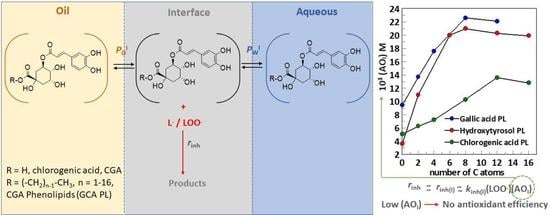
Unexpected Antioxidant Efficiency of Chlorogenic Acid Phenolipids in Fish Oil-in-Water Nanoemulsions: An Example of How Relatively Low Interfacial Concentrations Can Make Antioxidants to Be Inefficient
Abstract
:1. Introduction
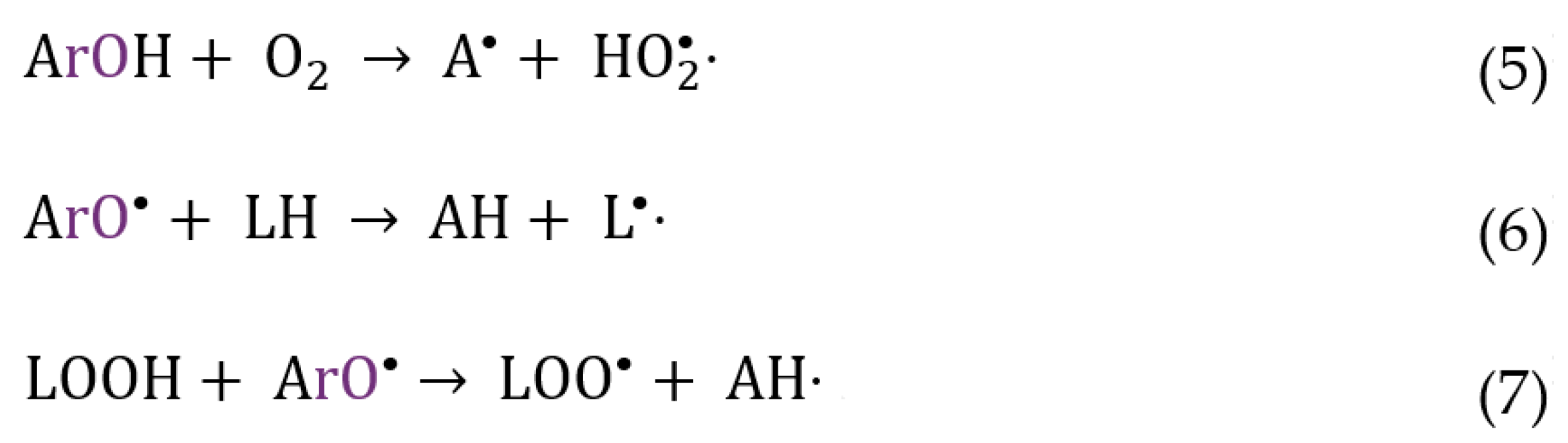
2. Results and Discussion
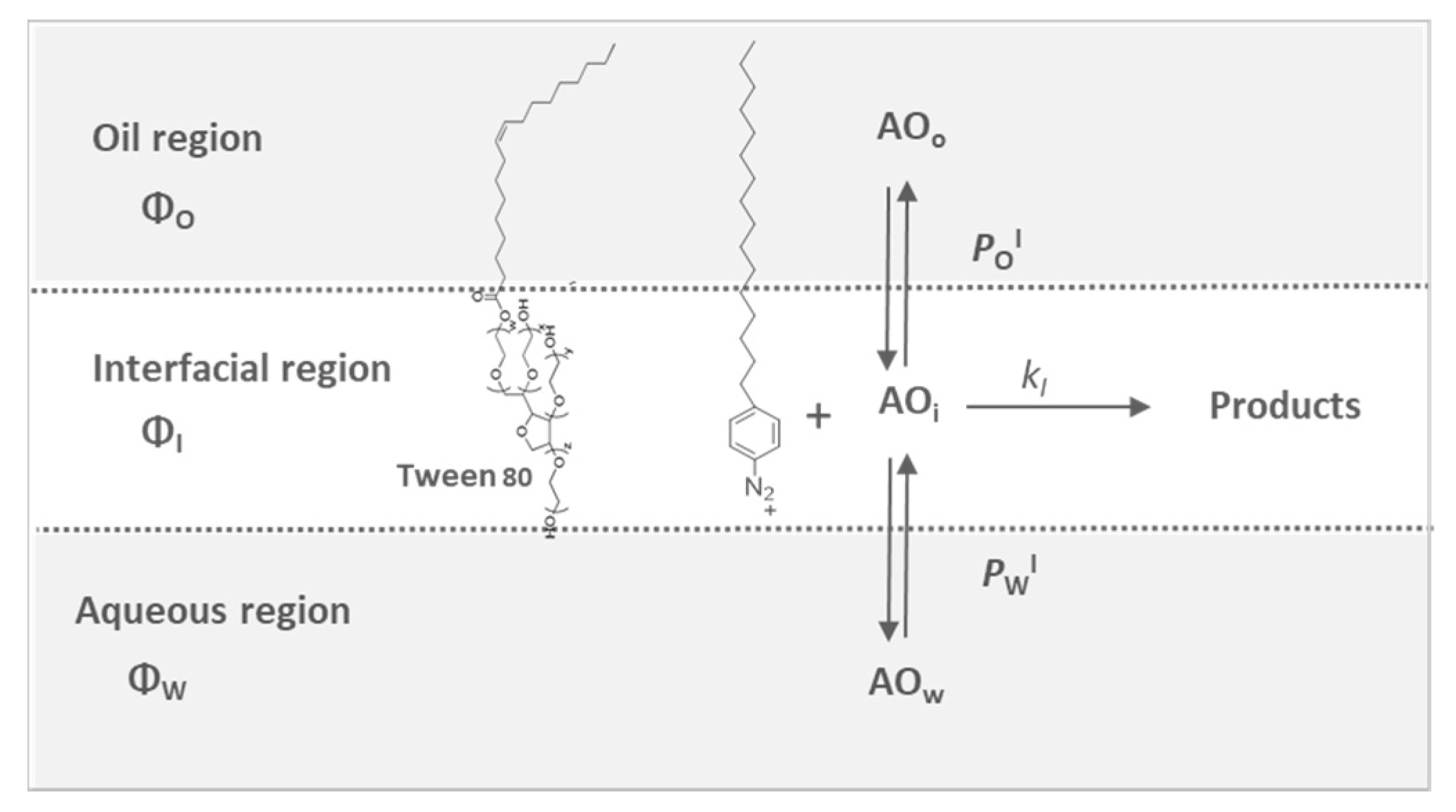
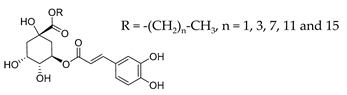
2.1. Partition Constants of Chlorogenic Acid and Its Esters in Binary Fish Oil-Water Mixtures, PWO
2.2. Determining the Partition Constants of Chlorogenic Acid and Its Esters in Intact Fish Oil Emulsified Systems
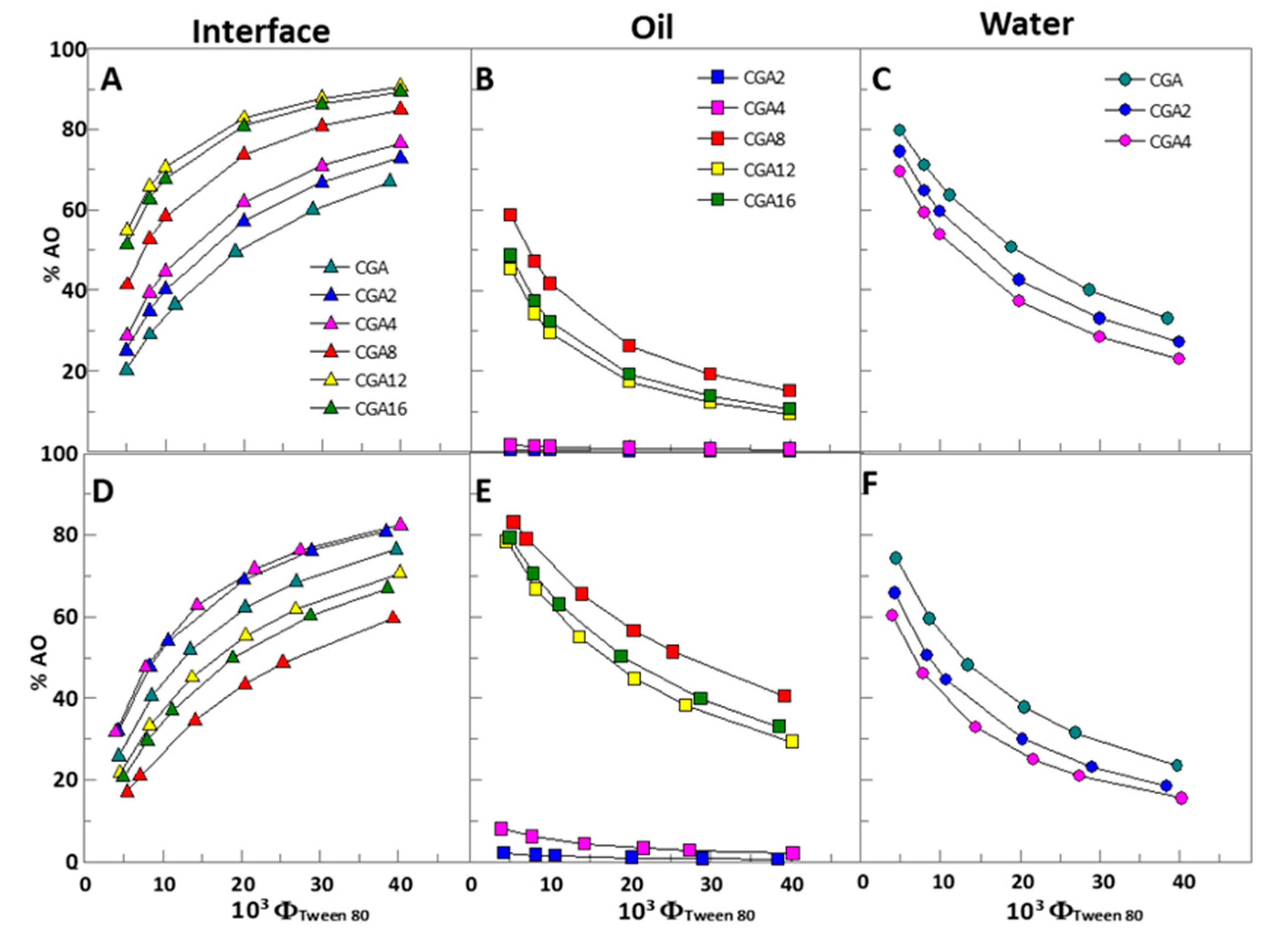
2.3. Distribution of Chlorogenic Acid and Its Esters in Fish Oil-in-Water Emulsified Systems
2.4. Effects of the Oil to Water Ratio, Droplet Size, and the Emulsifier Volume Fraction on the Effective Interfacial Concentrations of Chlorogenic Acid and Its Esters in Fish Oil Emulsified Systems
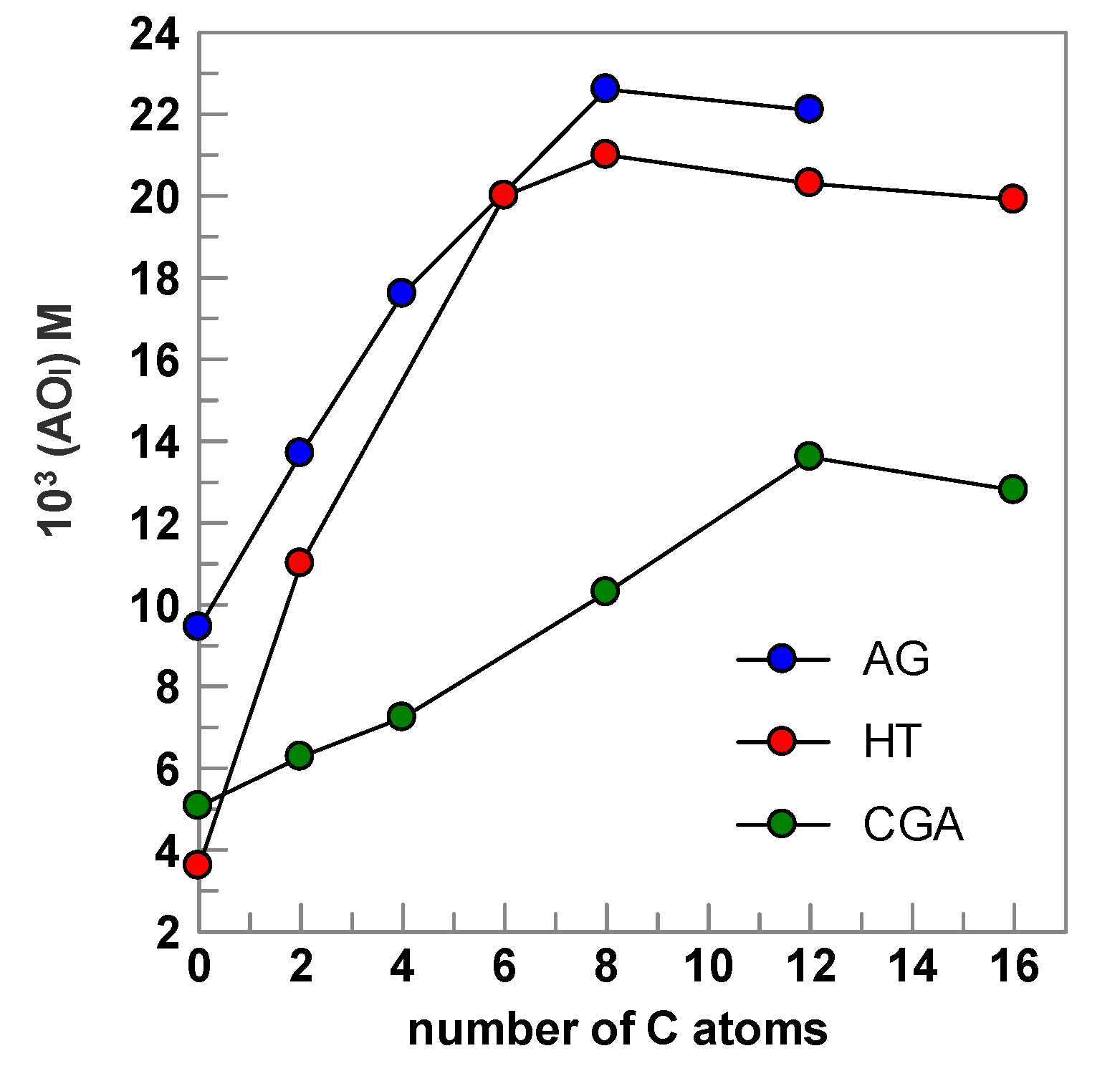
2.5. Antioxidant Efficiency of Chlorogenic Acid and Its Esters in Fish Oil Emulsified Systems
3. Materials and Methods
3.1. Materials
3.2. Preparation of Emulsions and Nanoemulsions
3.3. Droplet Size and Size Distributions of Fish Oil-In Water Emulsified Systems
3.4. Antioxidant Efficiency of Chlorogenic Acid and Its Esters in Fish Oil Emulsified Systems
3.5. Determining the Partition Constant, PWO, of Chlorogenic Acid and Its Esters in Binary Fish Oil–Water Mixtures
3.6. Determining Antioxidant Distribution and Local Concentrations in Intact Fish Oil Emulsified Systems
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jacobsen, C.; Nielsen, N.S.; Horn, A.F.; Sørensen, A.-D.M. Food Enrichment with Omega-3 Fatty Acids; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 0857098861. [Google Scholar]
- Fell, G.L.; Nandivada, P.; Gura, K.M.; Puder, M. Intravenous lipid emulsions in parenteral nutrition. Adv. Nutr. 2015, 6, 600–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural Design Principles for Delivery of Bioactive Components in Nutraceuticals and Functional Foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef] [PubMed]
- Arab-Tehrany, E.; Jacquot, M.; Gaiani, C.; Imran, M.; Desobry, S.; Linder, M. Beneficial effects and oxidative stability of omega-3 long-chain polyunsaturated fatty acids. Trends Food Sci. Technol. 2012, 25, 24–33. [Google Scholar] [CrossRef]
- Mason, R.P.; Sherratt, S.C.R. Omega-3 fatty acid fish oil dietary supplements contain saturated fats and oxidized lipids that may interfere with their intended biological benefits. Biochem. Biophys. Res. Commun. 2017, 483, 425–429. [Google Scholar] [CrossRef]
- Taşbozan, O.; Gökçe, M.A. Fatty Acids in Fish. In Fatty Acids; Catala, A., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 8; ISBN 978-953-51-3302-5. [Google Scholar]
- Mori, T.A. Omega-3 fatty acids and hypertension in humans. Clin. Exp. Pharmacol. Physiol. 2006, 33, 842–846. [Google Scholar] [CrossRef]
- Calder, P.C. 1-Nutritional benefits of omega-3 fatty acids. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Jacobsen, C., Nielsen, N.S., Horn, A.F., Moltke Sorensen, A.-D., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 3–26. ISBN 9780857094285. [Google Scholar]
- Secci, G.; Parisi, G. From farm to fork: Lipid oxidation in fish products. A review. Ital. J. Anim. Sci. 2016, 15, 124–136. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Serini, S.; Donato, V.; Piccioni, E.; Trombino, S.; Monego, G.; Toesca, A.; Innocenti, I.; Missori, M.; De Spirito, M.; Celleno, L. Docosahexaenoic acid reverts resistance to UV-induced apoptosis in human keratinocytes: Involvement of COX-2 and HuR. J. Nutr. Biochem. 2011, 22, 874–885. [Google Scholar] [CrossRef]
- Erdmann, M.E.; Lautenschlaeger, R.; Schmidt, H.; Zeeb, B.; Gibis, M.; Brüggemann, D.A.; Weiss, J. Influence of droplet size on the antioxidant efficacy of oil-in-water emulsions loaded with rosemary in raw fermented sausages. Eur. Food Res. Technol. 2017, 243, 1415–1427. [Google Scholar] [CrossRef]
- Kanner, J. Dietary advanced lipid oxidation endproducts are risk factors to human health. Mol. Nutr. Food Res. 2007, 51, 1094–1101. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation: Measurement methods. In Bailey’s Industrial Oil and Fat Products, 6th ed.; Shahidi, F., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005; pp. 357–385. [Google Scholar]
- Barclay, L.R.C.; Vinqvist, M.R. The Chemistry of Phenols; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Pokorny, J.; Yanishlieva, N.; Gordon, M. Antioxidants in Food: Practical Applications; Elsevier: Amsterdam, The Netherlands, 2001; ISBN 185573463X. [Google Scholar]
- Shahidi, F. Handbook of Antioxidants for Food Preservation; Woodhead Publishing: Cambridge, UK, 2015; ISBN 1782420975. [Google Scholar]
- Wanasundara, P.K.J.P.D.; Shahidi, F. Antioxidants: Science, Technology, and Applications. In Bailey’s Industrial Oil and Fat Products; Wiley & Sons: New York, NY, USA, 2005; pp. 431–489. [Google Scholar]
- Bravo-Díaz, C.; Romsted, L.S.; Liu, C.; Losada-Barreiro, S.; Pastoriza-Gallego, M.J.; Gao, X.; Gu, Q.; Krishnan, G.; Sánchez-Paz, V.; Zhang, Y.; et al. To Model Chemical Reactivity in Heterogeneous Emulsions, Think Homogeneous Microemulsions. Langmuir 2015, 31, 8961–8979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C.; Romsted, L.S. A direct correlation between the antioxidant efficiencies of caffeic acid and its alkyl esters and their concentrations in the interfacial region of olive oil emulsions. The pseudophase model interpretation of the “cut-off” effect. Food Chem. 2015, 175, 233–242. [Google Scholar] [CrossRef]
- Costa, M.; Losada-Barreiro, S.; Bravo-Díaz, C.; Vicente, A.A.; Monteiro, L.S.; Paiva-Martins, F. Influence of AO chain length, droplet size and oil to water ratio on the distribution and on the activity of gallates in fish oil-in-water emulsified systems: Emulsion and nanoemulsion comparison. Food Chem. 2020, 310, 125716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meireles, M.; Losada-Barreiro, S.; Costa, M.; Paiva-Martins, F.; Bravo-Díaz, C.; Monteiro, L.S. Control of antioxidant efficiency of chlorogenates in emulsions: Modulation of antioxidant interfacial concentrations. J. Sci. Food Agric. 2019, 99, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Losada-Barreiro, S.; Costa, M.; Bravo-Díaz, C.; Paiva-Martins, F. Distribution and antioxidant efficiency of resveratrol in stripped corn oil emulsions. Antioxidants 2014, 3, 212–228. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Modulating the interfacial concentration of gallates to improve the oxidative stability of fish oil-in-water emulsions. Food Res. Int. 2018, 112, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Freiría-Gándara, J.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Effects of droplet size on the interfacial concentrations of antioxidants in fish and olive oil-in-water emulsions and nanoemulsions and on their oxidative stability. J. Colloid Interface Sci. 2020, 562, 352–562. [Google Scholar] [CrossRef]
- Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Polyphenolic antioxidants in lipid emulsions: Partitioning effects and interfacial phenomena. Foods 2021, 10, 539. [Google Scholar] [CrossRef]
- Bravo-Díaz, C.; Romsted, L.S.; Losada-Barreiro, S.; Paiva-Martins, F. Using a pseudophase model to determine AO distributions in emulsions: Why dynamic equilibrium matters. Eur. J. Lipid Sci. Technol. 2017, 119, 1600277. [Google Scholar] [CrossRef]
- Costa, M.; Losada-Barreiro, S.; Bravo-Díaz, C.; Monteiro, L.S.; Paiva-Martins, F. Interfacial Concentrations of Hydroxytyrosol Derivatives in Fish Oil-in-Water Emulsions and Nanoemulsions and Its Influence on Their Lipid Oxidation: Droplet Size Effects. Foods 2020, 9, 1897. [Google Scholar] [CrossRef]
- Almeida, J.; Losada-Barreiro, S.; Costa, M.; Paiva-Martins, F.; Bravo-Díaz, C.; Romsted, L.S. Interfacial Concentrations of Hydroxytyrosol and Its Lipophilic Esters in Intact Olive Oil-in-Water Emulsions: Effects of Antioxidant Hydrophobicity, Surfactant Concentration, and the Oil-to-Water Ratio on the Oxidative Stability of the Emulsions. J. Agric. Food Chem. 2016, 64, 5274–5283. [Google Scholar] [CrossRef] [PubMed]
- Freiría-Gándara, J.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Differential partitioning of bioantioxidants in edible oil–water and octanol–water systems: Linear free energy relationships. J. Chem. Eng. Data 2018, 63, 2999–3007. [Google Scholar] [CrossRef]
- Costa, M.; Freiría-Gándara, J.; Losada-Barreiro, S.; Paiva-Martins, F.; Aliaga, C.; Bravo-Díaz, C. Interfacial kinetics in olive oil-in-water nanoemulsions: Relationships between rates of initiation of lipid peroxidation, induction times and effective interfacial antioxidant concentrations. J. Colloid Interface Sci. 2021, 604, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Partitioning and antioxidative effect of protocatechuates in soybean oil emulsions: Relevance of emulsifier concentration. Eur. J. Lipid Sci. Technol. 2017, 119, 1600274. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Solvent Effects on the Rates and Mechanisms of Reaction of Phenols with Free Radicals. Acc. Chem. Res. 2007, 40, 222–230. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Bravo-Diaz, C.; Paiva Martins, F.; Romsted, L.S. A Maximum in Antioxidant Distributions and Efficiencies with Increasing Hydrophobicity of Gallic Acid and its Alkyl Esters. The Pseudophase Model Interpretation of the “Cut-Off” Effect. J. Agric. Food. Chem. 2013, 61, 6533. [Google Scholar] [CrossRef]
- Laguerre, M.; López Giraldo, L.J.; Lecomte, J.; Figueroa-Espinoza, M.-C.; Baréa, B.; Weiss, J.; Decker, E.A.; Villeneuve, P. Chain Length Affects Antioxidant Properties of Chlorogenate Esters in Emulsion: The Cutoff Theory Behind the Polar Paradox. J. Agric. Food Chem. 2009, 57, 11335–11342. [Google Scholar] [CrossRef]
- Laguerre, M.; López Giraldo, L.J.; Lecomte, J.; Figueroa-Espinoza, M.-C.; Baréa, B.; Weiss, J.; Decker, E.A.; Villeneuve, P. Relationship between Hydrophobicity and Antioxidant Ability of “Phenolipids” in Emulsion: A Parabolic Effect of the Chain Length of Rosmarinate Esters. J. Agric. Food Chem. 2010, 58, 2869–2876. [Google Scholar] [CrossRef]
- Sørensen, A.-D.; Nielsen, N.S.; Decker, E.A.; Let, M.B.; Xu, X.; Jacobsen, C. The efficacy of compounds with different polarities as antioxidants in emulsions with omega-3 lipids. J. Am. Oil Chem. Soc. 2011, 88, 489–502. [Google Scholar] [CrossRef]
- Losada Barreiro, S.; Bravo Diaz, C.; Costa, M.; Paiva-Martins, F. Distribution of catechol in emulsions. J. Phys. Org. Chem. 2014, 27, 290–296. [Google Scholar] [CrossRef]
- Costa, M.; Losada-Barreiro, S.; Magalhães, J.; Monteiro, L.S.; Bravo-Díaz, C.; Paiva-Martins, F. Effects of the Reactive Moiety of Phenolipids on Their Antioxidant Efficiency in Model Emulsified Systems. Foods 2021, 10, 1028. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.; Frankel, E.N. Preface. In Lipid Oxidation, 2nd ed.; Oily Press Lipid Library, Series; Frankel, E.N., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 5–10. ISBN 9780953194988. [Google Scholar]
- Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Effects of Surfactant Volume Fraction on the Antioxidant Efficiency and on The Interfacial Concentrations of Octyl and Tetradecyl p-Coumarates in Corn Oil-in-Water Emulsions. Molecules 2021, 26, 6058. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C.; Let, M.B.; Nielsen, N.S.; Meyer, A.S. Antioxidant strategies for preventing oxidative flavour deterioration of foods enriched with n-3 polyunsaturated lipids: A comparative evaluation. Trends Food Sci. Technol. 2008, 19, 76–93. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.; Paiva-Martins, F.; Losada-Barreiro, S.; Bravo-Díaz, C. Modeling Chemical Reactivity at the Interfaces of Emulsions: Effects of Partitioning and Temperature. Molecules 2021, 26, 4703. [Google Scholar] [CrossRef]
- Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Effects of acidity, temperature and emulsifier concentration on the distribution of caffeic acid in stripped corn and olive oil-in-water emulsions. J. Am. Oil Chem. Soc. 2013, 90, 1629–1636. [Google Scholar] [CrossRef]
- Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Optimizing the efficiency of antioxidants in emulsions by lipophilization: Tuning interfacial concentrations. RSC Adv. 2016, 6, 91483–91493. [Google Scholar] [CrossRef]

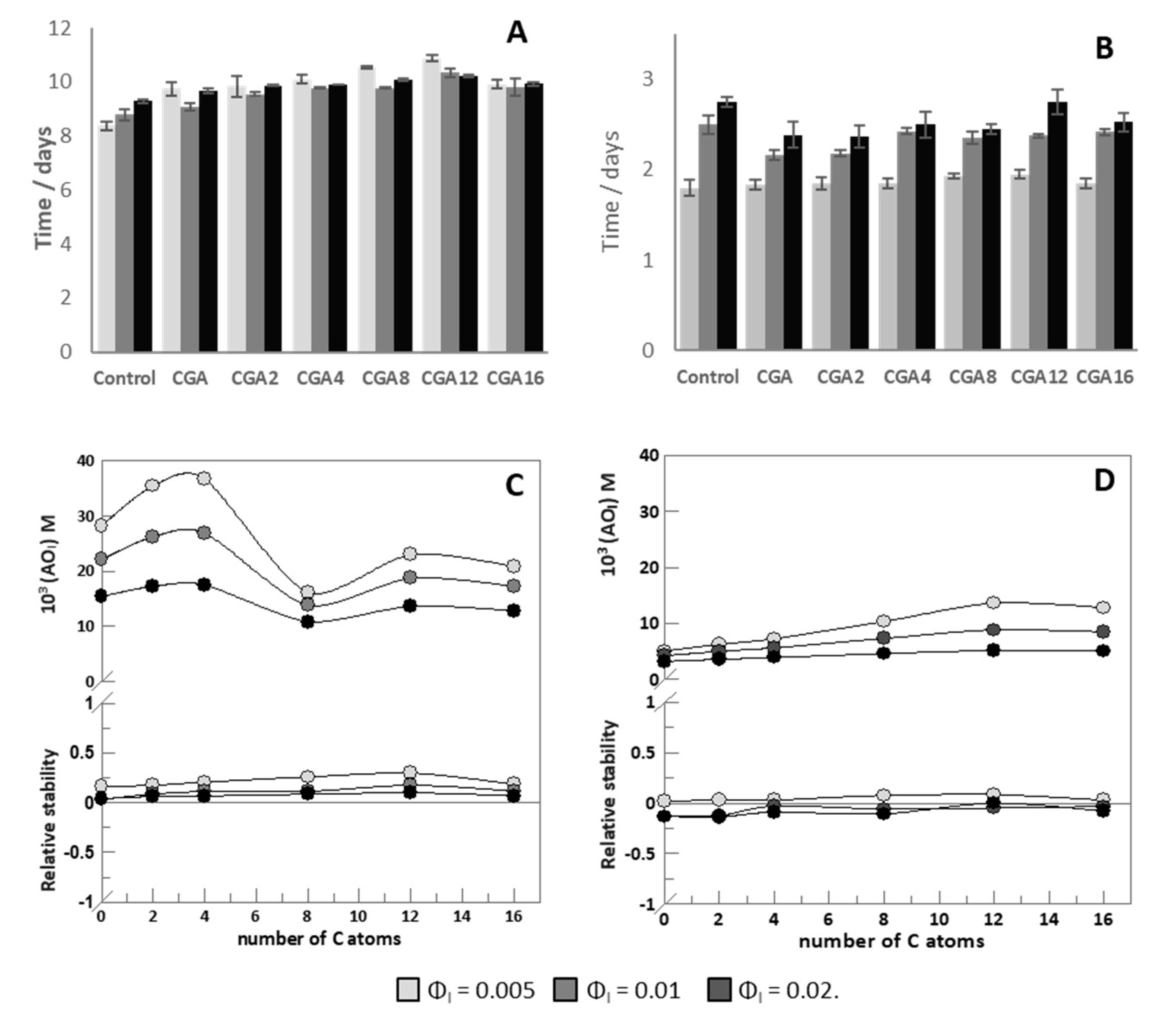
 —ΦI = 0.005,
—ΦI = 0.005,  —ΦI = 0.01,
—ΦI = 0.01,  —ΦI = 0.02) at different emulsifier volume fractions. (B) Effect of O/W ratio on interfacial AOs concentration in nanoemulsions at ΦI = 0.005. In both graphs, [AOT] = 0.125 mM.
—ΦI = 0.02) at different emulsifier volume fractions. (B) Effect of O/W ratio on interfacial AOs concentration in nanoemulsions at ΦI = 0.005. In both graphs, [AOT] = 0.125 mM.
 —ΦI = 0.005,
—ΦI = 0.005,  —ΦI = 0.01,
—ΦI = 0.01,  —ΦI = 0.02) at different emulsifier volume fractions. (B) Effect of O/W ratio on interfacial AOs concentration in nanoemulsions at ΦI = 0.005. In both graphs, [AOT] = 0.125 mM.
—ΦI = 0.02) at different emulsifier volume fractions. (B) Effect of O/W ratio on interfacial AOs concentration in nanoemulsions at ΦI = 0.005. In both graphs, [AOT] = 0.125 mM.
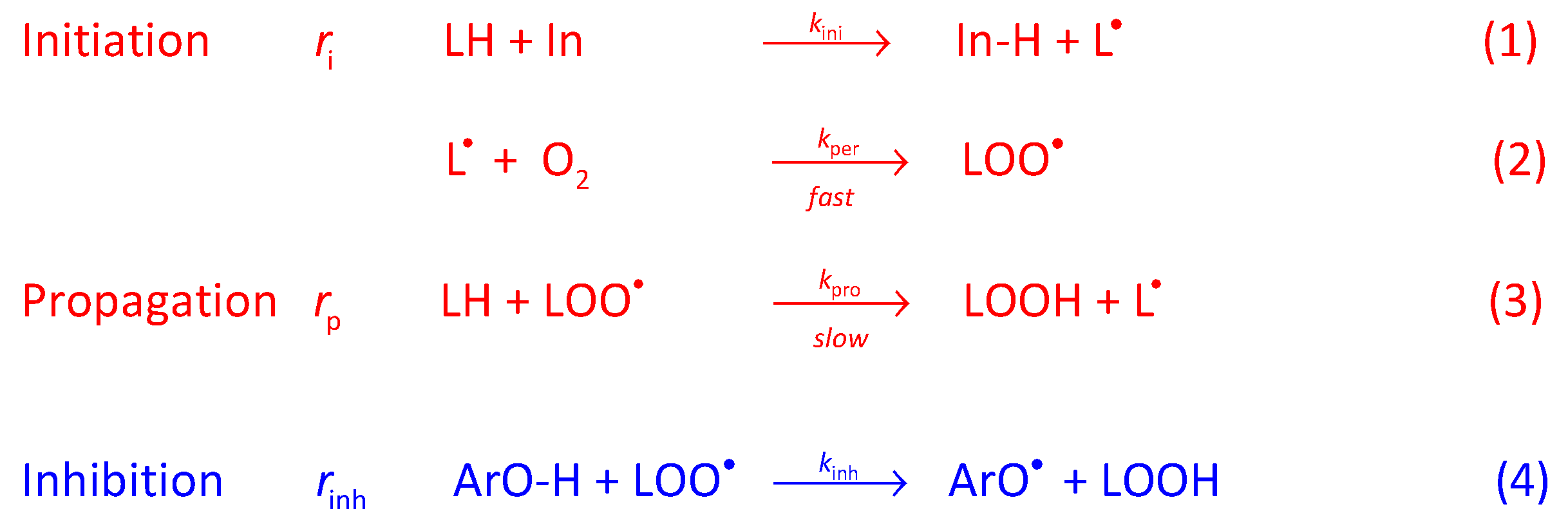
| Compound | CGA | CGA2 | CGA4 | CGA8 | CGA12 | CGA16 | |
|---|---|---|---|---|---|---|---|
| Binary System | (%AOW) | 99.4 ± 0.15 | 97.1 ± 0.52 | 87.5 ± 0.53 | ≈ 0 | ≈ 0 | ≈ 0 |
| PWO | 0.009 ± 0.002 | 0.05 ± 0.01 | 0.2 ± 0.01 | --- | --- | --- | |
| Emulsion O/W | PWI | 47 ± 3 | 67 ± 5 | 78 ± 9 | --- | --- | --- |
| POI | --- | 1344 ± 94 | 390 ± 44 | 15 ± 7 | 24 ± 3 | 21 ± 5 | |
| 102 kI (M−1s−1) | 1.9 ± 0.7 | 2.8 ± 0.4 | 3.6 ± 0.7 | 3.7 ± 0.1 | 3.4 ± 0.1 | 3.5 ± 0.1 | |
| Nanoemulsion O/W | PWI | 46 ± 3 | 60 ± 8 | 74 ± 5 | --- | --- | --- |
| POI | --- | 1195 ± 166 | 369 ± 26 | 14 ± 1 | 24 ± 4 | 21 ± 3 | |
| 102 kI (M−1s−1) | 4.4 ± 0.1 | 5.5 ± 1.2 | 7.7 ± 0.9 | 6.8 ± 0.1 | 7.2 ± 0.1 | 6.4 ± 0.1 | |
| EC50 * ± σa | 5 min | 0.243 ± 0.004 | 0.243 ± 0.003 | 0.248 ± 0.003 | 0.244 ± 0.003 | 0.249 ± 0.006 | 0.237 ± 0.006 |
| 15 min | 0.235 ± 0.005 | 0.217 ± 0.005 | 0.234 ± 0.002 | 0.234 ± 0.004 | 0.238 ± 0.004 | 0.223 ± 0.006 | |
| 60 min | 0.234 ± 0.006 | 0.172 ± 0.012 | 0.179 ± 0.005 | 0.184 ± 0.008 | 0.189 ± 0.005 | 0.161 ± 0.005 | |
| Epa * (V) | 0% Tween 80 | 0.391 | 0.410 | 0.397 | 0.406 | 0.398 | 0.390 |
| 2% Tween 80 | 0.398 | 0.403 | 0.400 | 0.409 | 0.407 | 0.400 |
| Nanoemulsions | Emulsions | |||||
|---|---|---|---|---|---|---|
| Φo | 1.0 | 1.0 | 1.0 | 4.0 | 4.0 | 4.0 |
| 102 ΦI | 0.5 | 1.0 | 2.0 | 0.5 | 1.0 | 2.0 |
| V-potential (mV) | −18.1 | −14.2 | −13.5 | −22.6 | nd | nd |
| 106 d (m) | 0.311 | 0.243 | 0.164 | 4.82 | 3.08 | 2.66 |
| 1012 Sdroplet (m2) | 0.30 | 0.18 | 0.09 | 71.3 | 30.2 | 22.8 |
| 1020 Vdroplet (m3) | 1.51 | 0.69 | 0.24 | 5343 | 1610 | 1038 |
| 10−12 Nd | 66.5 | 147 | 405 | 0.75 | 2.43 | 3.66 |
| Stotal (m2) | 19.2 | 24.7 | 36.5 | 4.98 | 7.80 | 8.57 |
| 102 mT80,droplet (g) | 0.51 | 0.65 | 0.96 | 0.13 | 0.20 | 0.22 |
| 102 mT80,excess (g) | −0.01 | 0.35 | 1.04 | 0.37 | 0.80 | 1.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, M.; Losada-Barreiro, S.; Vicente, A.; Bravo-Díaz, C.; Paiva-Martins, F. Unexpected Antioxidant Efficiency of Chlorogenic Acid Phenolipids in Fish Oil-in-Water Nanoemulsions: An Example of How Relatively Low Interfacial Concentrations Can Make Antioxidants to Be Inefficient. Molecules 2022, 27, 861. https://doi.org/10.3390/molecules27030861
Costa M, Losada-Barreiro S, Vicente A, Bravo-Díaz C, Paiva-Martins F. Unexpected Antioxidant Efficiency of Chlorogenic Acid Phenolipids in Fish Oil-in-Water Nanoemulsions: An Example of How Relatively Low Interfacial Concentrations Can Make Antioxidants to Be Inefficient. Molecules. 2022; 27(3):861. https://doi.org/10.3390/molecules27030861
Chicago/Turabian StyleCosta, Marlene, Sonia Losada-Barreiro, António Vicente, Carlos Bravo-Díaz, and Fátima Paiva-Martins. 2022. "Unexpected Antioxidant Efficiency of Chlorogenic Acid Phenolipids in Fish Oil-in-Water Nanoemulsions: An Example of How Relatively Low Interfacial Concentrations Can Make Antioxidants to Be Inefficient" Molecules 27, no. 3: 861. https://doi.org/10.3390/molecules27030861
APA StyleCosta, M., Losada-Barreiro, S., Vicente, A., Bravo-Díaz, C., & Paiva-Martins, F. (2022). Unexpected Antioxidant Efficiency of Chlorogenic Acid Phenolipids in Fish Oil-in-Water Nanoemulsions: An Example of How Relatively Low Interfacial Concentrations Can Make Antioxidants to Be Inefficient. Molecules, 27(3), 861. https://doi.org/10.3390/molecules27030861