Potential Antiviral Action of Alkaloids
Abstract
1. Introduction
2. Research Methodology
3. Natural Products as a Source for Antiviral Drugs Development
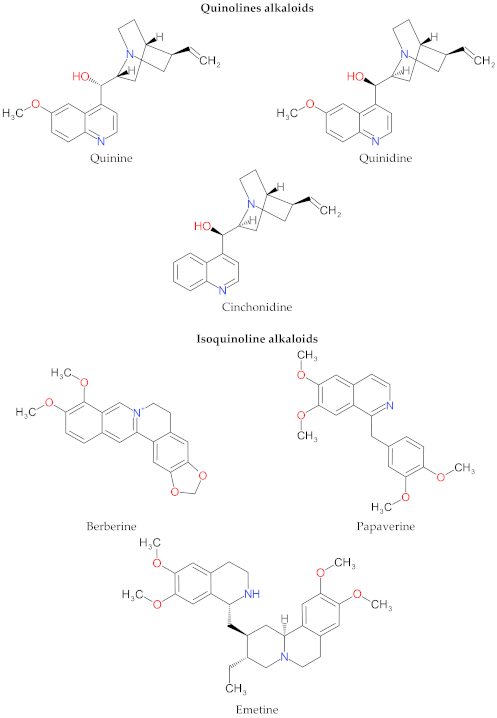
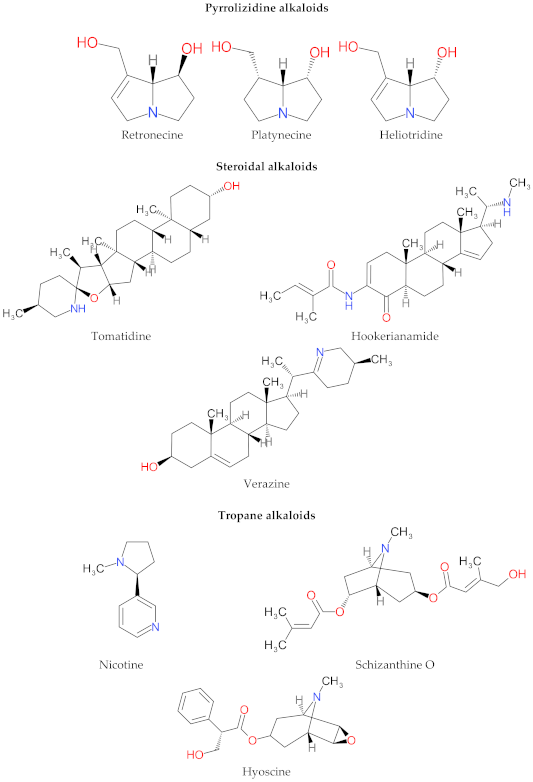
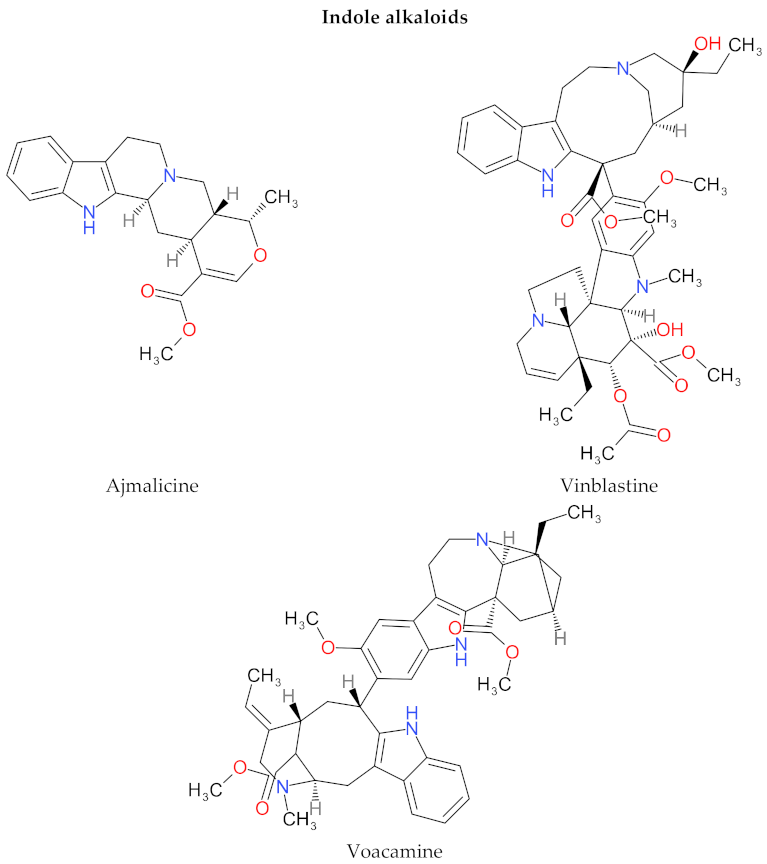
4. Structure and Characteristics of Alkaloids
5. Targets for Antiviral Alkaloids
5.1. Viral Attachment and Cell Entry Target
5.2. Viral Replication Target
5.2.1. Inhibition of DNA and RNA Synthesis
5.2.2. Inhibitors of Protein Synthesis
6. Toxicology and Side Effects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHIKV | Chikungunya virus |
| CVB3 | Coxsackievirus B3 |
| DENV | Dengue virus |
| EV71 | Enterovirus 71 |
| EVD | Ebola virus disease |
| H1N1 | Influenza A virus |
| H5N1 | Asian avian influenza A virus |
| H7N7 | Avian influenza A virus |
| HCMV | Human cytomegalovirus |
| HCV | Hepatitis C virus |
| HIV | Human immunodeficiency virus |
| HSV | Herpes simplex virus |
| MERS | Middle East respiratory syndrome |
| MLV | Murine leukemia viruses |
| MMTV | Mouse mammary tumor viruses |
| SARS | Severe acute respiratory syndrome |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| VACV | Vaccinia virus |
| ZIKAV | Zika virus |
References
- WHO. Global HIV & AIDS Statistics—2020 Fact Sheet. 2019. Available online: https://www.unaids.org/en/resources/fact-sheet#:~:text=In 2019%2C around 690 000,1.6 million%5D people in 2010 (accessed on 25 April 2020).
- Desselberger, U. Emerging and re-emerging infectious diseases. J. Infect. 2000, 40, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Devadasan, V.; Raman, P. Plant-derived alkaloids as anti-viral agents. Int. J. Res. Pharm. Sci. 2020, 11, 6174–6182. [Google Scholar] [CrossRef]
- Martinez, J.P.; Sasse, F.; Brönstrup, M.; Diez, J.; Meyerhans, A. Antiviral drug discovery: Broad-spectrum drugs from nature. Nat. Prod. Rep. 2015, 32, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, M.; Gaunt, E. Ecological Origins of Novel Human Pathogens. Crit. Rev. Microbiol. 2007, 33, 231–242. [Google Scholar] [CrossRef]
- Worldometers. COVID-19 Coronavirus Outbreak. Worldometers. 2020. Available online: https://www.worldometers.info/coronavirus/#countries (accessed on 27 August 2021).
- De Clercq, E. Chemotherapy of Viral Infections. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products As Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Ain, Q.-U.; Khan, H.; Mubarak, M.S.; Pervaiz, A. Plant Alkaloids as Antiplatelet Agent: Drugs of the Future in the Light of Recent Developments. Front. Pharmacol. 2016, 7, 292. [Google Scholar] [CrossRef]
- Lu, J.-J.; Bao, J.-L.; Chen, X.-P.; Huang, M.; Wang, Y.-T. Alkaloids Isolated from Natural Herbs as the Anticancer Agents. Evid.-Based Complement. Altern. Med. 2012, 2012, 921430. [Google Scholar] [CrossRef]
- Huang, M.; Gao, H.; Chen, Y.; Zhu, H.; Cai, Y.; Zhang, X.; Miao, Z.; Jiang, H.; Zhang, J.; Shen, H.; et al. Chimmitecan, a Novel 9-Substituted Camptothecin, with Improved Anticancer Pharmacologic Profiles In vitro and In vivo. Clin. Cancer Res. 2007, 13, 1298–1307. [Google Scholar] [CrossRef]
- Li, W.; Shao, Y.; Hu, L.; Zhang, X.; Chen, Y.; Tong, L.; Li, C.; Shen, X.; Ding, J. BM6, a new semi-synthetic vinca alkaloid, exhibits its potent in vivo anti-tumor activities via its high binding affinity for tubulin and improved pharmacokinetic profiles. Cancer Biol. Ther. 2007, 6, 787–794. [Google Scholar] [CrossRef]
- Alasvand, M.; Assadollahi, V.; Ambra, R.; Hedayatinezhad, E.; Kooti, W.; Peluso, I. Antiangiogenic Effect of Alkaloids. Oxidative Med. Cell. Longev. 2019, 2019, 9475908. [Google Scholar] [CrossRef]
- Gupta, A.P.; Pandotra, P.; Kushwaha, M.; Khan, S.; Sharma, R.; Gupta, S. Alkaloids: A source of anticancer agents from nature. In Studies in Natural Products Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2015; Volume 46, pp. 341–445. [Google Scholar]
- Peng, J.; Zheng, T.-T.; Li, X.; Liang, Y.; Wang, L.-J.; Huang, Y.-C.; Xiao, H.-T. Plant-Derived Alkaloids: The Promising Disease-Modifying Agents for Inflammatory Bowel Disease. Front. Pharmacol. 2019, 10, 351. [Google Scholar] [CrossRef]
- Yan, T.; Xu, M.; Wan, S.; Wang, M.; Wu, B.; Xiao, F.; Bi, K.; Jia, Y. Schisandra chinensis produces the antidepressant-like effects in repeated corticosterone-induced mice via the BDNF/TrkB/CREB signaling pathway. Psychiatr. Res. 2016, 243, 135–142. [Google Scholar] [CrossRef]
- Burrell, C.J.; Howard, C.R.; Murphy, F.A. Antiviral Chemotherapy. In Fenner and White’s Medical Virology; Academic Press: Cambridge, MA, USA, 2017; pp. 169–183. [Google Scholar] [CrossRef]
- Siddiqui, A.A. Role of Natural Products in Drug Discovery Process. Int. J. Drug Dev. Res. 2014, 6, 172–204. [Google Scholar]
- Rishton, G.M. Natural Products as a Robust Source of New Drugs and Drug Leads: Past Successes and Present Day Issues. Am. J. Cardiol. 2008, 101, S43–S49. [Google Scholar] [CrossRef]
- Andersen, D.O.; Weber, N.D.; Wood, S.G.; Hughes, B.G.; Murray, B.K.; North, J.A. In vitro virucidal activity of selected anthraquinones and anthraquinone derivatives. Antivir. Res. 1991, 16, 185–196. [Google Scholar] [CrossRef]
- Li, J.W.-H.; Vederas, J.C. Drug Discovery and Natural Products: End of an Era or an Endless Frontier? Available online: http://science.sciencemag.org/ (accessed on 21 August 2021).
- Chantrill, B.H.; Coulthard, C.E.; Dickinson, L.; Inkley, G.W.; Morris, W.; Pyle, A.H. The Action of Plant Extracts on a Bacteriophage of Pseudornonas pyocyanea and on Influenza A Virus. Microbiology 1952, 6, 74–84. [Google Scholar]
- Anani, K.; Hudson, J.B.; De Souza, C.; Akpagana, K.; Tower, G.H.N.; Arnason, J.T.; Gbeassor, M. Investigation of medicinal plants of Togo for antiviral and antimicrobial activities. Pharm. Biol. 2000, 38, 40–45. [Google Scholar] [CrossRef]
- Hudson, J.; Lee, M.; Rasoanaivo, P. Antiviral Activities in Plants Endemic to Madagascar. Pharm. Biol. 2000, 38, 36–39. [Google Scholar] [CrossRef]
- Sohail, M.N.; Rasul, F.; Karim, A.; Kanwal, U.; Attitalla, I.H. Plant as a Source of Natural Antiviral Agents. Asian J. Anim. Vet. Adv. 2011, 6, 1125–1152. [Google Scholar] [CrossRef][Green Version]
- Koparde, A.A.; Doijad, R.C.; Magdum, C.S. Natural products in drug discovery. In Pharmacognosy—Medicinal Plants; Perveen Al-Taweel, A., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Kapoor, R.S.; Sharma, B.; Kanwar, S.S. Antiviral Phytochemicals: An Overview. Biochem. Physiol. Open Access 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Moradi, M.T.; Karimi, A.; Lorigooini, Z. Alkaloids as the natural anti-influenza virus agents: A systematic review. Toxin. Rev. 2018, 37, 11–18. [Google Scholar] [CrossRef]
- Wink, M. Inrference of alkaloids with neuroreceptors and ion channels. Stud. Nat. Prod. Chem. 2000, 21, 3–122. [Google Scholar] [CrossRef]
- Ramawat, K.G.M.J. Biotechnology: Secondary Metabolites. Science, 2nd ed.; Science Publishers: Enfield, UK, 2007. [Google Scholar]
- Kukula-Koch, W.A.; Widelski, J. Chapter 9—Alkaloids. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 163–198. [Google Scholar]
- Verpoorte, R. Alkaloids. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2005; pp. 56–61. [Google Scholar]
- Cordell, G.A.; Quinn-Beattie, M.L.; Farnsworth, N.R. The potential of alkaloids in drug discovery. Phytother. Res. 2001, 15, 183–205. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development q Settings. 2001. Available online: www.elsevier.com/locate/drugdeliv (accessed on 15 May 2021).
- Ramanathan, S.; Parthasarathy, S.; Murugaiyah, V.; Magosso, E.; Tan, S.C.; Mansor, S.M. Understanding the Physicochemical Properties of Mitragynine, a Principal Alkaloid of Mitragyna speciosa, for Preclinical Evaluation. Molecules 2015, 20, 4915–4927. [Google Scholar] [CrossRef]
- Stockwell, B.R. Exploring biology with small organic molecules. Nature 2004, 432, 846–854. [Google Scholar] [CrossRef]
- Rao, K.N.; Venkatachalam, S.R. Inhibition of Dihydrofolate Reductase and Cell Growth Activity by the Phenanthroindolizidine Alkaloids Pergularinine and Tylophorinidine: The In Vitro Cytotoxicity of These Plant Alkaloids and their Potential as Antimicrobial and Anticancer Agents. Available online: www.elsevier.com/locate/toxinvit (accessed on 15 May 2021).
- Naithani, R.; Mehta, R.G.; Shukla, D.; Chandersekera, S.N.; Moriarty, R.M. Antiviral Activity of Phytochemicals: A Current Perspective. In Dietary Components and Immune Function; Humana Press: Totowa, NJ, USA, 2010; pp. 421–468. [Google Scholar] [CrossRef]
- Quintana, V.; Selisko, B.; Brunetti, J.; Eydoux, C.; Guillemot, J.; Canard, B.; Damonte, E.; Julander, J.; Castilla, V. Antiviral activity of the natural alkaloid anisomycin against dengue and Zika viruses. Antivir. Res. 2020, 176, 104749. [Google Scholar] [CrossRef]
- Luganini, A.; Mercorelli, B.; Messa, L.; Palù, G.; Gribaudo, G.; Loregian, A. The isoquinoline alkaloid berberine inhibits human cytomegalovirus replication by interfering with the viral Immediate Early-2 (IE2) protein transactivating activity. Antivir. Res. 2019, 164, 52–60. [Google Scholar] [CrossRef]
- Song, S.; Qiu, M.; Chu, Y.; Chen, D.; Wang, X.; Su, A.; Wu, Z. Downregulation of Cellular c-Jun N-Terminal Protein Kinase and NF-κB Activation by Berberine May Result in Inhibition of Herpes Simplex Virus Replication. Antimicrob. Agents Chemother. 2014, 58, 5068–5078. [Google Scholar] [CrossRef]
- Cecil, C.E.; Davis, J.; Cech, N.B.; Laster, S.M. Inhibition of H1N1 influenza A virus growth and induction of inflammatory mediators by the isoquinoline alkaloid berberine and extracts of goldenseal (Hydrastis canadensis). Int. Immunopharmacol. 2011, 11, 1706–1714. [Google Scholar] [CrossRef]
- McCormick, J.L.; McKee, T.C.; Cardellina, J.H.; Boyd, M.R. HIV Inhibitory Natural Products. 26. 1 Quinoline Alkaloids from Euodia roxburghiana. J. Nat. Prod. 1928, 59, 469–471. [Google Scholar] [CrossRef]
- Horwitz, S.B.; Chang, C.-K.; Grollman, A.P. Antiviral Action of Camptothecin. 1972. Available online: http://aac.asm.org/ (accessed on 19 March 2021).
- Wu, K.X.; Chu, J.J.-H. Antiviral screen identifies EV71 inhibitors and reveals camptothecin-target, DNA topoisomerase 1 as a novel EV71 host factor. Antivir. Res. 2017, 143, 122–133. [Google Scholar] [CrossRef]
- Wink, M. Potential of DNA Intercalating Alkaloids and Other Plant Secondary Metabolites against SARS-CoV-2 Causing COVID-19. Diversity 2020, 12, 175. [Google Scholar] [CrossRef]
- Khandelwal, N.; Chander, Y.; Rawat, K.D.; Riyesh, T.; Nishanth, C.; Sharma, S.; Jindal, N.; Tripathi, B.N.; Barua, S.; Kumar, N. Emetine inhibits replication of RNA and DNA viruses without generating drug-resistant virus variants. Antivir. Res. 2017, 144, 196–204. [Google Scholar] [CrossRef]
- Yang, S.; Xu, M.; Lee, E.M.; Gorshkov, K.; Shiryaev, S.A.; He, S.; Sun, W.; Cheng, Y.-S.; Hu, X.; Tharappel, A.M.; et al. Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: Inhibiting viral replication and decreasing viral entry. Cell Discov. 2018, 4, 31. [Google Scholar] [CrossRef]
- Valadão, A.L.C.; Abreu, C.M.; Dias, J.Z.; Arantes, P.R.; Verli, H.; Tanuri, A.; De Aguiar, R.S. Natural Plant Alkaloid (Emetine) Inhibits HIV-1 Replication by Interfering with Reverse Transcriptase Activity. Molecules 2015, 20, 11474–11489. [Google Scholar] [CrossRef]
- Bleasel, M.D.; Peterson, G.M. Emetine, Ipecac, Ipecac Alkaloids and Analogues as Potential Antiviral Agents for Coronaviruses. Pharmaceuticals 2020, 13, 51. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Roy, S.; Venkatadri, R.; Su, Y.-P.; Ye, W.; Barnaeva, E.; Griner, L.M.; Southall, N.; Hu, X.; Wang, A.Q.; et al. Efficacy and Mechanism of Action of Low Dose Emetine against Human Cytomegalovirus. PLoS Pathog. 2016, 12, e1005717. [Google Scholar] [CrossRef]
- Choy, K.-T.; Wong, A.Y.-L.; Kaewpreedee, P.; Sia, S.F.; Chen, D.; Hui, K.P.Y.; Chu, D.K.W.; Chan, M.C.W.; Cheung, P.H.P.; Huang, X.; et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020, 178, 104786. [Google Scholar] [CrossRef]
- Kim, D.E.; Min, J.S.; Jang, M.S.; Lee, J.Y.; Shin, Y.S.; Park, C.M.; Song, J.H.; Kim, H.R.; Kim, S.; Jin, Y.-H.; et al. Natural Bis-Benzylisoquinoline Alkaloids-Tetrandrine, Fangchinoline, and Cepharanthine, Inhibit Human Coronavirus OC43 Infection of MRC-5 Human Lung Cells. Biomolecules 2019, 9, 696. [Google Scholar] [CrossRef]
- Aljofan, M.; Sganga, M.L.; Lo, M.K.; Rootes, C.L.; Porotto, M.; Meyer, A.G.; Saubern, S.; Moscona, A.; Mungall, B.A. Antiviral activity of gliotoxin, gentian violet and brilliant green against Nipah and Hendra virus in vitro. Virol. J. 2009, 6, 187. [Google Scholar] [CrossRef]
- Houghton, P.J.; Woldemariam, T.Z.; Khan, A.I.; Burke, A.; Mahmood, N. Antiviral activity of natural and semi-synthetic chromone alkaloids. Antivir. Res. 1994, 25, 235–244. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Chen, M.-H.; Guo, Q.-L.; Lin, S.; Xu, C.-B.; Jiang, Y.-P.; Li, Y.-H.; Jiang, J.-D.; Shi, J.-G. Antiviral glycosidic bisindole alkaloids from the roots ofIsatis indigotica. J. Asian Nat. Prod. Res. 2015, 17, 689–704. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lao, Z.; Xu, J.; Li, Z.; Long, H.; Li, D.; Lin, L.; Liu, X.; Yu, L.; Liu, W.; et al. Antiviral activity of lycorine against Zika virus in vivo and in vitro. Virology 2020, 546, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cai, J.; Cheng, J.; Jing, C.; Yin, J.; Jiang, J.; Peng, Z.; Hao, X. Design, Synthesis and Structure-Activity Relationship Optimization of Lycorine Derivatives for HCV Inhibition. Sci Rep. 2015, 5, 14972. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qi, W.-B.; Wang, L.; Tian, J.; Jiao, P.; Liu, G.-Q.; Ye, W.-C.; Liao, M. Amaryllidaceae alkaloids inhibit nuclear-to-cytoplasmic export of ribonucleoprotein (RNP) complex of highly pathogenic avian influenza virus H5N1. Influ. Other Respir. Viruses 2013, 7, 922–931. [Google Scholar] [CrossRef]
- Wang, P.; Li, L.; Wang, Q.-Y.; Shang, L.-Q.; Shi, P.-Y.; Yin, Z. Anti-Dengue-Virus Activity and Structure-Activity Relationship Studies of Lycorine Derivatives. ChemMedChem 2014, 9, 1522–1533. [Google Scholar] [CrossRef]
- Li, S.-Y.; Chen, C.; Zhang, H.-Q.; Guo, H.-Y.; Wang, H.; Wang, L.; Zhang, X.; Hua, S.-N.; Yu, J.; Xiao, P.-G.; et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 2005, 67, 18–23. [Google Scholar] [CrossRef]
- Palem, J.; Bedadala, G.; El Sayed, K.; Hsia, S.-C. Manzamine A as a Novel Inhibitor of Herpes Simplex Virus Type-1 Replication in Cultured Corneal Cells. Planta Med. 2010, 77, 46–51. [Google Scholar] [CrossRef]
- McMahon, J.B.; Currens, M.J.; Gulakowski, R.J.; Buckheit, R.W., Jr.; Lackman-Smith, C.; Hallock, Y.F.; Boyd, M.R. Michellamine B, a Novel Plant Alkaloid, Inhibits Human Immunodeficiency Virus-Induced Cell Killing by at Least Two Distinct Mechanisms. Antimicrob. Agents Chemother. 1995, 39, 484–488. [Google Scholar] [CrossRef]
- Boyd, M.R.; Hallock, Y.F.; Cardellina, J.H.; Manfredi, K.P.; Blunt, J.W.; McMahon, J.B.; Buckheit, R.W., Jr.; Bringmann, G.; Schäffer, M.; Cragg, G.M.; et al. Anti-HIV Michellamines from Ancistrocladus korupensisl. J. Med. Chem. 1994, 37, 1740–1745. [Google Scholar] [CrossRef]
- Montanha, J.A.; Amoros, M.; Boustie, J.; Girre, L. Anti-Herpes Virus Activity of Aporphine Alkaloids. Planta Med. 1995, 61, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Loya, S.; Rudi, A.; Kashman, Y.; Hizi, A. Polycitone A, a novel and potent general inhibitor of retroviral reverse transcriptases and cellular DNA polymerases. Biochem. J. 1999, 344, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Jan, J.-T.; Ma, S.-H.; Kuo, C.-J.; Juan, H.-F.; Cheng, Y.-S.E.; Hsu, H.-H.; Huang, H.-C.; Wu, D.; Brik, A.; et al. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc. Natl. Acad. Sci. USA 2004, 101, 10012–10017. [Google Scholar] [CrossRef] [PubMed]
- Serkedjieva, J.; Velcheva, M. In Vitro Anti-Influenza Virus Activity of the Pavine Alkaloid (-)-Thalimonine Isolated from Thalictrum Simplex L. Antivir. Chem. Chemother. 2003, 14, 75–80. [Google Scholar] [CrossRef][Green Version]
- Troost, B.; Mulder, L.M.; Diosa-Toro, M.; Van De Pol, D.; Rodenhuis-Zybert, I.A.; Smit, J.M. Tomatidine, a natural steroidal alkaloid shows antiviral activity towards chikungunya virus in vitro. Sci. Rep. 2020, 10, 6364. [Google Scholar] [CrossRef]
- Diosa-Toro, M.; Troost, B.; van de Pol, D.; Heberle, A.M.; Urcuqui-Inchima, S.; Thedieck, K.; Smit, J.M. Tomatidine, a novel antiviral compound towards dengue virus. Antivir. Res. 2019, 161, 90–99. [Google Scholar] [CrossRef]
- Silva, E.M.; Cirne-Santos, C.C.; Frugulhetti, I.C.P.P.; Galvão-Castro, B.; Saraiva, E.M.B.; Kuehne, M.E.; Bou-Habib, D.C. Anti-HIV-1 Activity of the Iboga Alkaloid Congener 18-Methoxycoronaridine. Planta Med. 2004, 70, 808–812. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.-C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef]
- Zhou, Y.; Simmons, G. Development of novel entry inhibitors targeting emerging viruses. Expert Rev. Anti-Infect. Ther. 2012, 10, 1129–1138. [Google Scholar] [CrossRef]
- Cohen, S.; Au, S.; Panté, N. How viruses access the nucleus. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011, 1813, 1634–1645. [Google Scholar] [CrossRef]
- Dimitrov, D.S. Virus entry: Molecular mechanisms and biomedical applications. Nat. Rev. Genet. 2004, 2, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, C.A. Glycosphingolipid Functions. Cold Spring Harb. Perspect. Biol. 2011, 3, a011874. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.C.; Freiberg, A.N.; Zhang, T.; Akyol-Ataman, Z.; Grock, A.; Hong, P.W.; Li, J.; Watson, N.F.; Fang, A.Q.; Aguilar, H.C.; et al. A broad-spectrum antiviral targeting entry of enveloped viruses. Proc. Natl. Acad. Sci. USA 2010, 107, 3157–3162. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Song, J.; Lu, X.; Deng, Y.-Q.; Musyoki, A.M.; Cheng, H.; Zhang, Y.; Yuan, Y.; Song, H.; Haywood, J.; et al. Structures of the Zika Virus Envelope Protein and Its Complex with a Flavivirus Broadly Protective Antibody. Cell Host Microbe 2016, 19, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Carette, J.E.; Raaben, M.; Wong, A.C.; Herbert, A.S.; Obernosterer, G.; Mulherkar, N.; Kuehne, A.I.; Kranzusch, P.J.; Griffin, A.M.; Ruthel, G.; et al. Ebola virus entry requires the cholesterol transporter Niemann–Pick C1. Nature 2011, 477, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.-C.; Jassey, A.; Liu, C.-H.; Lin, C.-J.; Lin, C.-C.; Wong, S.H.; Wang, J.Y.; Yen, M.-H.; Lin, L.-T. Berberine inhibits hepatitis C virus entry by targeting the viral E2 glycoprotein. Phytomedicine 2018, 53, 62–69. [Google Scholar] [CrossRef]
- Xia, S.; Zhu, Y.; Liu, M.; Lan, Q.; Xu, W.; Wu, Y.; Ying, T.; Liu, S.; Shi, Z.; Jiang, S.; et al. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell. Mol. Immunol. 2020, 17, 765–767. [Google Scholar] [CrossRef]
- Maurya, V.K.; Kumar, S.; Prasad, A.K.; Bhatt, M.L.B.; Saxena, S.K. Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. VirusDisease 2020, 31, 179–193. [Google Scholar] [CrossRef]
- Fielding, B.C.; Filho, C.D.S.M.B.; Ismail, N.S.M.; De Sousa, D.P. Alkaloids: Therapeutic Potential against Human Coronaviruses. Molecules 2020, 25, 5496. [Google Scholar] [CrossRef]
- Gyebi, G.A.; Adegunloye, A.P.; Ibrahim, I.M.; Ogunyemi, O.M.; Afolabi, S.O.; Ogunro, O.B. Prevention of SARS-CoV-2 cell entry: Insight from in silico interaction of drug-like alkaloids with spike glycoprotein, human ACE2, and TMPRSS2. J. Biomol. Struct. Dyn. 2020, 1–25. [Google Scholar] [CrossRef]
- Lebecque, S.; Crowet, J.-M.; Lins, L.; Delory, B.M.; du Jardin, P.; Fauconnier, M.-L.; Deleu, M. Interaction between the barley allelochemical compounds gramine and hordenine and artificial lipid bilayers mimicking the plant plasma membrane. Sci. Rep. 2018, 8, 9784. [Google Scholar] [CrossRef] [PubMed]
- Lundbæk, J.A.; Birn, P.; Tape, S.E.; Toombes, G.E.S.; Søgaard, R.; Koeppe, R.; Gruner, S.M.; Hansen, A.J.; Andersen, O.S. Capsaicin Regulates Voltage-Dependent Sodium Channels by Altering Lipid Bilayer Elasticity. Mol. Pharmacol. 2005, 68, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Torrecillas-Sanchez, A.; Schneider, M.; Fernández-Martínez, A.M.; Ausili, A.; De Godos, A.M.; Corbalan, S.; Gomez-Fernandez, J.C. Capsaicin Fluidifies the Membrane and Localizes Itself near the Lipid–Water Interface. ACS Chem. Neurosci. 2015, 6, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Wink, M.; Schmeller, T.; Latz-Brüning, B. Modes of Action of Allelochemical Alkaloids: Interaction with Neuroreceptors, DNA, and Other Molecular Targets. J. Chem. Ecol. 1998, 24, 1881–1937. [Google Scholar] [CrossRef]
- Zidovetzki, R.; Sherman, I.W.; Atiya, A.; De Boeck, H. A nuclear magnetic resonance study of the interactions of the antimalarials chloroquine, quinacrine, quinine and mefloquine with dipalmitoylphosphatidylcholine bilayers. Mol. Biochem. Parasitol. 1989, 35, 199–207. [Google Scholar] [CrossRef]
- Chen, X.; Si, L.; Liu, D.; Proksch, P.; Zhang, L.; Zhou, D.; Lin, W. Neoechinulin B and its analogues as potential entry inhibitors of influenza viruses, targeting viral hemagglutinin. Eur. J. Med. Chem. 2015, 93, 182–195. [Google Scholar] [CrossRef]
- Ho, Y.-J.; Lu, J.-W.; Huang, Y.-L.; Lai, Z.-Z. Palmatine inhibits Zika virus infection by disrupting virus binding, entry, and stability. Biochem. Biophys. Res. Commun. 2019, 518, 732–738. [Google Scholar] [CrossRef]
- Matsuda, K.; Hattori, S.; Komizu, Y.; Kariya, R.; Ueoka, R.; Okada, S. Cepharanthine inhibited HIV-1 cell–cell transmission and cell-free infection via modification of cell membrane fluidity. Bioorganic Med. Chem. Lett. 2014, 24, 2115–2117. [Google Scholar] [CrossRef]
- Shao, J.; Zeng, D.; Tian, S.; Liu, G.; Fu, J. Identification of the natural product berberine as an antiviral drug. AMB Express 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Whitby, K.; Pierson, T.C.; Geiss, B.; Lane, K.; Engle, M.; Zhou, Y.; Doms, R.W.; Diamond, M.S. Castanospermine, a Potent Inhibitor of Dengue Virus Infection In Vitro and In Vivo. J. Virol. 2005, 79, 8698–8706. [Google Scholar] [CrossRef]
- Basu, A.; Kumar, G.S. Nucleic acids binding strategies of small molecules: Lessons from alkaloids. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2018, 1862, 1995–2016. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Mohr, I. Viral subversion of the host protein synthesis machinery. Nat. Rev. Microbiol. 2011, 9, 860–875. [Google Scholar] [CrossRef] [PubMed]
- Said, Z.; Abdelwahab, K. Antiviral replication agents. In Viral Replication; Rosas-Acosta, G., Ed.; InTech Open Science: London, UK, 2013; p. 144. [Google Scholar]
- Payne, S. Introduction to RNA Viruses. Viruses 2017, 97–105. [Google Scholar] [CrossRef]
- Rampersad, S.; Tennant, P. Replication and Expression Strategies of Viruses. Viruses 2018, 55–82. [Google Scholar] [CrossRef]
- Esposito, F.; Corona, A.; Tramontano, E. HIV-1 Reverse Transcriptase Still Remains a New Drug Target: Structure, Function, Classical Inhibitors, and New Inhibitors with Innovative Mechanisms of Actions. Mol. Biol. Int. 2012, 2012, 586401. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Beardsley, G.P.; Coen, D.M. Mechanism of ganciclovir-induced chain termination revealed by resistant viral polymerase mutants with reduced exonuclease activity. Proc. Natl. Acad. Sci. USA 2014, 111, 17462–17467. [Google Scholar] [CrossRef]
- Dial, C.N.; Tate, P.M.; Kicmal, T.M.; Mounce, B.C. Coxsackievirus B3 Responds to Polyamine Depletion via Enhancement of 2A and 3C Protease Activity. Viruses 2019, 11, 403. [Google Scholar] [CrossRef]
- Mounce, B.C.; Cesaro, T.; Moratorio, G.; Hooikaas, P.J.; Yakovleva, A.; Werneke, S.W.; Smith, E.C.; Poirier, E.Z.; Simon-Loriere, E.; Prot, M.; et al. Inhibition of Polyamine Biosynthesis Is a Broad-Spectrum Strategy against RNA Viruses. J. Virol. 2016, 90, 9683–9692. [Google Scholar] [CrossRef]
- Chang, K.-O.; Kim, Y.; Lovell, S.; Rathnayake, A.D.; Groutas, W.C. Antiviral Drug Discovery: Norovirus Proteases and Development of Inhibitors. Viruses 2019, 11, 197. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, C.Z.; Gorshkov, K.; Xu, M.; Lo, D.C.; Zheng, W. RNA-Dependent RNA Polymerase as a Target for COVID-19 Drug Discovery. SLAS Discov. Adv. Sci. Drug Discov. 2020, 25, 1141–1151. [Google Scholar] [CrossRef]
- Almaqwashi, A.A.; Paramanathan, T.; Rouzina, I.; Williams, M.C. Mechanisms of small molecule–DNA interactions probed by single-molecule force spectroscopy. Nucleic Acids Res. 2016, 44, 3971–3988. [Google Scholar] [CrossRef] [PubMed]
- Palchaudhuri, R.; Hergenrother, P.J. DNA as a target for anticancer compounds: Methods to determine the mode of binding and the mechanism of action. Curr. Opin. Biotechnol. 2007, 18, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.H.; Sun, N.-J.; Cassady, J.M.; Snapka, R.M. Topoisomerase II inhibition by aporphine alkaloids. Biochem. Pharmacol. 1999, 57, 1141–1145. [Google Scholar] [CrossRef]
- Krstin, S.; Mohamed, T.; Wang, X.; Wink, M. How do the alkaloids emetine and homoharringtonine kill trypanosomes? An insight into their molecular modes of action. Phytomedicine 2016, 23, 1771–1777. [Google Scholar] [CrossRef]
- Sinha, R.; Kumar, G.S. Interaction of Isoquinoline Alkaloids with an RNA Triplex: Structural and Thermodynamic Studies of Berberine, Palmatine, and Coralyne Binding to Poly(U).Poly(A)*Poly(U). J. Phys. Chem. B 2009, 113, 13410–13420. [Google Scholar] [CrossRef]
- Schmeller, T.; Latz-Brüning, B.; Wink, M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores. Phytochemistry 1997, 44, 257–266. [Google Scholar] [CrossRef]
- Sethi, M.L. Enzyme Inhibition VIII: Mode of Inhibition of Reverse Transcriptase Activity by Analogues, Isomers, and Related Alkaloids of Coralyne. J. Pharm. Sci. 1985, 74, 889–891. [Google Scholar] [CrossRef]
- Low, J.S.Y.; Chen, K.C.; Wu, K.X.; Ng, M.M.-L.; Chu, J.J.H. Antiviral Activity of Emetine Dihydrochloride Against Dengue Virus Infection. J. Antivir. Antiretrovir. 2009, 1, 62–71. [Google Scholar] [CrossRef]
- Contrerast, A.; Carrascot, L. Selective Inhibition of Protein Synthesis in Virus-Infected Mammalian Cells. 1979. Available online: http://jvi.asm.org/ (accessed on 7 August 2021).
- Grollman, A.P. No Tinhibitor of protein biosynthesisitle. J. Biol. Chem. 1968, 245, 4089–4094. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; O’Brien, S.; Cortes, J. Homoharringtonine/Omacetaxine Mepesuccinate: The Long and Winding Road to Food and Drug Administration Approval. Clin. Lymphoma Myeloma Leuk. 2013, 13, 530–533. [Google Scholar] [CrossRef]
- Gupta, R.S.; Siminovitch, L. The molecular basis of emetine resistance in chinese hamster ovary cells: Alteration in the 40S ribosomal subunit. Cell 1977, 10, 61–66. [Google Scholar] [CrossRef]
- Jimenez, A.; Carrasco, L.; Vazquez, D. Enzymic and Nonenzymic Translocation by Yeast Polysomes. Site of Action of a Number of Inhibitors? Biochemistry 1977, 16, 4727–4730. [Google Scholar] [CrossRef] [PubMed]
- Fresno, M.; Jimenez, A.; Vazquez, D. Inhibition of Translation in Eukaryotic Systems by Harringtonine. Eur. J. Biochem. 1977, 72, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Ramabhadran, T.V.; Thach, R.E. Specificity of protein synthesis inhibitors in the inhibition of encephalomyocarditis virus replication. J. Virol. 1980, 34, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, Y.; Xu, Y.; Ma, C.; Qin, C.; Zhang, L. Lycorine reduces mortality of human enterovirus 71-infected mice by inhibiting virus replication. Virol. J. 2011, 8, 483–483. [Google Scholar] [CrossRef]
- Jimenez, A.; Santos, A.; Alonso, G.; Vazquez, D. Inhibitors of protein synthesis in eukaryotic cells: Comparative effects of some Amaryllidaceae alkaloids. Biochim. Biophys. Acta (BBA)-Nucleic Acids Protein Synth. 1976, 425, 342–348. [Google Scholar] [CrossRef]
- Kukhanova, M.; Victorova, L.; Krayevsky, A. Peptidyltransferase center of ribosomes: On the mechanism of action of alkaloid lycorine. FEBS Lett. 1983, 160, 129–133. [Google Scholar] [CrossRef]
- Baroni, A.; Paoletti, I.; Ruocco, E.; Ayala, F.; Corrado, F.; Wolf, R.; Tufano, M.A.; Donnarumma, G. Antiviral effects of quinine sulfate on HSV-1 HaCat cells infected: Analysis of the molecular mechanisms involved. J. Dermatol. Sci. 2007, 47, 253–255. [Google Scholar] [CrossRef]
- Malakar, S.; Sreelatha, L.; Dechtawewat, T.; Noisakran, S.; Yenchitsomanus, P.T.; Chu, J.J.H.; Limjindaporn, T. Drug repurposing of quinine as antiviral against dengue virus infection. Virus Res. 2018, 255, 171–178. [Google Scholar] [CrossRef]
- Moertel, C.G.; Schutt, A.J.; Reitemeier, R.J.; Hahn, R.G. Phase II study of camptothecin (NSC-100880) in the treatment of advanced gastrointestinal cancer. Cancer Chemother. Rep. 1972, 65, 95–101. [Google Scholar]
- Bennett, R.P.; Stewart, R.A.; Hogan, P.A.; Ptak, R.G.; Mankowski, M.K.; Hartman, T.L.; Buckheit, R.W.; Snyder, B.A.; Salter, J.D.; Morales, G.; et al. An analog of camptothecin inactive against Topoisomerase I is broadly neutralizing of HIV-1 through inhibition of Vif-dependent APOBEC3G degradation. Antivir. Res. 2016, 136, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Kozielski, A.; Liu, X.; Wang, Y.; Vardeman, D.; Giovanella, B. Crystalline Camptothecin-20( S )-O-Propionate Hydrate: A Novel Anticancer Agent with Strong Activity against 19 Human Tumor Xenografts. Cancer Res. 2009, 69, 4742–4749. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov. Phase I Clinical Trial of Camptothecin-20-O-Propionate Hydrate (CZ48). Available online: https://clinicaltrials.gov/ct2/show/study/NCT02575638 (accessed on 22 October 2021).
- Lancelot, A.; Clavería-Gimeno, R.; Velazquez-Campoy, A.; Abian, O.; Serrano, J.L.; Sierra, T. Nanostructures based on ammonium-terminated amphiphilic Janus dendrimers as camptothecin carriers with antiviral activity. Eur. Polym. J. 2017, 90, 136–1497. [Google Scholar] [CrossRef]
- Garner-Wizard, M.; Henson, S.; Milot, B.; Minigh, J.; Oliff, H.S.; Oppel, M. Update on Plant-Derived Compounds Used on HIV Treatment. 2009. Available online: https://www.herbalgram.org/media/4849/373_review110685-373-110685.pdf (accessed on 25 October 2021).
- Cragg, G.M.; Newman, D.J.; Kingston, D.G.I. Terrestrial plants as a source of novel pharmaceutical agents. Compr. Nat. Prod. II Chem. Biol. 2010, 2, 5–39. [Google Scholar] [CrossRef]
- Lu, Y. The Design and Synthesis of Novel Michellamine B Analogues Targeting HIV; University of Wollongong: Wollongong, Australia, 2015. [Google Scholar]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. DrugBank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef]
- Nadia Bouabdallaoui, F.; Tardif, J.C. The Role of Colchicine in Recent Clinical Trials—A Focused Review on Pericardial Disease. American College of Cardiology. 2020. Available online: https://www.acc.org/latest-in-cardiology/articles/2020/08/13/13/11/the-role-of-colchicine-in-recent-clinical-trials (accessed on 29 October 2021).
- Deftereos, S.G.; Giannopoulos, G.; Vrachatis, D.A.; Siasos, G.D.; Giotaki, S.G.; Gargalianos, P.; Metallidis, S.; Sianos, G.; Baltagiannis, S.; Panagopoulos, P.; et al. Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized With Coronavirus Disease 2019: The GRECCO-19 randomized clinical trial. JAMA Netw. Open 2020, 3, e2013136. [Google Scholar] [CrossRef]
- Lopes, M.I.; Bonjorno, L.P.; Giannini, M.C.; Amaral, N.B.; Menezes, P.I.; Dib, S.M.; Gigante, S.L.; Benatti, M.N.; Rezek, U.C.; Emrich-Filho, L.L.; et al. Beneficial effects of colchicine for moderate to severe COVID-19: A randomised, double-blinded, placebo-controlled clinical trial. RMD Open 2021, 7, e001455. [Google Scholar] [CrossRef]
- Rubiaceae, S. Ipecacuanha, emetine, and dehydroemetine. Meyler’s Side Eff. Drugs 2016, 311–312. [Google Scholar] [CrossRef]
- Zbinden, G.; Kleinert, R.; Rageth, B. Assessment of Emetine Cardiotoxicity in a Subacute Toxicity Experiment in Rats. J. Cardiovasc. Pharmacol. 1980, 2, 155–164. [Google Scholar] [CrossRef]
- Matthews, H.; Deakin, J.; Rajab, M.; Idris-Usman, M.; Nirmalan, N.J. Investigating antimalarial drug interactions of emetine dihydrochloride hydrate using CalcuSyn-based interactivity calculations. PLoS ONE 2017, 12, e0173303. [Google Scholar] [CrossRef]
- Kretzing, S.; Abraham, G.; Seiwert, B.; Ungemach, F.R.; Krügel, U.; Regenthal, R. Dose-dependent emetic effects of the Amaryllidaceous alkaloid lycorine in beagle dogs. Toxicon 2011, 57, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Liang, L.; Xiao, X.; Feng, P.; Ye, M.; Liu, J. Lycorine: A prospective natural lead for anticancer drug discovery. Biomed. Pharmacother. 2018, 107, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yuan, X.; Yu, T.; Huang, H.; Yang, C.; Zhang, L.; Yang, S.; Luo, X.; Luo, J. Lycorine inhibits cell proliferation, migration and invasion, and primarily exerts in vitro cytostatic effects in human colorectal cancer via activating the ROS/p38 and AKT signaling pathways. Oncol. Rep. 2021, 45, 19. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Lipton, J.H.; Rea, D.; Digumarti, R.; Chuah, C.; Nanda, N.; Benichou, A.-C.; Craig, A.R.; Michallet, M.; Nicolini, F.E.; et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation. Blood 2012, 120, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, F.E.; Khoury, H.J.; Akard, L.; Rea, D.; Kantarjian, H.; Baccarani, M.; Leonoudakis, J.; Craig, A.; Benichou, A.C.; Cortes, J. Omacetaxine mepesuccinate for patients with accelerated phase chronic myeloid leukemia with resistance or intolerance to two or more tyrosine kinase inhibitors. Haematologica 2013, 98, 78–79. [Google Scholar] [CrossRef]
| Compound | Source | Virus | Dose IC50 or EC50 | Activity | Experiment | Ref |
|---|---|---|---|---|---|---|
| Anisomycin | Pure | DENV, ZIKAV | 7.6 nM 15.9 nM | Inhibits replication | In vitro/In vivo | [39] |
| Berberine | Berberis vulgaris (Berberidaceae) | HCMV | 0.68 μM | Inhibits the virus replication cycle between viral attachment/entry and genomic DNA replication | In vitro | [40] |
| HSV-1 and 2 | 6.77 and 5.04 μM | [41] | ||||
| Hydrastis Canadensis | H1N1 | 0.01 μM | Inhibited production of proteins | [42] | ||
| Buchapine, | Euodia roxburghiana | HIV-1 | 0.94 μM | Inhibit reverse transcriptase | In vitro | [43] |
| Ophiorrhiza mungos | VACV | 10 μM, | Inhibition of viral DNA synthesis | In vitro | [44] | |
| Camptothecin | Camptotheca acuminata | EV71 | 10 μM | Inhibits viral RNA replication and translation | In vitro, | [45] |
| Colchicine | Haplophyllum tuberculatum, Colchicum autumnale | HIV 1 | 1–10 | Inhibit replication by DNA intercalation | In vitro | [46] |
| Emetine | Cephaelis ipecacuanha A. | RNA, DNA viruses | 3.03 ng | Inhibition of viral protein synthesis | In vitro/In vivo | [47] |
| ZIKAV, EVD | 10 μM | Inhibition of ZIKAV NS5 polymerase activity and disruption of lysosomal function, inhibits EBOV entry andreplication of DNA viruses | In vitro/In vivo | [48] | ||
| HIV | 0.72 μM | Inhibits HIV-1 Replication by interfering with reverse transcriptase activity | In vitro | [49] | ||
| SARS, MERS | 0.0135 μM | Replication inhibition | In vitro | [50] | ||
| HCMV | 0.0087–0.04 μM | Inhibition on ribosomal processing S14 (RPS14) binding | In vitro | [51] | ||
| SARS-CoV-2 | 0.46 μM | Replication inhibition | In vitro | [52] | ||
| Fangchinolin | Stephania tetrandra | HCV | 10 µM | Suppressed the replication and inhibited viral S and N protein expression | In vitro | [53] |
| Gliotoxin | Pure | HI | 10 μM | Inhibits intracellular replication | In vitro | [54] |
| Hemanthamene | H5N1 | 4·15 μM | Inhibits the translocation of the ribonucleoprotein complex | In vitro | [55] | |
| Homorringtonine | Cephalotoxus fortune | SARS-CoV-2 | 2.55 μM | Replication inhibition | In vitro | [52] |
| Isatindigobisindoloside F. | Isatis indigotica | CVB3 | 8.4 μM | Inhibit the replication | In vitro | [56] |
| Lycorine | Clivia miniate | ZIKAV | 0.01 to 10 μM | Inhibits viral replication by restraining RdRp activity | In vitro/In vivo | [57] |
| HCV | 6.10 μM | Inhibits viral replication by the expression of hsc70 in host cells | In vitro | [58] | ||
| H5N1 | 0·52 μM | Inhibits the translocation of the ribonucleoprotein complex | In vitro | [59] | ||
| DENV | 0.4 μM | Suppression of viral RNA replication | In vitro | [60] | ||
| L.radiata | SARS-CoV | 15.7 nM | Viral inhibition | In vitro | [61] | |
| Manzamine | Acanthostrongylophora | HSV-1 | 1 µM | Repressed ICP0 transcription | In vitro | [62] |
| Michellamines B | A. korupensis | HIV | 1 μM | Inhibiting cellular fusion and syncytium formation | In vitro | [63] |
| Michellamines A, B, and C | Ancistrocladus korupensis | HIV1, HIV2 | 2–10 μM | Complete inhibition of the replication and cytopathic effects of HIV | In vitro/In vivo | [64] |
| Oliverine | Polyathia oliveri; | HSV-1 | 7.5 μM | Inhibition of viral DNA synthesis | In vitro | [65] |
| Polycitone A | Polycitor sp | HIV, MLV, MMTV | 295 nM | Potent inhibitory activity on both RNA- and DNA-directed DNA polymerases | In vitro | [66] |
| Reserpine | Pure | SARS | 3.4 μM | Inhibits 3CL protease and viral entry | In vitro | [67] |
| Schumannificine | Schumanniophyton magnificum; | HSV | 1.6 μM | Irreversible binding to gp120 | In vitro | [53] |
| Tetrandrine, | Stephania tetrandra | HCV | 10 µM | Suppressed the replication and inhibited viral S and N protein expression | In vitro | [53] |
| Thalimonine | Thalictrum simplex | H7N7 | 0.1 µM | Inhibits viral reproduction | In vitro | [68] |
| Tomatidine | Skin and leaves of tomatoes | CHIKV | 1.3 µM | Inhibits infection of three different CHIKV genotypes | In vitro | [69] |
| DENV | 0.82 μM | Inhibit replication | In vitro | [70] | ||
| 18-methoxycoronaidine | Tabernanthe iboga | HIV-1 | 9.5 μM | Inhibit the replication of primary isolates of HIV | In vitro | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abookleesh, F.L.; Al-Anzi, B.S.; Ullah, A. Potential Antiviral Action of Alkaloids. Molecules 2022, 27, 903. https://doi.org/10.3390/molecules27030903
Abookleesh FL, Al-Anzi BS, Ullah A. Potential Antiviral Action of Alkaloids. Molecules. 2022; 27(3):903. https://doi.org/10.3390/molecules27030903
Chicago/Turabian StyleAbookleesh, Frage L., Bader S. Al-Anzi, and Aman Ullah. 2022. "Potential Antiviral Action of Alkaloids" Molecules 27, no. 3: 903. https://doi.org/10.3390/molecules27030903
APA StyleAbookleesh, F. L., Al-Anzi, B. S., & Ullah, A. (2022). Potential Antiviral Action of Alkaloids. Molecules, 27(3), 903. https://doi.org/10.3390/molecules27030903








