morrison, H.G.; Sun, C.C.; Neervannan, S. Characterization of thermal behavior of deep eutectic solvents and their potential as drug solubilization vehicles. Int. J. Pharm. 2009, 378, 136–139. [Google Scholar] [CrossRef]
- Li, Z.; Lee, P.I. Investigation on drug solubility enhancement using deep eutectic solvents and their derivatives. Int. J. Pharm. 2016, 505, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Bevernage, J.; Brouwers, J.; Brewster, M.E.; Augustijns, P. Evaluation of gastrointestinal drug supersaturation and precipitation: Strategies and issues. Int. J. Pharm. 2013, 453, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, H.R.; Tawa, M.; Zhang, Z.; Ratanabanangkoon, P.; Shaw, P.; Gardner, C.R.; Chen, H.; Moreau, J.-P.; Almarsson, O.; Remenar, J.F. Combined use of crystalline salt forms and precipitation inhibitors to improve oral absorption of celecoxib from solid oral formulations. J. Pharm. Sci. 2007, 96, 2686–2702. [Google Scholar] [CrossRef] [PubMed]
- Bevernage, J.; Forier, T.; Brouwers, J.; Tack, J.; Annaert, P.; Augustijns, P. Excipient-mediated supersaturation stabilization in human intestinal fluids. Mol. Pharm. 2011, 8, 564–570. [Google Scholar] [CrossRef]
- Rahman, M.S.; Roy, R.; Jadhav, B.; Hossain, N.; Halim, M.A.; Raynie, D.E. Formulation, structure, and applications of therapeutic and amino acid-based deep eutectic solvents: An overview. J. Mol. Liq. 2021, 321, 114745. [Google Scholar] [CrossRef]
- Stott, P.W.; Williams, A.C.; Barry, B.W. Transdermal delivery from eutectic systems: Enhanced permeation of a model drug, ibuprofen. J. Cont. Rel. 1998, 50, 297–308. [Google Scholar] [CrossRef]
- Aroso, I.V.; Craveiro, R.; Rocha, A.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Design of controlled release systems for THEDES—Therapeutic deep eutectic solvents, using supercritical fluid technology. Int. J. Pharm. 2015, 492, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Shen, Q.; Li, X.; Li, W.; Zhao, X. Enhanced intestinal absorption of daidzein by borneol/menthol eutectic mixture and microemulsion. AAPS PharmSciTech 2011, 12, 1044–1049. [Google Scholar] [CrossRef]
- Stott, P.W.; Williams, A.C.; Barry, B.W. Mechanistic study into the enhanced transdermal permeation of a model β-blocker, propranolol, by fatty acids: A melting point depression effect. Int. J. Pharm. 2001, 219, 161–176. [Google Scholar] [CrossRef]
- Park, C.W.; Mansour, H.M.; Oh, T.O.; Kim, J.Y.; Ha, J.M.; Lee, B.J.; Chi, S.C.; Rhee, Y.S.; Park, E.S. Phase behavior of itraconazole-phenol mixtures and its pharmaceutical applications. Int. J. Pharm. 2012, 436, 652–658. [Google Scholar] [CrossRef]
- Bica, K.; Shamshina, J.; Hough, W.L.; MacFarlane, D.R.; Rogers, R.D. Liquid forms of pharmaceutical co-crystals: Exploring the boundaries of salt formation. Chem. Commun. 2011, 47, 2267–2269. [Google Scholar] [CrossRef] [PubMed]
- Aroso, I.M.; Silva, J.C.; Mano, F.; Ferreira, A.S.D.; Dionísio, M.; Sá-Nogueira, I.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Dissolution enhancement of active pharmaceutical ingredients by therapeutic deep eutectic systems. Eur. J. Pharm. Biopharm. 2016, 98, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Design of functional therapeutic deep eutectic solvents based on choline chloride and ascorbic acid. ACS Sustain. Chem. Eng. 2018, 6, 10355–10363. [Google Scholar] [CrossRef]
- Abbott, A.P.; Ahmed, E.I.; Prasad, K.; Qader, I.B.; Ryder, K.S. Liquid pharmaceuticals formulation by eutectic formation. Fluid Phase Equilib. 2017, 448, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Boscariol, R.; Caetano, E.A.; Silva, E.C.; Oliveira, T.J.; Rosa-Castro, R.M.; Vila, M.M.D.C.; Balcão, V.M. Performance of Choline Geranate Deep Eutectic Solvent as Transdermal Permeation Enhancer: An In Vitro Skin Histological Study. Pharmaceutics 2021, 13, 540. [Google Scholar] [CrossRef]
- Khafagy, E.S.; Morishita, M.; Onuki, Y.; Takayama, K. Current challenges in non-invasive insulin delivery systems: A comparative review. Adv. Drug Deliv. Rev. 2007, 59, 1521–1546. [Google Scholar] [CrossRef]
- Lassmann-Vague, V.; Raccah, D. Alternatives routes of insulin delivery. Diabetes Metab. 2006, 32, 513–522. [Google Scholar] [CrossRef]
- Roy, N.; Agrawal, M.; Chaudhary, S.; Tirkey, V.; Dhwaj, A.; Mishra, N. Review article on permeation enhancers: A major breakthrough in drug delivery technology. Int. J. Pharm. Sci. Res. 2017, 8, 1001–1011. [Google Scholar]
- Jorge, L.R.; Harada, L.K.; Silva, E.C.; Campos, W.F.; Moreli, F.C.; Shimamoto, G.; Pereira, J.F.B.; Oliveira, J.M., Jr.; Tubino, M.; Vila, M.M.D.C.; et al. Non-invasive Transdermal Delivery of Human Insulin Using Ionic Liquids: In vitro Studies. Front. Pharmacol. 2020, 11, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.C.; Oliveira, T.J.; Moreli, F.C.; Harada, L.K.; Vila, M.M.D.C.; Balcão, V.M. Newly isolated lytic bacteriophages for Staphylococcus intermedius, structurally and functionally stabilized in a hydroxyethylcellulose gel containing choline geranate: Potential for transdermal permeation in veterinary phage therapy. Res. Vet. Sci. 2020. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Baby, A.R.; Araújo, M.E.M.; Fernandes, A.S.; Costa, J.G.; de Almeida, T.S. Choline-Amino Acid Ionic Liquids as Green Functional Excipients to Enhance Drug Solubility. Pharmaceutics 2018, 10, 288. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.; Das, K.; Singh, V.; Deenadayalu, N.; Gardas, R.L. Volumetric and compressibility studies of monosaccharides in aqueous cholinium propanoate [Chl][Pro] solutions at different temperatures. J. Mol. Liq. 2020, 298, 111955. [Google Scholar] [CrossRef]
- Singh, V.; Chhotaray, P.K.; Gardas, R.L. Volumetric and ultrasonic properties of ternary (sucrose + water + protic ionic liquid) solutions. J. Chem. Thermodyn. 2015, 89, 60–68. [Google Scholar] [CrossRef]
- Patel, N.A.; Patel, N.J.; Patel, R.P. Design and Evaluation of Transdermal Drug Delivery System for Curcumin as an Anti-Inflammatory. Drug. Dev. Ind. Pharm. 2009, 35, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Sintov, A.C. Transdermal delivery of curcumin via microemulsion. Intern. J. Pharm. 2015, 481, 97–103. [Google Scholar] [CrossRef]
- Eckert, R.W.; Wiemann, S.; Keck, C.M. Improved Dermal and Transdermal Delivery of Curcumin with SmartFilms and Nanocrystals. Molecules 2021, 26, 1633. [Google Scholar] [CrossRef]
- Kriplani, P.; Guarve, K.; Baghel, U.S. Formulation optimization and characterization of transdermal film of curcumin by response surface methodology. Chin. Herbal Med. 2021, 13, 274–285. [Google Scholar] [CrossRef]
- Yin, T.; Wua, J.; Yuan, J.; Wang, X. Therapeutic deep eutectic solvent based on osthole and paeonol: Preparation, characterization, and permeation behavior. J. Mol. Liq. 2021, 346, 117133. [Google Scholar] [CrossRef]
- Feng, H.; Hu, J.J.; Wang, Y.; Pei, L.; Chen, X. Osthole inhibited TGF beta-induced epithelial-mesenchymal transition (EMT) by supressing NF-kappaB mediated Snail activation in lung cancer A549 cells. Cell Adh. Migr. 2017, 11, 464–475. [Google Scholar] [CrossRef] [Green Version]
- Adki, K.M.; Kulkarni, Y.A. Chemistry, pharmacokinetics, pharmacology and recent novel drug delivery systems of paeonol. Life Sci. 2020, 250, 117544. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Adwan, S.; Khanfer, M.; Idkaidek, N.; Al-Remawi1, M. A Novel Eutectic-Based Transdermal Delivery System for Risperidone. AAPS PharmSciTech 2021, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Mannens, G.; Meuldermans, W.; Snoeck, E.; Heykants, J. Plasma protein binding of risperidone and its distribution in blood. Psychopharmacology 1994, 114, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Mandal, A.; Dhawan, S.; Shevachman, M.; Mitragotri, S.; Joshi, N. Clinical translation of choline and geranic acid deep eutectic solvent. Bioeng. Transl. Med. 2021, 6, 10191. [Google Scholar] [CrossRef] [PubMed]
- Two, A.M.; del Rosso, J.Q. Kallikrein 5-mediated inflammation in rosacea: Clinically relevant correlations with acute and chronic manifestations in rosacea and how individual treatments may provide therapeutic benefit. J. Clin. Aesthet. Dermatol. 2014, 7, 20–25. [Google Scholar] [PubMed]
- Jarmuda, S.; O’Reilly, N.; Zaba, R.; Jakubowicz, O.; Szkaradkiewicz, A.; Kavanagh, K. Potential role of Demodex mites and bacteria in the induction of rosacea. J. Med. Microbiol. 2012, 61, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Santos, F.; Matias, A.A.; Paivaa, A.; Duartea, A.R.C. Design and processing of drug delivery formulations of therapeutic deep eutectic systems for tuberculosis. J. Supercrit. Fluids 2020, 161, 104826. [Google Scholar] [CrossRef]
- Santos, F.; Duarte, A.R.C. Therapeutic deep eutectic systems for the enhancement of drug bioavailability. In Deep Eutectic Solvents for Medicine, Gas Solubilization and Extraction of Natural Substances; Environmental Chemistry for a Sustainable World; Fourmentin, S., Gomes, M.C., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2021; Volume 56, pp. 103–129. [Google Scholar]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Santos, L.B.; Assis, R.S.; Barreto, J.A.; Bezerra, M.A.; Novaes, C.G.; Lemos, V.A. Deep eutectic solvents in liquid-phase microextraction: Contribution to green chemistry. TrAC Trend. Anal. Chem. 2022, 146, 116478. [Google Scholar] [CrossRef]
- Ortega-Zamora, C.; González-Sálamo, J.; Hernández-Borges, J. Deep Eutectic Solvents Application in Food Analysis. Molecules 2021, 26, 6846. [Google Scholar] [CrossRef]
- Hackl, K.; Kunz, W. Some aspects of green solvents. Comptes Rendus Chim. 2018, 21, 572–580. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Lomba, L.; García, C.B.; Ribate, M.P.; Giner, B.; Zuriaga, E. Applications of Deep Eutectic Solvents Related to Health, Synthesis, and Extraction of Natural Based Chemicals. Appl. Sci. 2021, 11, 10156. [Google Scholar] [CrossRef]
- Fuad, F.M.; Nadzir, M.M.; Harun, A. Hydrophilic natural deep eutectic solvent: A review on physicochemical properties and extractability of bioactive compounds. J. Mol. Liq. 2021, 339, 116923. [Google Scholar] [CrossRef]
- van Osch, D.J.G.P.; Dietz, C.H.J.T.; van Spronsen, J.; Kroon, M.C.; Gallucci, F.; van Sint Annaland, M.; Tuinier, R. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain. Chem. Eng. 2019, 3, 2933–2942. [Google Scholar] [CrossRef]
- Wu, Y.C.; Wu, P.; Li, Y.B.; Liu, T.C.; Zhang, L.; Zhou, Y.H. Natural deep eutectic solvents as new green solvents to extract anthraquinones from Rheum palmatum L. RSC Adv. 2018, 27, 15069–15077. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Yang, J.; Huang, Y.; Zhang, Y.; Wan, H.; Li, C. Green and Efficient Ultrasonic-Assisted Extraction of Bioactive Components from Salvia miltiorrhiza by Natural Deep Eutectic Solvents. Molecules 2020, 25, 140. [Google Scholar] [CrossRef] [Green Version]
- Panic’, M.; Gunjevic´, V.; Cravotto, G.; Redovnikovic, I.R. Enabling technologies for the extraction of grape-pomace anthocyanins using natural deep eutectic solvents in up-to-half-litre batches extraction of grape-pomace anthocyanins using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, X.; Liu, P.; Huang, J.; Wang, C.; Pan, M.; Kuang, Z. Enhanced phenolic compounds extraction from Morus alba L. leaves by deep eutectic solvents combined with ultrasonic-assisted extraction. Ind. Crops Prod. 2018, 120, 147–154. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Optimization of ultrasound-assisted extraction of oil from olive pomace using response surface technology: Oil recovery, unsaponifiable matter, total phenol content and antioxidant activity. LWT-Food Sci. Technol. 2017, 79, 178–189. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Srček, V.G.; Bubalo, M.C.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Aryati, D.W.; Nadhira, A.; Febianli, D.; Fransisca, F.; Mun’im, A. Natural deep eutectic solvents ultrasound-assisted extraction (NADES-UAE) of trans-cinnamaldehyde and coumarin from cinnamon bark [Cinnamomum burmannii (Nees & T. Nees) Blume]. J. Res. Pharm. 2020, 24, 389–398. [Google Scholar]
- Naseem, Z.; Zahid, M.; Hanif, M.A.; Shahid, M. Environmentally Friendly Extraction of Bioactive Compounds from Mentha arvensis Using Deep Eutectic Solvent as Green Extraction Media. Pol. J. Environ. Stud. 2020, 5, 3749–3757. [Google Scholar] [CrossRef]
- Wei, Z.F.; Qi, X.L.; Li, T.T.; Luo, M.; Wang, W.; Zu, Y.G.; Fu, Y.J. Application of natural deep eutectic solvents for extraction and determination of phenolics in Cajanus cajan leaves by ultra performance liquid chromatography. Sep. Purif. Technol. 2015, 149, 237–244. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Marques, C.; Igarashi-Mafra, L.; Coutinho, J.A.P.; Mafra, M.R. Extraction of phenolic compounds from rosemary using choline chloride—Based Deep Eutectic Solvents. Sep. Purif. Technol. 2021, 258, 117975. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xu, Y.; Li, L.; Luo, Z. Sonication-synergistic natural deep eutectic solvent as a green and efficient approach for extraction of phenolic compounds from peels of Carya cathayensis Sarg. Food Chem. 2021, 355, 129577. [Google Scholar] [CrossRef]
- Zannou, O.; Koca, I.; Aldawoud, T.M.S.; Galanakis, C.M. Recovery and Stabilization of Anthocyanins and Phenolic Antioxidants of Roselle (Hibiscus sabdariffa L.) with Hydrophilic Deep Eutectic Solvents. Molecules 2020, 25, 3715. [Google Scholar] [CrossRef]
- Zannou, O.; Koca, I. Optimization and stabilization of the antioxidant properties from Alkanet (Alkanna tinctoria) with natural deep eutectic solvents. Arab. J. Chem. 2020, 8, 6437–6450. [Google Scholar] [CrossRef]
- Li, Y.; Pan, Z.; Wang, B.; Yu, W.; Song, S.; Feng, H.; Zhao, W. Ultrasound-assisted extraction of bioactive alkaloids from Phellodendri amurensis cortex using deep eutectic solvent aqueous solutions. New J. Chem. 2020, 22, 9172–9178. [Google Scholar] [CrossRef]
- Shang, X.; Dou, Y.; Zhang, Y.; Tan, J.-N.; Liu, X.; Zhang, Z. Tailor-made natural deep eutectic solvents for green extraction of isoflavones from chickpea (Cicer arietinum L.) sprouts. Ind. Crops Prod. 2019, 140, 111724. [Google Scholar] [CrossRef]
- Unlu, A.E. Green and Non-conventional Extraction of Bioactive Compounds from Olive Leaves: Screening of Novel Natural Deep Eutectic Solvents and Investigation of Process Parameters. Waste Biomass Valorization 2021, 12, 5329–5346. [Google Scholar] [CrossRef] [PubMed]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- García, A.; Rodríguez-Juan, E.; Rodríguez-Gutiérrez, G.; Rios, J.J.; Fernández-Bolaños, J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Mouratoglou, E.; Malliou, V.; Makris, D.P. Novel glycerol-based natural eutectic mixtures and their efficiency in the ultrasound assisted extraction of antioxidant polyphenols from agri-food waste biomass. Waste Biomass Valorization 2016, 7, 1377–1387. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A. Natesan Subramanian Jayakumar New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef] [PubMed]
- García-Lafuente, A.; Guillamon, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- De Luna, S.R.R.; Ramirez-Garza, R.E.; Saldivar, S.O.S. Environmentally Friendly Methods for Flavonoid Extraction from Plant Material: Impact of Their Operating Conditions on Yield and Antioxidant Properties. Sci. World J. 2020, 2020, 6792069. [Google Scholar]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids from Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Soxhlet extraction of phenolic compounds fromVernonia cinereal leaves and its antioxidant activity. J. Appl. Res. Med. Aromat. Plants 2018, 11, 12–17. [Google Scholar]
- Um, M.; Han, T.H.; Lee, J.W. Ultrasound-assisted extraction and antioxidant activity of phenolic and flavonoid compounds and ascorbic acid from rugosa rose (Rosa rugosa Thunb.) fruit. Food Sci. Biotechnol. 2018, 27, 375–382. [Google Scholar] [CrossRef]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible deep eutectic solvents based on choline chloride: Characterization and application to the extraction of rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Qi, X.-L.; Peng, X.; Huang, Y.-Y.; Li, L.; Wei, Z.-F.; Zu, Y.-G.; Fu, Y.-J. Green and efficient extraction of bioactive flavonoids from Equisetum palustre L. by deep eutectic solvents-based negative pressure cavitation method combined with macroporous resin enrichment. Ind. Crops Prod. 2015, 70, 142–148. [Google Scholar] [CrossRef]
- Wei, Z.-F.; Wang, X.-Q.; Peng, X.; Wang, W.; Zhao, C.-J.; Zu, Y.-G.; Fu, Y.-J. Fast and green extraction and separation of main bioactive flavonoids from Radix Scutellariae. Ind. Crops Prod. 2015, 63, 175–181. [Google Scholar] [CrossRef]
- Kalyniukova, A.; Holusa, J.; Musiolek, D.; Sedlakova-Kadukova, J.; Płotka-Wasylka, J.; Andruch, V. Application of deep eutectic solvents for separation and determination of bioactive compounds in medicinal plants. Ind. Crops Prod. 2021, 172, 114047. [Google Scholar] [CrossRef]
- Mulia, K.; Fauzia, F.; Krisanti, E.A. Polyalcohols as hydrogen-bonding donors in choline chloride-based deep eutectic solvents for extraction of xanthones from the pericarp of Garcinia mangostana L. Molecules 2019, 24, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grudniewska, A.; Popłonski, J. Simple and green method for the extraction of xanthohumol from spent hops using deep eutectic solvents. Sep. Purif. Technol. 2020, 250, 117196. [Google Scholar] [CrossRef]
- Yang, M.; Cao, J.; Cao, F.; Lu, C.; Su, E. Efficient extraction of bioactive flavonoids from Ginkgo biloba leaves using deep eutectic solvent/water mixture as green media. Chem. Biochem. Eng. Q. 2018, 32, 315–324. [Google Scholar] [CrossRef]
- Wu, D.-T.; Feng, K.-L.; Huang, L.; Gan, R.-Y.; Hu, Y.-C.; Zou, L. Deep Eutectic Solvent-Assisted Extraction, Partially Structural Characterization, and Bioactivities of Acidic Polysaccharides from Lotus Leaves. Foods 2021, 10, 2330. [Google Scholar] [CrossRef]
- Ma, X.; Wang, X.; Fan, S.; Chen, J. Study on Extraction Process and Activity of Plant Polysaccharides. AIP Conf. Proc. 2017, 1890, 040122. [Google Scholar]
- Guo, Y.; Li, Y.; Li, Z.; Yan, W.; Chen, P.; Yao, S. Extraction assisted by far infrared radiation and hot air circulation with deep eutectic solvent for bioactive polysaccharides from Poria cocos (Schw.) wolf. Green Chem. 2021, 23, 7170–7192. [Google Scholar] [CrossRef]
- Nava-Ocampo, M.F.; Fuhaid, L.A.; Verpoorte, R.; Choi, Y.H.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S.; Witkamp, G.J.; Farinha, A.S.F.; Bucs, S.S. Natural deep eutectic solvents as biofilm structural breakers. Water Res. 2021, 201, 117323. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T. Biofilms. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Maloy, S., Hughes, K., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 335–337. [Google Scholar]
- Zeng, Q.; Wang, Y.; Huang, Y.; Ding, X.; Chen, J.; Xu, K. Deep eutectic solvents as novel extraction media for protein partitioning. Analyst 2014, 139, 2565–2573. [Google Scholar] [CrossRef]
- Wahlström, R.; Rommi, K.; Willberg-Keyriläinen, P.; Ercili-Cura, D.; Holopainen-Mantila, U.; Hiltunen, J.; Mäkinen, O.; Nygren, H.; Mikkelson, A.; Kuutti, L. High Yield Protein Extraction from Brewer’s Spent Grain with Novel Carboxylate Salt—Urea Aqueous Deep Eutectic Solvents. ChemistrySelect 2017, 2, 9355–9363. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Y.; Huang, Y.; Li, N.; Wen, Q. A green deep eutectic solvent-based aqueous two-phase system for protein extracting. Anal. Chim. Acta 2015, 864, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Grudniewska, A.; de Melo, E.M.; Chan, A.; Gniłka, R.; Boratyński, F.; Matharu, A.S. Enhanced Protein Extraction from Oilseed Cakes Using Glycerol–Choline Chloride Deep Eutectic Solvents: A Biorefinery Approach. ACS Sustain. Chem. Eng. 2018, 6, 15791–15800. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Xu, K.; Huang, Y.; Wen, Q.; Ding, X. Development of green betaine-based deep eutectic solvent aqueous two-phase system for the extraction of protein. Talanta 2016, 152, 23–32. [Google Scholar] [CrossRef]
- Nakhle, L.; Kfoury, M.; Mallard, I.; Landy, D.; Greige-Gerges, H. Microextraction of bioactive compounds using deep eutectic solvents: A review. Enviorn. Chem. Lett. 2021, 19, 3747–3759. [Google Scholar] [CrossRef]
- Du, Z.; Yu, Y.-L.; Wang, J.-H. Extraction of proteins from biological fluids by use of an ionic liquid/aqueous two-phase system. Chem. Eur. J. 2007, 13, 2130–2137. [Google Scholar] [CrossRef]
- Álvareza, M.S.; Zhang, Y. Sketching neoteric solvents for boosting drugs bioavailability. J. Control. Release 2019, 311–312, 225–232. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.-Y.; Zhou, R.-R.; Fang, L.Z.; Zhao, D.; Cai, P.; Yu, R.; Zhang, S.-H.; Huang, J.H. The extraction of phenolic acids and polysaccharides from Lilium lancifolium Thunb. using a deep eutectic solvent. Anal. Methods 2021, 13, 1226–1231. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ijardar, S.P.; Singh, V.; Gardas, R.L. Revisiting the Physicochemical Properties and Applications of Deep Eutectic Solvents. Molecules 2022, 27, 1368. https://doi.org/10.3390/molecules27041368
Ijardar SP, Singh V, Gardas RL. Revisiting the Physicochemical Properties and Applications of Deep Eutectic Solvents. Molecules. 2022; 27(4):1368. https://doi.org/10.3390/molecules27041368
Chicago/Turabian StyleIjardar, Sushma P., Vickramjeet Singh, and Ramesh L. Gardas. 2022. "Revisiting the Physicochemical Properties and Applications of Deep Eutectic Solvents" Molecules 27, no. 4: 1368. https://doi.org/10.3390/molecules27041368
APA StyleIjardar, S. P., Singh, V., & Gardas, R. L. (2022). Revisiting the Physicochemical Properties and Applications of Deep Eutectic Solvents. Molecules, 27(4), 1368. https://doi.org/10.3390/molecules27041368







