Antileishmanial Effects of Acetylene Acetogenins from Seeds of Porcelia macrocarpa (Warm.) R.E. Fries (Annonaceae) and Semisynthetic Derivatives
Abstract
:1. Introduction
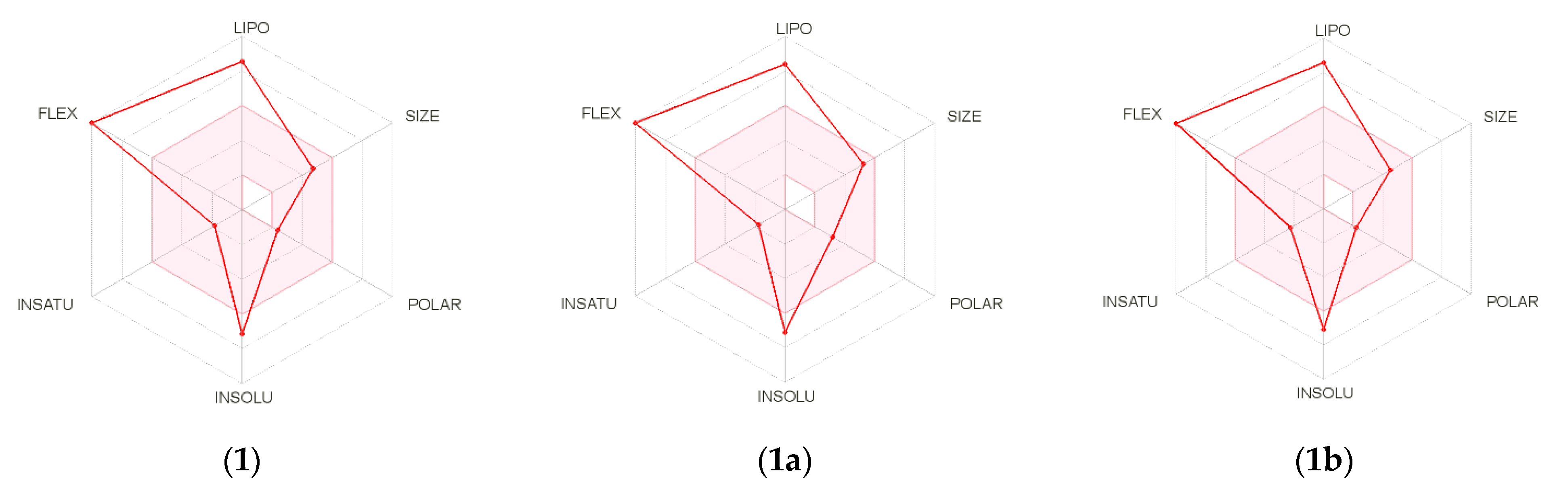
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
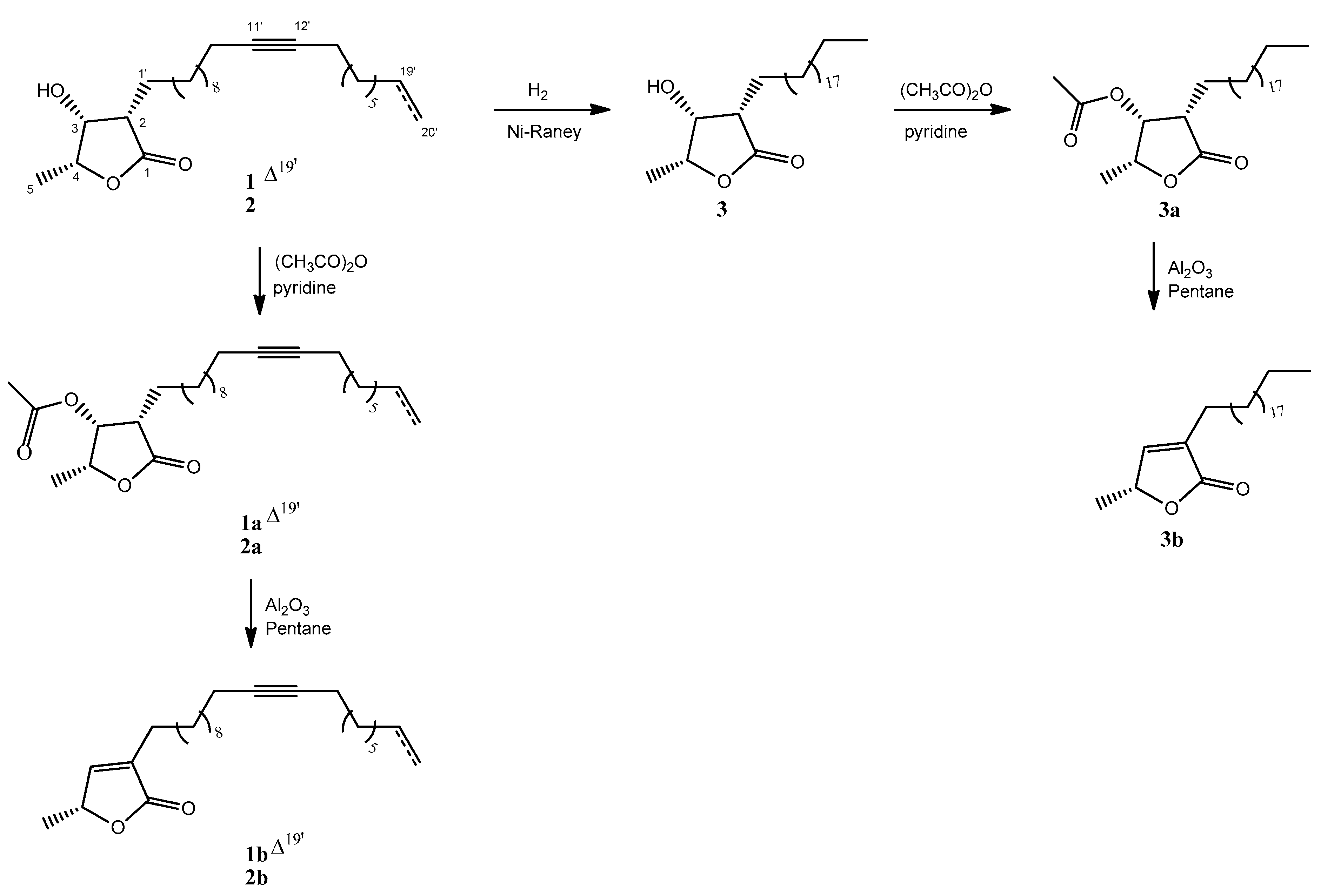
3.4. Preparation of Semisynthetic Compounds
3.4.1. Hydrogenation (Compound 3)
3.4.2. Acetylation (Compounds 1a–3a)
3.4.3. Elimination (Compounds 1b–3b)
3.5. In Silico Studies
3.6. Bioassay Procedures
3.6.1. Animals
3.6.2. Parasite Maintenance
3.6.3. Mammalian Cells
3.6.4. Determination of the Activity against L. (L.) infantum—Intracellular Amastigotes
3.6.5. Determination of the Cytotoxicity against Mammalian Cells
3.6.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sasidharan, S.; Saudagar, P. Leishmaniasis: Where are we and where are we heading? Parasitol. Res. 2021, 120, 1541–1554. [Google Scholar] [CrossRef]
- Sakyi, P.O.; Amewu, R.K.; Devine, R.N.; Ismaila, E.; Miller, W.A.; Kwofie, S.K. The search for putative hits in combating leishmaniasis: The contributions of natural products over the last decade. Nat. Prod. Bioprospect. 2021, 11, 489–544. [Google Scholar] [CrossRef] [PubMed]
- Passero, L.F.D.; dos Santos Brunelli, E.; Sauini, T.; Amorim Pavani, T.F.; Jesus, J.A.; Rodrigues, E. The Potential of Traditional Knowledge to Develop Effective Medicines for the Treatment of Leishmaniasis. Front. Pharmacol. 2021, 12, 1408. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.A. Revision of Cymbopetalum and Porcelia (Annonaceae). Syst. Bot. Monogr. 1993, 40, 89–121. [Google Scholar] [CrossRef]
- Santos, L.D.Á.; Cavalheiro, A.J.; Tempone, A.G.; Correa, D.S.; Alexandre, T.R.; Quintiliano, N.F.; Rodrigues-Oliveira, A.F.; Oliveira-Silva, D.; Martins, R.C.C.; Lago, J.H.G. Antitrypanosomal acetylene fatty acid derivatives from the seeds of Porcelia macrocarpa (Annonaceae). Molecules 2015, 20, 8168–8180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Londero, V.S.; da Costa-Silva, T.A.; Gomes, K.D.S.; Ferreira, D.D.; Mesquita, J.T.; Tempone, A.; Young, M.C.M.; Jerz, G.; Lago, J.H.G. Acetylenic fatty acids from Porcelia macrocarpa (Annonaceae) against trypomastigotes of Trypanosoma cruzi: Effect of octadec-9-ynoic acid in plasma membrane electric potential. Bioorg. Chem. 2018, 78, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.A.; Brito, I.A.; Lima, M.L.; Romanelli, M.; Moreira-Filho, J.T.; Neves, B.J.; Andrade, C.H.; Sartorelli, P.; Tempone, A.G.; Costa-Silva, T.A.; et al. Antitrypanosomal Activity of acetogenins isolated from the seeds of Porcelia macrocarpa is associated with alterations in both plasma membrane electric potential and mitochondrial membrane potential. J. Nat. Prod. 2019, 82, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Brito, I.; Oliveira, E.; Chaves, M.; Thevenard, F.; Rodrigues-Oliveira, A.; Barbosa-Reis, G.; Sartorelli, P.; Oliveira-Silva, D.; Tempone, A.; Costa-Silva, T.; et al. Antileishmanial acetylene fatty acid and acetogenins from seeds of Porcelia macrocarpa. J. Braz. Chem. Soc. 2021, 32, 447–453. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Chaves, M.H.; Ayres, M.C.C.; Agripino, D.G.; Young, M.C.M. Evaluation of antifungal and DNA-damaging activities of alkaloids from branches of Porcelia macrocarpa. Planta Med. 2007, 73, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.B.P.; Soares, M.G.; Mariane, B.; Vallim, M.A.; Pascon, R.C.; Sartorelli, P.; Lago, J.H.G. The seasonal variation of the chemical composition of essential oils from Porcelia macrocarpa R.E. Fries (Annonaceae) and their antimicrobial activity. Molecules 2013, 18, 13574–13587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves, M.H.; Roque, N.F. Acetogenins from Porcelia macrocarpa: Stereochemical determination of 2-alkyl-3-hydroxy-4-methyl γ-lactones by 13C NMR spectroscopy. Phytochemistry 1997, 44, 523–528. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stauber, L.A.; Franchino, E.M.; Grun, J. An eight-day method for screening compounds against Leishmania donovani in the golden hamster. J. Protozool. 1958, 5, 269–273. [Google Scholar] [CrossRef]
- Grecco, S.S.; Costa-Silva, T.A.; Sousa, F.S.; Cargnelutti, S.B.; Umehara, E.; Mendonça, P.S.; Tempone, A.; Lago, J.H.G. Neolignans isolated from twigs of Nectandra leucantha Ness & Mart (Lauraceae) displayed in vitro antileishmanial activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 27. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Shiho, O.; Kuroshima, K.-I.; Koyama, M.; Tsukamoto, K. An improved colorimetric assay for interleukin 2. J. Immunol. Methods 1986, 93, 157–165. [Google Scholar] [CrossRef]

| Compound | IC50/μM L. (L.) Infantum | CC50/μM NCTC | SI |
|---|---|---|---|
| 1 | 29.9 ± 9.7 | >200 | >6.7 |
| 2 | NA | >200 | - |
| mixtures of 1 and 2 * | |||
| 2:1 | 8.4 ± 3.6 | >200 | >23.8 |
| 1:1 | 13.6 ± 4.3 | >200 | >14.4 |
| 1:2 | 19.4 ± 7.8 | >200 | >10.3 |
| 1a | 43.4 ± 3.9 | >200 | >4.6 |
| 2a | NA | >200 | - |
| mixtures of 1a and 2a * | |||
| 2:1 | 12.0 ± 2.0 | >200 | >16.7 |
| 1:1 | 23.1 ± 6.5 | >200 | >8.7 |
| 1:2 | 38.4 ± 6.2 | >200 | >5.2 |
| 1b | 23.1 ± 5.4 | >200 | >8.6 |
| 2b | NA | >200 | - |
| mixtures of 1b and 2b * | |||
| 2:1 | 7.9 ± 4.4 | >200 | >25.3 |
| 1:1 | 10.5 ± 7.1 | >200 | >19.0 |
| 1:2 | 18.2 ± 9.0 | >200 | >11.0 |
| 3 | NA | >200 | - |
| 3a | NA | >200 | - |
| 3b | NA | >200 | - |
| Miltefosine | 17.8 ± 1.4 | 116.0 ± 5.3 | 6.5 |
| Physicochemical Properties | 1 | 1a | 1b |
|---|---|---|---|
| Num. heavy atoms | 28 | 31 | 27 |
| Fraction Csp3 | 0.77 | 0.78 | 0.72 |
| Num. rotatable bonds | 16 | 18 | 16 |
| Num. H-bond acceptors | 2 | 4 | 2 |
| Num. H-bond donors | 1 | 0 | 0 |
| log Po/w (iLOGP) | 6.15 | 5.64 | 5.80 |
| Water Solubility | Poorly | Poorly | Poorly |
| GI absorption | Low | Low | Low |
| BBB permeant | No | No | No |
| CYP1A2 inhibitor | Yes | Yes | Yes |
| CYP2C19 inhibitor | No | No | No |
| CYP2C9 inhibitor | No | Yes | Yes |
| CYP2D6 inhibitor | No | No | No |
| CYP3A4 inhibitor | No | No | No |
| Lipinski | One violation (log p > 4.15) | One violation (log p > 4.15) | One violation (log p > 4.15) |
| Bioavailability Score | 0.55 | 0.55 | 0.55 |
| PAINS alert | No | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, I.A.; Thevenard, F.; Costa-Silva, T.A.; Oliveira, S.S.; Cunha, R.L.O.R.; de Oliveira, E.A.; Sartorelli, P.; Guadagnin, R.C.; Romanelli, M.M.; Tempone, A.G.; et al. Antileishmanial Effects of Acetylene Acetogenins from Seeds of Porcelia macrocarpa (Warm.) R.E. Fries (Annonaceae) and Semisynthetic Derivatives. Molecules 2022, 27, 893. https://doi.org/10.3390/molecules27030893
Brito IA, Thevenard F, Costa-Silva TA, Oliveira SS, Cunha RLOR, de Oliveira EA, Sartorelli P, Guadagnin RC, Romanelli MM, Tempone AG, et al. Antileishmanial Effects of Acetylene Acetogenins from Seeds of Porcelia macrocarpa (Warm.) R.E. Fries (Annonaceae) and Semisynthetic Derivatives. Molecules. 2022; 27(3):893. https://doi.org/10.3390/molecules27030893
Chicago/Turabian StyleBrito, Ivanildo A., Fernanda Thevenard, Thais A. Costa-Silva, Samuel S. Oliveira, Rodrigo L. O. R. Cunha, Emerson A. de Oliveira, Patricia Sartorelli, Rafael C. Guadagnin, Maiara M. Romanelli, Andre G. Tempone, and et al. 2022. "Antileishmanial Effects of Acetylene Acetogenins from Seeds of Porcelia macrocarpa (Warm.) R.E. Fries (Annonaceae) and Semisynthetic Derivatives" Molecules 27, no. 3: 893. https://doi.org/10.3390/molecules27030893
APA StyleBrito, I. A., Thevenard, F., Costa-Silva, T. A., Oliveira, S. S., Cunha, R. L. O. R., de Oliveira, E. A., Sartorelli, P., Guadagnin, R. C., Romanelli, M. M., Tempone, A. G., & Lago, J. H. G. (2022). Antileishmanial Effects of Acetylene Acetogenins from Seeds of Porcelia macrocarpa (Warm.) R.E. Fries (Annonaceae) and Semisynthetic Derivatives. Molecules, 27(3), 893. https://doi.org/10.3390/molecules27030893








