Recent Advances in C5 and C6 Sugar Alcohol Synthesis by Hydrogenation of Monosaccharides and Cellulose Hydrolytic Hydrogenation over Non-Noble Metal Catalysts
Abstract
:1. Introduction
2. Hydrogenation of Glucose to Sorbitol
3. Fructose Hydrogenation
4. Hydrogenation of Other C5 and C6 Sugars
5. Hydrolytic Cellulose Hydrogenation to Hexitols
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Werpy, T.A.; Holladay, J.E.; White, J.F. Top Value Added Chemicals From Biomass: I. Results of Screening for Potential Candidates from Sugars and Synthesis Gas; PNNL-14808; Northwest National Lab. (PNNL): Richland, WA, USA, 2004; p. 926125. [Google Scholar]
- Sánchez-Bastardo, N.; Delidovich, I.; Alonso, E. From Biomass to Sugar Alcohols: Purification of Wheat Bran Hydrolysates Using Boronic Acid Carriers Followed by Hydrogenation of Sugars over Ru/H-ZSM-5. ACS Sustain. Chem. Eng. 2018, 6, 11930–11938. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Sharma, B. Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef] [Green Version]
- Galán, G.; Martín, M.; Grossmann, I.E. Integrated Renewable Production of Sorbitol and Xylitol from Switchgrass. Ind. Eng. Chem. Res. 2021, 60, 5558–5573. [Google Scholar] [CrossRef] [PubMed]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of Biomass into Chemicals over Metal Catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef] [PubMed]
- Van der Waal, J.C. Process for Preparing Ethylene Glycol from a Carbohydrate. U.S. Patent 10,131,600, 20 November 2018. [Google Scholar]
- Shing, T.K.M.; So, K.H.; Kwok, W.S. Carbocyclization of Carbohydrates: Diastereoselective Synthesis of (+)-Gabosine F, (−)-Gabosine O, and (+)-4- Epi -Gabosine O. Org. Lett. 2009, 11, 5070–5073. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Nieto-Márquez, A.; Alonso, E. Bimetallic Ru:Ni/MCM-48 Catalysts for the Effective Hydrogenation of d -Glucose into Sorbitol. Appl. Catal. A Gen. 2017, 529, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Bonnin, I.; Méreau, R.; Tassaing, T.; Jérôme, F.; De Oliveira Vigier, K. Hydrogenation of Sugars to Sugar Alcohols in the Presence of a Recyclable Ru/Al2O3 Catalyst Commercially Available. ACS Sustain. Chem. Eng. 2021, 9, 9240–9247. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Taran, O.P.; Timofeeva, M.N.; Said-Aizpuru, O.; Panchenko, V.N.; Gerasimov, E.Y.; Kozhevnikov, I.V.; Parmon, V.N. The Main Factors Affecting the Catalytic Properties of Ru/Cs-HPA Systems in One-Pot Hydrolysis-Hydrogenation of Cellulose to Sorbitol. Appl. Catal. A Gen. 2020, 595, 117489. [Google Scholar] [CrossRef]
- Najarnezhadmashhadi, A.; Eränen, K.; Engblom, S.; Aho, A.; Murzin, D.; Salmi, T. Continuous Hydrogenation of Monomeric Sugars and Binary Sugar Mixtures on a Ruthenium Catalyst Supported by Carbon-Coated Open-Cell Aluminum Foam. Ind. Eng. Chem. Res. 2020, 59, 13450–13459. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Li, H.-W.; Bin, W.; Dou, B.-J.; Chen, D.-S.; Cheng, X.-P.; Li, M.; Wang, H.-Y.; Chen, K.-Q.; Jin, L.-Q.; et al. Efficient Synthesis of Sugar Alcohols under Mild Conditions Using a Novel Sugar-Selective Hydrogenation Catalyst Based on Ruthenium Valence Regulation. J. Agric. Food Chem. 2020, 68, 12393–12399. [Google Scholar] [CrossRef]
- Simakova, I.L.; Demidova, Y.S.; Murzina, E.V.; Aho, A.; Murzin, D.Y. Structure Sensitivity in Catalytic Hydrogenation of Galactose and Arabinose over Ru/C Catalysts. Catal. Lett. 2016, 146, 1291–1299. [Google Scholar] [CrossRef]
- Kolarić, S.; Šunjić, V. Comparative Study of Homogeneous Hydrogenation of D-Glucose and d-Mannose Catalyzed by Water Soluble [Ru(Tri(m-Sulfophenyl) Phosphine)] Complex. J. Mol. Catal. A Chem. 1996, 110, 189–193. [Google Scholar] [CrossRef]
- Hameršak, Z.; Pavlović, N.; Delić, V.; Šunjić, V. Regio- and Stereo-Selectivity in Homogeneous Catalytic Hydrogenation of 2,5-Diketo-d-Threo-Hexonic Acid. Carbohydr. Res. 1997, 302, 245–249. [Google Scholar] [CrossRef]
- Tindall, D.J.; Mader, S.; Kindler, A.; Rominger, F.; Hashmi, A.S.K.; Schaub, T. Selective and Scalable Synthesis of Sugar Alcohols by Homogeneous Asymmetric Hydrogenation of Unprotected Ketoses. Angew. Chem. Int. Ed. 2021, 60, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal Structure and Hydrogen-Bonding System in Cellulose Iβ from Synchrotron X-Ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Fu, Y. Hydrolysis of Cellulose to Glucose by Solid Acid Catalysts. Green Chem. 2013, 15, 1095. [Google Scholar] [CrossRef]
- Mikkola, J.-P.; Vainio, H.; Salmi, T.; Sjöholm, R.; Ollonqvist, T.; Väyrynen, J. Deactivation Kinetics of Mo-Supported Raney Ni Catalyst in the Hydrogenation of Xylose to Xylitol. Appl. Catal. A Gen. 2000, 196, 143–155. [Google Scholar] [CrossRef]
- Lazaridis, P.A.; Karakoulia, S.; Delimitis, A.; Coman, S.M.; Parvulescu, V.I.; Triantafyllidis, K.S. D-Glucose Hydrogenation/Hydrogenolysis Reactions on Noble Metal (Ru, Pt)/Activated Carbon Supported Catalysts. Catal. Today 2015, 257, 281–290. [Google Scholar] [CrossRef]
- Rodiansono, R.; Astuti, M.D.; Mujiyanti, D.R.; Santoso, U.T. Selective Hydrogenation of Sucrose into Sugar Alcohols over Supported Raney Nickel-Based Catalysts. Indones. J. Chem. 2019, 19, 183. [Google Scholar] [CrossRef] [Green Version]
- Esteban, J.; Yustos, P.; Ladero, M. Catalytic Processes from Biomass-Derived Hexoses and Pentoses: A Recent Literature Overview. Catalysts 2018, 8, 637. [Google Scholar] [CrossRef] [Green Version]
- Dong, Q.; Huang, Y.; Yang, H.; Pei, J.; Li, K.; Yuan, M.; Xiao, W.; Ni, W.; Hou, Z. The Catalytic Hydrogenation of Biomass Platform Molecules by Ni–Co Nanoalloy Catalysts. Top. Catal. 2017, 60, 666–676. [Google Scholar] [CrossRef]
- Guo, J.; Hou, Y.; Yang, C.; Wang, Y.; He, H.; Li, W. Preparation of Ni–B Amorphous Alloy Catalyst from Nickel Hydrazine Complex with Ultrasonic Assistance. Catal. Commun. 2011, 16, 86–89. [Google Scholar] [CrossRef]
- Singh, H.; Rai, A.; Yadav, R.; Sinha, A.K. Glucose Hydrogenation to Sorbitol over Unsupported Mesoporous Ni/NiO Catalyst. Mol. Catal. 2018, 451, 186–191. [Google Scholar] [CrossRef]
- Silvester, L.; Ramos, F.; Thuriot-Roukos, J.; Heyte, S.; Araque, M.; Paul, S.; Wojcieszak, R. Fully Integrated High-Throughput Methodology for the Study of Ni- and Cu-Supported Catalysts for Glucose Hydrogenation. Catal. Today 2019, 338, 72–80. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.; Liu, Y.; Li, B. Hydrogenation of Glucose over Reduced Ni/Cu/Al Hydrotalcite Precursors. Catal. Commun. 2013, 35, 23–26. [Google Scholar] [CrossRef]
- Fu, Y.; Ding, L.; Singleton, M.L.; Idrissi, H.; Hermans, S. Synergistic Effects Altering Reaction Pathways: The Case of Glucose Hydrogenation over Fe-Ni Catalysts. Appl. Catal. B Environ. 2021, 288, 119997. [Google Scholar] [CrossRef]
- Upare, P.P.; Hwang, Y.K.; Kim, J.C.; Lee, J.H.; Kwak, S.K.; Hwang, D.W. A Robust and Highly Selective Catalytic System of Copper–Silica Nanocomposite and 1-Butanol in Fructose Hydrogenation to Mannitol. ChemSusChem 2020, 13, 5050–5057. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, S.; Wu, S.; Liu, Y. Hydrogenation of Fructose over Magnetic Catalyst Derived from Hydrotalcite Precursor. Chem. Eng. Sci. 2013, 99, 171–176. [Google Scholar] [CrossRef]
- Zelin, J.; Regenhardt, S.A.; Meyer, C.I.; Duarte, H.A.; Sebastian, V.; Marchi, A.J. Selective Aqueous-Phase Hydrogenation of D-Fructose into D-Mannitol Using a Highly Efficient and Reusable Cu-Ni/SiO2 Catalyst. Chem. Eng. Sci. 2019, 206, 315–326. [Google Scholar] [CrossRef]
- Morales, R.; Campos, C.H.; Fierro, J.L.G.; Fraga, M.A.; Pecchi, G. Stable Reduced Ni Catalysts for Xylose Hydrogenation in Aqueous Medium. Catal. Today 2018, 310, 59–67. [Google Scholar] [CrossRef]
- Audemar, M.; Ramdani, W.; Junhui, T.; Raluca Ifrim, A.; Ungureanu, A.; Jérôme, F.; Royer, S.; Oliveira Vigier, K. Selective Hydrogenation of Xylose to Xylitol over Co/SiO2 Catalysts. ChemCatChem 2020, 12, 1973–1978. [Google Scholar] [CrossRef]
- Sadier, A.; Shi, D.; Mamede, A.-S.; Paul, S.; Marceau, E.; Wojcieszak, R. Selective Aqueous Phase Hydrogenation of Xylose to Xylitol over SiO2-Supported Ni and Ni-Fe Catalysts: Benefits of Promotion by Fe. Appl. Catal. B Environ. 2021, 298, 120564. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Mizugaki, T.; Mitsudome, T. Efficient D-Xylose Hydrogenation to D-Xylitol over a Hydrotalcite-Supported Nickel Phosphide Nanoparticle Catalyst. Eur. J. Inorg. Chem. 2021, 2021, 3327–3331. [Google Scholar] [CrossRef]
- Scholz, D.; Aellig, C.; Mondelli, C.; Pérez-Ramírez, J. Continuous Transfer Hydrogenation of Sugars to Alditols with Bioderived Donors over Cu-Ni-Al Catalysts. ChemCatChem 2015, 7, 1551–1558. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Shahrestani, R.G. Innovative Strategies for Engineering Mannitol Production. Trends Food Sci. Technol. 2009, 20, 263–270. [Google Scholar] [CrossRef]
- Heinen, A.W.; Peters, J.A.; van Bekkum, H. Hydrogenation of Fructose on Ru/C Catalysts. Carbohydr. Res. 2000, 328, 449–457. [Google Scholar] [CrossRef]
- Kuusisto, J.; Mikkola, J.-P.; Casal, P.P.; Karhu, H.; Väyrynen, J.; Salmi, T. Kinetics of the Catalytic Hydrogenation of D-Fructose over a CuO-ZnO Catalyst. Chem. Eng. J. 2005, 115, 93–102. [Google Scholar] [CrossRef]
- Vieira, J.L.; Almeida-Trapp, M.; Mithöfer, A.; Plass, W.; Gallo, J.M.R. Rationalizing the Conversion of Glucose and Xylose Catalyzed by a Combination of Lewis and Brønsted Acids. Catal. Today 2020, 344, 92–101. [Google Scholar] [CrossRef]
- Lee, G.; Jeong, Y.; Takagaki, A.; Jung, J.C. Sonication Assisted Rehydration of Hydrotalcite Catalyst for Isomerization of Glucose to Fructose. J. Mol. Catal. A Chem. 2014, 393, 289–295. [Google Scholar] [CrossRef]
- Zelin, J.; Meyer, C.I.; Regenhardt, S.A.; Sebastian, V.; Garetto, T.F.; Marchi, A.J. Selective Liquid-Phase Hydrogenation of Fructose to d-Mannitol over Copper-Supported Metallic Nanoparticles. Chem. Eng. J. 2017, 319, 48–56. [Google Scholar] [CrossRef]
- Ruddlesden, J.F.; Stewart, A.; Thompson, D.J.; Whelan, R. Diastereoselective Control in Ketose Hydrogenations with Supported Copper and Nickel Catalysts. Faraday Discuss. Chem. Soc. 1981, 72, 397. [Google Scholar] [CrossRef]
- Sifontes Herrera, V.A.; Saleem, F.; Kusema, B.; Eränen, K.; Salmi, T. Hydrogenation of L-Arabinose and d-Galactose Mixtures Over a Heterogeneous Ru/C Catalyst. Top. Catal. 2012, 55, 550–555. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Fujita, S.; Nakajima, K.; Yamazoe, S.; Yamasaki, J.; Mizugaki, T.; Mitsudome, T. Support-Boosted Nickel Phosphide Nanoalloy Catalysis in the Selective Hydrogenation of Maltose to Maltitol. ACS Sustain. Chem. Eng. 2021, 9, 6347–6354. [Google Scholar] [CrossRef]
- Sifontes Herrera, V.A.; Rivero Mendoza, D.E.; Leino, A.-R.; Mikkola, J.-P.; Zolotukhin, A.; Eränen, K.; Salmi, T. Sugar Hydrogenation in Continuous Reactors: From Catalyst Particles towards Structured Catalysts. Chem. Eng. Processing-Process Intensif. 2016, 109, 1–10. [Google Scholar] [CrossRef]
- Eisenbeis, C.; Guettel, R.; Kunz, U.; Turek, T. Monolith Loop Reactor for Hydrogenation of Glucose. Catal. Today 2009, 147, S342–S346. [Google Scholar] [CrossRef]
- Najarnezhadmashhadi, A.; Wärnå, J.; Eränen, K.; Trajano, H.L.; Murzin, D.; Salmi, T. Modelling of Kinetics, Mass Transfer and Flow Pattern on Open Foam Structures in Tubular Reactors: Hydrogenation of Arabinose and Galactose on Ruthenium Catalyst. Chem. Eng. Sci. 2021, 233, 116385. [Google Scholar] [CrossRef]
- García, B.; Orozco-Saumell, A.; López Granados, M.; Moreno, J.; Iglesias, J. Catalytic Transfer Hydrogenation of Glucose to Sorbitol with Raney Ni Catalysts Using Biomass-Derived Diols as Hydrogen Donors. ACS Sustain. Chem. Eng. 2021, 9, 14857–14867. [Google Scholar] [CrossRef]
- Liang, G.; He, L.; Cheng, H.; Li, W.; Li, X.; Zhang, C.; Yu, Y.; Zhao, F. The Hydrogenation/Dehydrogenation Activity of Supported Ni Catalysts and Their Effect on Hexitols Selectivity in Hydrolytic Hydrogenation of Cellulose. J. Catal. 2014, 309, 468–476. [Google Scholar] [CrossRef]
- Xin, H.; Hu, X.; Cai, C.; Wang, H.; Zhu, C.; Li, S.; Xiu, Z.; Zhang, X.; Liu, Q.; Ma, L. Catalytic Production of Oxygenated and Hydrocarbon Chemicals From Cellulose Hydrogenolysis in Aqueous Phase. Front. Chem. 2020, 8, 333. [Google Scholar] [CrossRef]
- Manaenkov, O.V.; Kislitsa, O.V.; Matveeva, V.G.; Sulman, E.M.; Sulman, M.G.; Bronstein, L.M. Cellulose Conversion Into Hexitols and Glycols in Water: Recent Advances in Catalyst Development. Front. Chem. 2019, 7, 834. [Google Scholar] [CrossRef]
- Li, Y.; Liao, Y.; Cao, X.; Wang, T.; Ma, L.; Long, J.; Liu, Q.; Xua, Y. Advances in Hexitol and Ethylene Glycol Production by One-Pot Hydrolytic Hydrogenation and Hydrogenolysis of Cellulose. Biomass Bioenergy 2015, 74, 148–161. [Google Scholar] [CrossRef]
- Liang, G.; He, L.; Cheng, H.; Zhang, C.; Li, X.; Fujita, S.; Zhang, B.; Arai, M.; Zhao, F. ZSM-5-Supported Multiply-Twinned Nickel Particles: Formation, Surface Properties, and High Catalytic Performance in Hydrolytic Hydrogenation of Cellulose. J. Catal. 2015, 325, 79–86. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X.; Wu, Q.; Zhang, C.; Yu, Y.; Lan, M.; Wei, X.; Ying, Z.; Liu, T.; Liang, G.; et al. Synthesis of Ni/Mesoporous ZSM-5 for Direct Catalytic Conversion of Cellulose to Hexitols: Modulating the Pore Structure and Acidic Sites via a Nanocrystalline Cellulose Template. Green Chem. 2016, 18, 3315–3323. [Google Scholar] [CrossRef]
- Liang, G.; Cheng, H.; Li, W.; He, L.; Yu, Y.; Zhao, F. Selective Conversion of Microcrystalline Cellulose into Hexitols on Nickel Particles Encapsulated within ZSM-5 Zeolite. Green Chem. 2012, 14, 2146. [Google Scholar] [CrossRef]
- Van de Vyver, S.; Geboers, J.; Dusselier, M.; Schepers, H.; Vosch, T.; Zhang, L.; Van Tendeloo, G.; Jacobs, P.A.; Sels, B.F. Selective Bifunctional Catalytic Conversion of Cellulose over Reshaped Ni Particles at the Tip of Carbon Nanofibers. ChemSusChem 2010, 3, 698–701. [Google Scholar] [CrossRef]
- Van de Vyver, S.; Geboers, J.; Schutyser, W.; Dusselier, M.; Eloy, P.; Dornez, E.; Seo, J.W.; Courtin, C.M.; Gaigneaux, E.M.; Jacobs, P.A.; et al. Tuning the Acid/Metal Balance of Carbon Nanofiber-Supported Nickel Catalysts for Hydrolytic Hydrogenation of Cellulose. ChemSusChem 2012, 5, 1549–1558. [Google Scholar] [CrossRef] [Green Version]
- Pang, J.; Wang, A.; Zheng, M.; Zhang, Y.; Huang, Y.; Chen, X.; Zhang, T. Catalytic Conversion of Cellulose to Hexitols with Mesoporous Carbon Supported Ni-Based Bimetallic Catalysts. Green Chem. 2012, 14, 614. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hosaka, Y.; Hara, K.; Feng, B.; Hirosaki, Y.; Fukuoka, A. Control of Selectivity, Activity and Durability of Simple Supported Nickel Catalysts for Hydrolytic Hydrogenation of Cellulose. Green Chem. 2014, 16, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.-N.; Wang, A.-Q.; Zheng, M.-Y.; Zhang, T. Selective Transformation of Cellulose into Sorbitol by Using a Bifunctional Nickel Phosphide Catalyst. ChemSusChem 2010, 3, 818–821. [Google Scholar] [CrossRef]
- Yang, P.; Kobayashi, H.; Hara, K.; Fukuoka, A. Phase Change of Nickel Phosphide Catalysts in the Conversion of Cellulose into Sorbitol. ChemSusChem 2012, 5, 920–926. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Li, N.; Ma, P.; Zhang, Y. Direct Synthesis of Hexitols from Microcrystalline Cellulose and Birch over Zirconium(iv) Phosphate Supported Nickel Catalysts and the Mechanism Study. Green Chem. 2021, 23, 1353–1360. [Google Scholar] [CrossRef]
- Luo, C.; Wang, S.; Liu, H. Cellulose Conversion into Polyols Catalyzed by Reversibly Formed Acids and Supported Ruthenium Clusters in Hot Water. Angew. Chem. Int. Ed. 2007, 46, 7636–7639. [Google Scholar] [CrossRef]
- He, M.; Guo, J.; Wang, X.; Song, Y.; Liu, S.; Wang, H.; Li, C. Direct Conversion of Cellulose into Isosorbide over Ni Doped NbOPO4 Catalysts in Water. New J. Chem. 2020, 44, 10292–10299. [Google Scholar] [CrossRef]
- Bonnin, I.; Mereau, R.; Tassaing, T.; De Oliveira Vigier, K. One-Pot Synthesis of Isosorbide from Cellulose or Lignocellulosic Biomass: A Challenge? Beilstein J. Org. Chem. 2020, 16, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
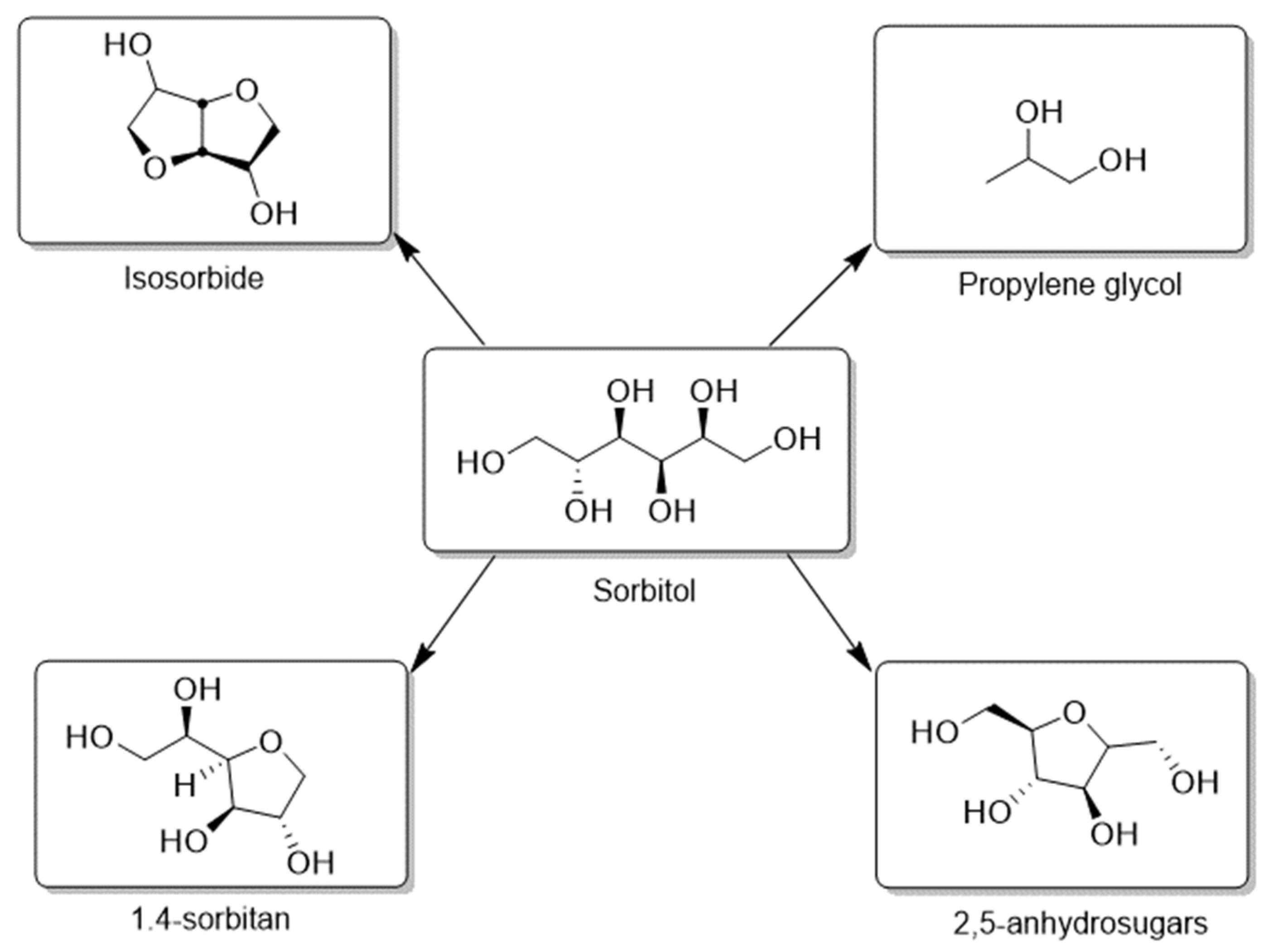

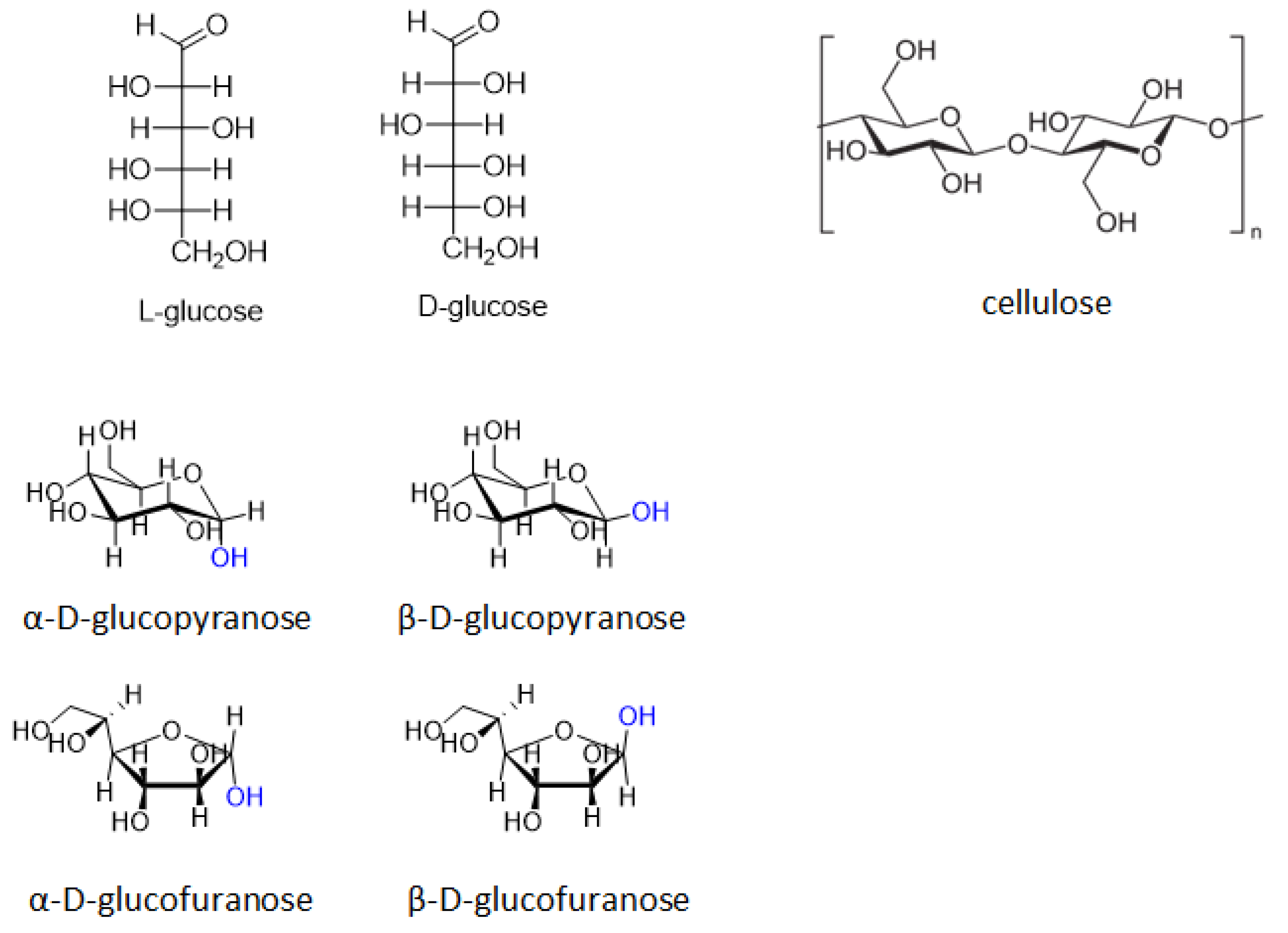

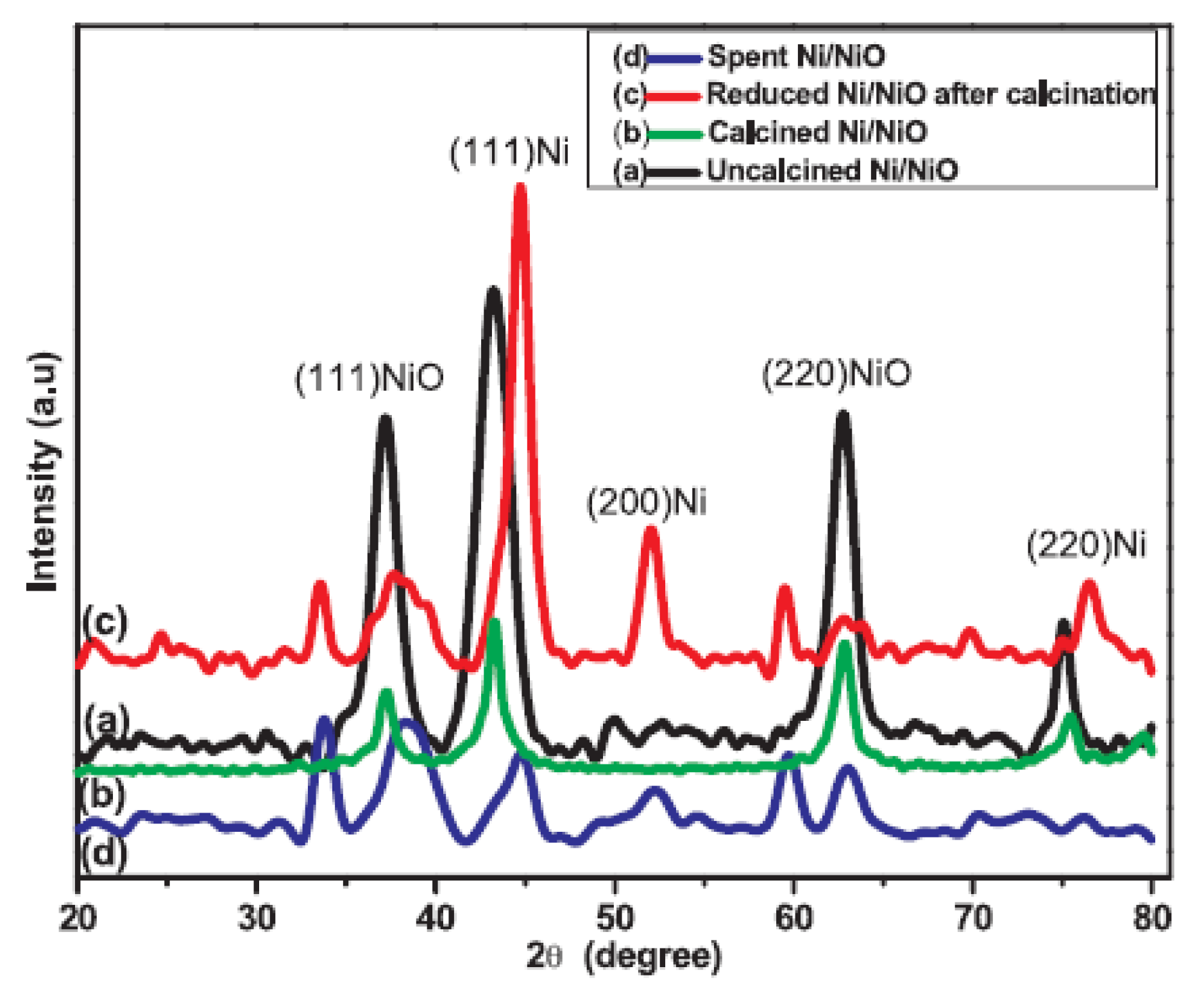
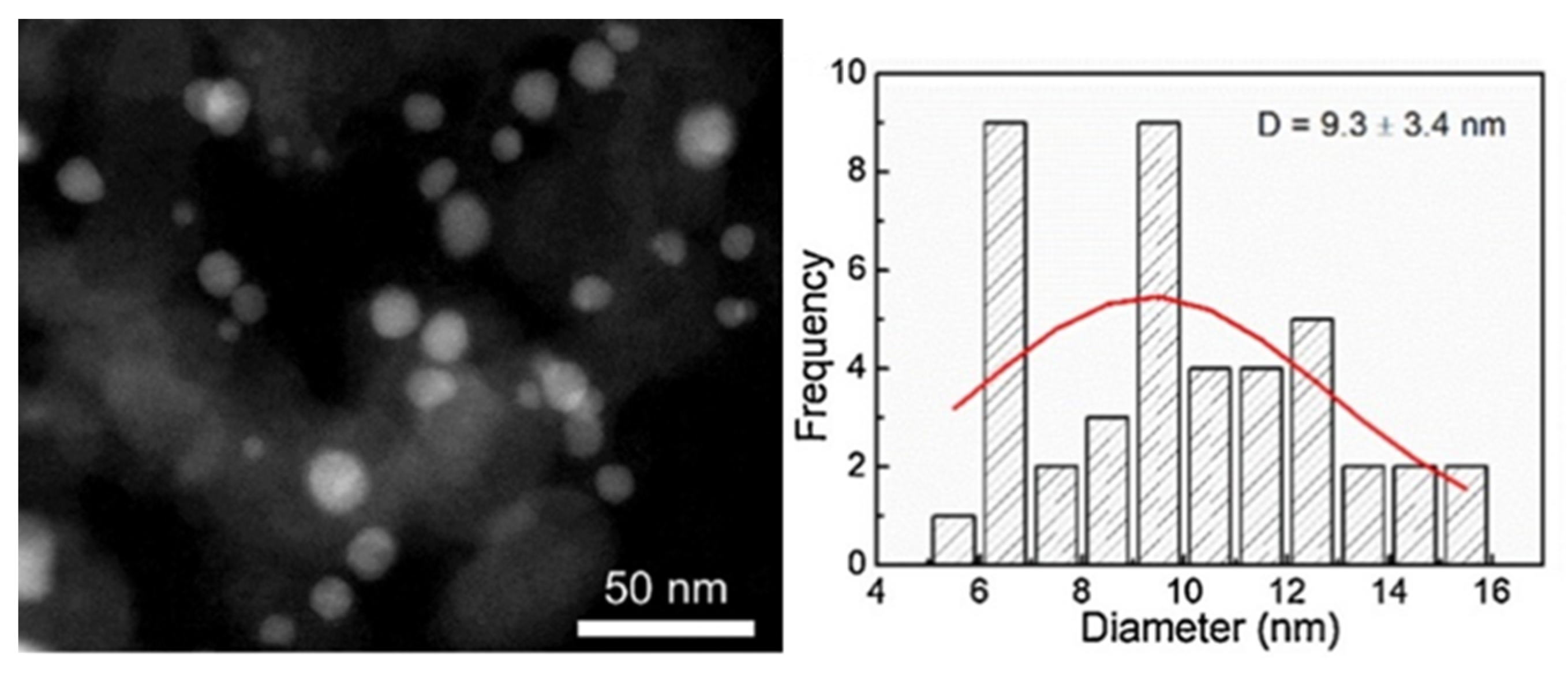
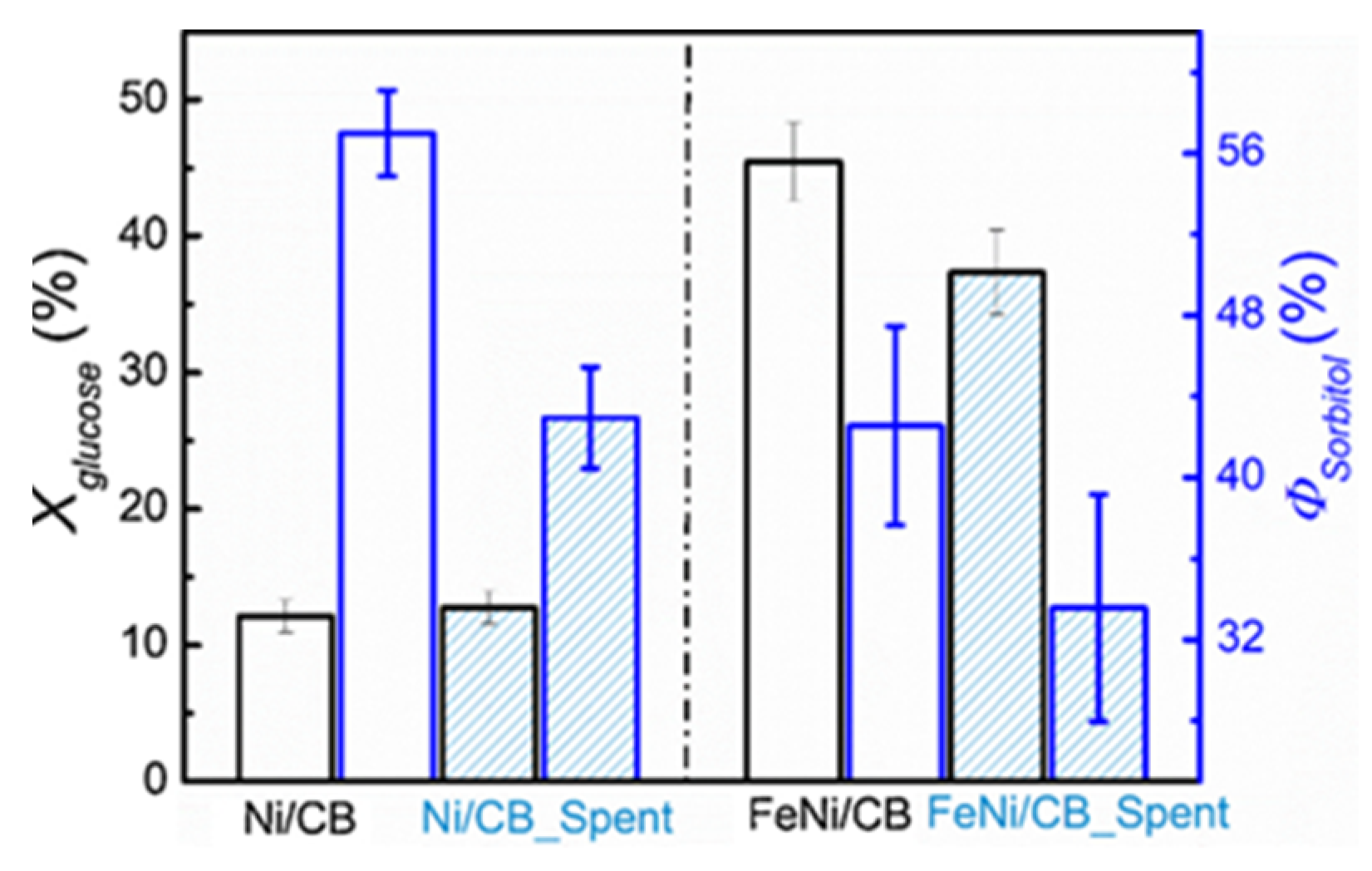
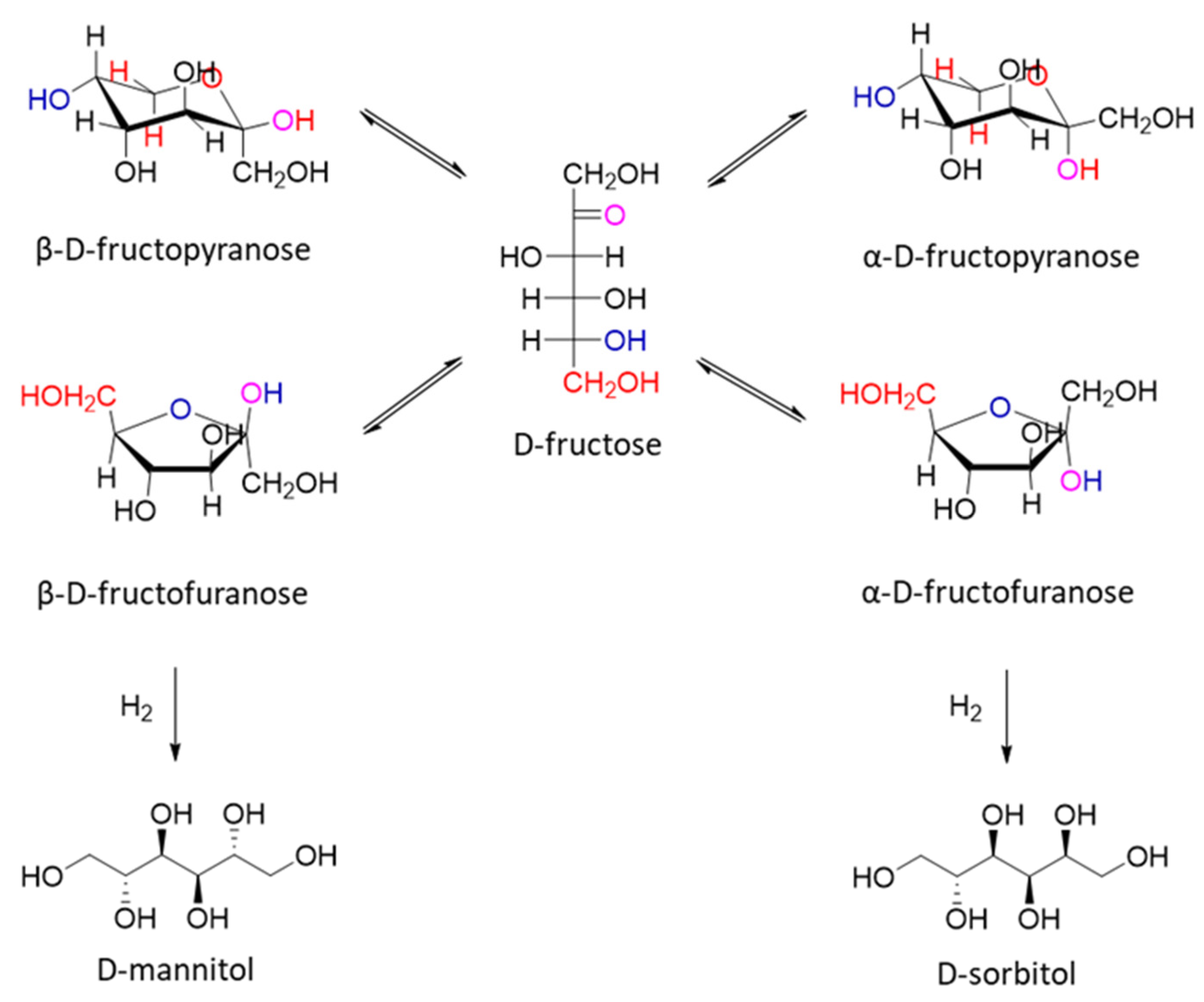

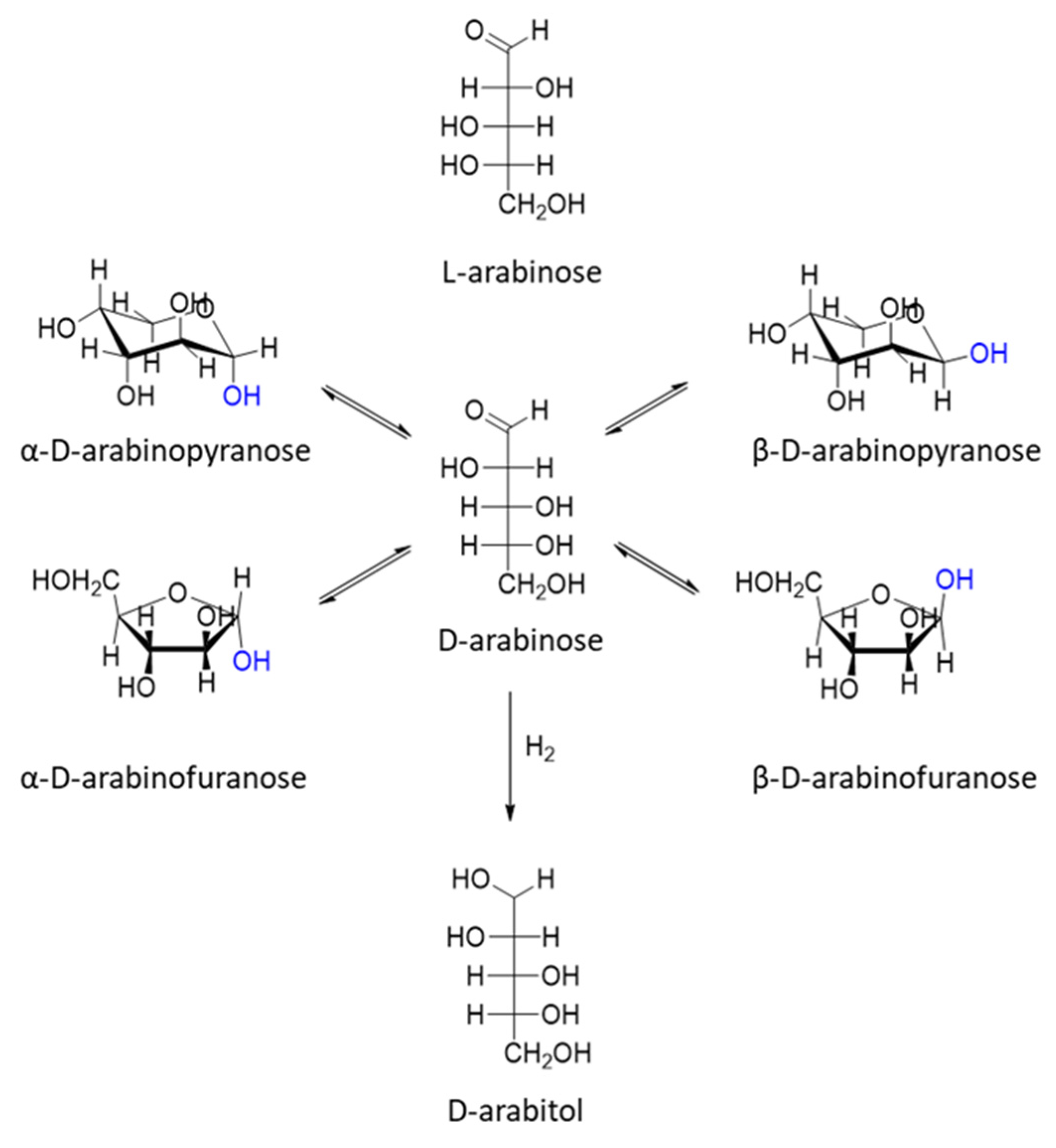
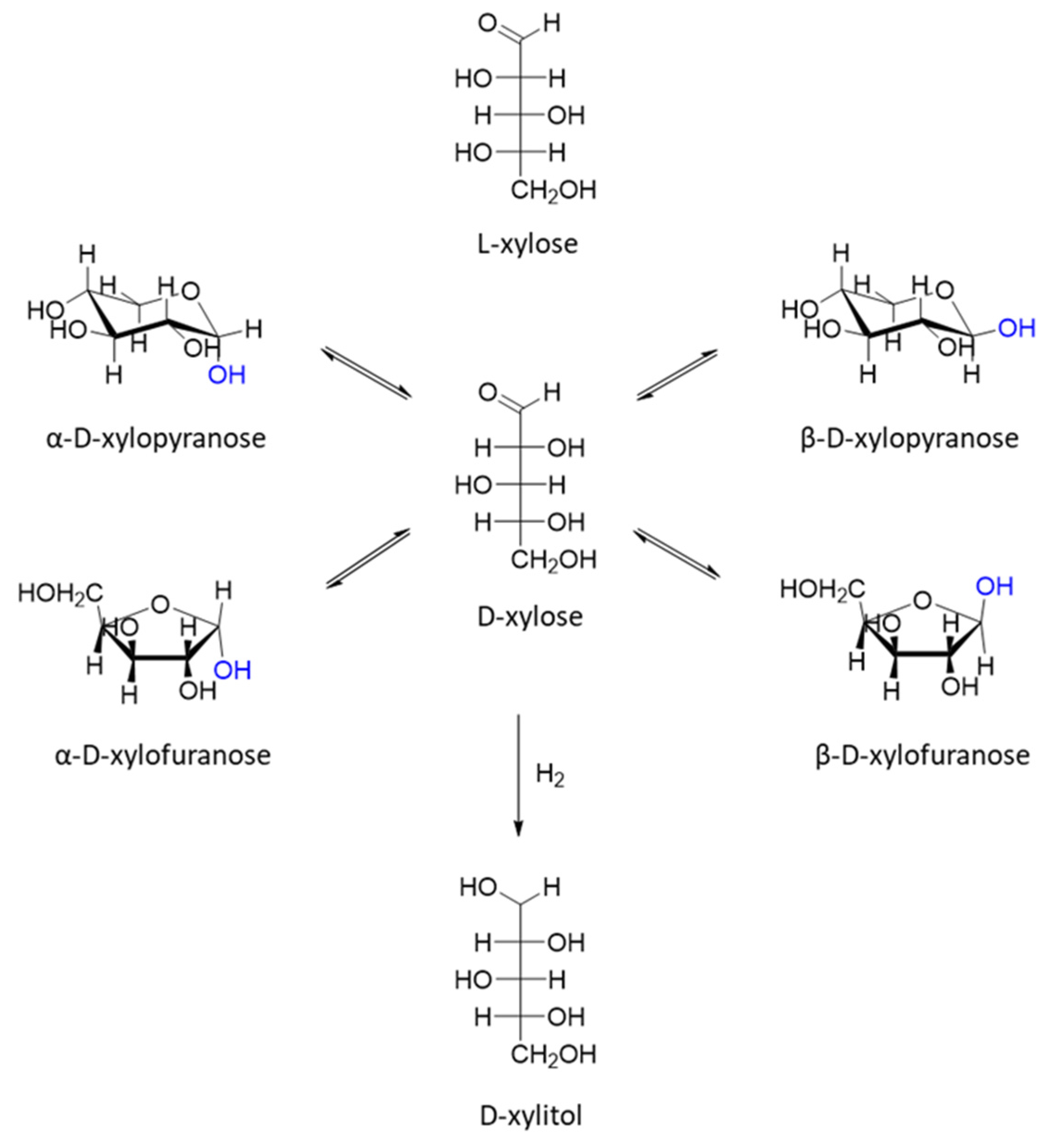
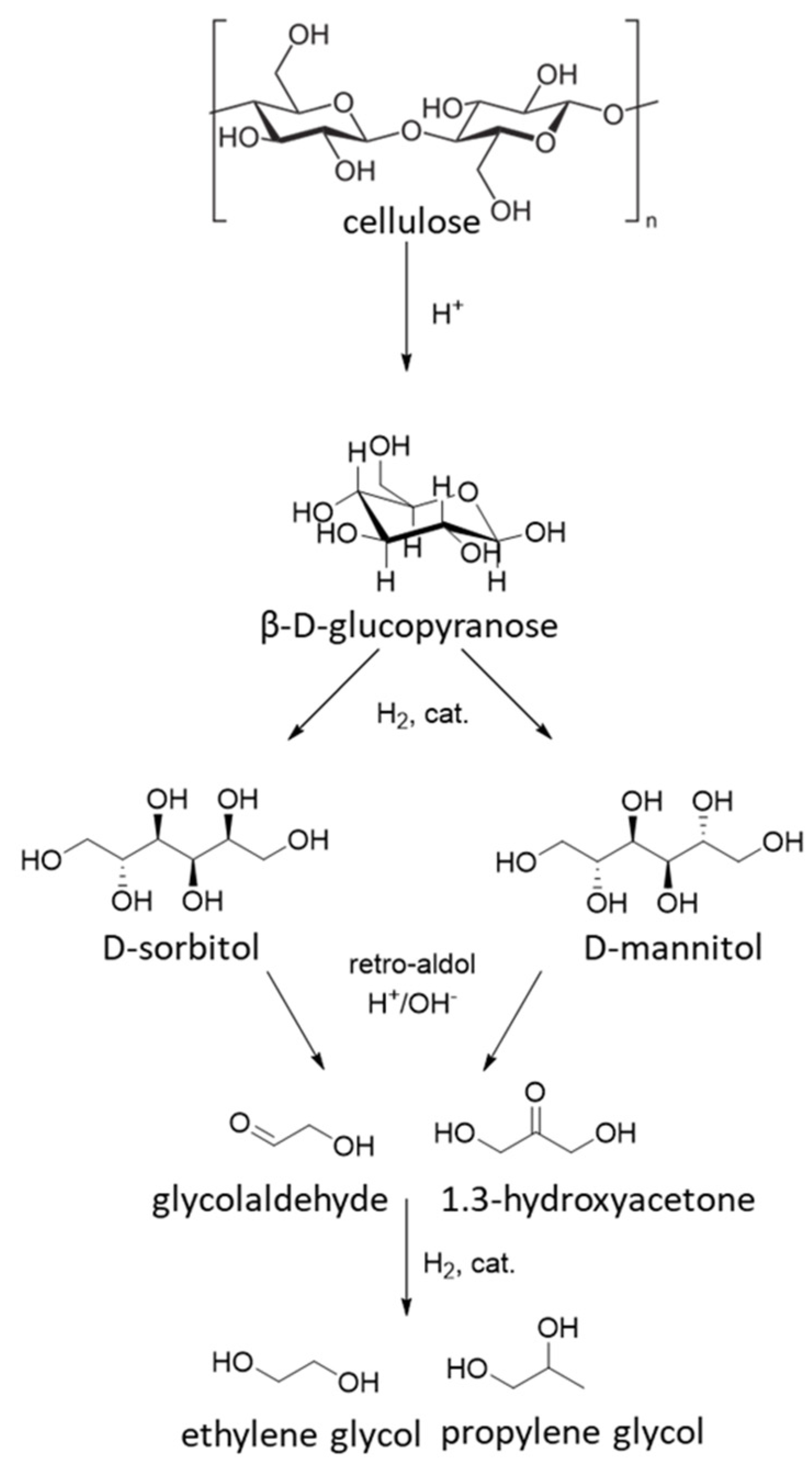
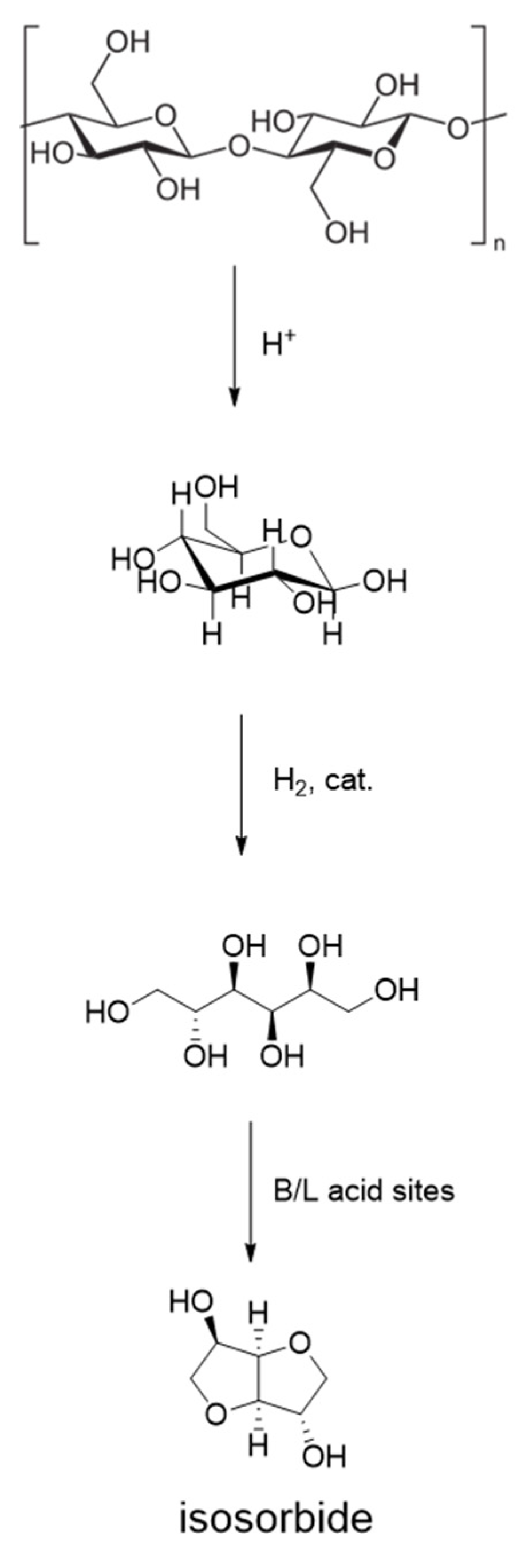
| Catalyst | Regime | Substrate | Product | t, °C | P (H2), Atm | Time, h | Solvent | Sub/Met | X, % | S, % | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni-Co nanoalloy (1:1) | Batch | D-glucose | D-sorbitol | 90 | 30 | 4 | H2O | 0.14 mol/g | 97 | 99 | [23] |
| Ni-B | Batch | D-glucose | D-sorbitol | 120 | 40 | 2 | H2O | 0.3 mol/g | 85 | n.d. | [24] |
| Ni/NiO Mesopore Catalyst | Batch | D-glucose | D-sorbitol | 130 | 50 | n.d. | H2O | 0.13 mol/g | 95 | 88 | [25] |
| 5%Ni/Al2O3 (Ni0) | Batch | D-glucose | D-sorbitol | 130 | 30 | 4 | H2O | 19.6 mol/mol (0.34 mol/g Ni) | 60 | 80 | [26] |
| 5%Cu/SiO2 (Cu0) | Batch | D-glucose | D-sorbitol | 130 | 30 | 4 | H2O | 19.6 mol/mol | 45 | 89 | [22] |
| Ni1.85Cu1Al1.15 | Batch | D-glucose | D-sorbitol | 120 | 30 | 3 | H2O | 0.03 mol/g | 80 | 87 | [27] |
| 8%Fe-8%Ni/CB | Batch | D-glucose | D-sorbitol | 140 | 30 | 6 | H2O | 16.5 mol/mol | 71 | 70 | [28] |
| 80%Cu/SiO2 | Batch | D-fructose | D-mannitol | 120 | 35 | 10 | 1-butanol | 2.2 mol/mol | >99 | 83 | [29] |
| Ni4.63Cu1Al1.82Fe0.79 | Batch | D-fructose | D-mannitol | 110 | 30 | 2 | H2O | 0.03 mol/g | >99 | 57 | [30] |
| 5%Cu-7%Ni/SiO2 | Batch | D-fructose | D-mannitol | 100 | 40 | 2 | H2O | 8.3 mol/mol | >99 | 73 | [31] |
| D-sorbitol | 24 | ||||||||||
| 11%Cu/SiO2 | Batch | D-fructose | D-mannitol | 100 | 40 | 6 | H2O | 9.5 mol/mol | 95 | 75 | [29] |
| D-sorbitol | 22 | ||||||||||
| 9%Ni/SiO2 | Batch | D-fructose | D-mannitol | 100 | 40 | 6 | H2O | 10.5 mol/mol | 55 | 55 | [29] |
| Nd1Al0.162Ni0.838O3 (20% wt Ni) | Batch | D-xylose | D-xylitol | 100 | 25 | 6 | H2O | 9.8 mol/mol | 70 | 53 | [32] |
| 10%Co/SiO2 | Batch | D-xylose | D-xylitol | 140 | 50 | 2 | H2O | 78.5 mol/mol | >99 | 90 | [33] |
| 26%Ni-16%Fe/SiO2 | Batch | D-xylose | D-xylitol | 80 | 20 | 4 | H2O | 10.6 mol/mol | >99 | 98 | [34] |
| Ni2P/HT (0.91% wt. of Ni) | Batch | D-xylose | D-xylitol | 100 | 20 | 2 | H2O | 16 mol/mol | >99 | >99 | [35] |
| Cu3Ni3Al2 | Flow | glucose | sorbitol | 150 | hydrogen donor 1,4-butanediol | 0.19 | H2O | 6-45 mol/g·min | 87 | 70 | [36] |
| fructose | mannitol/sorbitol | 85 | 70/19 | ||||||||
| xylose | xylitol | 85 | 71 | ||||||||
| arabinose | arabinitol | 90 | 73 |
| Catalyst | Substrate | Product | T, °C | P (H2), Atm | Time, h | Solvent | sub/cat | X, % | S, % | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|
| Ni MTPs/ZSM-5 | Cellulose | Sorbitol + Mannitol | 240 | 40 | 2.5 | H2O | 0.3 g/100 mg | 94 | 67 | [54] |
| Ni/ZSM-5 (Ni 40 % wt.) | Cellobiose | Sorbitol + Mannitol | 240 | 40 | 4 | H2O | 0.2 g/100 mg | >99 | 82 | [50] |
| Ni/Al2O3 | >99 | 29 | ||||||||
| Ni/SiO2 | >99 | 44 | ||||||||
| Ni/NCC-ZSM-5 nanocrystalline cellulose-templated mesoporous ZSM-5 | Microcrystalline Cellulose (MCC) | Sorbitol + Mannitol | 240 | 40 | 2.5 | H2O | 0.1 g/50 mg | 87 | 68 | [55] |
| 17%Ni/ZSM-5 | Microcrystalline Cellulose (MCC) | Sorbitol + Mannitol | 240 | 40 | 2.5 | H2O | 0.2 g/100 mg | 87 | 67 | [56] |
| 40%Ni/ZSM-5 | 86 | 57 | ||||||||
| 40%Ni/Al2O3 | 85 | 16 | ||||||||
| 40%Ni/SiO2 | 84 | 19 | ||||||||
| 3%Ni/CNF/Al2O3 | Ball-Milled Cellulose at 190 °C for 24 h | Sorbitol + Mannitol | 230 | 60 | 4 | H2O | 1 g/500 mg | 92 | 61 | [57] |
| 3%Ni/CNF/Al2O3 | 190 | 60 | 24 | 88 | 63 | |||||
| 7.5%Ni/CNF HNO3 treated (Ni surface atom 26.9 μmol/g cat) | Ball-Milled Cellulose at 190 °C for 24 h | Sorbitol + Mannitol | 190 | 60 | 24 | H2O | 1 g/500 mg | 93 | 82 | [58] |
| 2.6%Ni/CNF HNO3 treated (Ni surface atom 8.1 μmol/g cat) | 91 | 54 | ||||||||
| 1%Ir-5%Ni/MC | Microcrystalline Cellulose (MCC) | Sorbitol + Mannitol | 245 | 60 | 0.5 | H2O | 0.5 g/150 mg | >99 | 58 | [59] |
| 1%Rh-5%Ni/MC | >99 | 60 | ||||||||
| 20%Ni/MC | 85 | 51 | ||||||||
| 20%Ni/AC | 62 | 32 | ||||||||
| 50%Ni/C | Ball-Milled Cellulose | Sorbitol + Mannitol | 210 | 50 | 6 | H2O | 0.3 g/50 mg | 90 | 71 | [60] |
| 16%Ni2P/AC | Cellulose | Sorbitol | 225 | 60 | 1.5 | H2O | 0.5 g/150 mg | >99 | 48 | [61] |
| 10%Ni-3.2%P/AC (Ni2P) | Ball-Milled Cellulose | Sorbitol | 230 | 40 | 0.7 | H2O | 0.162 g/50 mg | 90 | 68 | [62] |
| 20%Ni/ZrP2 | Cellulose | Sorbitol | 200 | 50 | 5 | H2O | 0.05 g/50 mg | 61% yield | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redina, E.; Tkachenko, O.; Salmi, T. Recent Advances in C5 and C6 Sugar Alcohol Synthesis by Hydrogenation of Monosaccharides and Cellulose Hydrolytic Hydrogenation over Non-Noble Metal Catalysts. Molecules 2022, 27, 1353. https://doi.org/10.3390/molecules27041353
Redina E, Tkachenko O, Salmi T. Recent Advances in C5 and C6 Sugar Alcohol Synthesis by Hydrogenation of Monosaccharides and Cellulose Hydrolytic Hydrogenation over Non-Noble Metal Catalysts. Molecules. 2022; 27(4):1353. https://doi.org/10.3390/molecules27041353
Chicago/Turabian StyleRedina, Elena, Olga Tkachenko, and Tapio Salmi. 2022. "Recent Advances in C5 and C6 Sugar Alcohol Synthesis by Hydrogenation of Monosaccharides and Cellulose Hydrolytic Hydrogenation over Non-Noble Metal Catalysts" Molecules 27, no. 4: 1353. https://doi.org/10.3390/molecules27041353
APA StyleRedina, E., Tkachenko, O., & Salmi, T. (2022). Recent Advances in C5 and C6 Sugar Alcohol Synthesis by Hydrogenation of Monosaccharides and Cellulose Hydrolytic Hydrogenation over Non-Noble Metal Catalysts. Molecules, 27(4), 1353. https://doi.org/10.3390/molecules27041353






