Sm0.5Sr0.5Co1−xNixO3−δ—A Novel Bifunctional Electrocatalyst for Oxygen Reduction/Evolution Reactions
Abstract
:1. Introduction
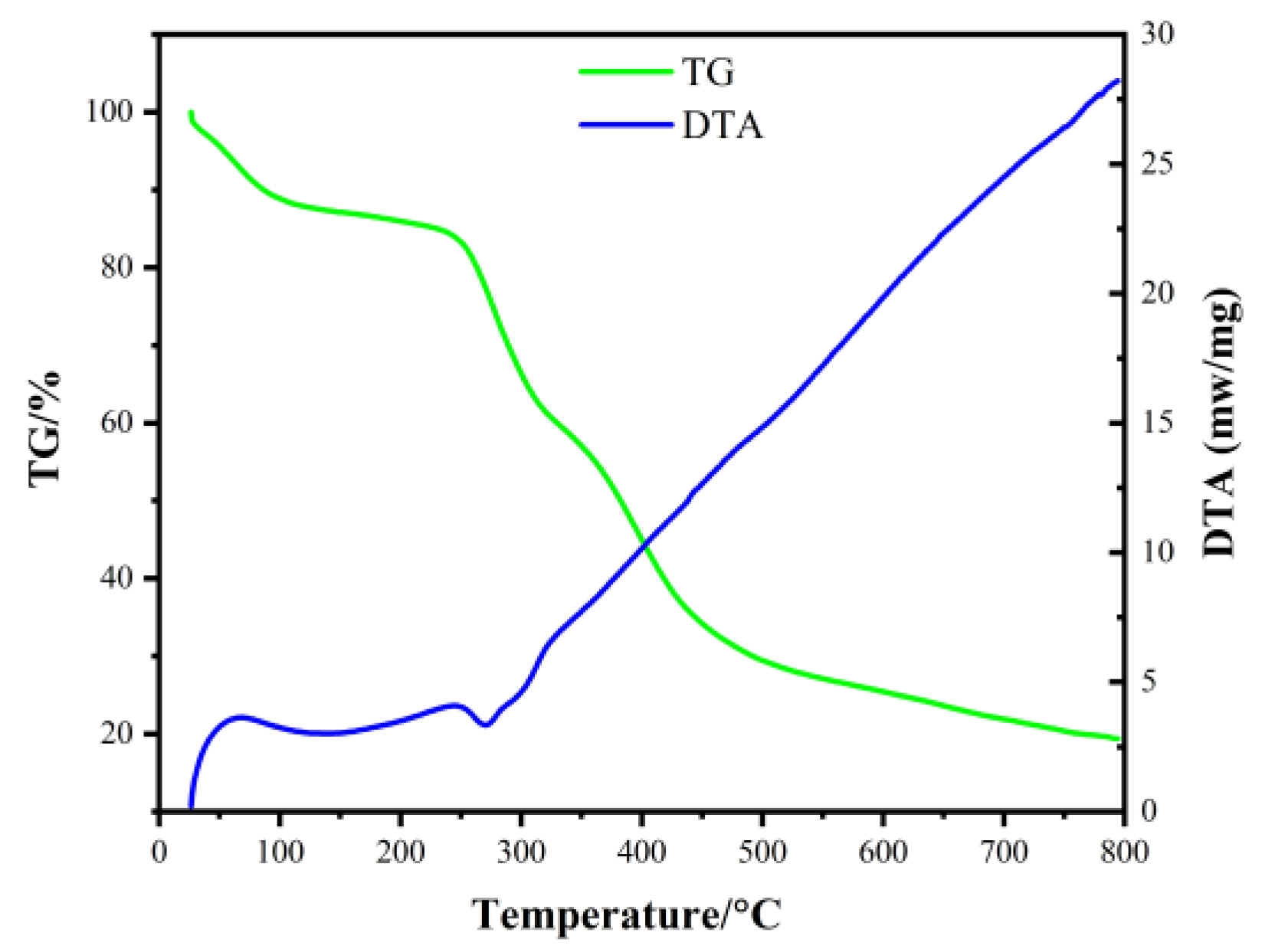
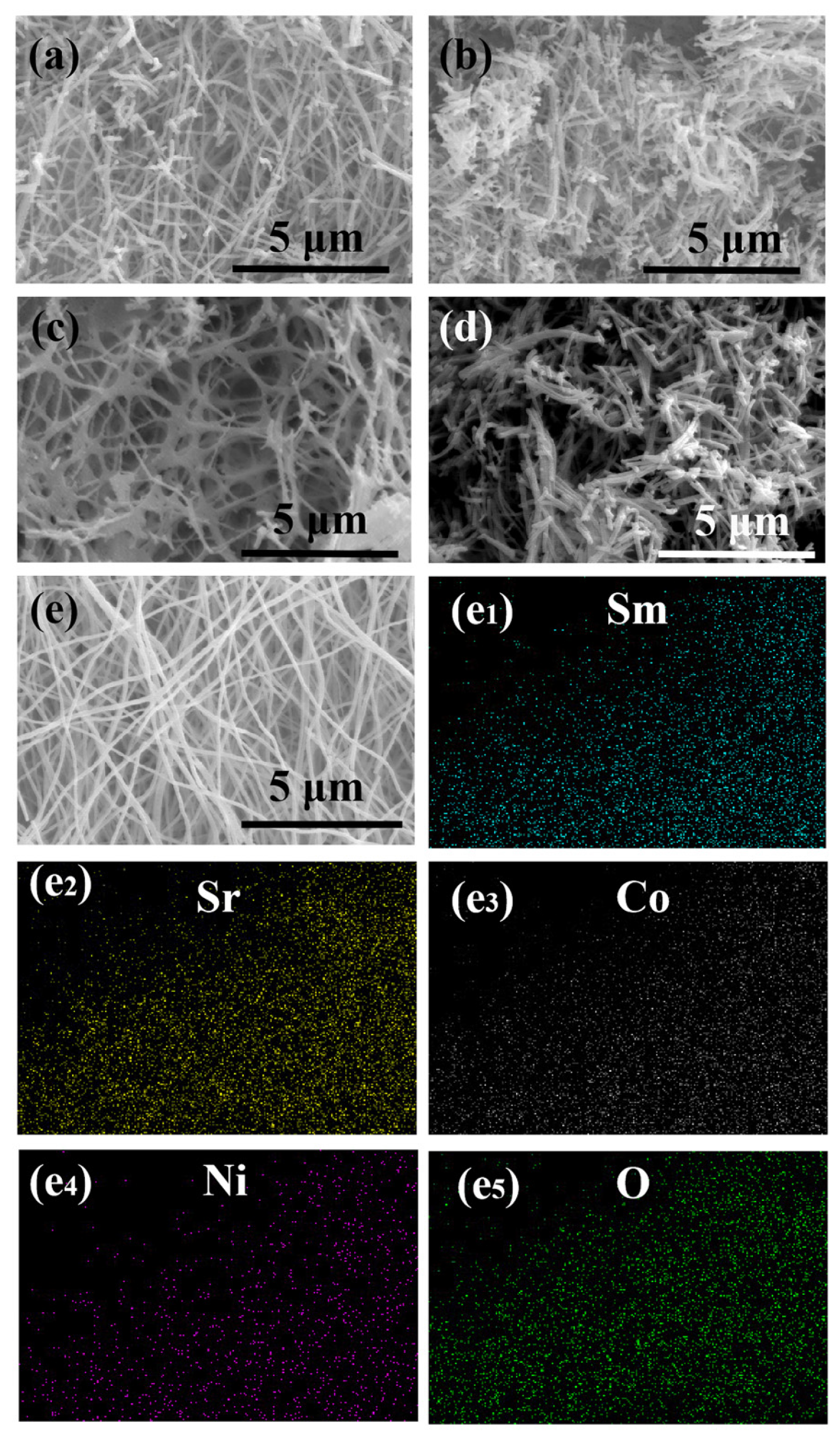
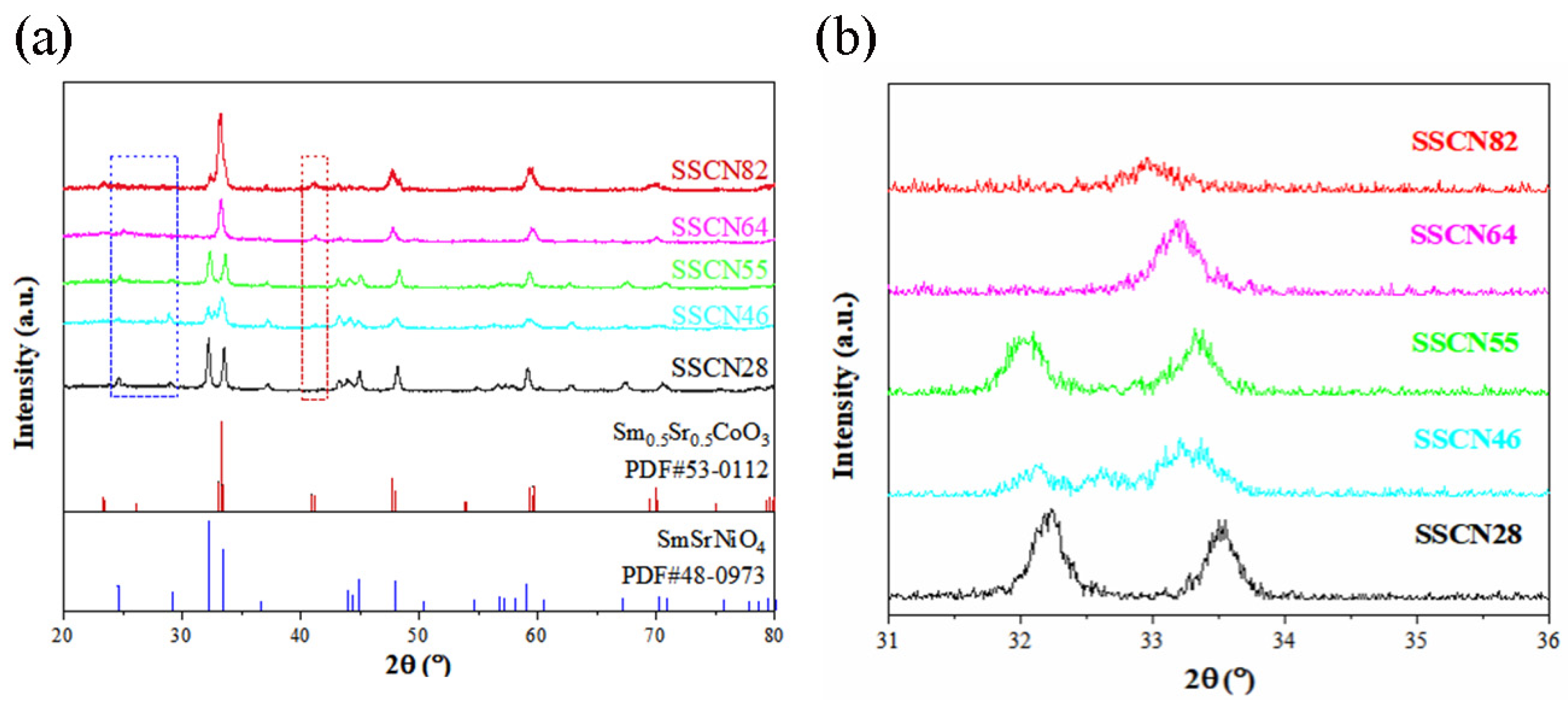
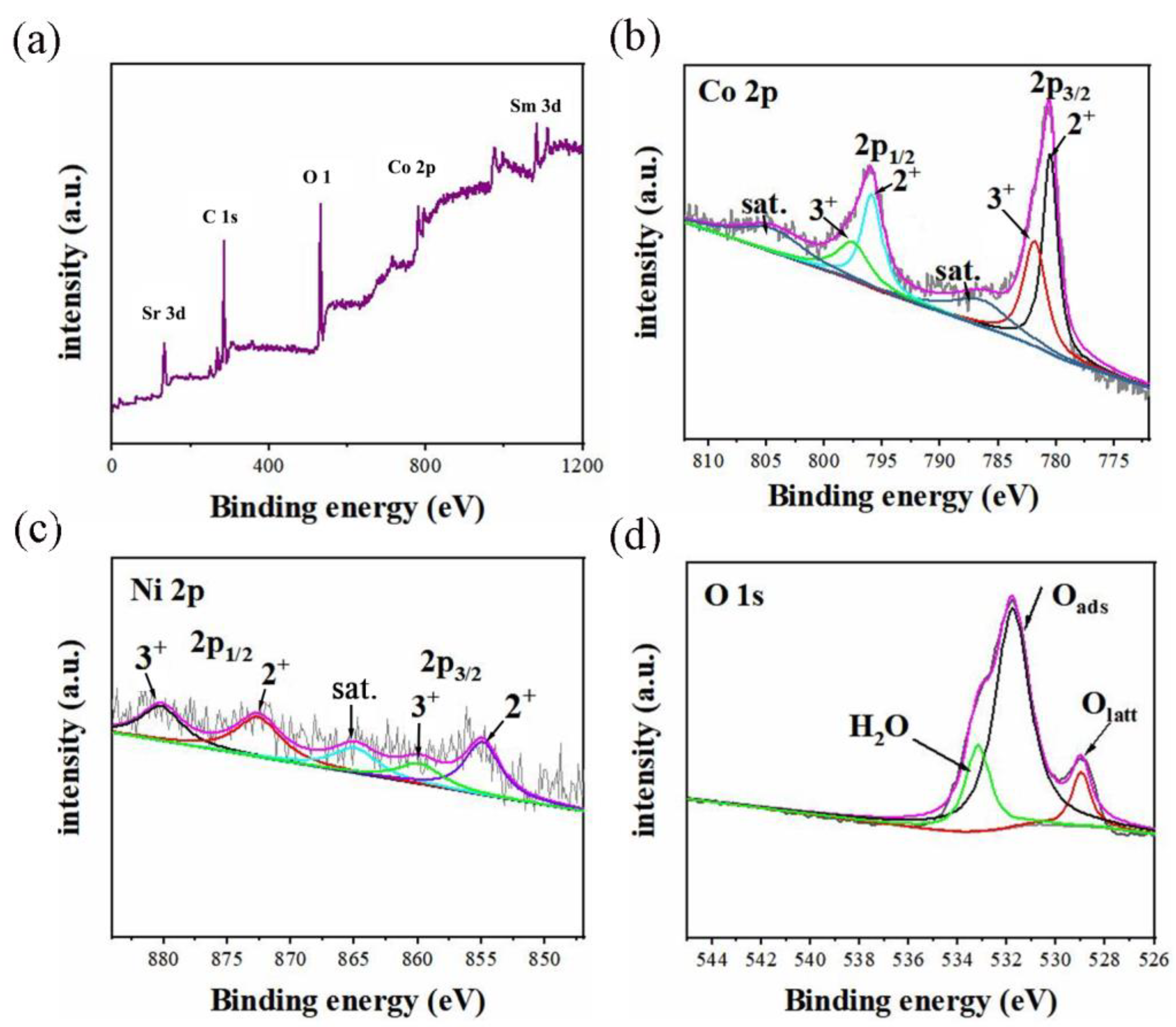
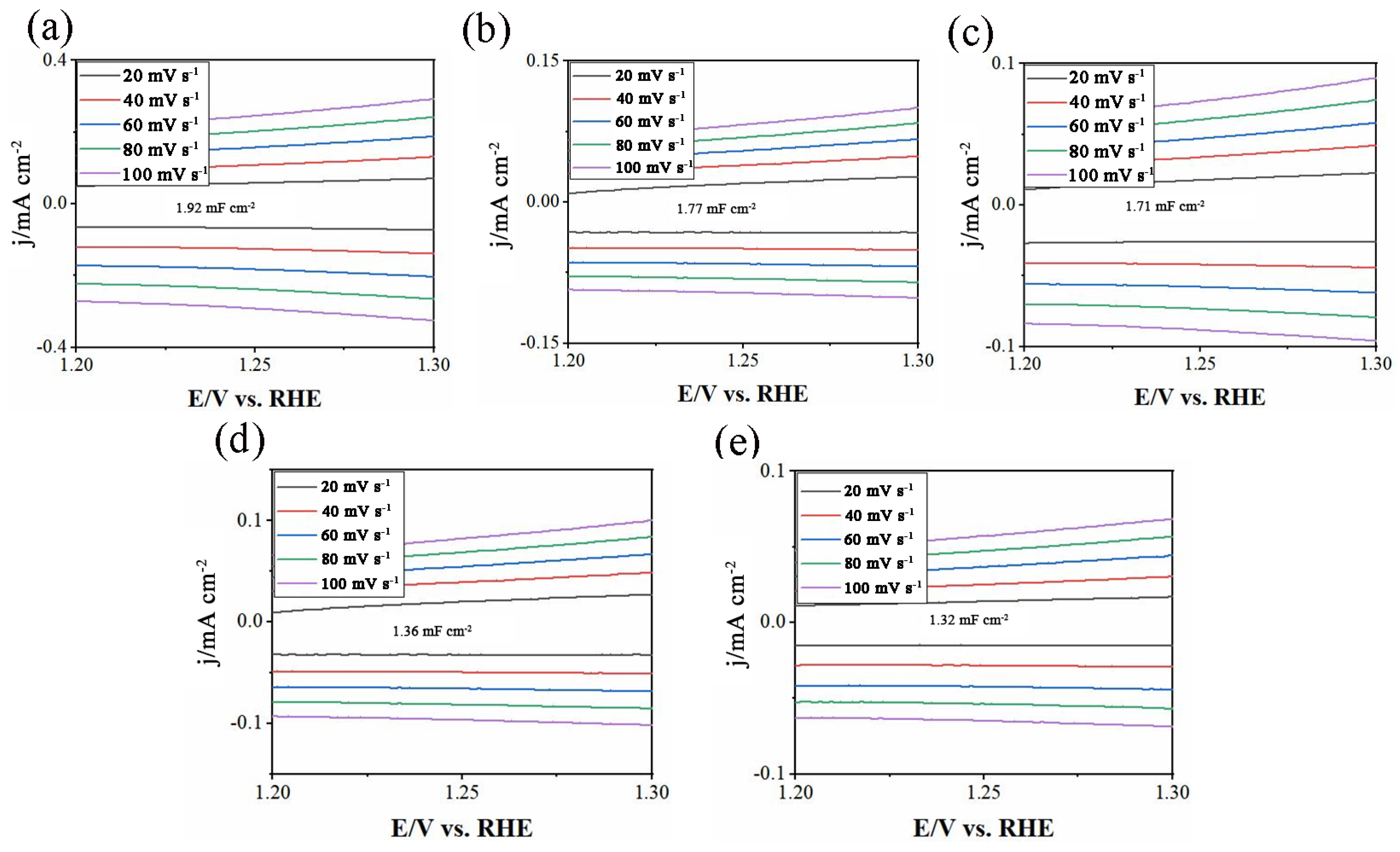
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chu, S.; Majumdar, A. Opportunities and Challenges for a Sustainable Energy Future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Armaroli, N.; Balzani, V. The Future of Energy Supply: Challenges and Opportunities. Angew. Chem. Int. Ed. 2007, 46, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Ivey, D.G.; Xie, Z.; Qu, W. Rechargeable Zn-Air Batteries: Progress in Electrolyte Development and Cell Configuration Advancement. J. Power Sources 2015, 283, 358–371. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Y.Y.; Yang, H.; Dong, Z.; Liu, H.; Xia, B.Y. Advanced Architectures and Relatives of Air Electrodes in Zn-Air Batteries. Adv. Sci. 2018, 5, 1700691. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xu, N.; Zhu, T.; Xu, Z.; Sun, M.; Geng, D. La0.4Sr0.6Co0.7Fe0.2Nb0.1O3-δ Perovskite Prepared by the Sol-Gel Method with Superior Performance as a Bifunctional Oxygen Electrocatalyst. Int. J. Hydrog. Energy 2020, 45, 30583–30591. [Google Scholar] [CrossRef]
- Eskandrani, A.A.; Ali, S.M.; Al-Otaibi, H.M. Study of the Oxygen Evolution Reaction at Strontium Palladium Perovskite Electrocatalyst in Acidic Medium. Int. J. Mol. Sci. 2020, 21, 3785. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, O.; Kim, C.; Gwon, O.; Jeong, H.Y.; Kim, K.-H.; Shin, J.; Kim, G. Strategy for Enhancing Interfacial Effect of Bifunctional Electrocatalyst: Infiltration of Cobalt Nanooxide on Perovskite. Adv. Mater. Interfaces 2018, 5, 1800123. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, M.; Jiang, Y.; Zou, L.; Gong, Y.; Chi, B.; Pu, J.; Jian, L. Perovskite La0.6Sr0.4Co0.2Fe0.8O3 as an Effective Electrocatalyst for Non-Aqueous Lithium Air Batteries. Electrochim. Acta 2016, 191, 106–115. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of Electrocatalysts for Oxygen- and Hydrogen-Involving Energy Conversion Reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Mohan, S.; Mao, Y. Molten Salt Synthesized Submicron Perovskite La1–XSrxCoO3 Particles as Efficient Electrocatalyst for Water Electrolysis. Front. Mater. 2020, 7, 259. [Google Scholar] [CrossRef]
- Kushwaha, H.S.; Halder, A.; Thomas, P.; Vaish, R. CaCu3Ti4O12: A Bifunctional Perovskite Electrocatalyst for Oxygen Evolution and Reduction Reaction in Alkaline Medium. Electrochim. Acta 2017, 252, 532–540. [Google Scholar] [CrossRef]
- Yu, J.; Li, C.; Li, B.; Zhu, X.; Zhang, R.; Ji, L.; Tang, D.; Asiri, A.M.; Sun, X.; Li, Q.; et al. A Perovskite La2Ti2O7 Nanosheet as an Efficient Electrocatalyst for Artificial N2 Fixation to NH3 in Acidic Media. Chem. Commun. 2019, 55, 6401–6404. [Google Scholar] [CrossRef] [PubMed]
- Velraj, S.; Zhu, J.H. Sm0.5Sr0.5CoO3−δ—A New Bi-Functional Catalyst for Rechargeable Metal-Air Battery Applications. J. Power Sources 2013, 227, 48–52. [Google Scholar] [CrossRef]
- Retuerto, M.; Calle-Vallejo, F.; Pascual, L.; Lumbeeck, G.; Fernandez-Diaz, M.T.; Croft, M.; Gopalakrishnan, J.; Peña, M.A.; Hadermann, J.; Greenblatt, M.; et al. La1.5Sr0.5NiMn0.5Ru0.5O6 Double Perovskite with Enhanced ORR/OER Bifunctional Catalytic Activity. ACS Appl. Mater. Interfaces 2019, 11, 21454–21464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azad, U.P.; Singh, M.; Ghosh, S.; Singh, A.K.; Ganesan, V.; Singh, A.K.; Prakash, R. Facile Synthesis of BSCF Perovskite Oxide as an Efficient Bifunctional Oxygen Electrocatalyst. Int. J. Hydrogen Energy 2018, 43, 20671–20679. [Google Scholar] [CrossRef]
- Bu, Y.; Nam, G.; Kim, S.; Choi, K.; Zhong, Q.; Lee, J.; Qin, Y.; Cho, J.; Kim, G. A Tailored Bifunctional Electrocatalyst: Boosting Oxygen Reduction/Evolution Catalysis via Electron Transfer Between N-Doped Graphene and Perovskite Oxides. Small 2018, 14, 1802767. [Google Scholar] [CrossRef]
- Yu, M.; Yin, Z.; Yan, G.; Wang, Z.; Guo, H.; Li, G.; Liu, Y.; Li, L.; Wang, J. Synergy of Interlayer Expansion and Capacitive Contribution Promoting Sodium Ion Storage in S, N-Doped Mesoporous Carbon Nanofiber. J. Power Sources 2020, 449, 227514. [Google Scholar] [CrossRef]
- Fan, L.; Xiong, Y.; Liu, L.; Wang, Y.; Brito, M.E. Preparation and Performance Study of One-Dimensional Nanofiber-Based Sm0.5Sr0.5CoO3-δ-Gd0.2Ce0.8O1.9 Composite Cathodes for Intermediate Temperature Solid Oxide Fuel Cells. Int. J. Electrochem. Sci. 2013, 8, 11. [Google Scholar]
- Fan, L.; Wang, Y.; Jia, Z.; Xiong, Y.; Brito, M.E. Nanofiber-Structured SSC–GDC Composite Cathodes for a LSGM Electrolyte Based IT-SOFCs. Ceram. Int. 2015, 41, 6583–6588. [Google Scholar] [CrossRef]
- Vignesh, A.; Vajeeston, P.; Pannipara, M.; Al-Sehemi, A.G.; Xia, Y.; Kumar, G.G. Bimetallic Metal-Organic Framework Derived 3D Hierarchical NiO/Co3O4/C Hollow Microspheres on Biodegradable Garbage Bag for Sensitive, Selective, and Flexible Enzyme-Free Electrochemical Glucose Detection. Chem. Eng. J. 2022, 430, 133157. [Google Scholar] [CrossRef]
- Li, L.; Song, J.; Lu, Q.; Tan, X. Synthesis of Nano-Crystalline Sm0.5Sr0.5Co(Fe)O3−δ Perovskite Oxides by a Microwave-Assisted Sol–Gel Combustion Process. Ceram. Int. 2014, 40, 1189–1194. [Google Scholar] [CrossRef]
- Gao, L.; Zhu, M.; Xia, T.; Li, Q.; Li, T.; Zhao, H. Ni-Doped BaFeO3—Perovskite Oxide as Highly Active Cathode Electrocatalyst for Intermediate-Temperature Solid Oxide Fuel Cells. Electrochim. Acta 2018, 289, 428–436. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, S.; Xiong, Y.; Wei, J. Effect of B Sites on the Catalytic Activities for Perovskite Oxides La0.6Sr0.4CoxFe1-XO3-δ as Metal-Air Batteries Catalysts. Prog. Nat. Sci. Mater. Int. 2018, 28, 399–407. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Liang, C.; Fan, L.; Han, J.; Xiong, Y. Effect of Morphology on the Oxygen Evolution Reaction for La0.8Sr0.2Co0.2Fe0.8O3−δ Electrochemical Catalyst in Alkaline Media. RSC Adv. 2016, 6, 69251–69256. [Google Scholar] [CrossRef]
- Baek, S.-W.; Kim, J.H.; Bae, J. Characteristics of ABO3 and A2BO4 (ASm, Sr; BCo, Fe, Ni) Samarium Oxide System as Cathode Materials for Intermediate Temperature-Operating Solid Oxide Fuel Cell. Solid State Ion. 2008, 179, 1570–1574. [Google Scholar] [CrossRef]
- Liu, S.; Luo, H.; Li, Y.; Liu, Q.; Luo, J.-L. Structure-Engineered Electrocatalyst Enables Highly Active and Stable Oxygen Evolution Reaction over Layered Perovskite LaSr3Co1.5Fe1.5O10-δ. Nano Energy 2017, 40, 115–121. [Google Scholar] [CrossRef]
- Da, Y.; Zeng, L.; Wang, C.; Gong, C.; Cui, L. A Simple Approach to Tailor OER Activity of SrxCo0.8Fe0.2O3 Perovskite Catalysts. Electrochim. Acta 2019, 300, 85–92. [Google Scholar] [CrossRef]
- Li, P.; Wei, B.; Lü, Z.; Wu, Y.; Zhang, Y.; Huang, X. La1.7Sr0.3Co0.5Ni0.5O4+δ Layered Perovskite as an Efficient Bifunctional Electrocatalyst for Rechargeable Zinc-Air Batteries. Appl. Surf. Sci. 2019, 464, 494–501. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Ponraj, J.; Mansour, S.A. Synthesis and Growth Mechanism of Bamboo like N-Doped CNT/Graphene Nanostructure Incorporated with Hybrid Metal Nanoparticles for Overall Water Splitting. Carbon 2020, 170, 452–463. [Google Scholar] [CrossRef]
- Cabello, A.; Gayán, P.; García-Labiano, F.; de Diego, L.F.; Abad, A.; Izquierdo, M.T.; Adánez, J. Relevance of the Catalytic Activity on the Performance of a NiO/CaAl2O4 Oxygen Carrier in a CLC Process. Appl. Catal. B Environ. 2014, 147, 980–987. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Zong, X.; Jin, Y.; Li, D.; Xiong, Y.; Wu, G. N & S Co-Doped Carbon Nanofiber Network Embedded with Ultrafine NiCo Nanoalloy for Efficient Oxygen Electrocatalysis and Zn-Air Battery. Nanoscale 2020, 12, 9581–9589. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, J.; Liu, Y.; Hu, X.; Geng, D. Assemblage of Perovskite LaNiO3 Connected With In Situ Grown Nitrogen-Doped Carbon Nanotubes as High-Performance Electrocatalyst for Oxygen Evolution Reaction. Phys. Status. Solidi. A 2018, 215, 1800380. [Google Scholar] [CrossRef]
- Favaro, M.; Drisdell, W.S.; Marcus, M.A.; Gregoire, J.M.; Crumlin, E.J.; Haber, J.A.; Yano, J. An Operando Investigation of (Ni–Fe–Co–Ce)Ox System as Highly Efficient Electrocatalyst for Oxygen Evolution Reaction. ACS Catal. 2017, 7, 1248–1258. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yin, F.; Li, G.; Chen, B.; Wang, Z. Preparation, Characterization and Bifunctional Catalytic Properties of MOF(Fe/Co) Catalyst for Oxygen Reduction/Evolution Reactions in Alkaline Electrolyte. Int. J. Hydrog. Energy 2014, 39, 16179–16186. [Google Scholar] [CrossRef]
- Lambert, T.N.; Vigil, J.A.; White, S.E.; Davis, D.J.; Limmer, S.J.; Burton, P.D.; Coker, E.N.; Beechem, T.E.; Brumbach, M.T. Electrodeposited NixCo3−xO4 Nanostructured Films as Bifunctional Oxygen Electrocatalysts. Chem. Commun. 2015, 51, 9511–9514. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xue, Y.; Sun, S.; Li, S.; Miao, H.; Liu, Z. La0.8Sr0.2Co1−XMnxO3 Perovskites as Efficient Bi-Functional Cathode Catalysts for Rechargeable Zinc-Air Batteries. Electrochim. Acta 2017, 254, 14–24. [Google Scholar] [CrossRef]
- Lin, H.; Xie, J.; Zhang, Z.; Wang, S.; Chen, D. Perovskite Nanoparticles@N-Doped Carbon Nanofibers as Robust and Efficient Oxygen Electrocatalysts for Zn-Air Batteries. J. Colloid. Interface Sci. 2021, 581, 374–384. [Google Scholar] [CrossRef]
- Wang, C.C.; Cheng, Y.; Ianni, E.; Jiang, S.P.; Lin, B. A Highly Active and Stable La0.5Sr0.5Ni0.4Fe0.6O3-δ Perovskite Electrocatalyst for Oxygen Evolution Reaction in Alkaline Media. Electrochim. Acta 2017, 246, 997–1003. [Google Scholar] [CrossRef]
- Bu, Y.; Gwon, O.; Nam, G.; Jang, H.; Kim, S.; Zhong, Q.; Cho, J.; Kim, G. A Highly Efficient and Robust Cation Ordered Perovskite Oxide as a Bifunctional Catalyst for Rechargeable Zinc-Air Batteries. ACS Nano 2017, 11, 11594–11601. [Google Scholar] [CrossRef]
- Luo, Q.; Lin, D.; Zhan, W.; Zhang, W.; Tang, L.; Luo, J.; Gao, Z.; Jiang, P.; Wang, M.; Hao, L.; et al. Hexagonal Perovskite Ba0.9Sr0.1Co0.8Fe0.1Ir0.1O3−δ as an Efficient Electrocatalyst towards the Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2020, 3, 7149–7158. [Google Scholar] [CrossRef]
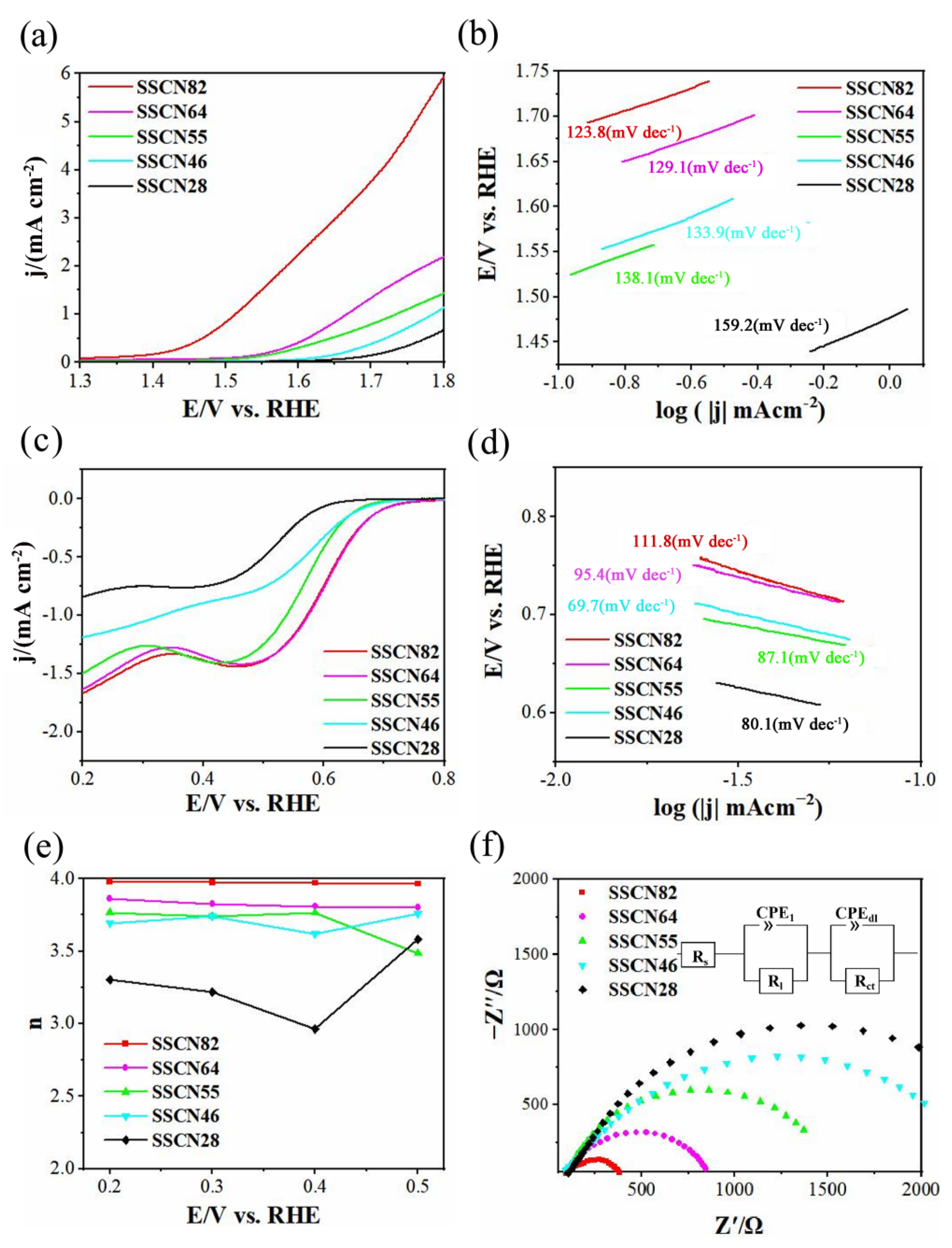
| Catalysts | OER Onset Potential (E/V vs. RHE) | Tafel Slope (mV dec−1) | Current Density at 1.8 V (mA cm−2) | References |
|---|---|---|---|---|
| SSCN82 | 1.39 | 123.8 | 6.01 | This work |
| SSCN64 | 1.51 | 129.1 | 2.2 | This work |
| SSCN55 | 1.52 | 133.9 | 1.4 | This work |
| SSCN46 | 1.61 | 138.1 | 1.12 | This work |
| SSCN28 | 1.63 | 159.2 | 0.65 | This work |
| BSCF900N2 | 143 | ca 7 | [15] | |
| SSC-HG | 1.53 | 115 | [16] | |
| LSM | 1.7 | 226 | 2 | [36] |
| LCNP@NCNF | 1.51 | 152 | 4.09 | [37] |
| LSNF-5546 | 1.56 | 76 | [38] | |
| IrO2 | 1.56 | 115 | - | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, Y.; Fan, L.; Zhang, W.; Cao, W.; Han, X.; Liu, X.; Jia, H. Sm0.5Sr0.5Co1−xNixO3−δ—A Novel Bifunctional Electrocatalyst for Oxygen Reduction/Evolution Reactions. Molecules 2022, 27, 1263. https://doi.org/10.3390/molecules27041263
Liu X, Wang Y, Fan L, Zhang W, Cao W, Han X, Liu X, Jia H. Sm0.5Sr0.5Co1−xNixO3−δ—A Novel Bifunctional Electrocatalyst for Oxygen Reduction/Evolution Reactions. Molecules. 2022; 27(4):1263. https://doi.org/10.3390/molecules27041263
Chicago/Turabian StyleLiu, Xingmei, Yuwei Wang, Liquan Fan, Weichao Zhang, Weiyan Cao, Xianxin Han, Xijun Liu, and Hongge Jia. 2022. "Sm0.5Sr0.5Co1−xNixO3−δ—A Novel Bifunctional Electrocatalyst for Oxygen Reduction/Evolution Reactions" Molecules 27, no. 4: 1263. https://doi.org/10.3390/molecules27041263
APA StyleLiu, X., Wang, Y., Fan, L., Zhang, W., Cao, W., Han, X., Liu, X., & Jia, H. (2022). Sm0.5Sr0.5Co1−xNixO3−δ—A Novel Bifunctional Electrocatalyst for Oxygen Reduction/Evolution Reactions. Molecules, 27(4), 1263. https://doi.org/10.3390/molecules27041263






