Screening of Acrylamide of Par-Fried Frozen French Fries Using Portable FT-IR Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. French Fry Material
3.2. Acrylamide Extraction and LC-MS/MS Analysis
3.3. FT-IR Spectroscopy
3.4. Chemometric Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mesías, M.; Morales, F.J. Acrylamide in commercial potato crisps from Spanish market: Trends from 2004 to 2014 and assessment of the dietary exposure. Food Chem. Toxicol. 2015, 81, 104–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, L.L.; Jia, Q.; Liu, C.; Wang, W.; Duan, L.; Yang, G.; Han, C.Q.; Li, H. Thin layer chromatography combined with surface-enhanced raman spectroscopy for rapid sensing aflatoxins. J. Chromatogr. A 2018, 1579, 115–120. [Google Scholar] [CrossRef]
- Yadav, A.; Sengar, N.; Issac, A.; Dutta, M.K. Image processing based acrylamide detection from fried potato chip images using continuous wavelet transform. Comput. Electron. Agric. 2018, 145, 349–362. [Google Scholar] [CrossRef]
- Wawrzyniak, R.; Jasiewicz, B. Straightforward and rapid determination of acrylamide in coffee beans by means of HS-SPME/GC-MS. Food Chem. 2019, 301, 125264. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Zhang, P.; Xiang, L.; Wang, H.; Rao, P.; Wang, S. Interaction of acrylamide with micelles in French fry aqueous extracts. Food Control 2020, 110, 106974. [Google Scholar] [CrossRef]
- Bušová, M.; Bencko, V.; Laktičová, K.V.; Holcátová, I.; Vargová, M. Risk of exposure to acrylamide. Cent. Eur. J. Public Health 2020, 28, S43–S46. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Internal Report 14/002. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/08/14-002.pdf (accessed on 15 August 2021).
- Gökmen, V.; Şenyuva, H.Z.; Acar, J.; Sarioǧlu, K. Determination of acrylamide in potato chips and crisps by high-performance liquid chromatography. J. Chromatogr. A 2005, 1088, 193–199. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Acrylamide in Food What is Acrylamide? Why is it Present in Food? An Overview of EFSA’ s Risk Assessment: What Are the Risks for Consumers of Acrylamide in Food? What Happens to Acrylamide in the Body? What Is the Margin of Exposure? 2015, pp. 1–4. Available online: https://www.efsa.europa.eu/sites/default/files/corporate_publications/files/acrylamide150604.pdf (accessed on 20 August 2021).
- Olesen, P.T.; Olsen, A.; Frandsen, H.; Frederiksen, K.; Overvad, K.; Tjønneland, A. Acrylamide exposure and incidence of breast cancer among postmenopausal women in the Danish Diet, Cancer and Health study. Int. J. Cancer 2008, 122, 2094–2100. [Google Scholar] [CrossRef]
- Svensson, K.; Abramsson, L.; Becker, W.; Glynn, A.; Hellenäs, K.E.; Lind, Y.; Rosén, J. Dietary intake of acrylamide in Sweden. Food Chem. Toxicol. 2003, 41, 1581–1586. [Google Scholar] [CrossRef]
- Romani, S.; Bacchiocca, M.; Rocculi, P.; Dalla Rosa, M. Effect of frying time on acrylamide content and quality aspects of French fries. Eur. Food Res. Technol. 2008, 226, 555–560. [Google Scholar] [CrossRef]
- Jackson, L.S.; Al-Taher, F. Effects of Consumer Food Preparation on Acrylamide Formation. In Chemistry and Safety of Acrylamide in Food. Advances in Experimental Medicine and Biology; Friedman, M., Mottram, D., Eds.; Springer: Boston, MA, USA, 2005; pp. 447–465. [Google Scholar]
- California Office of Environmental Health Hazard Assessment Proposition 65. Available online: https://oehha.ca.gov/proposition-65 (accessed on 22 August 2021).
- Mills, C.; Mottram, D.S.; Wedzicha, B.L. Acrylamide. In Process-Induced Food Toxicants Occurance, Formation, Mitigation, and Health Risks; Stadler, R.H., Lineback, D.R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 23–50. [Google Scholar]
- Pedreschi, F.; León, J.; Mery, D.; Moyano, P.; Pedreschi, R.; Kaack, K.; Granby, K. Color development and acrylamide content of pre-dried potato chips. J. Food Eng. 2007, 79, 786–793. [Google Scholar] [CrossRef]
- Chuang, W.H.; Chiu, C.P.; Chen, B.H. Analysis and Formation of Acrylamide in French Fries and Chicken Legs During Frying. J. Food Biochem. 2005, 30, 497–507. [Google Scholar] [CrossRef]
- Katz, J.M.; Winter, C.K.; Buttrey, S.E.; Fadel, J.G. Comparison of acrylamide intake from Western and guideline based diets using probabilistic techniques and linear programming. Food Chem. Toxicol. 2012, 50, 877–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EU Commission Regulation (EU) 2017/2158: Establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. Off. J. Eur. Union 2017, 304, 24–44.
- Rodriguez-Saona, L.; Aykas, D.P.; Borba, K.R.; Urtubia, A. Miniaturization of optical sensors and their potential for high-throughput screening of foods. Curr. Opin. Food Sci. 2020, 31, 136–150. [Google Scholar] [CrossRef]
- Pedreschi, F.; Segtnan, V.H.; Knutsen, S.H. On-line monitoring of fat, dry matter and acrylamide contents in potato chips using near infrared interactance and visual reflectance imaging. Food Chem. 2010, 121, 616–620. [Google Scholar] [CrossRef]
- Ayvaz, H.; Rodriguez-Saona, L.E. Application of handheld and portable spectrometers for screening acrylamide content in commercial potato chips. Food Chem. 2015, 174, 154–162. [Google Scholar] [CrossRef]
- Adedipe, O.E.; Johanningsmeier, S.D.; Truong, V.D.; Yencho, G.C. Development and Validation of a Near-Infrared Spectroscopy Method for the Prediction of Acrylamide Content in French-Fried Potato. J. Agric. Food Chem. 2016, 64, 1850–1860. [Google Scholar] [CrossRef]
- Gökmen, V.; Palazoǧlu, T.K.; Şenyuva, H.Z. Relation between the acrylamide formation and time-temperature history of surface and core regions of French fries. J. Food Eng. 2006, 77, 972–976. [Google Scholar] [CrossRef]
- Becalski, A.; Lau, B.P.Y.; Lewis, D.; Seaman, S.W. Acrylamide in foods: Occurrence, sources, and modeling. J. Agric. Food Chem. 2003, 51, 802–808. [Google Scholar] [CrossRef]
- Mesias, M.; Delgado-Andrade, C.; Holgado, F.; Morales, F.J. Acrylamide content in French fries prepared in food service establishments. LWT Food Sci. Technol. 2019, 100, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Claeys, W.L.; De Vleeschouwer, K.; Hendrickx, M.E. Kinetics of acrylamide formation and elimination during heating of an asparagine-sugar model system. J. Agric. Food Chem. 2005, 53, 9999–10005. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.I.F.S.; Van Boekel, M.A.J.S. A kinetic model for the glucose/glycine Maillard reaction pathways. Food Chem. 2005, 90, 257–269. [Google Scholar] [CrossRef]
- Knol, J.J.; Van Loon, W.A.M.; Linssen, J.P.H.; Ruck, A.L.; Van Boekel, M.A.J.S.; Voragen, A.G.J. Toward a kinetic model for acrylamide formation in a glucose-asparagine reaction system. J. Agric. Food Chem. 2005, 53, 6133–6139. [Google Scholar] [CrossRef] [PubMed]
- Ścibisz, I.; Reich, M.; Bureau, S.; Gouble, B.; Causse, M.; Bertrand, D.; Renard, C.M.G.C. Mid-infrared spectroscopy as a tool for rapid determination of internal quality parameters in tomato. Food Chem. 2011, 125, 1390–1397. [Google Scholar] [CrossRef]
- Santos, F.B.; Miranda, N.T.; Schiavon, M.I.R.B.; Fregolente, L.V.; Wolf Maciel, M.R. Thermal degradation kinetic of poly(acrylamide-co-sodium acrylate) hydrogel applying isoconversional methods. J. Therm. Anal. Calorim. 2021, 146, 2503–2514. [Google Scholar] [CrossRef]
- Murugan, R.; Mohan, S.; Bigotto, A. FTIR and Polarised Raman Spectra of Acrylamide and Polyacrylamide. J. Korean Phys. Soc. 1998, 32, 505–512. [Google Scholar]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiroma, C.; Rodriguez-Saona, L. Application of NIR and MIR spectroscopy in quality control of potato chips. J. Food Compos. Anal. 2009, 22, 596–605. [Google Scholar] [CrossRef]
- Cruz, G.; Cruz-Tirado, J.P.; Delgado, K.; Guzman, Y.; Castro, F.; Rojas, M.L.; Linares, G. Impact of pre-drying and frying time on physical properties and sensorial acceptability of fried potato chips. J. Food Sci. Technol. 2018, 55, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.D.; Cabo, N. Infrared spectroscopy in the study of edible oils and fats. J. Sci. Food Agric. 1997, 75, 1–11. [Google Scholar] [CrossRef]
- Derevyashkin, S.V.; Soboleva, E.A.; Shelkovnikov, V.V.; Orlova, N.A.; Malakhov, I.A.; Berezhnaya, V.N.; Savina, E.D.; Tsentalovich, Y.P. Phototransformations of acrylamide derivatives of piperazine-substituted polyfluorinated chalcones. J. Photochem. Photobiol. A Chem. 2021, 406, 112973. [Google Scholar] [CrossRef]
- Czarnecka, E.; Nowaczyk, J. Synthesis and characterization superabsorbent polymers made of starch, acrylic acid, acrylamide, poly(Vinyl alcohol), 2-hydroxyethyl methacrylate, 2-acrylamido-2-methylpropane sulfonic acid. Int. J. Mol. Sci. 2021, 22, 4325. [Google Scholar] [CrossRef] [PubMed]
- Moyano, P.C.; Pedreschi, F. Kinetics of oil uptake during frying of potato slices: Effect of pre-treatments. LWT Food Sci. Technol. 2006, 39, 285–291. [Google Scholar] [CrossRef]
- Segtnan, V.H.; Kita, A.; Mielnik, M.; Jørgensen, K.; Knutsen, S.H. Screening of acrylamide contents in potato crisps using process variable settings and near-infrared spectroscopy. Mol. Nutr. Food Res. 2006, 50, 811–817. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Allendorf, M.E. Use of FTIR for rapid authentication and detection of adulteration of food. Annu. Rev. Food Sci. Technol. 2011, 2, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Sivakesava, S.; Irudayaraj, J. Rapid determination of tetracycline in milk by FT-MIR and FT-NIR spectroscopy. J. Dairy Sci. 2002, 85, 487–493. [Google Scholar] [CrossRef]
- Lu, R.; Mizaikoff, B.; Li, W.W.; Qian, C.; Katzir, A.; Raichlin, Y.; Sheng, G.P.; Yu, H.Q. Determination of chlorinated hydrocarbons in water using highly sensitive mid-infrared sensor technology. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Pejcic, B.; Myers, M.; Ross, A. Mid-infrared sensing of organic pollutants in aqueous environments. Sensors 2009, 9, 6232–6253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kos, G.; Sieger, M.; McMullin, D.; Zahradnik, C.; Sulyok, M.; Öner, T.; Mizaikoff, B.; Krska, R. A novel chemometric classification for FTIR spectra of mycotoxin-contaminated maize and peanuts at regulatory limits. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2016, 33, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Mastovska, K.; Lehotay, S.J. Rapid sample preparation method for LC-MS/MS or GC-MS analysis of acrylamide in various food matrices. J. Agric. Food Chem. 2006, 54, 7001–7008. [Google Scholar] [CrossRef] [PubMed]
- Abdi, H. Partial least squares regression and projection on latent structure regression (PLS Regression). Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 97–106. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Hawkins, D.M. The Problem of Overfitting. J. Chem. Inf. Comput. Sci. 2004, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D.; Kwiatkowski, M.J.; Parker, M.; Cynkar, W.U.; Dambergs, R.G.; Gishen, M.; Herderich, M.J. Prediction of phenolic compounds in red wine fermentations by visible and near infrared spectroscopy. Anal. Chim. Acta 2004, 513, 73–80. [Google Scholar] [CrossRef]
- Williams, P. The RPD Statistic: A Tutorial Note. NIR News 2014, 25, 22–26. [Google Scholar] [CrossRef]
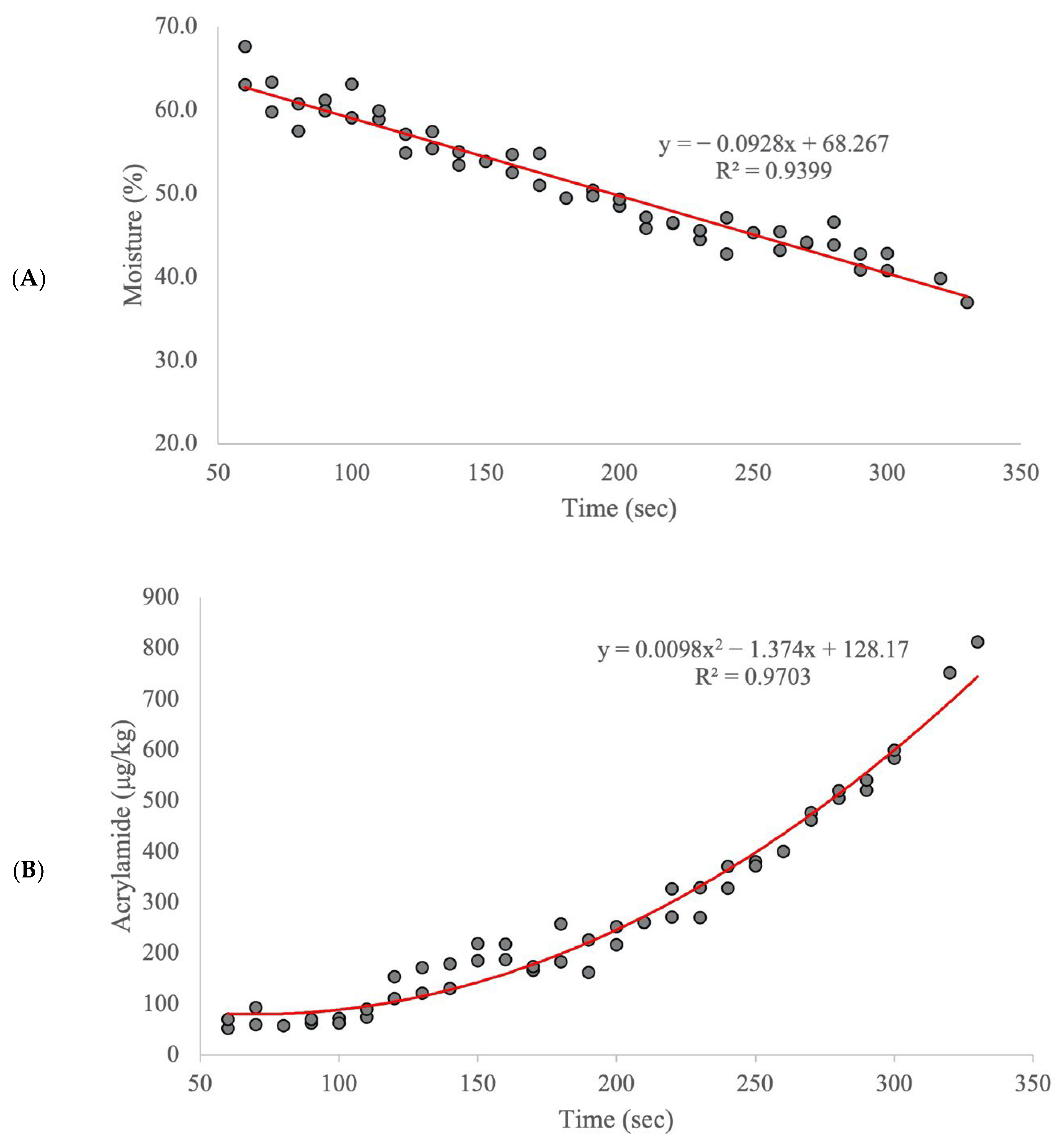
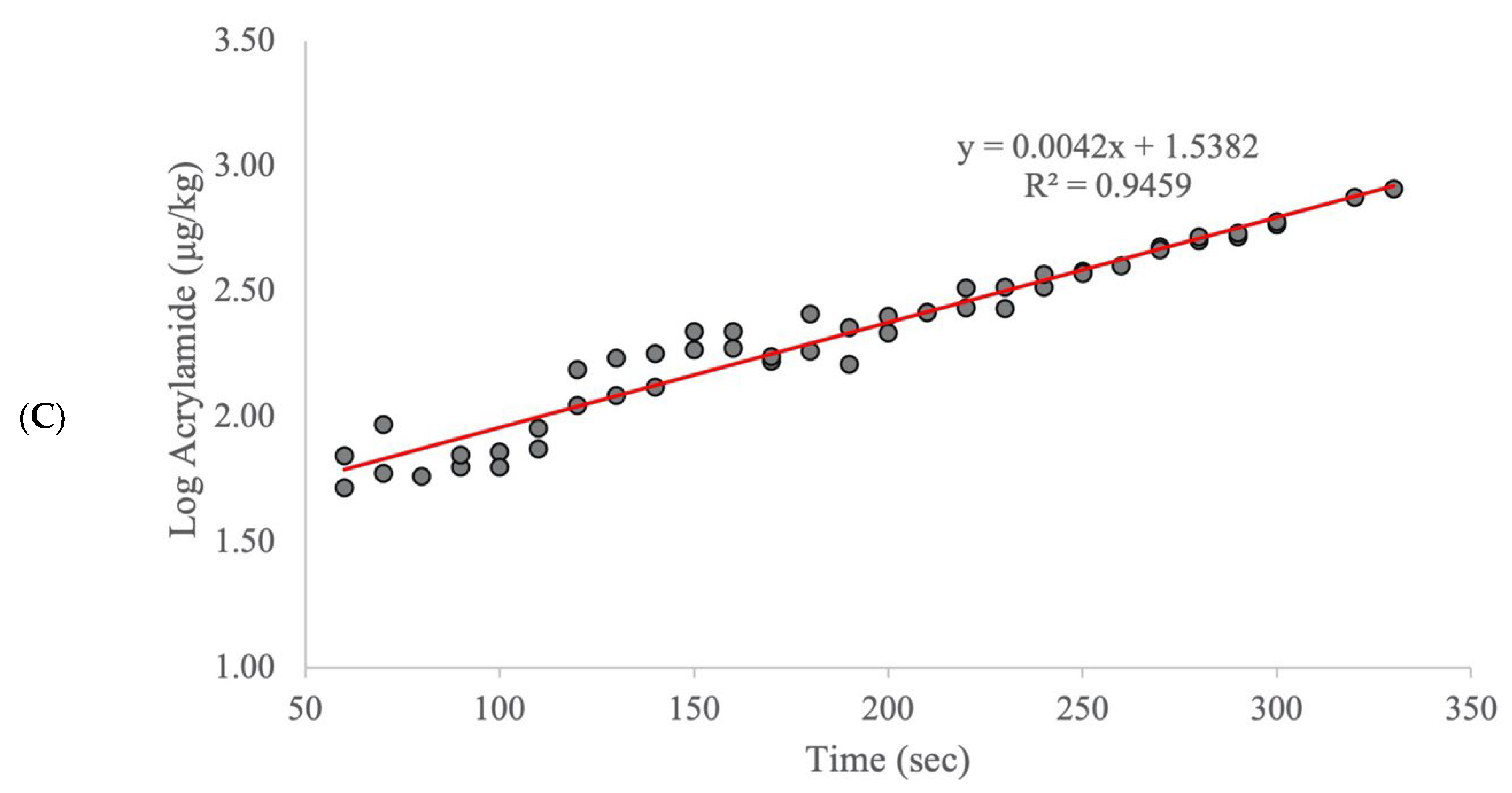
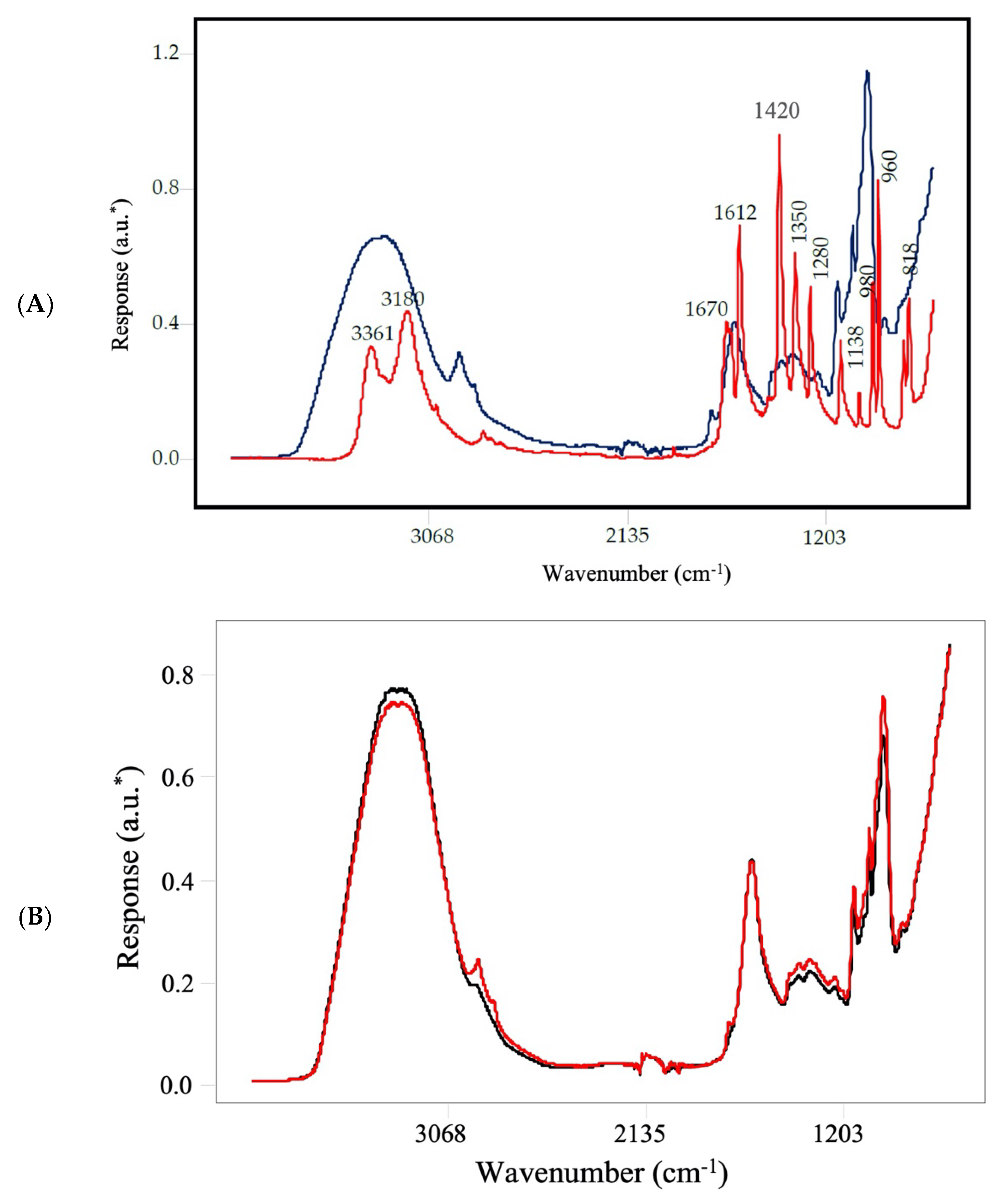
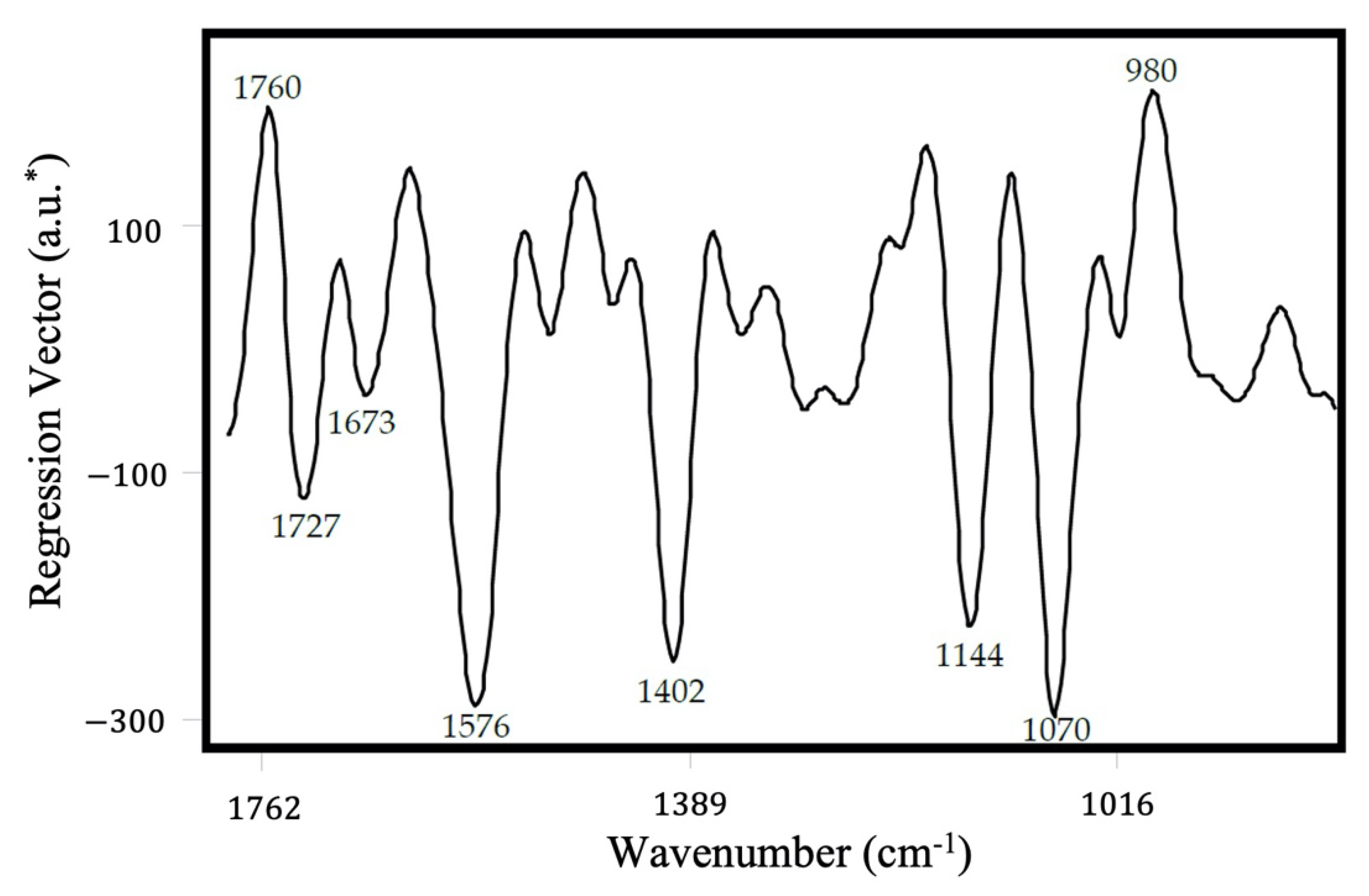
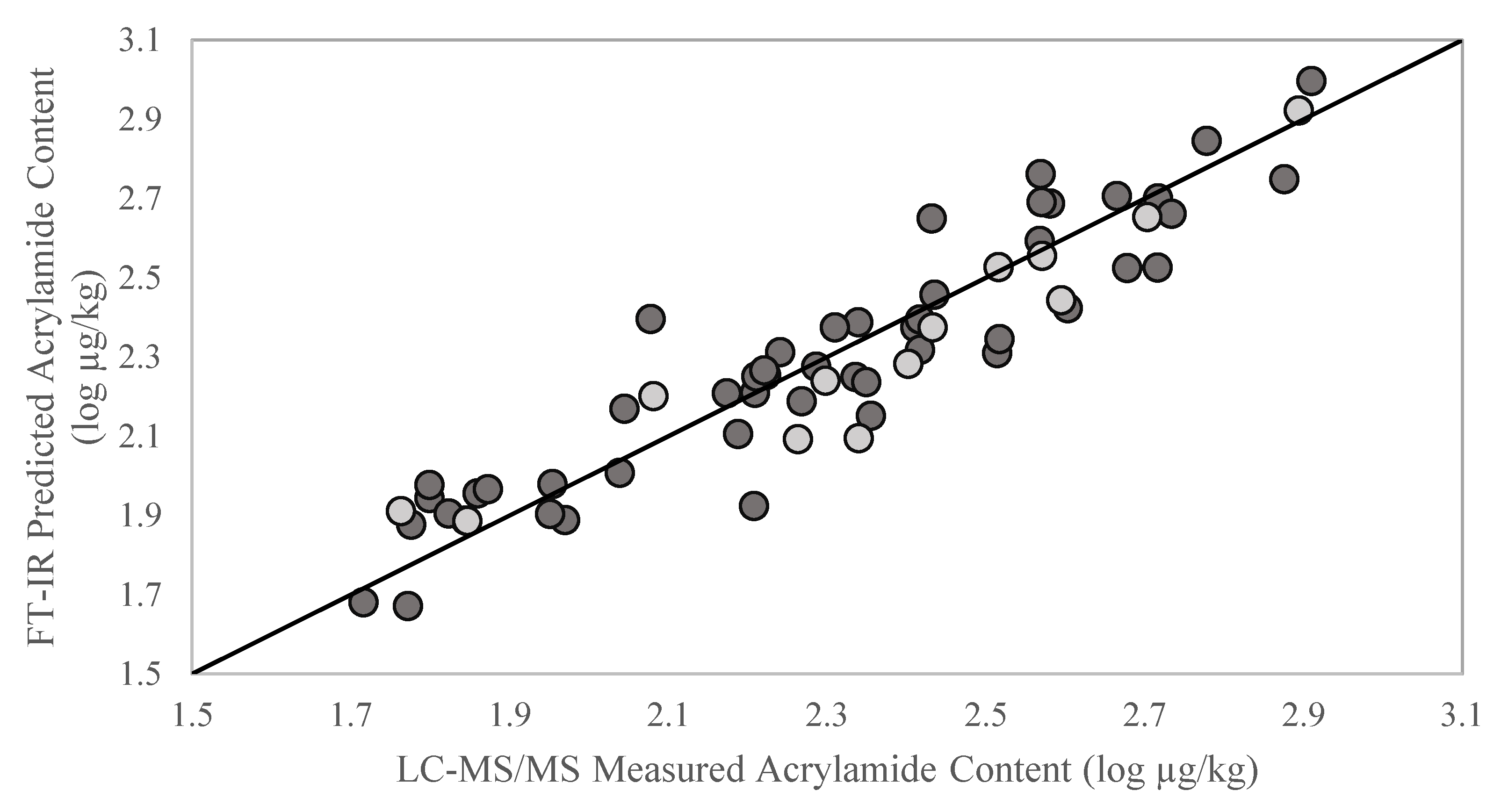
| Calibration Model | External Validation Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Range | N a | Factor | SECV b | RCV c | Range | n d | SEP e | Rpre f | RPD g |
| 52.0–812.8 | 53 | 7 | 58.7 | 0.93 | 57.9–783.4 | 13 | 55.1 | 0.94 | 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aykas, D.P.; Urtubia, A.; Wong, K.; Ren, L.; López-Lira, C.; Rodriguez-Saona, L.E. Screening of Acrylamide of Par-Fried Frozen French Fries Using Portable FT-IR Spectroscopy. Molecules 2022, 27, 1161. https://doi.org/10.3390/molecules27041161
Aykas DP, Urtubia A, Wong K, Ren L, López-Lira C, Rodriguez-Saona LE. Screening of Acrylamide of Par-Fried Frozen French Fries Using Portable FT-IR Spectroscopy. Molecules. 2022; 27(4):1161. https://doi.org/10.3390/molecules27041161
Chicago/Turabian StyleAykas, Didem P., Alejandra Urtubia, Kevin Wong, Luju Ren, Claudia López-Lira, and Luis E. Rodriguez-Saona. 2022. "Screening of Acrylamide of Par-Fried Frozen French Fries Using Portable FT-IR Spectroscopy" Molecules 27, no. 4: 1161. https://doi.org/10.3390/molecules27041161
APA StyleAykas, D. P., Urtubia, A., Wong, K., Ren, L., López-Lira, C., & Rodriguez-Saona, L. E. (2022). Screening of Acrylamide of Par-Fried Frozen French Fries Using Portable FT-IR Spectroscopy. Molecules, 27(4), 1161. https://doi.org/10.3390/molecules27041161









