Biocatalytic System Made of 3D Chitin, Silica Nanopowder and Horseradish Peroxidase for the Removal of 17α-Ethinylestradiol: Determination of Process Efficiency and Degradation Mechanism †
Abstract
:1. Introduction
2. Results and Discussion
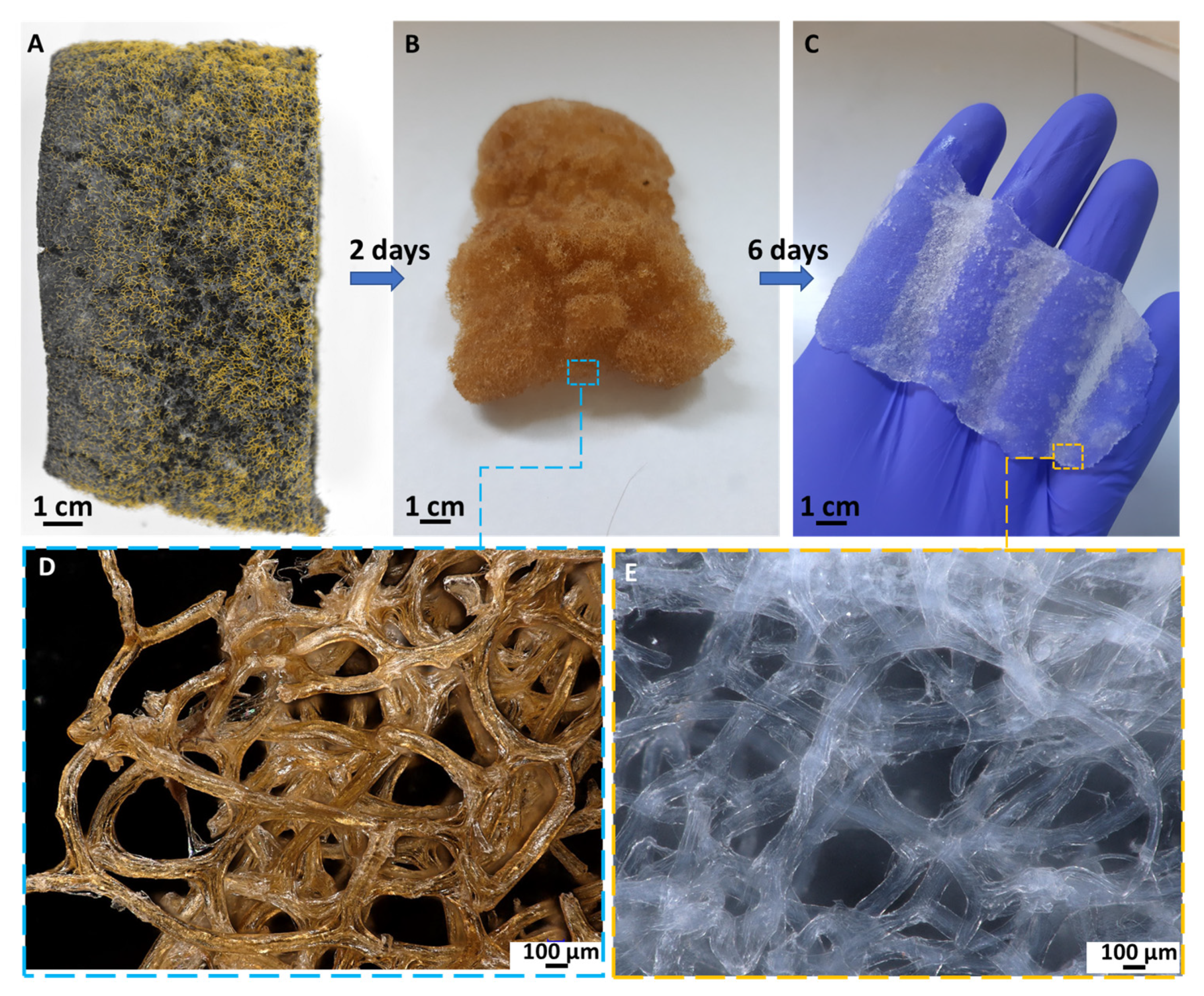
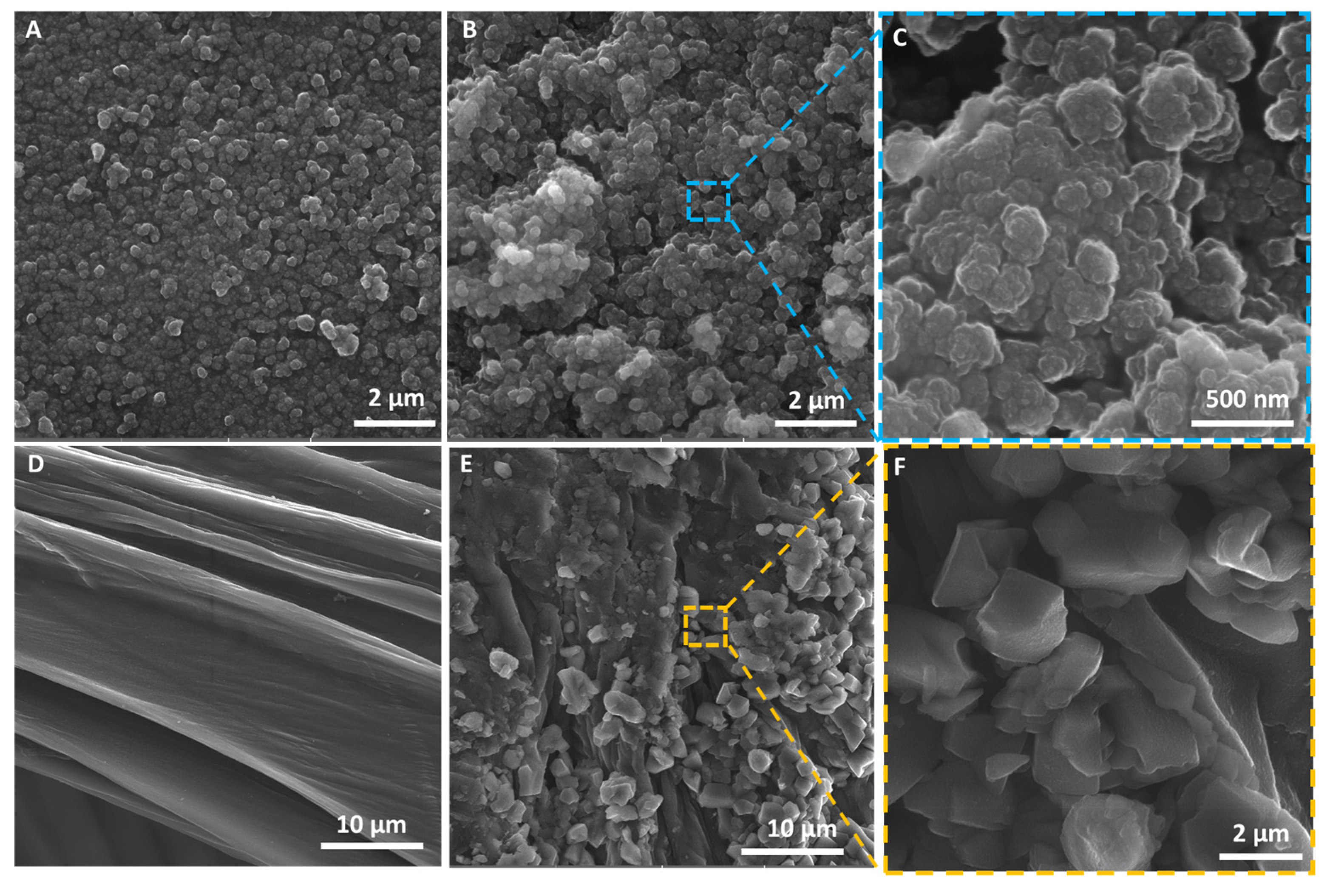
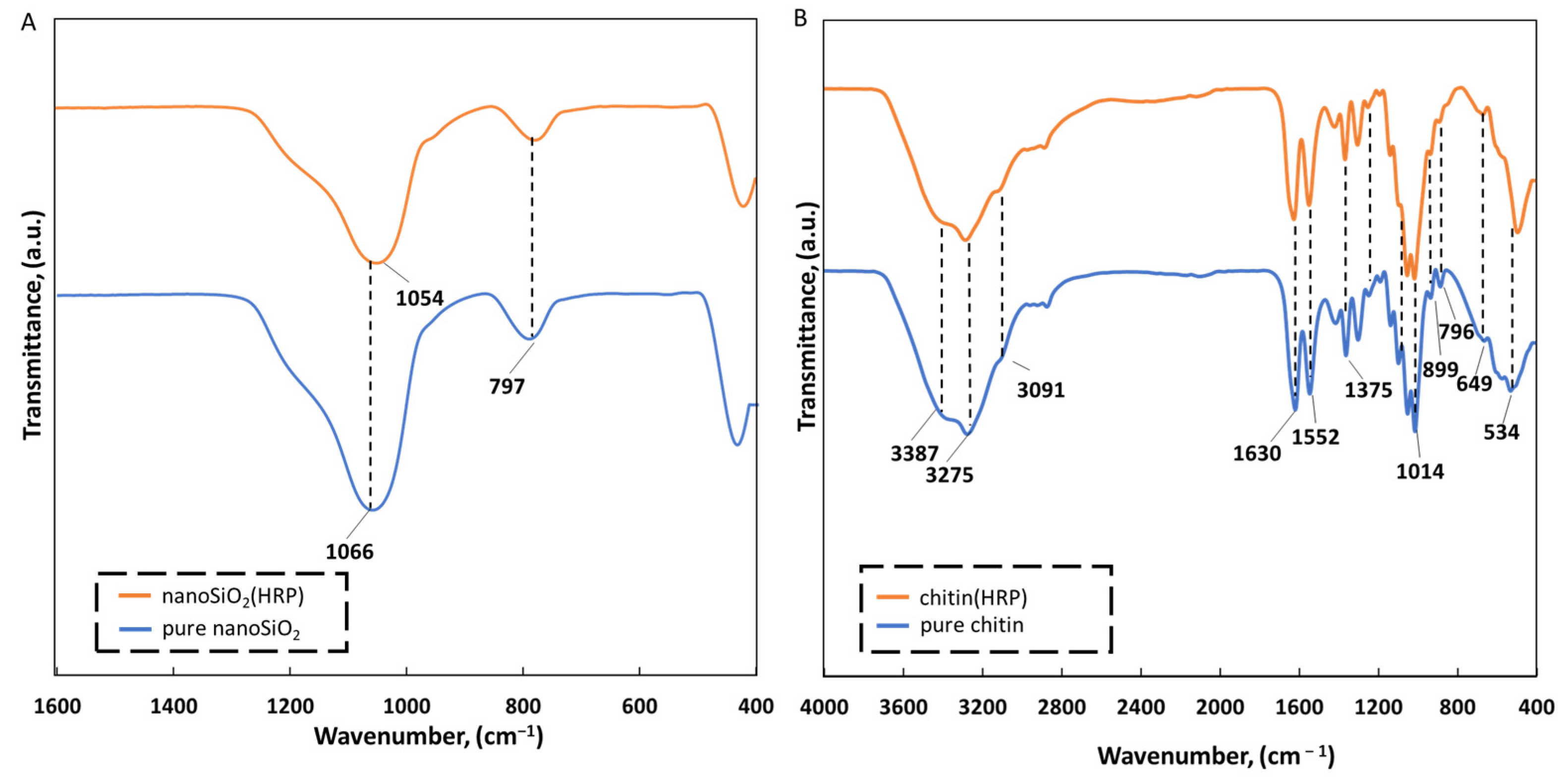
2.1. Physicochemical Characterization of the EE2 Removal System
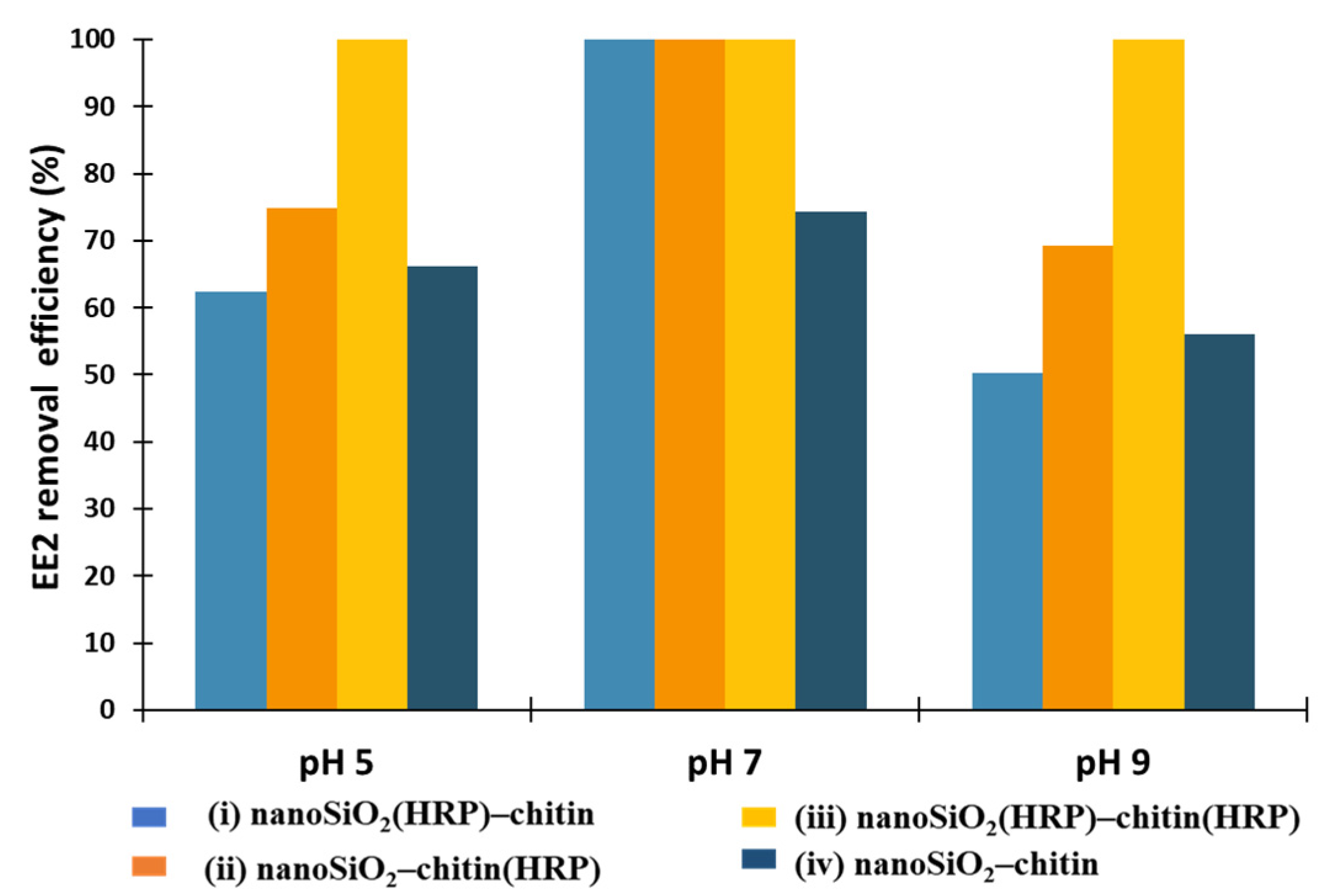
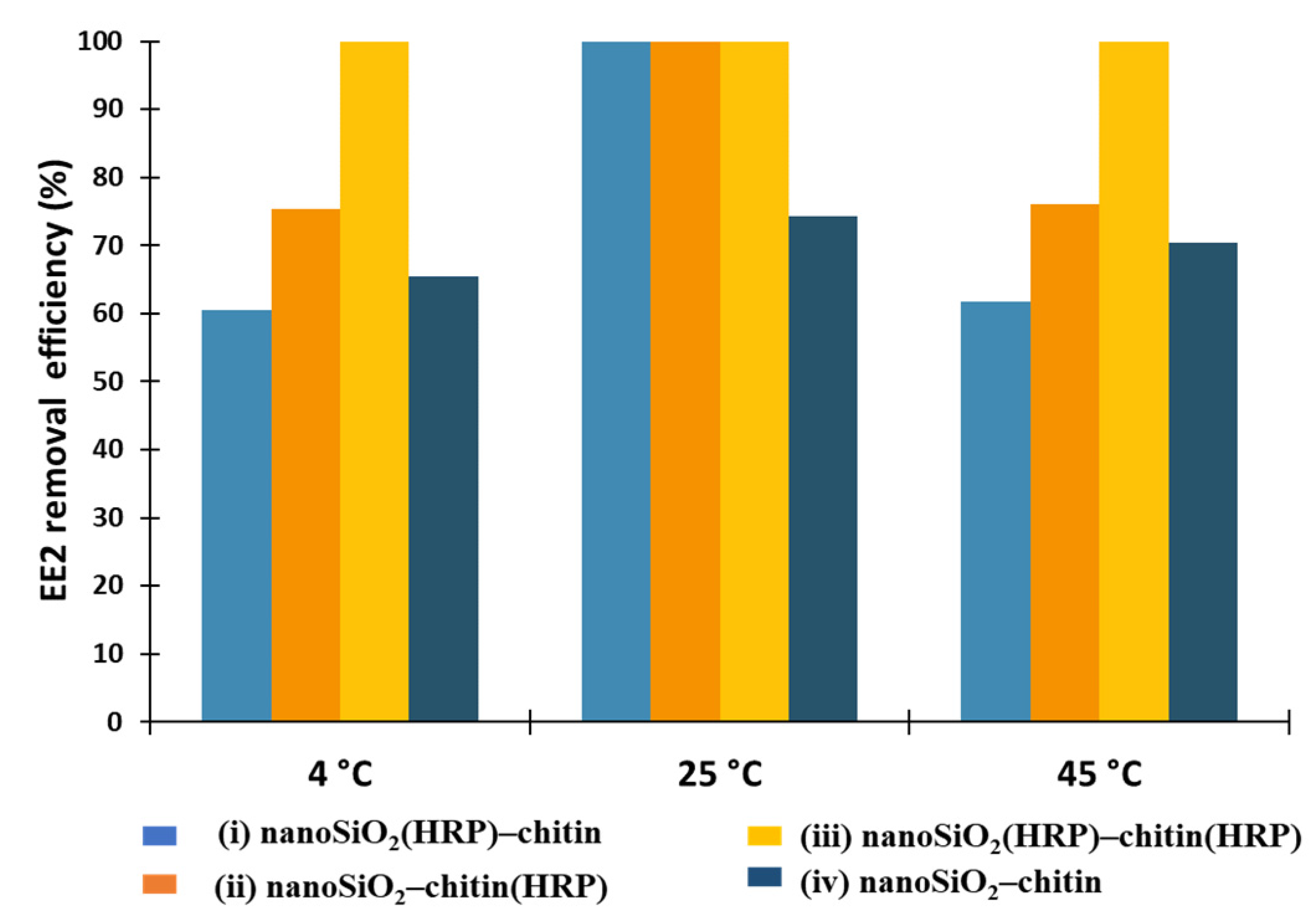
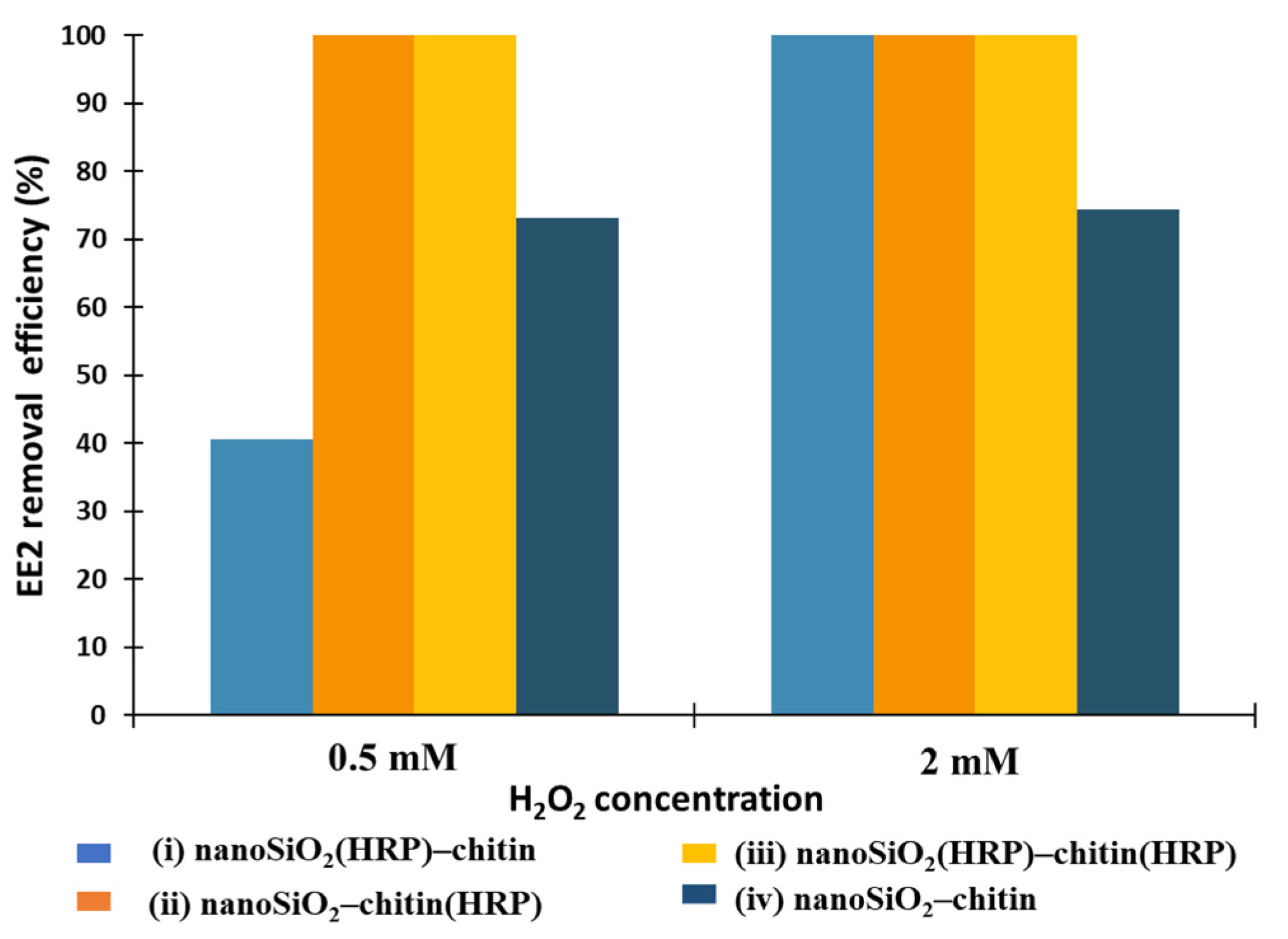
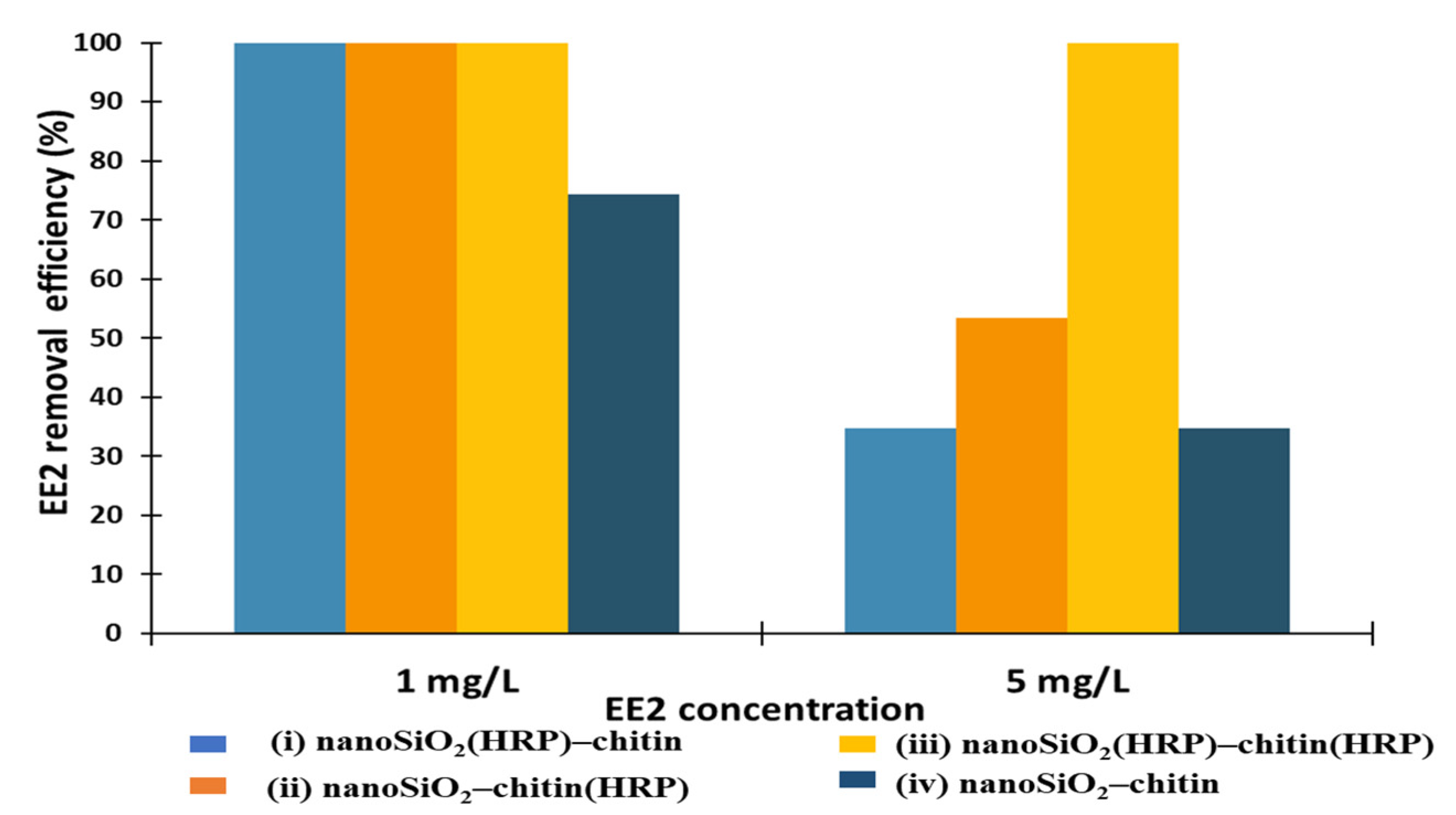
2.2. EE2 Removal Study
2.3. Mechanism of 17α-Ethinylestradiol Removal
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Chitinous Scaffold Isolation from A. fistularis Marine Sponge
3.3. Horseradish Peroxidase Immobilization onto Chitin and Nanosilica and Amount of Immobilized Enzyme
3.4. Removal of EE2 by Simultaneous Adsorption and Catalytic Conversion
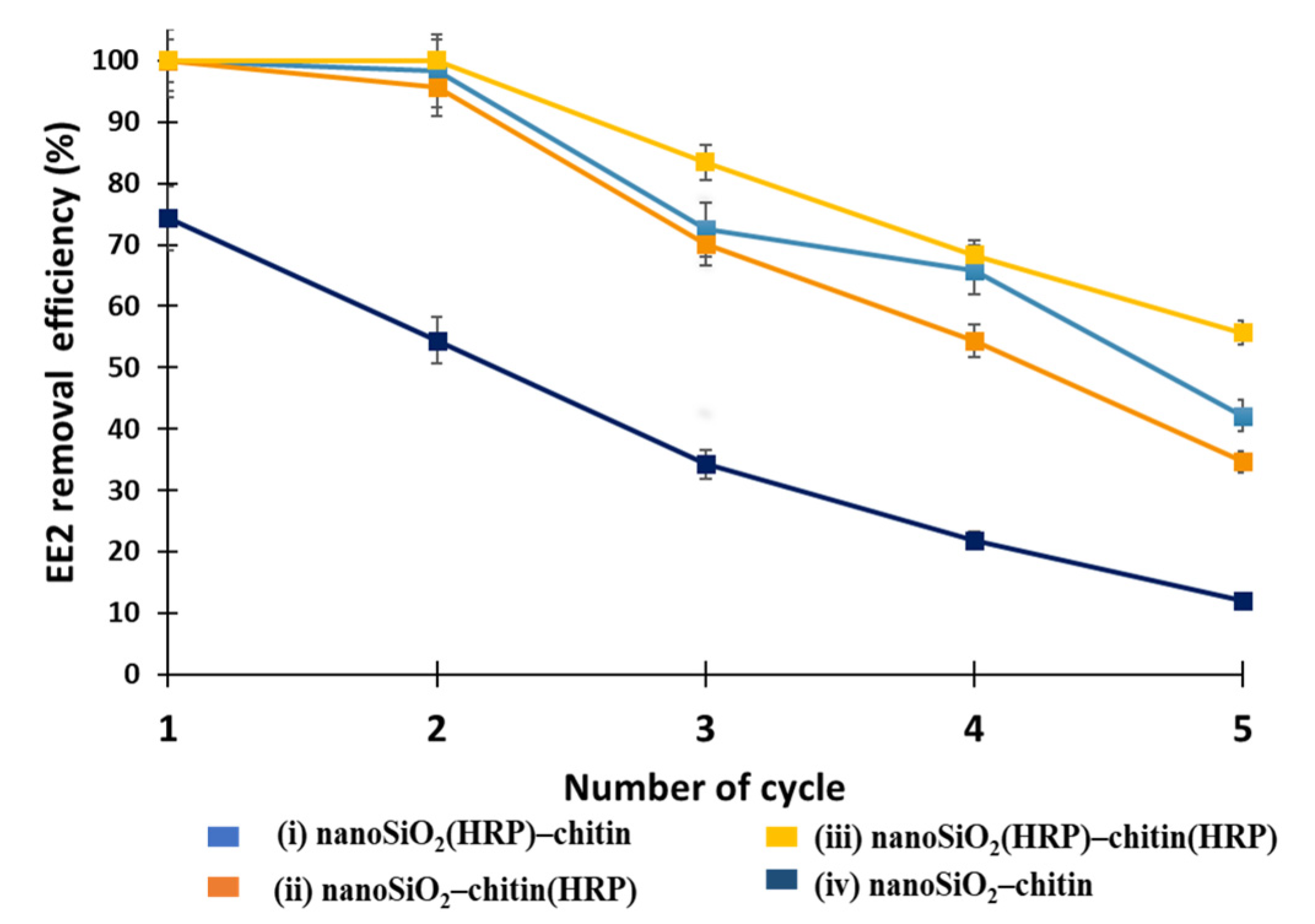
3.5. Reusability of the Proposed EE2 Removal Systems
3.6. Characterization Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zdarta, J.; Jankowska, K.; Bachosz, K.; Degórska, O.; Kaźmierczak, K.; Nguyen, L.N.; Nghiem, L.D.; Jesionowski, T. Enhanced wastewater treatment by immobilized enzymes. Curr. Pollut. Rep. 2021, 7, 167–179. [Google Scholar] [CrossRef]
- Xu, R.; Yuan, J.; Si, Y.; Li, F.; Zhang, B. Estrone removal by horseradish peroxidase immobilized on a nanofibrous support with Fe3O4 nanoparticles. RSC Adv. 2016, 6, 3927–3933. [Google Scholar] [CrossRef]
- Snyder, S.; Westerhoff, P.; Yoon, Y.; Sedlak, D. Disruptors in water: Implications for the water industry. Environ. Eng. Sci. 2003, 20, 449–469. [Google Scholar] [CrossRef]
- Nazari, E.; Suja, F. Effects of 17β-estradiol (E2) on aqueous organisms and its treatment problem: A review. Rev. Environ. Health 2016, 31, 465–491. [Google Scholar] [CrossRef]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-pour, A.; Verma, M.; Surampalli, R.Y. Removal of pharmaceutical compounds in water and wastewater using fungal oxidoreductase enzymes. Environ. Pollut. 2018, 234, 190–213. [Google Scholar] [CrossRef]
- Lacerda, M.F.A.R.; Lopes, F.M.; Sartoratto, A.; Ponezi, A.N.; Thomaz, D.V.; Schimidt, F.; Santiago, M.F. Stability of immobilized laccase on Luffa cylindrica fibers and assessment of synthetic hormone degradation. Prep. Biochem. Biotechnol. 2019, 49, 58–63. [Google Scholar] [CrossRef]
- Zdarta, J.; Machałowski, T.; Degórska, O.; Bachosz, K.; Fursov, A.; Hermann Ehrlich, V.; Ivanenko, V.N.; Jesionowski, T. 3D chitin scaffolds from the marine demosponge Aplysina archeri as a support for laccase immobilization and its use in the removal of pharmaceuticals. Biomolecules 2020, 10, 646. [Google Scholar] [CrossRef]
- Azevedo, A.M.; Martins, V.C.; Prazeres, D.M.F.; Vojinović, V.; Cabral, J.M.S.; Fonseca, L.P. Horseradish peroxidase: A valuable tool in biotechnology. Biotechnol. Annu. Rev. 2003, 9, 199–247. [Google Scholar]
- Li, J.; Chen, X.; Xu, D.; Pan, K. Immobilization of horseradish peroxidase on electrospun magnetic nanofibers for phenol removal. Ecotoxicol. Environ. Saf. 2019, 170, 716–721. [Google Scholar] [CrossRef]
- Jankowska, K.; Zdarta, J.; Grzywaczyk, A.; Degórska, O.; Kijeńska-Gawrońska, E.; Pinelo, M.; Jesionowski, T. Horseradish peroxidase immobilised onto electrospun fibres and its application in decolourisation of dyes from model sea water. Process Biochem. 2021, 102, 10–21. [Google Scholar] [CrossRef]
- Ai, J.; Zhang, W.; Liao, G.; Xia, H.; Wang, D. NH2-Fe3O4@SiO2 supported peroxidase catalyzed H2O2 for degradation of endocrine disrupter from aqueous solution: Roles of active radicals and NOMs. Chemosphere 2017, 186, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Selvankumar, T.; Paray, B.A.; Rehman, M.U.; Kamala-Kannan, S.; Govarthanan, M.; Kim, W.; Selvam, K. Chlorpyrifos degradation efficiency of Bacillus sp. laccase immobilized on iron magnetic nanoparticles. 3 Biotech 2020, 10, 366. [Google Scholar] [CrossRef]
- Khatoon, H.; Rai, J.P.N. Augmentation of Atrazine biodegradation by two Bacilli immobilized on α-Fe2O3 magnetic nanoparticles. Sci. Rep. 2018, 8, 17831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Liu, X.; Zhang, X.; Wu, J.; Chai, C.; Ma, D.; Chen, Q.; Xiang, D.; Ge, W. Immobilized lignin peroxidase on Fe3O4@SiO2@polydopamine nanoparticles for degradation of organic pollutants. Int. J. Biol. Macromol. 2019, 138, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Costantini, A.; Venezia, V.; Pota, G.; Bifulco, A.; Califano, V.; Sannino, F. Adsorption of cellulase on wrinkled silica nanoparticles with enhanced inter-wrinkle distance. Nanomaterials 2020, 10, 1799. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Park, G.D.; Patel, S.K.S.; Kondaveeti, S.; Otari, S.; Anwar, M.Z.; Kalia, V.C.; Singh, Y.; Kim, S.C.; Cho, B.K.; et al. SiO2 microparticles with carbon nanotube-derived mesopores as an efficient support for enzyme immobilization. Chem. Eng. J. 2018, 359, 1252–1264. [Google Scholar] [CrossRef]
- Luo, Y.; Jin, D.; He, W.; Huang, J.; Chen, A.; Qi, F. A SiO2 microcarrier with an opal-like structure for cross-linked enzyme immobilization. Langmuir 2021, 37, 14147–14156. [Google Scholar] [CrossRef]
- Mondal, S.; Malik, S.; Sarkar, R.; Roy, D.; Saha, S.; Mishra, S.; Sarkar, A.; Chatterjee, M.; Mandal, B. Exuberant immobilization of urease on an inorganic SiO2 support enhances the enzymatic activities by threefold for perennial utilization. Bioconjug. Chem. 2019, 30, 134–147. [Google Scholar] [CrossRef]
- Libertino, S.; Aiello, V.; Scandurra, A.; Renis, M.; Sinatra, F. Immobilization of the enzyme glucose oxidase on both bulk and porous SiO2 surfaces. Sensors 2008, 8, 5637–5648. [Google Scholar] [CrossRef]
- Zhang, Q.; Qian, J.; Guo, H.; Zhang, W.; Kuang, C. Utilization of nano-SiO2 as a supporting material for immobilization of porcine pancreatic lipase. J. Nanosci. Nanotechnol. 2018, 18, 5837–5841. [Google Scholar] [CrossRef] [PubMed]
- Klinger, C.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Petrenko, I.; Machałowski, T.; Ereskovsky, A.; Martinović, R.; Muzychka, L.; et al. Express method for isolation of ready-to-use 3D chitin scaffolds from Aplysina archeri (Aplysineidae: Verongiida) Demosponge. Mar. Drugs 2019, 17, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubert, M.; Binnewerg, B.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; Bechmann, N.; et al. Naturally prefabricated marine biomaterials: Isolation and applications of flat chitinous 3D scaffolds from Ianthella labyrinthus (Demospongiae: Verongiida). Int. J. Mol. Sci. 2019, 20, 5105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kertmen, A.; Ehrlich, H. Patentology of chitinous biomaterials. Part I: Chitin. Carbohydr. Polym. 2022, 282, 119102. [Google Scholar] [CrossRef]
- Khrunyk, Y.; Lach, S.; Petrenko, I.; Ehrlich, H. Progress in modern marine biomaterials research. Mar. Drugs 2020, 18, 589. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Bazhenov, V.V.; Stawski, D.; Petrenko, I.; Ehrlich, A.; Behm, T.; Kljajic, Z.; Stelling, A.L.; Jesionowski, T.; et al. Poriferan chitin as a template for hydrothermal zirconia deposition. Front. Mater. Sci. 2013, 7, 248–260. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Beyer, J.; Makarova, A.; Stöcker, H.; Walter, J.; Galli, R.; Kaiser, S.; Vyalikh, D.; Bazhenov, V.V.; et al. Extreme biomimetic approach for developing novel chitin-GeO2 nanocomposites with photoluminescent properties. Nano Res. 2015, 8, 2288–2301. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Rafaja, D.; Koltsov, I.; Stocker, H.; Szalaty, T.J.; Bazhenov, V.V.; Stelling, A.L.; Beyer, J.; Heitmann, J.; et al. Extreme biomimetic approach for synthesis of nanocrystalline chitin-(Ti,Zr)O2 multiphase composites. Mater. Chem. Phys. 2017, 188, 115–124. [Google Scholar] [CrossRef]
- Schleuter, D.; Günther, A.; Paasch, S.; Ehrlich, H.; Kljajić, Z.; Hanke, T.; Bernhard, G.; Brunner, E. Chitin-based renewable materials from marine sponges for uranium adsorption. Carbohydr. Polym. 2013, 92, 712–718. [Google Scholar] [CrossRef]
- Machałowski, T.; Rusak, A.; Wiatrak, B.; Haczkiewicz-Leśniak, K.; Popie, A.; Jaroszewicz, J.; Żak, A.; Podhorska-Okołów, M.; Jesionowski, T. Naturally formed chitinous skeleton isolated from the marine demosponge Aplysina fistularis as a 3D scaffold for tissue engineering. Materials 2021, 14, 2992. [Google Scholar] [CrossRef]
- Machałowski, T.; Wysokowski, M.; Żółtowska-Aksamitowska, S.; Bechmann, N.; Binnewerg, B.; Schubert, M.; Guan, K.; Bornstein, S.R.; Czaczyk, K.; Pokrovsky, O.; et al. Spider chitin. The biomimetic potential and applications of Caribena versicolor tubular chitin. Carbohydr. Polym. 2019, 226, 115301. [Google Scholar] [CrossRef]
- Mutsenko, V.; Gryshkov, O.; Rogulska, O.; Lode, A.; Petrenko, A.Y.; Gelinsky, M.; Glasmacher, B.; Ehrlich, H. Chitinous scaffolds from marine sponges for tissue engineering. In Marine-Derived Biomaterials for Tissue Engineering Applications Chitinous; Choi, A., Ben-Nissan, B., Eds.; Springer Nature: Singapore, 2019; pp. 285–307. [Google Scholar]
- Mutsenko, V.V.; Bazhenov, V.V.; Rogulska, O.; Tarusin, D.N.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; Meißner, H.; Lode, A.; et al. 3D chitinous scaffolds derived from cultivated marine demosponge Aplysina aerophoba for tissue engineering approaches based on human mesenchymal stromal cells. Int. J. Biol. Macromol. 2017, 104, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Binnewerg, B.; Schubert, M.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Djurović, M.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; et al. Marine biomaterials: Biomimetic and pharmacological potential of cultivated Aplysina aerophoba marine demosponge. Mater. Sci. Eng. C 2020, 109, 110566. [Google Scholar] [CrossRef] [PubMed]
- Tsurkan, M.V.; Voronkina, A.; Khrunyk, Y.; Wysokowski, M.; Petrenko, I.; Ehrlich, H. Progress in chitin analytics. Carbohydr. Polym. 2021, 252, 117204. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef]
- Stanisz, M.; Klapiszewski, Ł.; Jesionowski, T. Recent advances in the fabrication and application of biopolymer-based micro- and nanostructures: A comprehensive review. Chem. Eng. J. 2020, 397, 125409. [Google Scholar] [CrossRef]
- Betancor, L.; López-Gallego, F.; Alonso-Morales, N.; Dellamora, G.; Mateo, C.; Fernandez-Lafuente, R.; Guisan, J.M. Glutaraldehyde in protein immobilization. In Methods in Biotechnology: Immobilization of Enzymes and Cells; Guisan, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 57–64. [Google Scholar]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.; Xiao, P.; Jiang, W.; Wang, D. Immobilization of horseradish peroxidase on Fe3O4 nanoparticles for enzymatic removal of endocrine disrupting chemicals. Environ. Sci. Pollut. Res. 2020, 27, 24357–24368. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Bai, S.; Guan, X.; Li, H.; Ou, J. Effect of the specific surface area of nano-silica particle on the properties of cement paste. Powder Technol. 2021, 392, 680–689. [Google Scholar] [CrossRef]
- Machałowski, T.; Idaszek, J.; Chlanda, A.; Heljak, M.; Piasecki, A.; Swięszkowski, W.; Jesionowski, T. Naturally prefabricated 3D chitinous skeletal scaffold of marine demosponge origin, biomineralized ex vivo as a functional biomaterial. Carbohydr. Polym. 2022, 275, 118750. [Google Scholar] [CrossRef] [PubMed]
- Voss, R.; Brook, M.A.; Thompson, J.; Chen, Y.; Pelton, R.H.; Brennan, J.D. Non-destructive horseradish peroxidase immobilization in porous silica nanoparticles. J. Mater. Chem. 2007, 17, 4854–4863. [Google Scholar] [CrossRef]
- Wysokowski, M.; Bazhenov, V.V.; Tsurkan, M.V.; Galli, R.; Stelling, A.L.; Stöcker, H.; Kaiser, S.; Niederschlag, E.; Gärtner, G.; Behm, T.; et al. Isolation and identification of chitin in three-dimensional skeleton of Aplysina fistularis marine sponge. Int. J. Biol. Macromol. 2013, 62, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Petrenko, I.; Stelling, A.L.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan chitin as a versatile template for extreme biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef] [Green Version]
- Arslan, M. Immobilization horseradish peroxidase on amine-functionalized glycidyl methacrylate-g-poly(ethylene terephthalate) fibers for use in azo dye decolorization. Polym. Bull. 2011, 66, 865–879. [Google Scholar] [CrossRef]
- Hernández-Ortiz, M.; Hernández-Padrón, G.; Bernal, R.; Cruz-Vázquez, C.; Castaño, M. Nanocrystalline mimetic opals: Synthesis and comparative characterization vs. natural stones. Int. J. Basic Appl. Sci. 2015, 4, 238–243. [Google Scholar] [CrossRef]
- Budiarti, H.A.; Puspitasari, R.N.; Hatta, A.M.; Risanti, D.D.; Risanti, S. Synthesis and characterization of TiO2@SiO2 and SiO2@TiO2 core-shell structure using lapindo mud extract via sol-gel method. Procedia Eng. 2017, 170, 65–71. [Google Scholar] [CrossRef]
- Solarska-Ściuk, K.; Adach, K.; Cyboran-Mikołajczyk, S.; Bonarska-Kujawa, D.; Rusak, A.; Cwynar-Zając, Ł.; Machałowski, T.; Jesionowski, T.; Grzywacz, K.; Fijałkowski, M. Are biogenic and pyrogenic mesoporous SiO2 nanoparticles safe for normal cells? Molecules 2021, 26, 1427. [Google Scholar] [CrossRef]
- El-Nahass, M.N.; El-keiy, M.M.; Ali, E.M.M. Immobilization of horseradish peroxidase into cubic mesoporous silicate, SBA-16 with high activity and enhanced stability. Int. J. Biol. Macromol. 2018, 116, 1304–1309. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Pomastowski, P.; Hornowska, M.; Król, A.; Rafińska, K.; Buszewski, B. Naturally organic functionalized 3D biosilica from diatom microalgae. Mater. Des. 2017, 132, 22–29. [Google Scholar] [CrossRef]
- Zdarta, J.; Jankowska, K.; Bachosz, K.; Kijeńska-Gawrońska, E.; Zgoła-Grześkowiak, A.; Kaczorek, E.; Jesionowski, T. A promising laccase immobilization using electrospun materials for biocatalytic degradation of tetracycline: Effect of process conditions and catalytic pathways. Catal. Today 2020, 348, 127–136. [Google Scholar] [CrossRef]
- Ben Samuel, J.; Julyes Jaisingh, S.; Sivakumar, K.; Mayakannan, A.V.; Arunprakash, V.R. Visco-elastic, thermal, antimicrobial and dielectric behaviour of areca fibre-reinforced nano-silica and neem oil-toughened epoxy resin bio composite. Silicon 2021, 13, 1703–1712. [Google Scholar] [CrossRef]
- Alshawafi, W.M.; Aldhahri, M.; Almulaiky, Y.Q.; Salah, N.; Moselhy, S.S.; Ibrahim, I.H.; El-Shishtawy, R.M.; Mohamed, S.A. Immobilization of horseradish peroxidase on PMMA nanofibers incorporated with nanodiamond. Artif. Cells Nanomed. Biotechnol. 2018, 46, S973–S981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Ruiz, J.; Arnao, M.B.; Hiner, A.N.P.; García-Cánovas, F.; Acosta, M. Catalase-like activity of horseradish peroxidase: Relationship to enzyme inactivation by H2O2. Biochem. J. 2001, 354, 107–114. [Google Scholar] [CrossRef]
- Patel, A.C.; Li, S.; Yuan, J.M.; Wei, Y. In situ encapsulation of horseradish peroxidase in electrospun porous silica fibers for potential biosensor applications. Nano Lett. 2006, 6, 1042–1046. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Z.; Wang, H.; Dang, Z.; Liu, Y. A review of 17α-ethynylestradiol (EE2) in surface water across 32 countries: Sources, concentrations, and potential estrogenic effects. J. Environ. Manag. 2021, 292, 112804. [Google Scholar] [CrossRef]
- Figueiredo, S.A.; Loureiro, J.M.; Boaventura, R.A. Natural waste materials containing chitin as adsorbents for textile dyestuffs: Batch and continuous studies. Water Res. 2005, 39, 4142–4152. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.; Liang, Z.; Zhao, Z.; Wang, W.; Yang, C.; Hu, B.; Cui, F. Enhanced adsorption of steroid estrogens by one-pot synthesized phenyl-modified mesoporous silica: Dependence on phenyl-organosilane precursors and pH condition. Chemosphere 2019, 234, 438–449. [Google Scholar] [CrossRef]
- Manoli, F.; Koutsopoulos, S.; Dalas, E. Crystallization of calcite on chitin. J. Cryst. Growth 1997, 182, 116–124. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gunatilake, S.R.; Clark, T.L.; Rodriguez, J.M.; Mlsna, T.E. Determination of five estrogens in wastewater using a comprehensive two-dimensional gas chromatograph. Anal. Methods 2014, 6, 5652–5658. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machałowski, T.; Jankowska, K.; Bachosz, K.; Smułek, W.; Ehrlich, H.; Kaczorek, E.; Zdarta, J.; Jesionowski, T. Biocatalytic System Made of 3D Chitin, Silica Nanopowder and Horseradish Peroxidase for the Removal of 17α-Ethinylestradiol: Determination of Process Efficiency and Degradation Mechanism. Molecules 2022, 27, 1354. https://doi.org/10.3390/molecules27041354
Machałowski T, Jankowska K, Bachosz K, Smułek W, Ehrlich H, Kaczorek E, Zdarta J, Jesionowski T. Biocatalytic System Made of 3D Chitin, Silica Nanopowder and Horseradish Peroxidase for the Removal of 17α-Ethinylestradiol: Determination of Process Efficiency and Degradation Mechanism. Molecules. 2022; 27(4):1354. https://doi.org/10.3390/molecules27041354
Chicago/Turabian StyleMachałowski, Tomasz, Katarzyna Jankowska, Karolina Bachosz, Wojciech Smułek, Hermann Ehrlich, Ewa Kaczorek, Jakub Zdarta, and Teofil Jesionowski. 2022. "Biocatalytic System Made of 3D Chitin, Silica Nanopowder and Horseradish Peroxidase for the Removal of 17α-Ethinylestradiol: Determination of Process Efficiency and Degradation Mechanism" Molecules 27, no. 4: 1354. https://doi.org/10.3390/molecules27041354
APA StyleMachałowski, T., Jankowska, K., Bachosz, K., Smułek, W., Ehrlich, H., Kaczorek, E., Zdarta, J., & Jesionowski, T. (2022). Biocatalytic System Made of 3D Chitin, Silica Nanopowder and Horseradish Peroxidase for the Removal of 17α-Ethinylestradiol: Determination of Process Efficiency and Degradation Mechanism. Molecules, 27(4), 1354. https://doi.org/10.3390/molecules27041354











