LC–MS3 Strategy for Quantification of Carbamazepine in Human Plasma and Its Application in Therapeutic Drug Monitoring
Abstract
:1. Introduction
2. Results
Assay Validation
3. Discussion
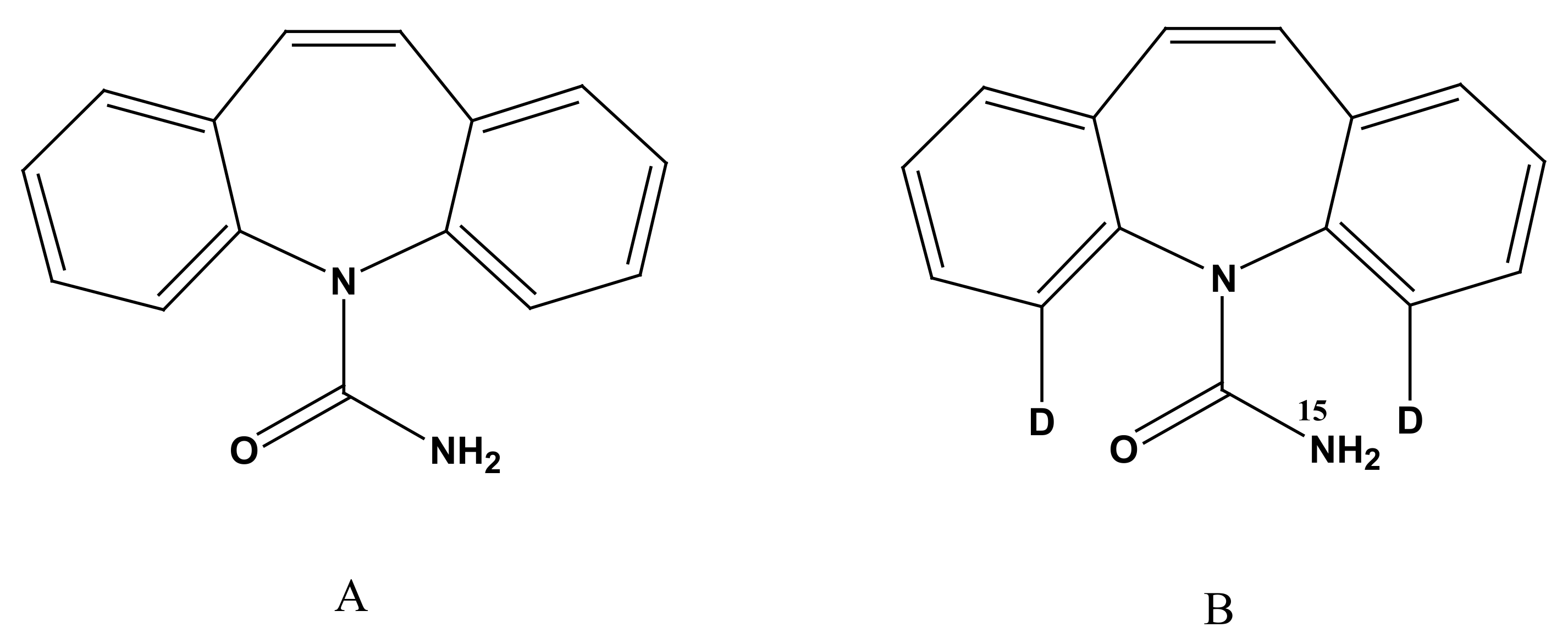
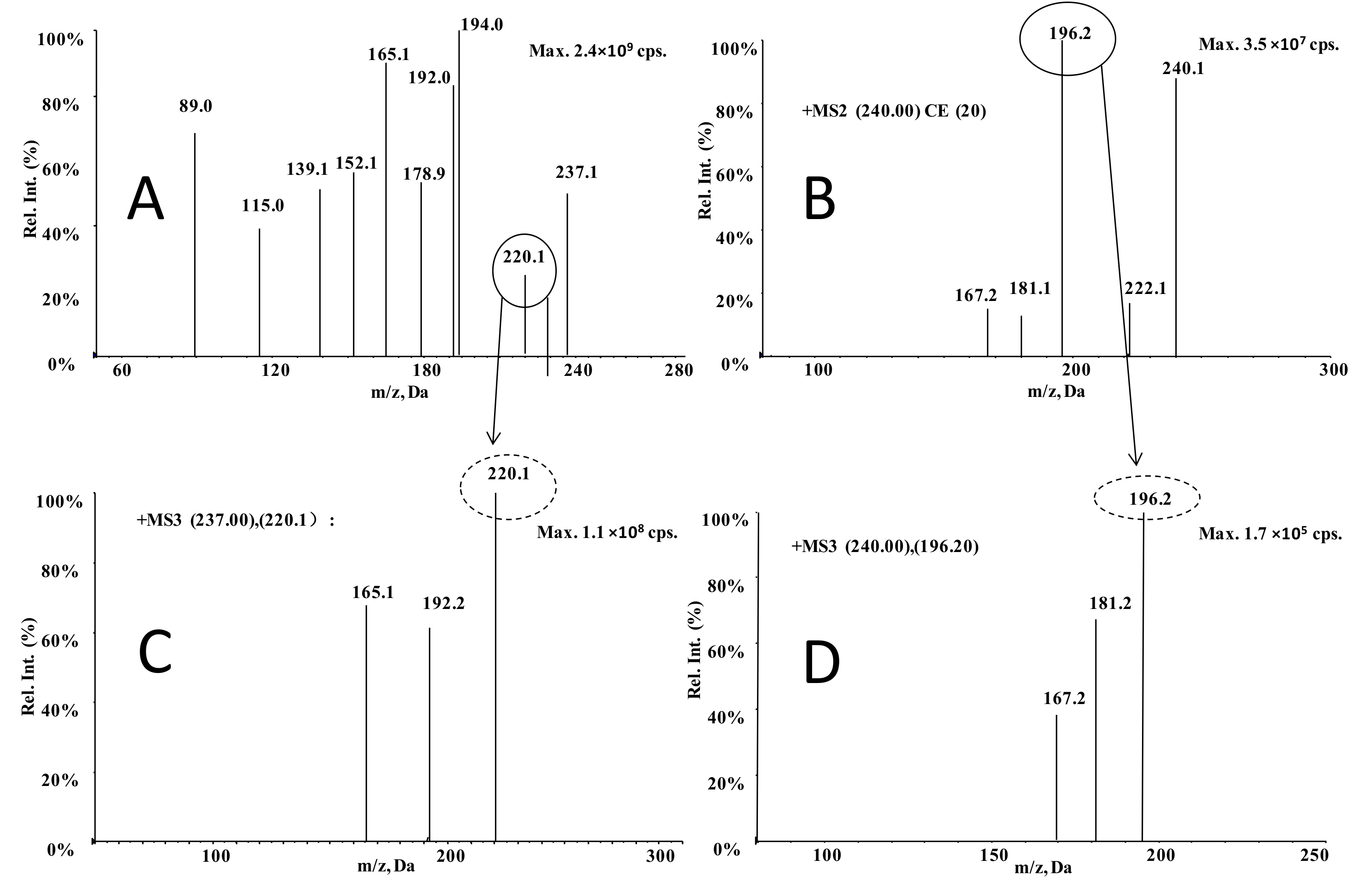
3.1. Optimization of MS Conditions
3.2. Optimization of LC Conditions
3.3. Optimization of Sample Processing
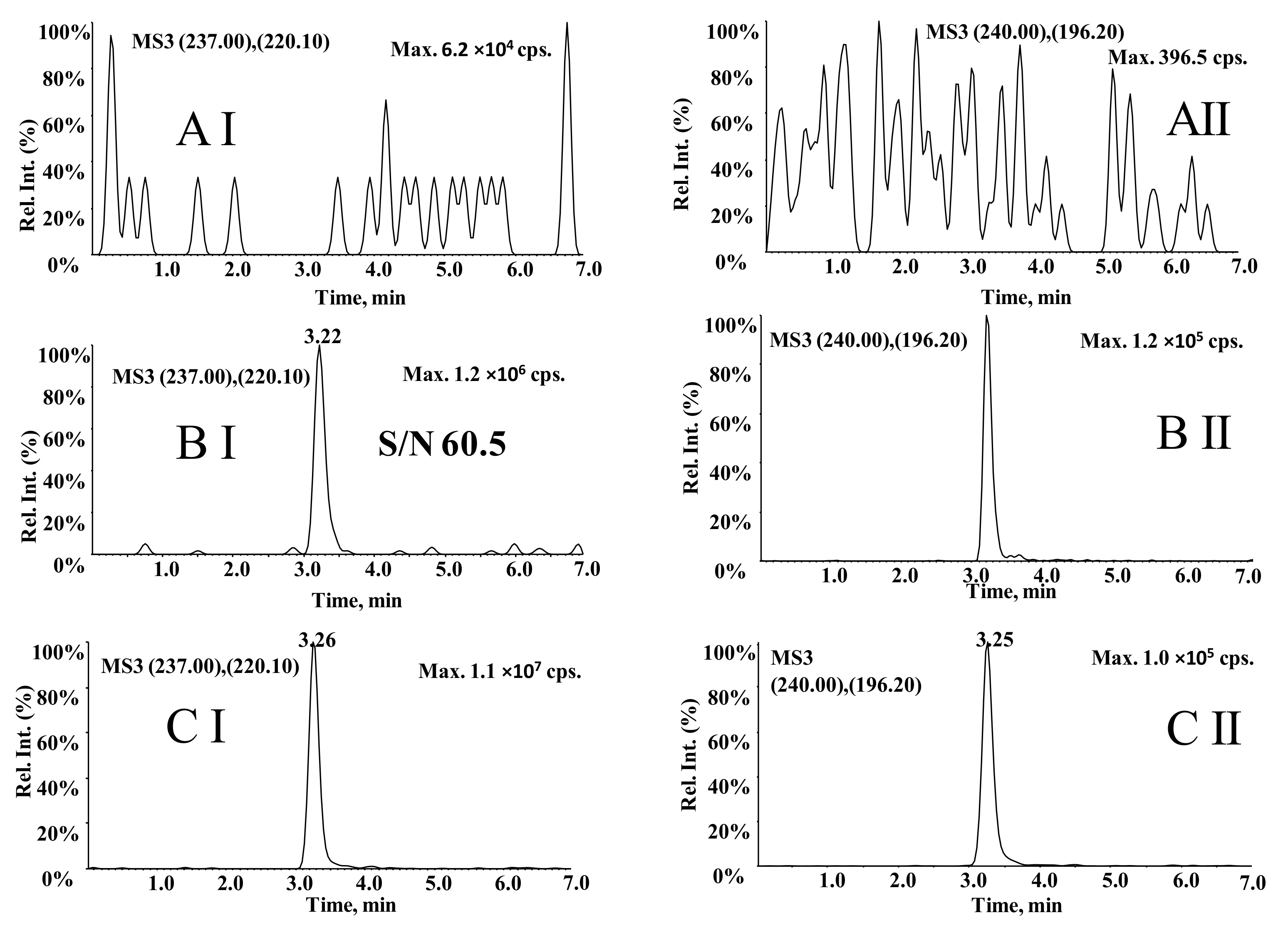
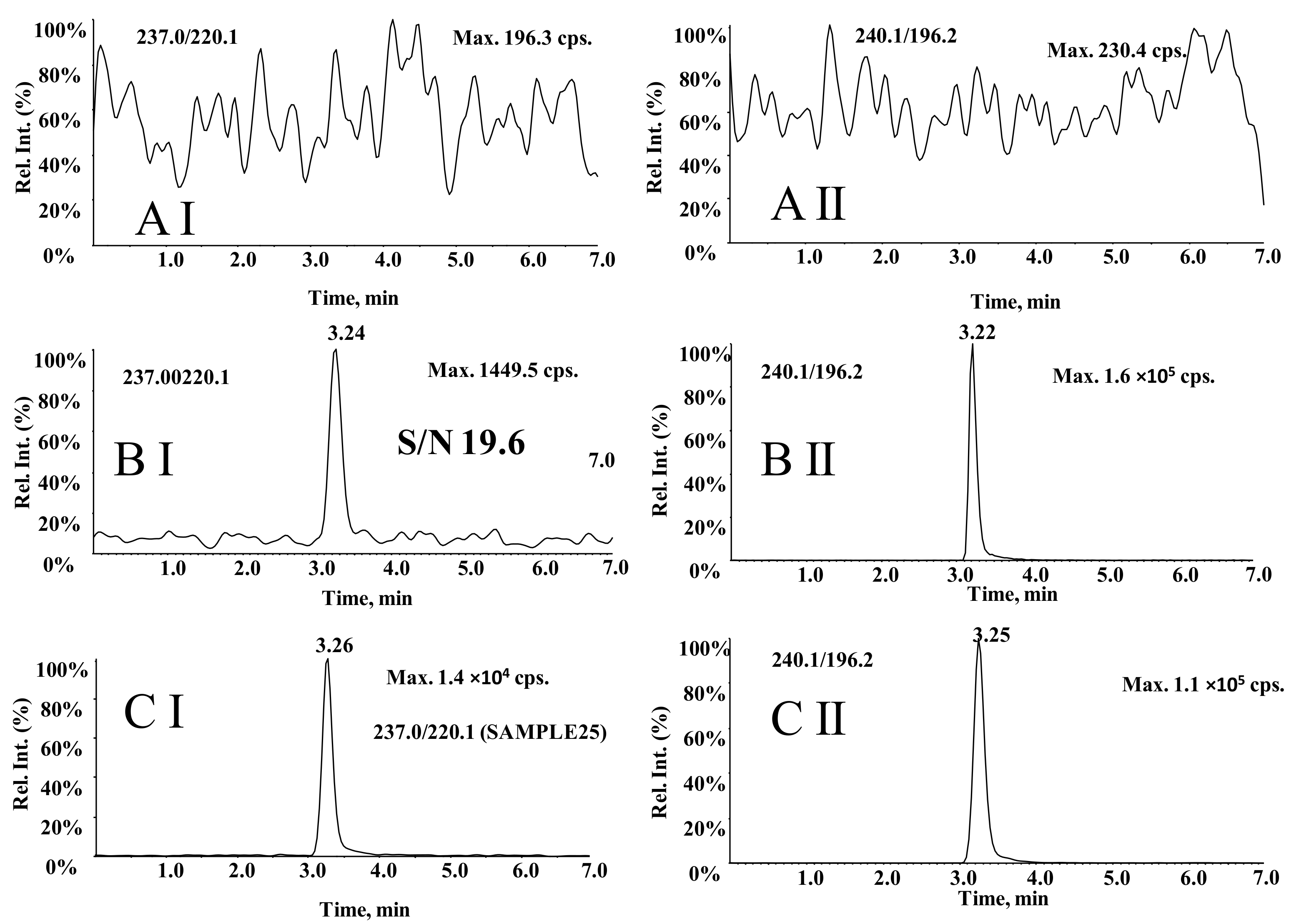
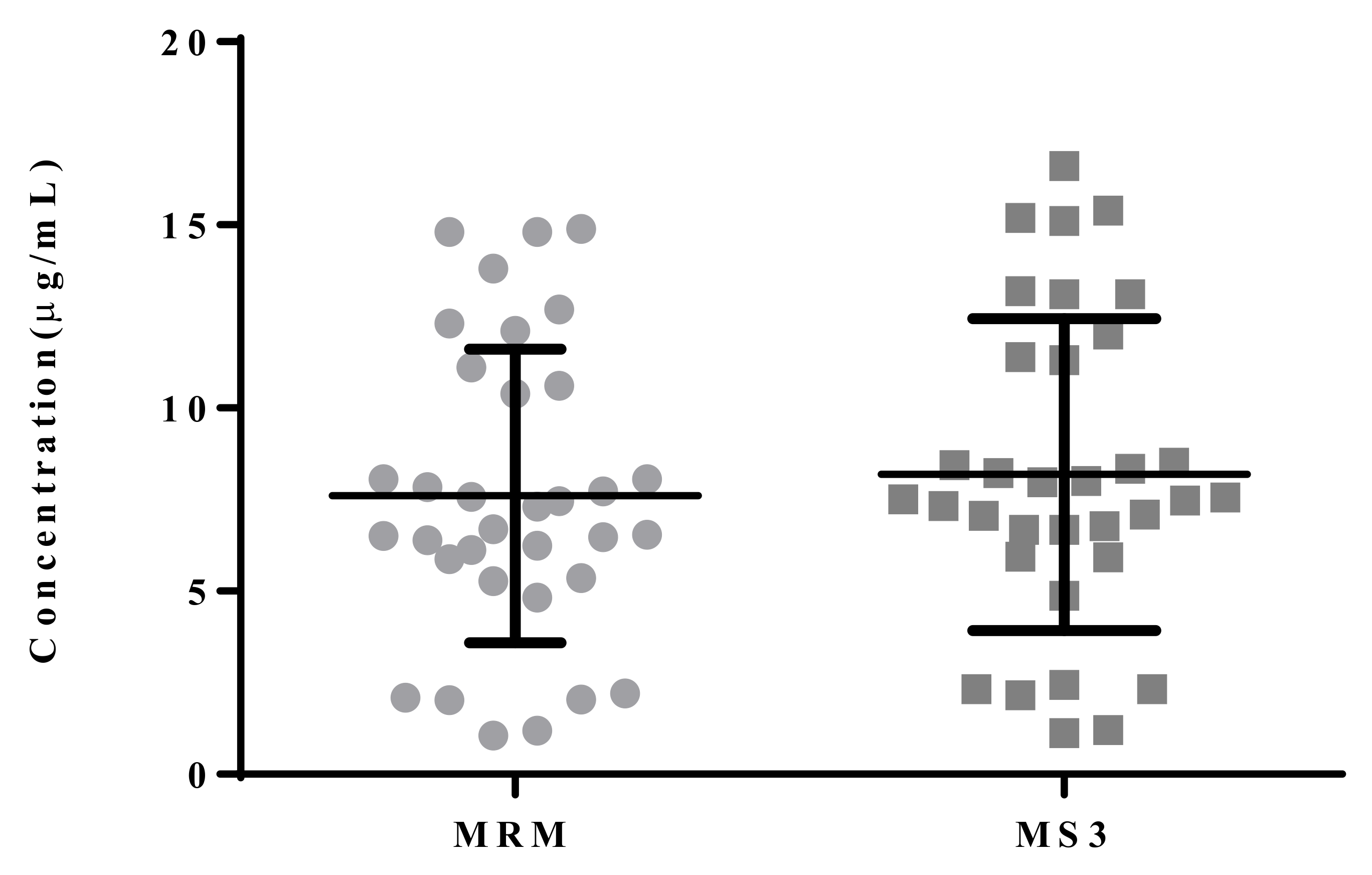
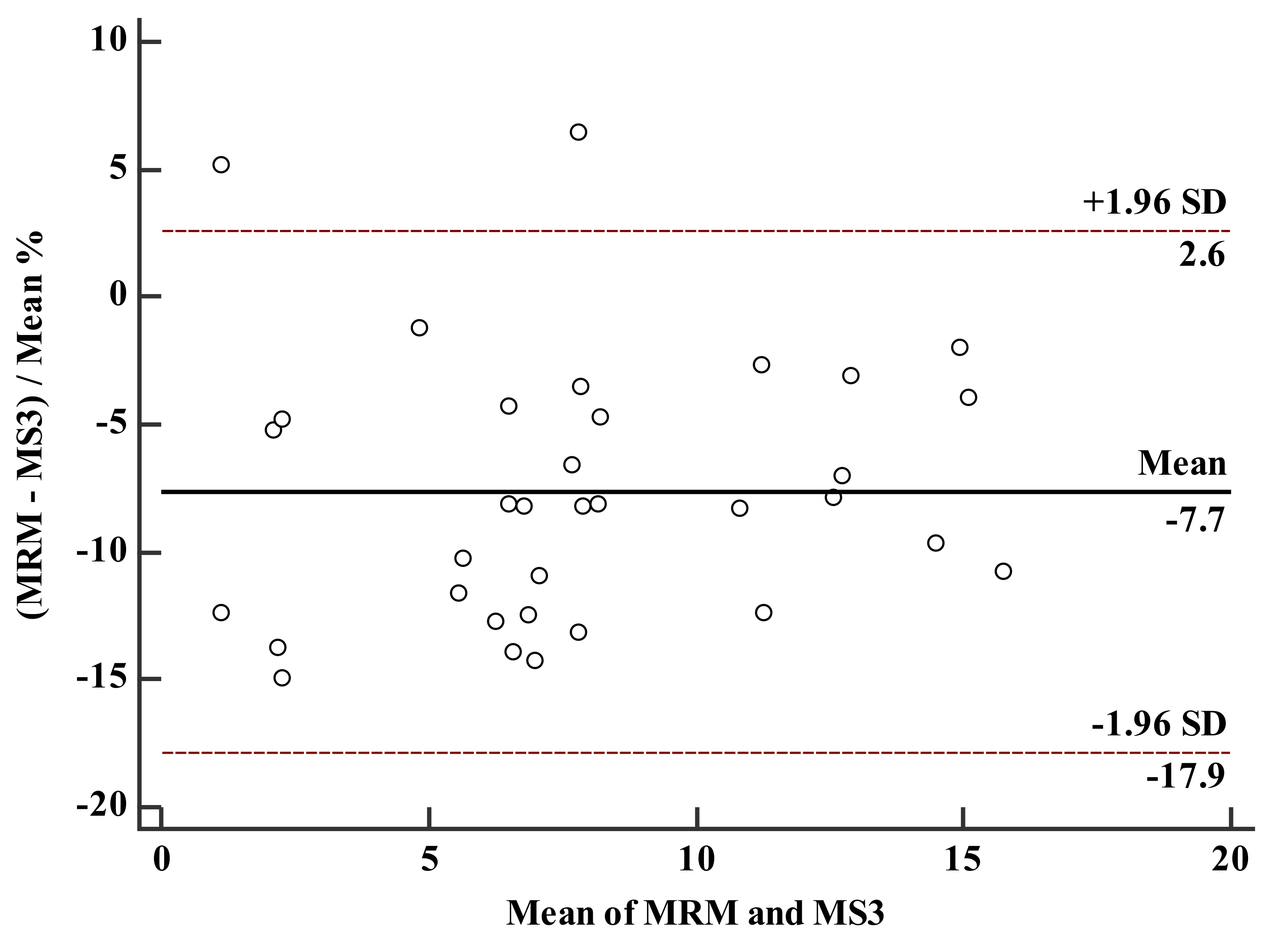
3.4. Comparison of LC–MS3 and LC-MRM Methods
4. Materials and Methods
4.1. Reagents
4.2. LC–MS3 Conditions
4.3. Calibration Standards and Preparing QC Samples
4.4. Sample Processing
4.5. Assay Validation
4.6. Blood Concentration Monitoring
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Carbamazepine for epilepsy. Drug Ther. Bull. 1967, 5, 83–84. [CrossRef]
- Poch, G.F. Carbamazepine in the treatment of epilepsy. Prensa Med. Argent. 1969, 56, 1263–1266. [Google Scholar]
- Elian, M. Treatment of epilepsy with carbamazepine. Harefuah. 1972, 82, 200–201. [Google Scholar] [PubMed]
- Cereghino, J.J.; Brock, J.T.; Van Meter, J.C.; Penry, J.K.; Smith, L.D.; White, B.G. Carbamazepine for epilepsy. A controlled prospective evaluation. Neurology 1974, 24, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Balogh, A.; Csanda, E. Clinical considerations in carbamazepine therapy of temporal lobe epilepsy. Orv. Hetil. 1982, 123, 3015–3020. [Google Scholar] [PubMed]
- Acharya, S.P.; Pundarikakshudu, K.; Panchal, A.; Lalwani, A. Development of carbamazepine transnasal microemulsion for treatment of epilepsy. Drug Deliv. Transl. Res. 2013, 3, 252–259. [Google Scholar] [CrossRef]
- Ullah, S.; Ali, N.; Khan, A.; Ali, S.; Nazish, H.R.; Uddin, Z. Epilepsy control with carbamazepine monotherapy from a genetic perspective. BMC Pharmacol. Toxicol. 2018, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- Keranen, T.; Sivenius, J. Side effects of carbamazepine, valproate and clonazepam during long-term treatment of epilepsy. Acta Neurol. Scand. Suppl. 1983, 97, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, A.; DuPont, S.; Zhou, D.; Matta, M.; Nagire, V.; Lagae, L. Current role of carbamazepine and oxcarbazepine in the management of epilepsy. Seizure 2020, 83, 251–263. [Google Scholar] [CrossRef]
- Lattanzi, S.; Belcastro, V. What place do carbamazepine-related antiepileptic drugs have in the modern day treatment of epilepsy? Expert Opin. Pharmacother. 2020, 21, 1655–1658. [Google Scholar] [CrossRef]
- Patel, V.H.; Schindlbeck, M.A.; Bryant, S.M. Delayed elevation in carbamazepine concentrations after overdose: A retrospective poison center study. Am. J. Ther. 2013, 20, 602–606. [Google Scholar] [CrossRef]
- Singh, H.; Nanjundappa, G.B.; Reddi, S.K.; Chandra, P.S. Carbamazepine induced asterixis with hyperammonemia: A case report with review of literature. Indian J. Psychol. Med. 2015, 37, 99–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, E.N.; Marks, A.; Lizer, M.H. Carbamazepine-induced hyperammonemia. Am. J. Health. Syst. Pharm. 2009, 66, 1468–1470. [Google Scholar] [CrossRef] [PubMed]
- Babaei, A.; Eslamai, M.H. Evaluation of therapeutic drug level monitoring of phenobarbital, phenytoin and carbamazepine in Iranian epileptic patients. Int. J. Clin. Pharmacol. Ther. 2007, 45, 121–125. [Google Scholar] [CrossRef]
- Descoeur, J.; Dupuy, A.M.; Bargnoux, A.S.; Cristol, J.P.; Mathieu, O. Comparison of four immunoassays to an HPLC method for the therapeutic drug monitoring of methotrexate: Influence of the hydroxylated metabolite levels and impact on clinical threshold. J. Oncol. Pharm. Pract. 2022, 28, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Afonso, J.; De Sousa, H.T.; Rosa, I.; Carvalho, J.; Dias, C.C.; Magro, F. Therapeutic drug monitoring of CT-P13: A comparison of four different immunoassays. Therap Adv. Gastroenterol. 2017, 10, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Shipkova, M.; Petrova, D.T.; Rosler, A.E.; Orth, M.; Engelmayer, J.; Wieland, E.; Brandhorst, G.; Oellerich, M. Comparability and imprecision of 8 frequently used commercially available immunoassays for therapeutic drug monitoring. Ther. Drug. Monit. 2014, 36, 433–441. [Google Scholar] [CrossRef]
- Haver, V.M.; Audino, N.; Burris, S.; Nelson, M. Four fluorescence polarization immunoassays for therapeutic drug monitoring evaluated. Clin. Chem. 1989, 35, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Blecka, L.J.; Jackson, G.J. Immunoassays in therapeutic drug monitoring. Clin. Lab. Med. 1987, 7, 357–370. [Google Scholar] [CrossRef]
- Marks, V. New developments in immunoassays for therapeutic drug monitoring. Antibiot. Chemother. 1979, 26, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Taibon, J.; Schmid, R.; Lucha, S.; Pongratz, S.; Tarasov, K.; Seger, C.; Timm, C.; Thiele, R.; Herlan, J.M.; Kobold, U. An LC-MS/MS based candidate reference method for the quantification of carbamazepine in human serum. Clin. Chim. Acta. 2017, 472, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Linder, C.; Andersson, M.; Wide, K.; Beck, O.; Pohanka, A. A LC-MS/MS method for therapeutic drug monitoring of carbamazepine, lamotrigine and valproic acid in DBS. Bioanalysis 2015, 7, 2031–2039. [Google Scholar] [CrossRef] [PubMed]
- El Hamd, M.A.; Wada, M.; Ikeda, R.; Kawakami, S.; Nakashima, K. Validation of an LC-MS/MS Method for the Determination of Propofol, Midazolam, and Carbamazepine in Rat Plasma: Application to Monitor Their Concentrations Following Co-administration. Biol. Pharm. Bull. 2015, 38, 1250–1253. [Google Scholar] [CrossRef] [Green Version]
- Calisto, V.; Bahlmann, A.; Schneider, R.J.; Esteves, V.I. Application of an ELISA to the quantification of carbamazepine in ground, surface and wastewaters and validation with LC-MS/MS. Chemosphere 2011, 84, 1708–1715. [Google Scholar] [CrossRef]
- McMillin, G.A.; Juenke, J.M.; Johnson, M.J.; Dasgupta, A. Discordant carbamazepine values between two immunoassays: Carbamazepine values determined by ADVIA Centaur correlate better with those determined by LC-MS/MS than PETINIA assay. Clin. Lab. Anal. 2011, 25, 212–216. [Google Scholar] [CrossRef]
- Carra, A.; Guidolin, V.; Dator, R.P.; Upadhyaya, P.; Kassie, F.; Villalta, P.W.; Balbo, S. Targeted High Resolution LC/MS3 Adductomics Method for the Characterization of Endogenous DNA Damage. Front. Chem. 2019, 7, 658. [Google Scholar] [CrossRef]
- Richards, K.H.; Monk, R.; Renko, K.; Rathmann, D.; Rijntjes, E.; Kohrle, J. A combined LC-MS/MS and LC-MS3 multi-method for the quantification of iodothyronines in human blood serum. Anal. Bioanal. Chem. 2019, 411, 5605–5616. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Zhang, Z.; Fawcett, J.P.; Sun, D.; Gu, J. Micro-solid phase extraction and LC-MS3 for the determination of triptorelin in rat plasma and application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2019, 166, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Dziadosz, M.; Klintschar, M.; Teske, J. Imatinib quantification in human serum with LC-MS3 as an effective way of protein kinase inhibitor analysis in biological matrices. Drug. Metab. Pers. Ther. 2017, 32, 147–150. [Google Scholar] [CrossRef]
- Dziadosz, M. The application of multiple analyte adduct formation in the LC-MS3 analysis of valproic acid in human serum. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2017, 1040, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Quinete, N.; Bertram, J.; Reska, M.; Lang, J.; Kraus, T. Highly selective and automated online SPE LC-MS3 method for determination of cortisol and cortisone in human hair as biomarker for stress related diseases. Talanta 2015, 134, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Szeitz, A.; Nguyen, T.A.; Riggs, K.W.; Rurak, D. A validated assay to quantitate serotonin in lamb plasma using ultrahigh-performance liquid chromatography-tandem mass spectrometry: Applications with LC/MS3. Anal. Bioanal. Chem. 2014, 406, 5055–5059. [Google Scholar] [CrossRef]
- Sanda, M.; Pompach, P.; Benicky, J.; Goldman, R. LC-MS3 quantification of O-glycopeptides in human serum. Electrophoresis 2013, 34, 2342–2349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, T.; Tatemori, S.; Muranaka, N.; Hirahara, Y.; Homma, S.; Nakane, T.; Takano, A.; Nomi, Y.; Otsuka, Y. The identification of vitamin E homologues in medicinal plant samples using ESI(+)-LC-MS3. J. Agric. Food Chem. 2012, 60, 9581–9588. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.C.; Hou, W.; Whitehead, S.N.; Ethier, M.; Bennett, S.A.; Figeys, D. Identification of lysophosphatidylcholine (LPC) and platelet activating factor (PAF) from PC12 cells and mouse cortex using liquid chromatography/multi-stage mass spectrometry (LC/MS3). Rapid Commun. Mass Spectrom. 2008, 22, 3579–3587. [Google Scholar] [CrossRef] [PubMed]

| Recovery (%) | |||
|---|---|---|---|
| Low QC | Medium QC | High QC | |
| Carbamazepine | 100.7 ± 9.20 | 110.5 ± 7.00 | 110.2 ± 4.00 |
| Compound | Nominal Conc. (μg/mL) | Long Term −70 °C | Short Term | Freeze– Thaw | Post- Preparative |
|---|---|---|---|---|---|
| Carbamazepine | 1.0 | 99.9 ± 3.77 | 105.0 ± 2.65 | 98.0± 5.75 | 101.5 ± 7.21 |
| 5.0 | 96.4 ± 1.51 | 101.5 ± 7.23 | 100.4 ± 5.25 | 91.6 ± 3.52 | |
| 15 | 94.4 ± 2.52 | 98.4 ±3.67 | 94.9 ± 3.01 | 89.3 ± 0.67 |
| Sample ID | MRM | MS3 | % |
|---|---|---|---|
| sample1 (74 years, female) | 6.39 | 6.67 | 95.8 |
| sample2 (34 years, female) | 8.06 | 7.56 | 106.6 |
| sample3 (40 years, male) | 2.03 | 2.33 | 87.1 |
| sample4 (29 years, male) | 1.06 | 1.2 | 88.3 |
| sample5 (23 years, male) | 11.1 | 11.4 | 97.4 |
| sample6 (61 years, male) | 12.1 | 13.1 | 92.4 |
| sample7 (34 years, female) | 13.8 | 15.2 | 90.8 |
| sample8 (37 years, female) | 14.8 | 15.4 | 96.1 |
| sample9 (30 years, male) | 5.87 | 6.67 | 88.0 |
| sample10 (33 years, female) | 7.31 | 8.34 | 87.6 |
| sample11 (27 years, male) | 2.1 | 2.44 | 86.1 |
| sample12 (29 years, male) | 2.05 | 2.16 | 94.9 |
| sample13 (25 years, male) | 5.36 | 5.94 | 90.2 |
| sample14 (57 years, female) | 14.9 | 16.6 | 89.8 |
| sample15 (23 years, male) | 6.47 | 7.33 | 88.3 |
| sample16 (30 years, female) | 7.84 | 8.51 | 92.1 |
| sample17 (34 years, male) | 6.13 | 7.05 | 87.0 |
| sample18 (23 years, female) | 6.5 | 7.5 | 86.7 |
| sample19 (33 years, female) | 7.46 | 7.97 | 93.6 |
| sample20 (39 years, female) | 2.21 | 2.32 | 95.3 |
| sample21 (9 years, male) | 1.19 | 1.13 | 105.3 |
| sample22 (14 years, male) | 10.6 | 12 | 88.3 |
| sample23 (58 years, female) | 12.7 | 13.1 | 96.9 |
| sample24 (20 years, female) | 5.27 | 5.92 | 89.0 |
| sample25 (40 years, female) | 6.24 | 6.77 | 92.2 |
| sample26 (36years, male) | 7.72 | 8 | 96.5 |
| sample27 (29 years, female) | 10.4 | 11.3 | 92.0 |
| sample28 (39 years, female) | 12.3 | 13.2 | 93.2 |
| sample29 (52 years, male) | 14.8 | 15.1 | 98.0 |
| sample30 (19 years, female) | 6.54 | 7.1 | 92.1 |
| sample31 (25 years, female) | 8.05 | 8.44 | 95.4 |
| sample32 (20 years, male) | 6.70 | 7.48 | 89.6 |
| sample33 (38 years, female) | 7.58 | 8.23 | 92.1 |
| sample34 (29 years, male) | 4.82 | 4.88 | 98.8 |
| mean | 7.60 | 8.19 | |
| SD | 4.01 | 4.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, D.; Ji, Z.; Cao, H.; Huang, J.; Zeng, L.; Yin, L. LC–MS3 Strategy for Quantification of Carbamazepine in Human Plasma and Its Application in Therapeutic Drug Monitoring. Molecules 2022, 27, 1224. https://doi.org/10.3390/molecules27041224
Ma D, Ji Z, Cao H, Huang J, Zeng L, Yin L. LC–MS3 Strategy for Quantification of Carbamazepine in Human Plasma and Its Application in Therapeutic Drug Monitoring. Molecules. 2022; 27(4):1224. https://doi.org/10.3390/molecules27041224
Chicago/Turabian StyleMa, Dongxiao, Zhengchao Ji, Haiwei Cao, Jing Huang, Lei Zeng, and Lei Yin. 2022. "LC–MS3 Strategy for Quantification of Carbamazepine in Human Plasma and Its Application in Therapeutic Drug Monitoring" Molecules 27, no. 4: 1224. https://doi.org/10.3390/molecules27041224
APA StyleMa, D., Ji, Z., Cao, H., Huang, J., Zeng, L., & Yin, L. (2022). LC–MS3 Strategy for Quantification of Carbamazepine in Human Plasma and Its Application in Therapeutic Drug Monitoring. Molecules, 27(4), 1224. https://doi.org/10.3390/molecules27041224






