Hybrid Cements with ZnO Additions: Hydration, Compressive Strength and Microstructure
Abstract
:1. Introduction
2. Results and Discussion
2.1. Setting Time
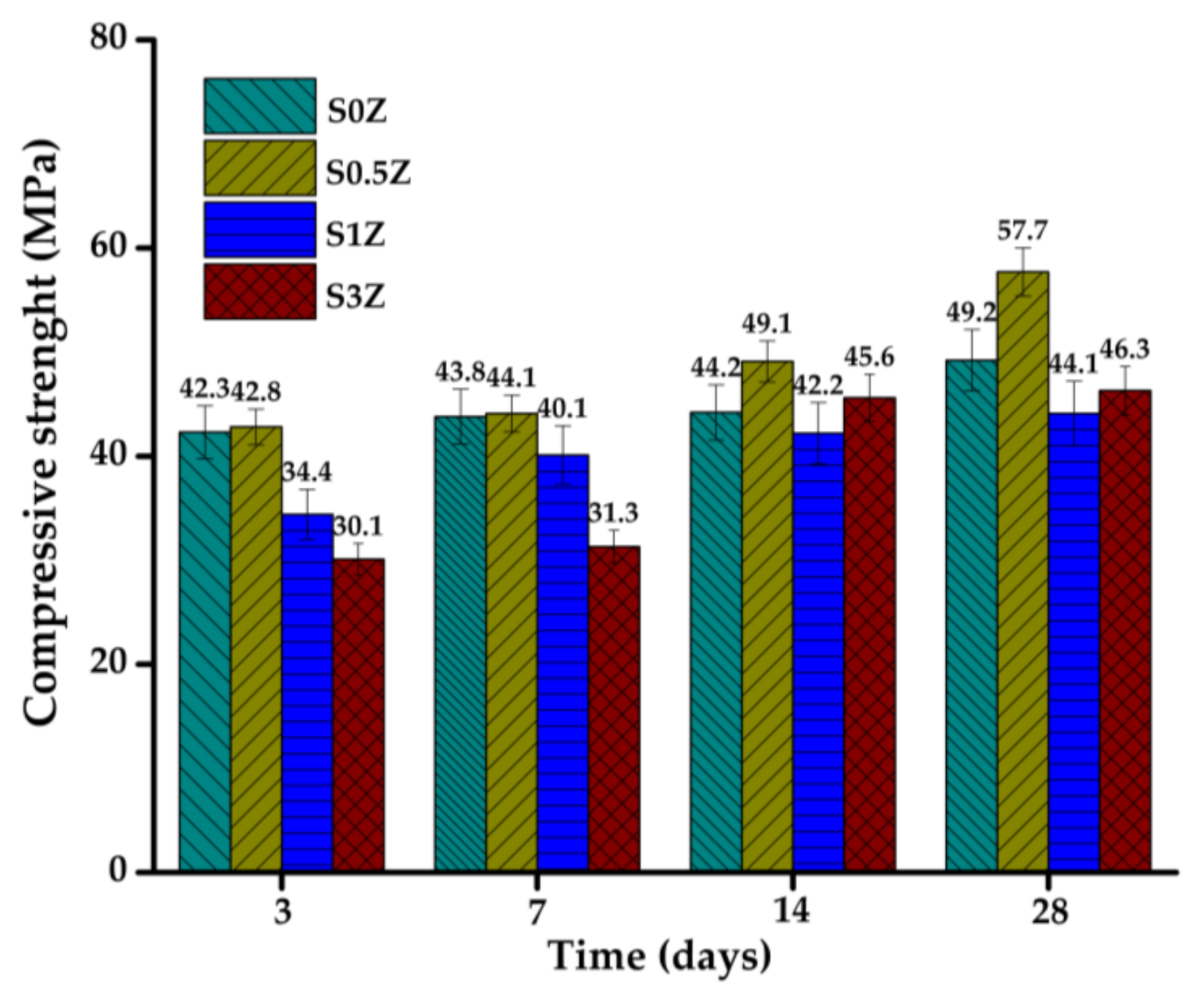
2.2. Compressive Strength
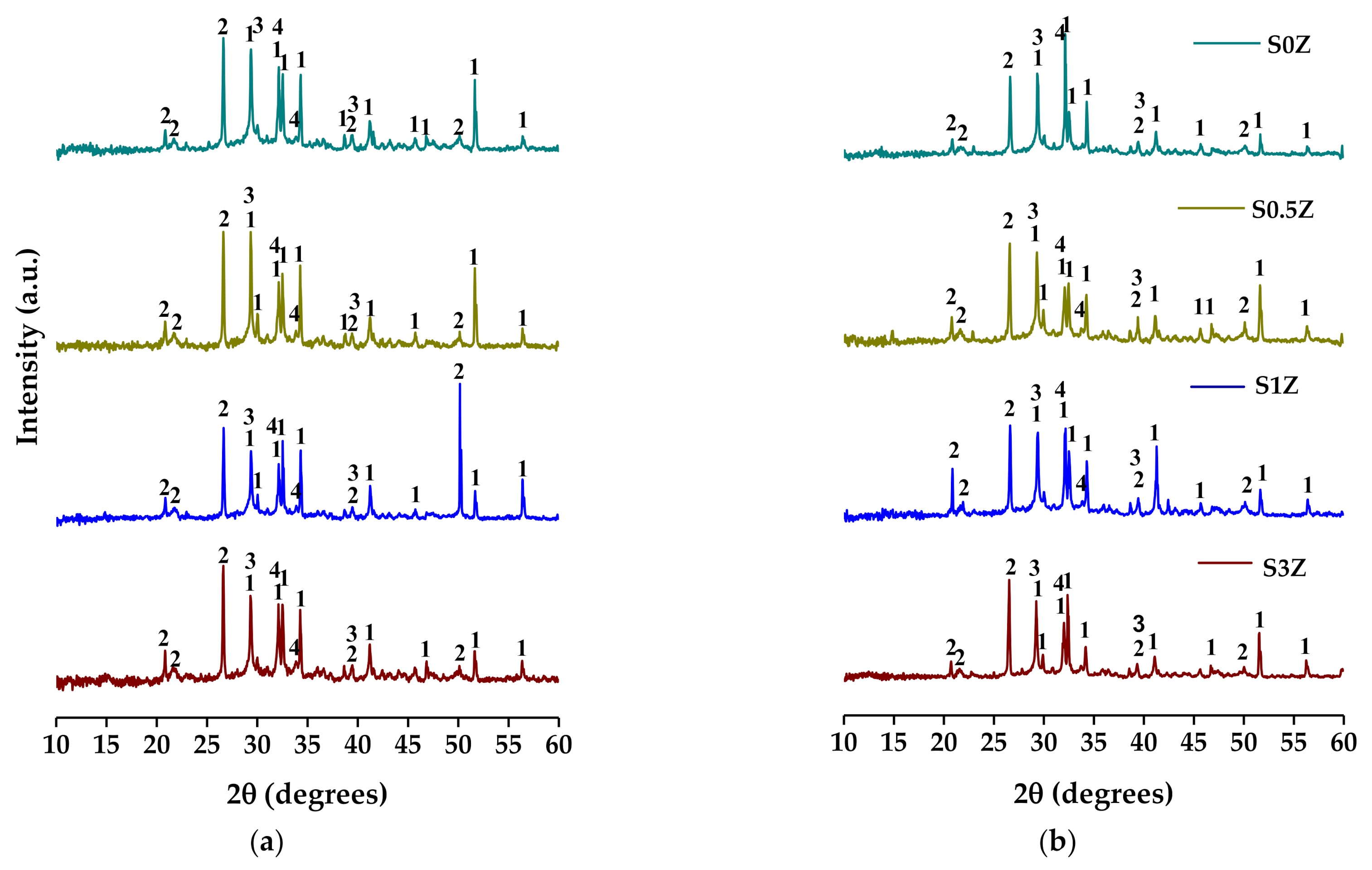
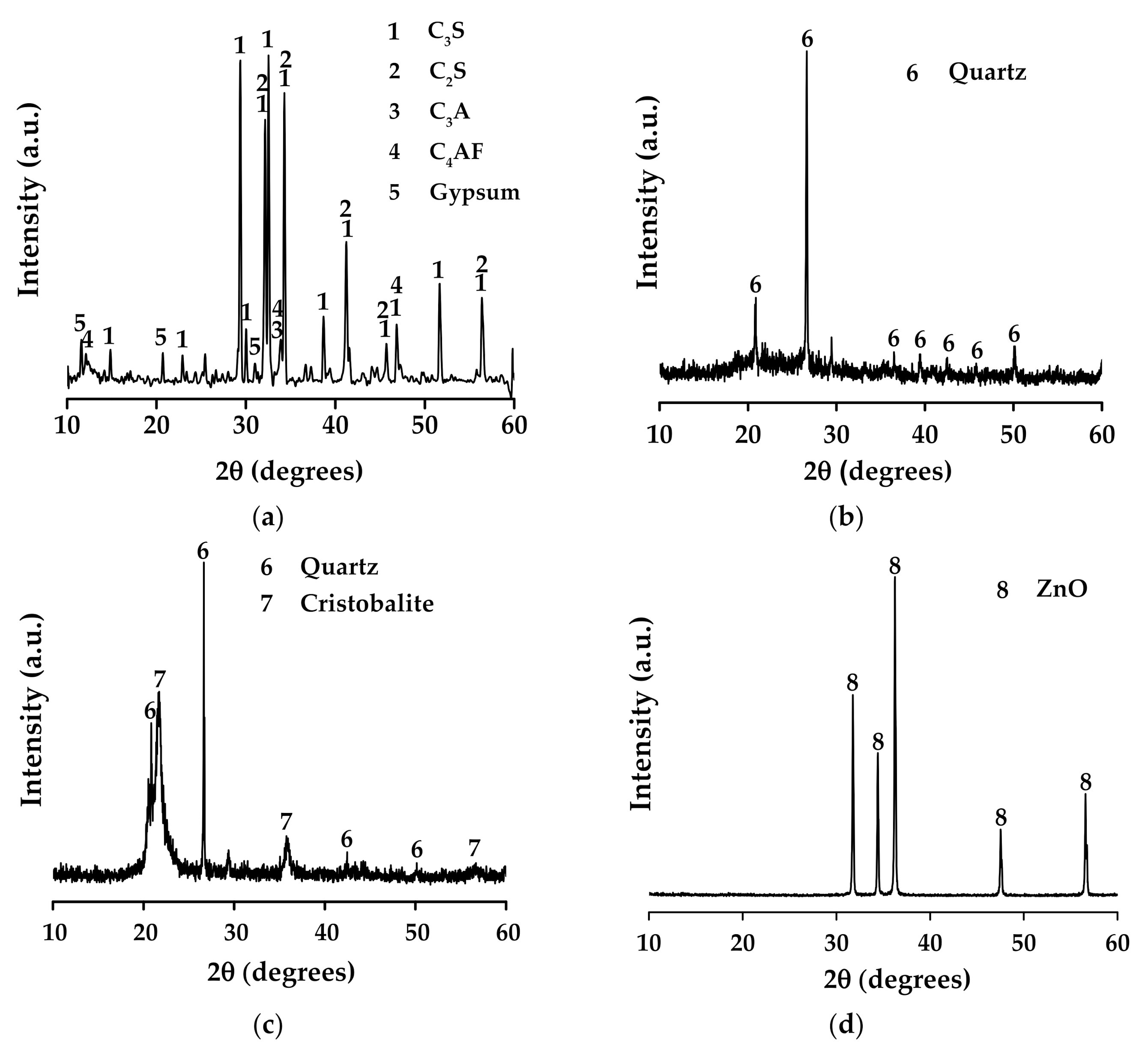
2.3. X-ray Diffraction
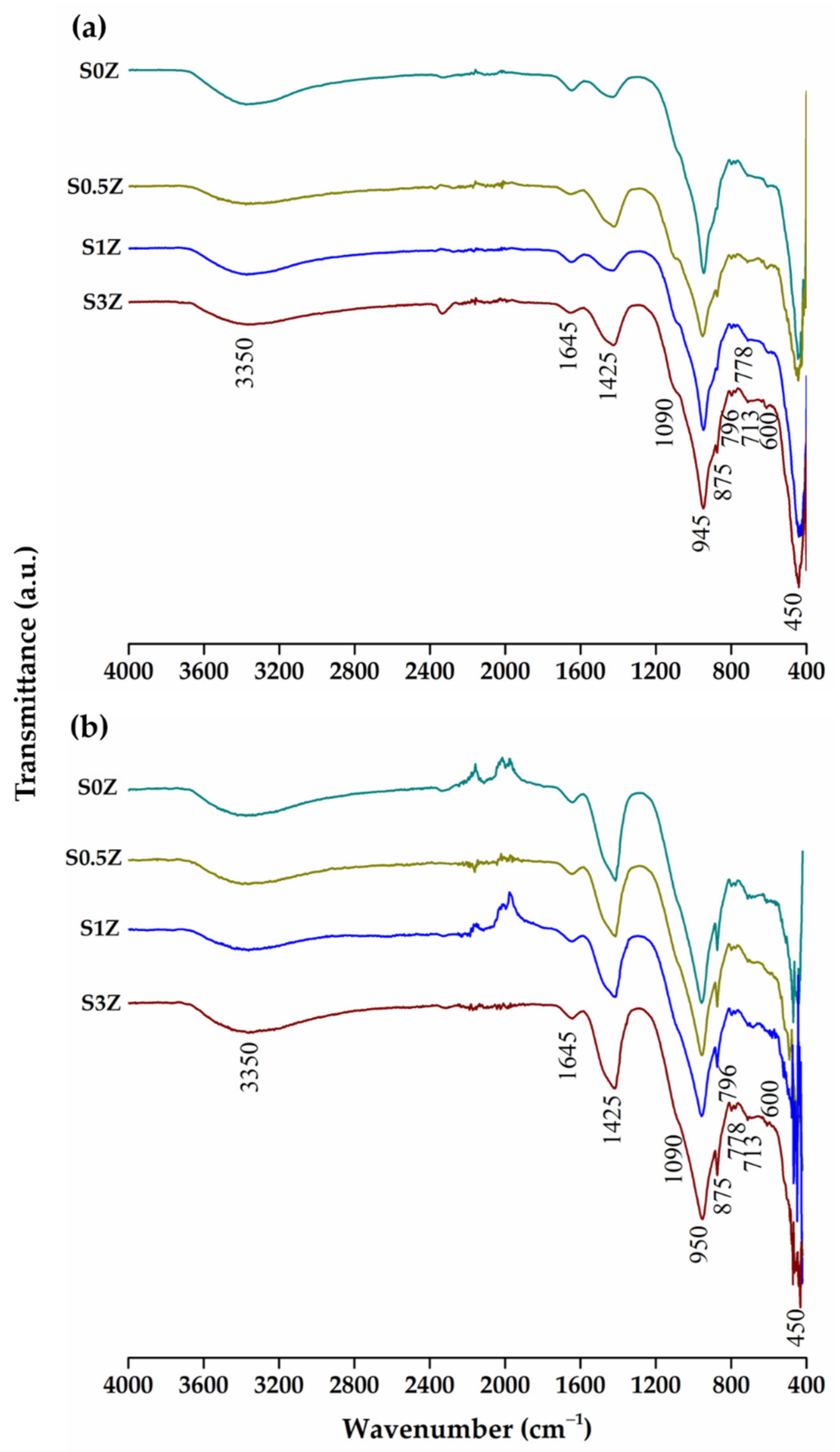
2.4. Fourier Transform Infrared Spectroscopy
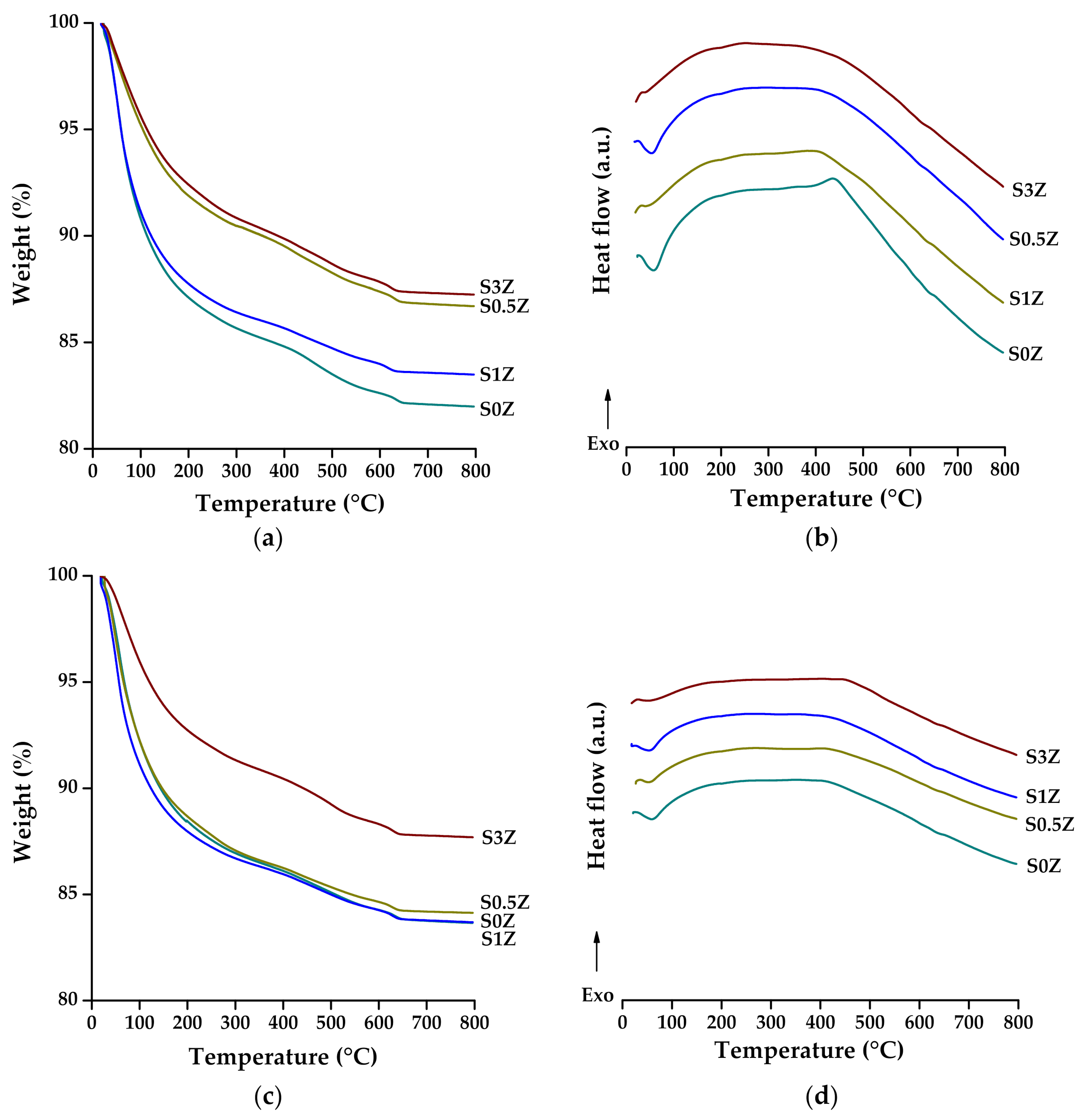
2.5. Thermogravimetric Analysis and Differential Scanning Calorimetry
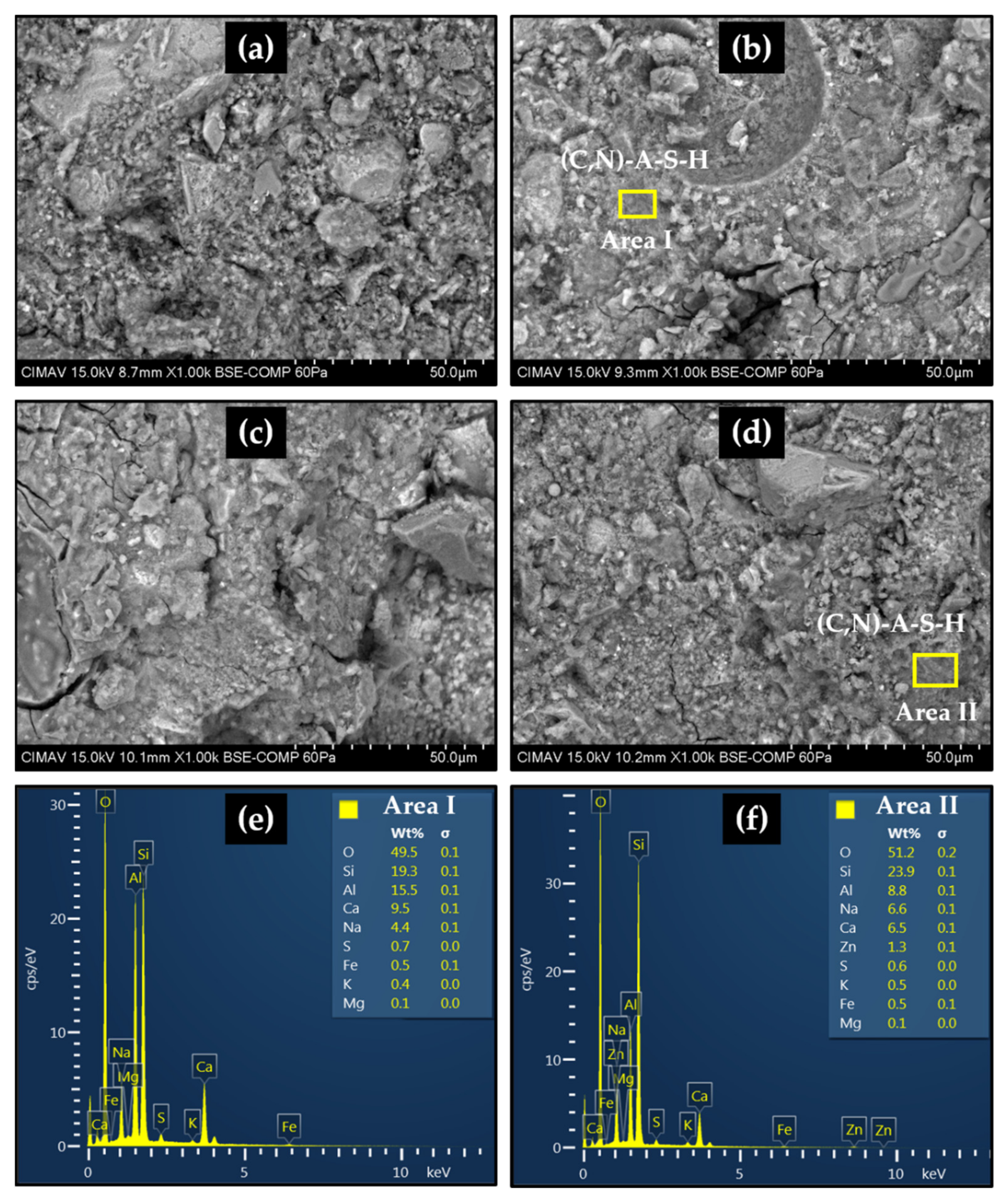
2.6. Scanning Electron Microscopy
3. Materials and Methods
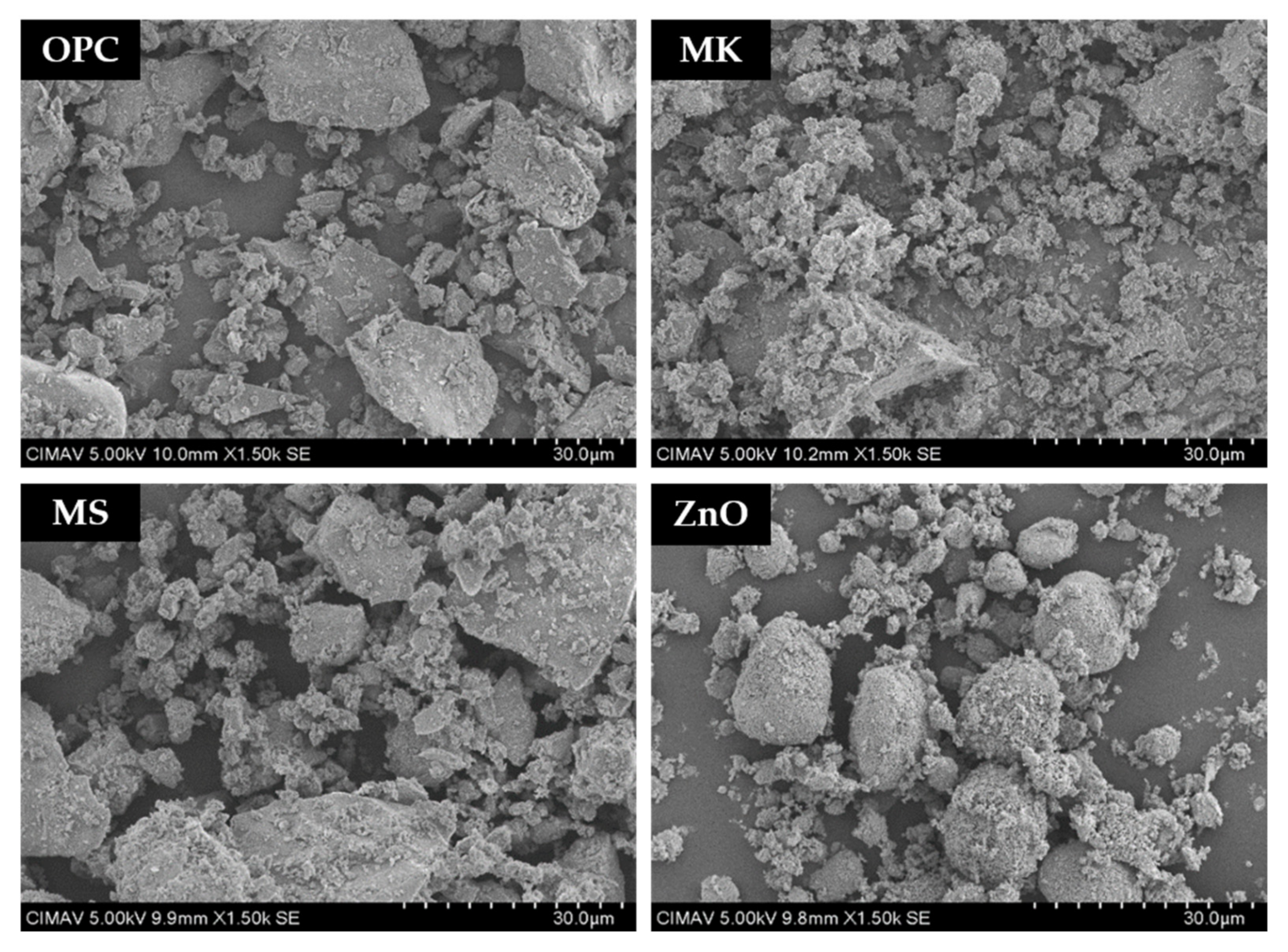
3.1. Materials
3.2. Preparation of Pastes
3.3. Tests Conducted
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Al-Chaar, K.G.; Alkadi, M.; Asteris, P.G. Natural Pozzolan as a Partial Substitute for Cement in Concrete. Open Constr. Build. Technol. J. 2013, 7, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Bapat, J.D. Mineral Admixtures in Cement and Concrete. CRC Press: Boca Ratón, FL, USA, 2012; pp. 1–285. [Google Scholar]
- Bumanis, G.; Vitola, L.; Stipniece, L.; Locs, J.; Korjakins, A.; Bajare, D. Evaluation of Industrial by-products as pozzolans: A road map for use in concrete production. Case Stud. Constr. Mater. 2020, 13, e00424. [Google Scholar] [CrossRef]
- Thomas, M. Supplementary Cementing Materials in Concrete; CRC Press: New York, NY, USA, 2014. [Google Scholar]
- Da Silva Andrade, D.; da Silva Rêgo, J.H.; Cesar Morais, P.; Frías Rojas, M. Chemical and mechanical characterization of ternary cement pastes containing metakaolin and nanosilica. Constr. Build. Mater. 2018, 159, 18–26. [Google Scholar] [CrossRef]
- Elahi, A.; Basheer, P.A.M.; Nanukuttan, S.V.; Khan, Q.U.Z. Mechanical and durability properties of high performance concretes containing supplementary cementitious materials. Constr. Build. Mater. 2010, 24, 292–299. [Google Scholar] [CrossRef]
- El-Diadamony, H.; Amer, A.A.; Sokkary, T.M.; El-Hoseny, S. Hydration and characteristics of metakaolin pozzolanic cement pastes. HBRC J. 2018, 14, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.M.; Karim, M.R.; Hasan, M.; Hossain, M.K.; Zain, M.F.M. Durability of mortar and concrete made up of pozzolans as a partial replacement of cement: A review. Constr. Build. Mater. 2016, 116, 128–140. [Google Scholar] [CrossRef]
- Siddique, R.; Klaus, J. Influence of metakaolin on the properties of mortar and concrete: A review. Appl. Clay Sci. 2009, 43, 392–400. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A. Variation in hybrid cements over time. Alkaline activation of fly ash-portland cement blends. Cem. Concr. Res. 2013, 52, 112–122. [Google Scholar] [CrossRef]
- Ali, M.B.; Saidur, R.; Hossain, M.S. A review on emission analysis in cement industries. Renew. Sustain. Energy Rev. 2011, 15, 2252–2261. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, G.; Chen, B.; Song, D.; Qi, J.; Liu, X. Analysis of CO2 Emission for the cement manufacturing with alternative raw materials: A LCA-based framework. Energy Procedia 2014, 61, 2541–2545. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Day, R.L. Pozzolanic reaction in the presence of chemical activators. Cem. Concr. Res. 2000, 30, 51–58. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Sun, W.; Yan, H.D. Hydration of high-volume fly ash cement pastes. Cem. Concr. Compos. 2000, 22, 445–452. [Google Scholar] [CrossRef]
- Mehdipour, I.; Khayat, K.H. Effect of particle-size distribution and specific surface area of different binder systems on packing density and flow characteristics of cement paste. Cem. Concr. Compos. 2017, 78, 120–131. [Google Scholar] [CrossRef]
- Bicer, A. Effect of fly ash particle size on thermal and mechanical properties of fly ash-cement composites. Therm. Sci. Eng. Prog. 2018, 8, 78–82. [Google Scholar] [CrossRef]
- Qudoos, A.; Kim, H.G.; Atta-ur-Rehman; Ryou, J.S. Effect of mechanical processing on the pozzolanic efficiency and the microstructure development of wheat straw ash blended cement composites. Constr. Build Mater. 2018, 193, 481–490. [Google Scholar] [CrossRef]
- Feng, Y.; Kero, J.; Yang, Q.; Chen, Q.; Engström, F.; Samuelsson, C.; Qi, C. Mechanical activation of granulated copper slag and its influence on hydration heat and compressive strength of blended cement. Materials 2019, 12, 772. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Hollanders, S.; Adriaens, R.; Skibsted, J.; Cizer, Ö.; Elsen, J. Pozzolanic reactivity of pure calcined clays. Appl. Clay Sci. 2016, 132–133, 552–560. [Google Scholar] [CrossRef]
- Alahrache, S.; Winnefeld, F.; Champenois, J.B.; Hesselbarth, F.; Lothenbach, B. Chemical activation of hybrid binders based on siliceous fly ash and Portland cement. Cem. Concr. Compos. 2016, 66, 10–23. [Google Scholar] [CrossRef]
- Karim, M.R.; Zain, M.F.M.; Jamil, M.; Lai, F.C. Development of a Zero-Cement Binder Using Slag, Fly Ash, and Rice Husk Ash with Chemical Activator. Adv. Mater. Sci Eng. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Ramirez, S.; Palomo, A. OPC hydration with highly alkaline solutions. Adv. Cem. Res. 2001, 13, 123–129. [Google Scholar] [CrossRef]
- Palomo, A.; Fernández-Jiménez, A.; Kovalchuk, G.; Ordoñez, L.M.; Naranjo, M.C. Opc-fly ash cementitious systems: Study of gel binders produced during alkaline hydration. J. Mater. Sci. 2007, 42, 2958–2966. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Maltseva, O.; Palomo, Á.; Fernández-Jiménez, A. Hybrid alkaline cements. Part I: Fundamentals. Rom J. Mater. 2012, 42, 330–335. [Google Scholar]
- Cristelo, N.; Garcia-Lodeiro, I.; Rivera, J.F.; Miranda, T.; Palomo, Á.; Coelho, J.; Fernández-Jiménez, A. One-part hybrid cements from fly ash and electric arc furnace slag activated by sodium sulphate or sodium chloride. J. Build. Eng. 2021, 44, 103298. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Boudissa, N.; Palomo, A. Use of clays in alkaline hybrid cement preparation. The role of bentonites. Mater. Lett. 2018, 233, 134–137. [Google Scholar] [CrossRef]
- Ouellet-Plamondon, C.; Scherb, S.; Köberl, M.; Thienel, K.C. Acceleration of cement blended with calcined clays. Constr Build. Mater. 2020, 245, 118439. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Donatello, S.; Fernández-Jiménez, A.; Palomo, Á. Hydration of hybrid alkaline cement containing a very large proportion of fly ash: A descriptive model. Materials. 2016, 9, 605. [Google Scholar] [CrossRef] [Green Version]
- García-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; MacPhee, D.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O-CaO-Al2O3-SiO 2-H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Garcia Lodeiro, I.; Fernandez-Jiménez, A.; Palomo, A.; Macphee, D.E. Effect on fresh C-S-H gels of the simultaneous addition of alkali and aluminium. Cem. Concr. Res. 2010, 40, 27–32. [Google Scholar] [CrossRef]
- Batuecas, E.; Ramón-Álvarez, I.; Sánchez-Delgado, S.; Torres-Carrasco, M. Carbon footprint and water use of alkali-activated and hybrid cement mortars. J. Clean Prod. 2021, 319, 128653. [Google Scholar] [CrossRef]
- Qu, B.; Martín, A.; Pastor, J.Y.; Palomo, A.; Fernández Jiménez, A. Microstructural characterisation of hybrid cement after exposure to high temperatures. Constr Build. Mater. 2020, 262, 1–10. [Google Scholar] [CrossRef]
- Fernández-jiménez, A.; Olga, I.G.; Angel, M. Hydration mechanisms of hybrid cements as a function of the way of addition of chemicals. J. Am. Ceram. Soc. 2018, 102, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Barboza-Chavez, A.C.; Gómez-Zamorano, L.Y.; Acevedo-Dávila, J.L. Synthesis and characterization of a hybrid cement based on fly ash, metakaolin and portland cement clinker. Materials 2020, 13, 1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, L.; Zhang, Z.; Wang, H. Hydration mechanisms and durability of hybrid alkaline cements (HACs): A review. Constr Build. Mater. 2021, 266, 121039. [Google Scholar] [CrossRef]
- María, A.; Reyes, M.D.L.; Suárez-navarro, J.A.; Alonso, M.; Gascó, C.; Sobrados, I.; Puertas, F. Hybrid Cements: Mechanical Properties, Microstructure and Radiological Behavior. Molecules 2022, 27, 498. [Google Scholar]
- Bonilla, A.; Villaquir, A.; Mej, R.; Guti, D. Novel Alkali-Activated Materials with Photocatalytic and Bactericidal Properties Based on Ceramic Tile Waste. Coatings 2022, 12, 35. [Google Scholar] [CrossRef]
- Gawlicki, M.; Czamarska, D. Effect of ZnO on the hydration of Portland cement. J. Therm Anal. 1992, 38, 2157–2161. [Google Scholar] [CrossRef]
- Ataie, F.F.; Juenger, M.C.G.; Taylor-Lange, S.C.; Riding, K.A. Comparison of the retarding mechanisms of zinc oxide and sucrose on cement hydration and interactions with supplementary cementitious materials. Cem. Concr. Res. 2015, 72, 128–136. [Google Scholar] [CrossRef]
- Puertas, F.; García-Díaz, I.; Palacios, M.; Gazulla, M.F.; Gómez, M.P.; Orduña, M. Clinkers and cements obtained from raw mix containing ceramic waste as a raw material. Characterization, hydration and leaching studies. Cem. Concr. Compos. 2010, 32, 175–186. [Google Scholar] [CrossRef]
- Dyer, T.; Jones, R.; Garvin, S. Exposure of Portland cement to multiple trace metal loadings. Mag Concr Res. 2009, 61, 57–65. [Google Scholar] [CrossRef]
- Liu, J.; Jin, H.; Gu, C.; Yang, Y. Effects of zinc oxide nanoparticles on early-age hydration and the mechanical properties of cement paste. Constr Build. Mater. 2019, 217, 352–362. [Google Scholar] [CrossRef]
- Garg, N.; White, C.E. Mechanism of zinc oxide retardation in alkali-activated materials: An: In situ X-ray pair distribution function investigation. J. Mater. Chem A. 2017, 5, 11794–11804. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, Y.; Wang, K.; Bu, Y.; Wang, C.; Ma, C.; Liu, H. Delaying the hydration of Portland cement by sodium silicate: Setting time and retarding mechanism. Constr. Build. Mater. 2019, 205, 543–548. [Google Scholar] [CrossRef]
- Yousuf, M.; Mollah, A.; Vempati, R.K.; Lin, T.C.; Cocke, D.L. The interfacial chemistry of solidification/stabilization of metals in cement and pozzolanic material systems. Waste Manag. 1995, 15, 137–148. [Google Scholar] [CrossRef]
- Yousuf, M.; Mollah, A.; Pargat, J.R.; Cocke, D.L. An infrared spectroscopic examination of cement-based solidification/stabilization systems - Portland types V and IP with zinc. J. Environ. Sci Heal. 1992, 27, 1503–1519. [Google Scholar] [CrossRef]
- Taylor-lange, S.C.; Riding, K.A.; Juenger, M.C.G. Cement & Concrete Composites Increasing the reactivity of metakaolin-cement blends using zinc oxide. Cem. Concr. Compos. 2012, 34, 835–847. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Trezza, M.A. Hydration study of ordinary portland cement in the presence of zinc ions. Mater. Res. 2007, 10, 331–334. [Google Scholar] [CrossRef]
- Ferone, C.; Colangelo, F.; Roviello, G.; Asprone, D.; Menna, C.; Balsamo, A.; Prota, A.; Cioffi, R.; Manfredi, G. Application-oriented chemical optimization of a metakaolin based geopolymer. Materials 2013, 6, 1920–1939. [Google Scholar] [CrossRef] [Green Version]
- Gallucci, E.; Zhang, X.; Scrivener, K.L. Effect of temperature on the microstructure of calcium silicate hydrate (C-S-H). Cem. Concr. Res. 2013, 53, 185–195. [Google Scholar] [CrossRef]
| Sample | Initial Setting Time | Final Setting Time |
|---|---|---|
| S0Z | 8 | 16 |
| S0.5Z | 11 | 25 |
| S1Z | 23 | 32 |
| S3Z | 30 | 55 |
| Sample | 7 Curing Days | 28 Curing Days |
|---|---|---|
| S0Z | 5.4 | 5.6 |
| S0.5Z | 5.2 | 5.4 |
| S1Z | 4.9 | 4.6 |
| S3Z | 5.1 | 5.0 |
| Sample | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | LOI | APS |
|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 20.7 | 4.3 | 2.9 | 63.7 | 2.3 | 0.2 | 0.1 | 2.9 | 2.7 | 13.6 |
| MK | 55.5 | 30.8 | 1.9 | 6.7 | 0.3 | 0.8 | 0.4 | 0.7 | 2.9 | 10.9 |
| MS | 82.2 | 6.9 | 1.3 | 5.7 | 0.1 | 0.3 | 0.1 | 0.9 | 3.0 | 12.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Felix, M.; Baldenebro-Lopez, F.J.; Carreño-Gallardo, C.; Herrera-Ramirez, J.M. Hybrid Cements with ZnO Additions: Hydration, Compressive Strength and Microstructure. Molecules 2022, 27, 1278. https://doi.org/10.3390/molecules27041278
Soto-Felix M, Baldenebro-Lopez FJ, Carreño-Gallardo C, Herrera-Ramirez JM. Hybrid Cements with ZnO Additions: Hydration, Compressive Strength and Microstructure. Molecules. 2022; 27(4):1278. https://doi.org/10.3390/molecules27041278
Chicago/Turabian StyleSoto-Felix, Magnolia, Francisco Javier Baldenebro-Lopez, Caleb Carreño-Gallardo, and Jose Martin Herrera-Ramirez. 2022. "Hybrid Cements with ZnO Additions: Hydration, Compressive Strength and Microstructure" Molecules 27, no. 4: 1278. https://doi.org/10.3390/molecules27041278
APA StyleSoto-Felix, M., Baldenebro-Lopez, F. J., Carreño-Gallardo, C., & Herrera-Ramirez, J. M. (2022). Hybrid Cements with ZnO Additions: Hydration, Compressive Strength and Microstructure. Molecules, 27(4), 1278. https://doi.org/10.3390/molecules27041278








