Microwave Synthesis of Copper Phyllosilicates as Effective Catalysts for Hydrogenation of C≡C Bonds
Abstract
:1. Introduction
2. Results
2.1. Physico-Chemical Properties of Catalysts
2.2. Catalytic Properties in the Selective Hydrogenation of Unsaturated Compounds
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kottappara, R.; Pillai, S.C.; Vijayan, B.K. Copper-based nanocatalysts for nitroarene reduction-A review of recent advances. Inorg. Chem. Commun. 2020, 121, 108181. [Google Scholar] [CrossRef]
- Yue, H.; Zhao, Y.; Zhao, S.; Wang, B.; Ma, X.; Gong, J. A copper-phyllosilicate core-sheath nanoreactor for carbon-oxygen hydrogenolysis reactions. Nat. Commun. 2013, 4, 2339. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Gao, X.; Zhu, Y.; Zhu, Y.; Zheng, H.; Li, Y. Promoting effect of boron oxide on Cu/SiO2 catalyst for glycerol hydrogenolysis to 1,2-propanediol. J. Catal. 2013, 303, 70–79. [Google Scholar] [CrossRef]
- Bozbag, S.E.; Sot, P.; Nachtegaal, M.; Ranocchiari, M.; van Bokhoven, J.A.; Mesters, C. Direct Stepwise Oxidation of Methane to Methanol over Cu-SiO2. ACS Catal. 2018, 8, 5721–5731. [Google Scholar] [CrossRef] [Green Version]
- Song, E.H.; Wen, Z.; Jiang, Q. CO Catalytic Oxidation on Copper-Embedded Graphene. J. Phys. Chem. C 2011, 115, 3678–3683. [Google Scholar] [CrossRef]
- To, D.-T.; Lin, Y.-C. Copper Phyllosilicates-Derived Catalysts in the Production of Alcohols from Hydrogenation of Carboxylates, Carboxylic Acids, Carbonates, Formyls, and CO2: A Review. Catalysts 2021, 11, 255. [Google Scholar] [CrossRef]
- Zhang, B.; Hui, S.; Zhang, S.; Ji, Y.; Li, W.; Fang, D. Effect of copper loading on texture, structure and catalytic performance of Cu/SiO2 catalyst for hydrogenation of dimethyl oxalate to ethylene glycol. J. Nat. Gas Chem. 2012, 21, 563–570. [Google Scholar] [CrossRef]
- Wang, X.; Ma, K.; Guo, L.; Tian, Y.; Cheng, Q.; Bai, X.; Huang, J.; Ding, T.; Li, X. Cu/ZnO/SiO2 catalyst synthesized by reduction of ZnO-modified copper phyllosilicate for dimethyl ether steam reforming. Appl. Catal. A Gen. 2017, 540, 37–46. [Google Scholar] [CrossRef]
- Dong, X.; Ma, X.; Xu, H.; Ge, Q. Comparative study of silica-supported copper catalysts prepared by different methods: Formation and transition of copper phyllosilicate. Catal. Sci. Technol. 2016, 6, 4151–4158. [Google Scholar] [CrossRef]
- Li, F.; Lu, C.-S.; Li, X.-N. The effect of the amount of ammonia on the Cu0/Cu+ ratio of Cu/SiO2 catalyst for the hydrogenation of dimethyl oxalate to ethylene glycol. Chin. Chem. Lett. 2014, 25, 1461–1465. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, X.; Chen, B.; Tu, Z.; Gutiérrez, O.Y.; Cui, Y.; Wang, J.; Huang, J.; Xu, Y.; Sun, H.; et al. Copper-Based Catalysts Confined in Carbon Nanocage Reactors for Condensed Ester Hydrogenation: Tuning Copper Species by Confined SiO2 and Methanol Resistance. ACS Sustain. Chem. Eng. 2021, 9, 16270–16280. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, N.; Wang, K.; Zhang, S.; Wu, J. Effect of copper nanoparticles dispersion on catalytic performance of Cu/SiO2 catalyst for hydrogenation of dimethyl oxalate to ethylene glycol. J. Nanomater. 2013, 2013, 629375. [Google Scholar]
- Van der Grift, C.J.G.; Elberse, P.A.; Mulder, A.; Geus, J.W. Preparation of silica-supported copper catalysts by means of deposition-precipitation. Appl. Catal. 1990, 59, 275–289. [Google Scholar] [CrossRef]
- Di, W.; Cheng, J.; Tian, S.; Li, J.; Chen, J.; Sun, Q. Synthesis and characterization of supported copper phyllosilicate catalysts for acetic ester hydrogenation to ethanol. Appl. Catal. A Gen. 2016, 510, 244–259. [Google Scholar] [CrossRef]
- Du, H.; Ma, X.; Yan, P.; Jiang, M.; Zhao, Z.; Zhang, Z.C. Catalytic furfural hydrogenation to furfuryl alcohol over Cu/SiO2 catalysts: A comparative study of the preparation methods. Fuel Process. Technol. 2019, 193, 221–231. [Google Scholar] [CrossRef]
- Pompe, C.E.; Slagter, M.; de Jongh, P.E.; de Jong, K.P. Impact of heterogeneities in silica-supported copper catalysts on their stability for methanol synthesis. J. Catal. 2018, 365, 1–9. [Google Scholar] [CrossRef]
- Kostyukhin, E.M.; Nissenbaum, V.D.; Abkhalimov, E.V.; Kustov, A.L.; Ershov, B.G.; Kustov, L.M. Microwave-Assisted Synthesis of Water-Dispersible Humate-Coated Magnetite Nanoparticles: Relation of Coating Process Parameters to the Properties of Nanoparticles. Nanomaterials 2020, 10, 1558. [Google Scholar] [CrossRef] [PubMed]
- Kostyukhin, E.M.; Kustov, A.L.; Evdokimenko, N.V.; Bazlov, A.I.; Kustov, L.M. Hydrothermal microwave-assisted synthesis of LaFeO3 catalyst for N2O decomposition. J. Am. Ceram. Soc. 2021, 104, 492–503. [Google Scholar] [CrossRef]
- Vikanova, K.; Redina, E.; Kapustin, G.; Nissenbaum, V.; Mishin, I.; Kostyukhin, E.; Kustov, L. Template-free one-step synthesis of micro-mesoporous CeO2–ZrO2 mixed oxides with a high surface area for selective hydrogenation. Ceram. Int. 2020, 46, 13980–13988. [Google Scholar] [CrossRef]
- Kirichenko, O.A.; Shuvalova, E.V.; Strekalova, A.A.; Davshan, N.A.; Kapustin, G.I.; Nissenbaum, V.D. Catalytic Activity of Cu and Cu–Fe Hydrosilicates in Hydrogenation with Molecular Hydrogen. Russ. J. Phys. Chem. A 2018, 92, 2417–2423. [Google Scholar] [CrossRef]
- McCusker, L.B.; Liebau, F.; Engelhardt, G. Nomenclature of structural and compositional characteristics of ordered microporous and mesoporous materials with inorganic hosts. Microporous Mesoporous Mater. 2003, 58, 3–13. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Ding, J.; Liu, T.; Shi, G.; Li, X.; Dang, W.; Cheng, Y.; Guo, R. Pore Structure and Fractal Characteristics of Different Shale Lithofacies in the Dalong Formation in the Western Area of the Lower Yangtze Platform. Minerals 2020, 10, 72. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Chen, X.; Ma, J.; He, H.; Wang, W. Characterization and catalytic performance of sol–gel derived Cu/SiO2 catalysts for hydrogenolysis of diethyl oxalate to ethylene glycol. J. Sol-Gel Sci. Technol. 2010, 55, 285–292. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-F.; Guo, P.-J.; Qiao, M.-H.; Yan, S.-R.; Li, H.-X.; Shen, W.; Xu, H.-L.; Fan, K.-N. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2008, 257, 172–180. [Google Scholar] [CrossRef]
- Shesterkina, A.A.; Strekalova, A.A.; Shuvalova, E.V.; Kapustin, G.I.; Tkachenko, O.P.; Kustov, L.M. CuO-Fe2O3 Nanoparticles Supported on SiO2 and Al2O3 for Selective Hydrogenation of 2-Methyl-3-Butyn-2-ol. Catalysts 2021, 11, 625. [Google Scholar] [CrossRef]
- Kirichenko, O.; Kapustin, G.; Nissenbaum, V.; Strelkova, A.; Shuvalova, E.; Shesterkina, A.; Kustov, L. Thermal decomposition and reducibility of silica-supported precursors of Cu, Fe and Cu–Fe nanoparticles. J. Therm. Anal. Calorim. 2018, 134, 233–251. [Google Scholar] [CrossRef]

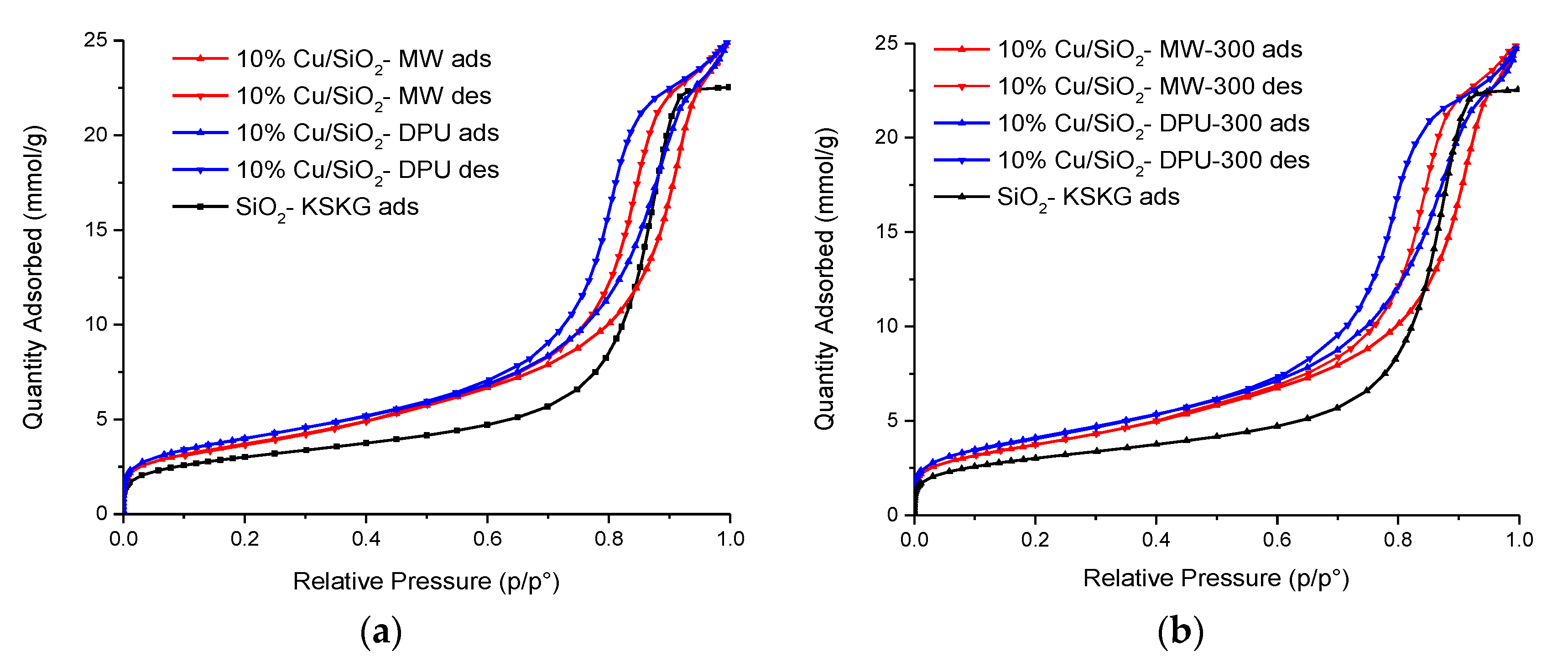
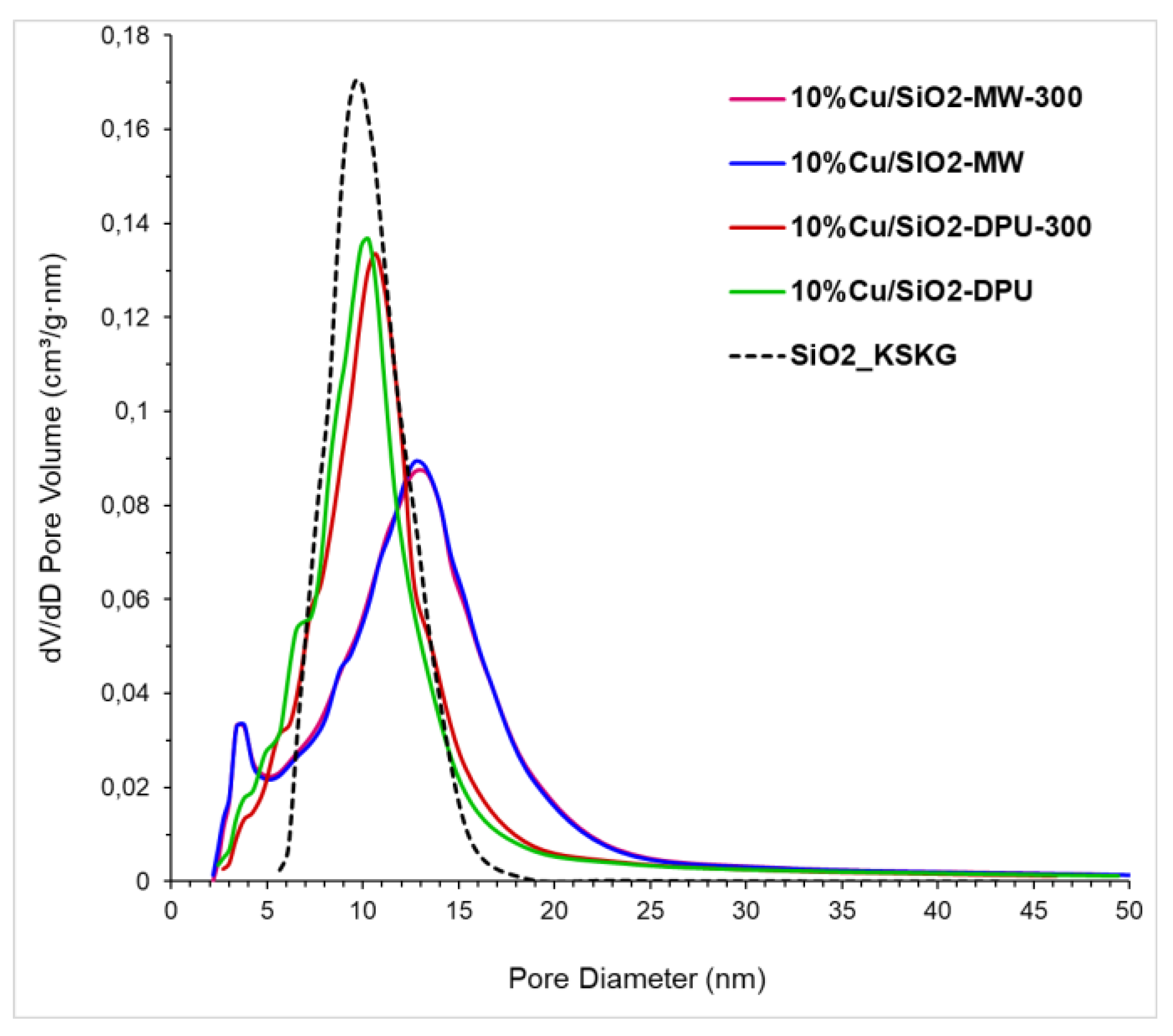

| Sample | SBET, m2/g | Vtot, cm3/g | Vmeso, cm3/g | Vmicro (t-Plot), cm3/g | Dav, nm |
|---|---|---|---|---|---|
| 10%Cu/SiO2-MW | 303 | 0.862 | 0.853 | 0.0021 | 1–2, 2–25 |
| 10%Cu/SiO2-MW-300 | 299 | 0.857 | 0.848 | 0.0047 | 1–2, 2–25 |
| 10%Cu/SiO2-DPU | 333 | 0.857 | 0.844 | 0.0017 | 1–2, 2–25 |
| 10%Cu/SiO2-DPU-300 | 323 | 0.863 | 0.851 | 0.0049 | 1–2, 2–25 |
| SiO2-KSKG | 244 | 0.782 | 0.773 | 0.0065 | 6–18 |
| Substrate | Catalyst | Reaction Temperature, °C | Reaction Time, h | Conversion, % | Selectivity to C=C Bond, % |
|---|---|---|---|---|---|
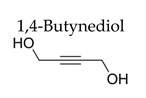 | 5%Cu/SiO2-MW | 150 | 2 4 | 41.8 100 | 95.6 90.2 |
| 5%Cu/SiO2-DPU | 120 | 0.5 | 0 | 0 | |
| 150 | 0.5 | 17.5 | 100 | ||
| 2 | 40.6 | 94.1 | |||
| 4 | 90.5 | 89.4 | |||
| 10%Cu/SiO2-MW | 150 | 3 | 100 | 96.5 | |
| 10%Cu/SiO2-MW-300 | 150 | 2 | 100 | 96.3 | |
| 10%Cu/SiO2-DPU | 150 | 2 3 | 63.8 98 | 95.1 92 | |
| 10%Cu/SiO2-DPU-300 | 150 | 2.5 | 100 | 93 | |
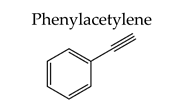 | 10%Cu/SiO2-MW-300 | 170 | 0.5 | 99.5 | 75.2 |
 | 10%Cu/SiO2-MW-300 | 140 160 170 | 0.5 0.5 0.5 3 | 3.9 10.2 20 52 | 35 20.5 24.8 11.9 |
 | 10%Cu/SiO2-MW-300 | 140 160 | 0.5 0.5 | 20 99.5 | 100 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shesterkina, A.; Vikanova, K.; Kostyukhin, E.; Strekalova, A.; Shuvalova, E.; Kapustin, G.; Salmi, T. Microwave Synthesis of Copper Phyllosilicates as Effective Catalysts for Hydrogenation of C≡C Bonds. Molecules 2022, 27, 988. https://doi.org/10.3390/molecules27030988
Shesterkina A, Vikanova K, Kostyukhin E, Strekalova A, Shuvalova E, Kapustin G, Salmi T. Microwave Synthesis of Copper Phyllosilicates as Effective Catalysts for Hydrogenation of C≡C Bonds. Molecules. 2022; 27(3):988. https://doi.org/10.3390/molecules27030988
Chicago/Turabian StyleShesterkina, Anastasiya, Kseniia Vikanova, Egor Kostyukhin, Anna Strekalova, Elena Shuvalova, Gennady Kapustin, and Tapio Salmi. 2022. "Microwave Synthesis of Copper Phyllosilicates as Effective Catalysts for Hydrogenation of C≡C Bonds" Molecules 27, no. 3: 988. https://doi.org/10.3390/molecules27030988
APA StyleShesterkina, A., Vikanova, K., Kostyukhin, E., Strekalova, A., Shuvalova, E., Kapustin, G., & Salmi, T. (2022). Microwave Synthesis of Copper Phyllosilicates as Effective Catalysts for Hydrogenation of C≡C Bonds. Molecules, 27(3), 988. https://doi.org/10.3390/molecules27030988







