A Green Extraction Method to Achieve the Highest Yield of Limonin and Hesperidin from Lime Peel Powder (Citrus aurantifolia)
Abstract
:1. Introduction
2. Results
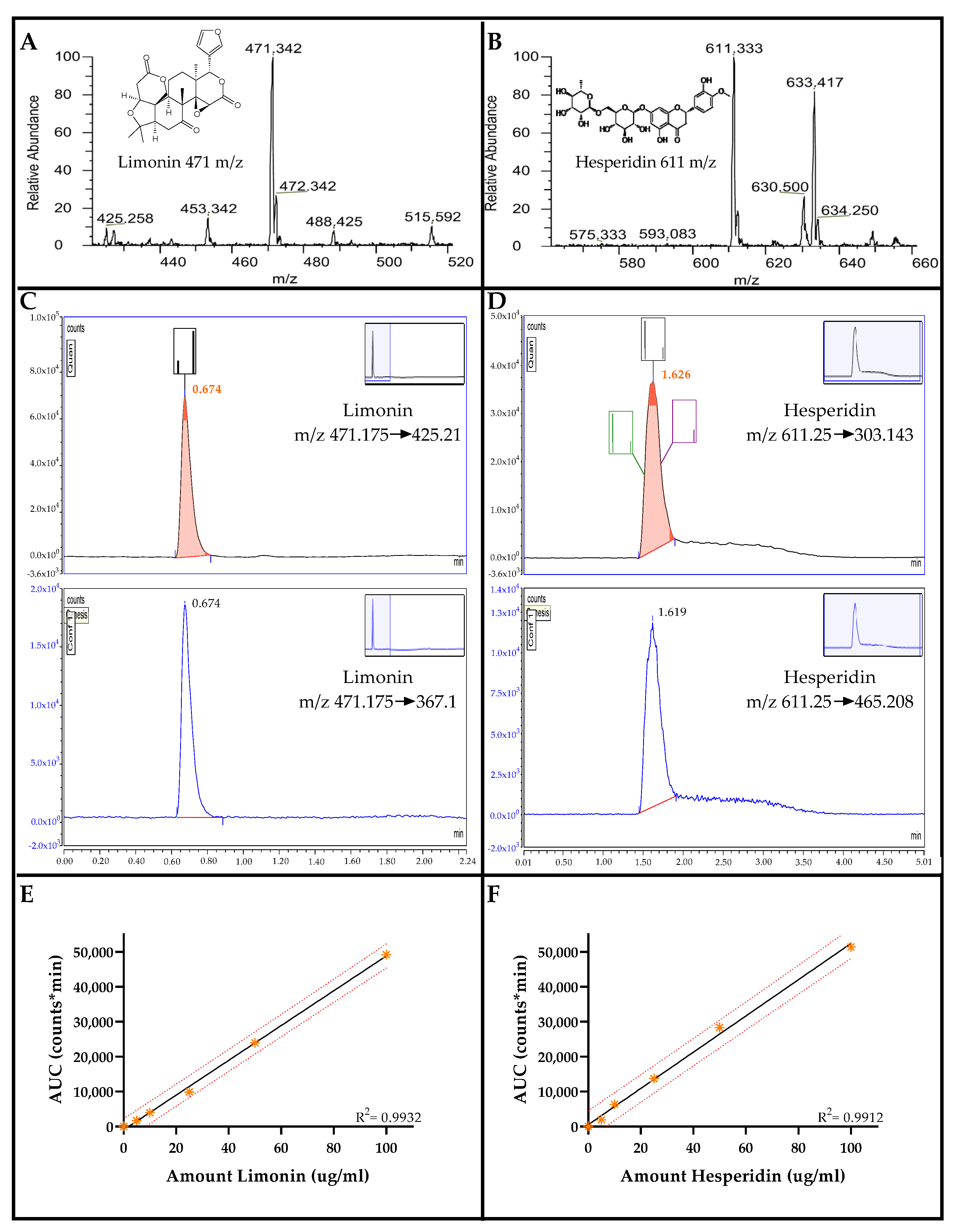
2.1. Chromatograms and Mass Spectra of Limonin and Hesperidin
2.2. Effect of Extraction Factors on Limonin and Hesperidin Concentration
2.3. Optimization of Extraction Conditions
2.4. Yields of Limonin and Hesperidin in Ethanolic-Aqueous Extracts of Lime Peel
3. Discussion
4. Materials and Methods
4.1. Chemicals and Raw Materials
4.2. Experimental Design
4.3. UHPLC-MS/MS Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahbandeh, M. Leading Fresh Lemon and Lime Producers Worldwide in 2020/2021. Available online: https://www.statista.com/statistics/1045016/world-lemons-and-limes-major-producers/ (accessed on 18 November 2021).
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern Extraction and Purification Techniques for Obtaining High Purity Food-Grade Bioactive Compounds and Value-Added Co-Products from Citrus Wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledesma-Escobar, C.; Castro, M. Towards a comprehensive exploitation of citrus. Trends Food Sci. Technol. 2014, 39, 63–74. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elgayed, S.; Elsayed, A.; ElGhonaimy, A.; Wahab, S. PLC characterization and potentiality of Citrus aurantium var. deliciosa fruits: Peel and seeds on plant-parasitic nematodes. J. Med. Plant Res. 2017, 11, 284–295. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, C.; Luo, T.; Wang, J.; Tang, Y.; Chen, Z.; Yu, L. Limonin: A Review of Its Pharmacology, Toxicity, and Pharmacokinetics. Molecules 2019, 24, 3679. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.R.; Park, W.H.; Suh, D.H.; No, J.H.; Kim, Y.B.; Kim, K. Role of limonin in anticancer effects of Evodia rutaecarpa on ovarian cancer cells. BMC Complement. Med. Ther. 2020, 20, 94. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Liu, J.; Zhao, W. By blocking hexokinase-2 phosphorylation, limonin suppresses tumor glycolysis and induces cell apoptosis in hepatocellular carcinoma. Onco. Targets Ther. 2018, 11, 3793–3803. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, S.; Miyamoto, S.; Fujii, G.; Nakanishi, R.; Onuma, W.; Ozaki, Y.; Fujimoto, K.; Yano, T.; Mutoh, M. Suppression of intestinal carcinogenesis in Apc-mutant mice by limonin. J. Clin. Biochem. Nutr. 2015, 57, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Fan, Y.-Y.; Smith, R.; Patil, B.; Jayaprakasha, G.K.; McMurray, D.N.; Chapkin, R.S. Dietary curcumin and limonin suppress CD4+ T-cell proliferation and interleukin-2 production in mice. J. Nutr. 2009, 139, 1042–1048. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.; Lv, C.; Wang, Q.; Zheng, Z.; Sun, X.; Tang, M.; Deng, F. Extraction, identification, and antioxidant property evaluation of limonin from pummelo seeds. Animal Nutr. 2018, 4, 281–287. [Google Scholar] [CrossRef]
- Balestrieri, E.; Pizzimenti, F.; Ferlazzo, A.; Giofrè, S.V.; Iannazzo, D.; Piperno, A.; Romeo, R.; Chiacchio, M.A.; Mastino, A.; Macchi, B. Antiviral activity of seed extract from Citrus bergamia towards human retroviruses. Bioorg. Med. Chem. 2011, 19, 2084–2089. [Google Scholar] [CrossRef]
- Iwata, H.; Tezuka, Y.; Kadota, S.; Hiratsuka, A.; Watabe, T. Mechanism-based inactivation of human liver microsomal CYP3A4 by rutaecarpine and limonin from Evodia fruit extract. Drug Metab. Pharmacokinet. 2005, 20, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, M.F.; Gamal, S.; El-Fayoumi, H.M. Limonin attenuates hepatocellular injury following liver ischemia and reperfusion in rats via toll-like receptor dependent pathway. Eur. J. Pharmacol. 2014, 740, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Vinothkumar, V.; Murali, R. Chapter 22-Antidiabetic Efficacy of Citrus Fruits with Special Allusion to Flavone Glycosides. In Bioactive Food as Dietary Interventions for Diabetes, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 335–346. [Google Scholar]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Estruel-Amades, S.; Massot-Cladera, M.; Garcia-Cerdà, P.; Pérez-Cano, F.J.; Franch, À.; Castell, M.; Camps-Bossacoma, M. Protective Effect of Hesperidin on the Oxidative Stress Induced by an Exhausting Exercise in Intensively Trained Rats. Nutrients 2019, 11, 783. [Google Scholar] [CrossRef] [Green Version]
- Banjerdpongchai, R.; Wudtiwai, B.; Khaw-On, P.; Rachakhom, W.; Duangnil, N.; Kongtawelert, P. Hesperidin from Citrus seed induces human hepatocellular carcinoma HepG2 cell apoptosis via both mitochondrial and death receptor pathways. Tumour Biol 2016, 37, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, V.; Tuli, H.S.; Thakral, F.; Singhal, P.; Aggarwal, D.; Srivastava, S.; Pandey, A.; Sak, K.; Varol, M.; Khan, M.A.; et al. Molecular mechanisms of action of hesperidin in cancer: Recent trends and advancements. Exp. Biol. Med. 2020, 245, 486–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanwar, A.A.; Badole, S.L.; Shende, P.S.; Hegde, M.V.; Bodhankar, S.L. Chapter 76-Cardiovascular Effects of Hesperidin: A Flavanone Glycoside. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 989–992. [Google Scholar]
- Mas-Capdevila, A.; Teichenne, J.; Domenech-Coca, C.; Caimari, A.; Del Bas, J.M.; Escoté, X.; Crescenti, A. Effect of Hesperidin on Cardiovascular Disease Risk Factors: The Role of Intestinal Microbiota on Hesperidin Bioavailability. Nutrients 2020, 12, 1488. [Google Scholar] [CrossRef]
- Jokić, S.; Šafranko, S.; Jakovljević, M.; Cikoš, A.-M.; Kajić, N.; Kolarević, F.; Babić, J.; Molnar, M. Sustainable Green Procedure for Extraction of Hesperidin from Selected Croatian Mandarin Peels. Processes 2019, 7, 469. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Liu, J.; Rong, Y.-h.; Liang, N.; Rong, L. Aqueous extraction of limonin from Citrus reticulate Blanco. Czech. J. Food Sci. 2012, 30, 364–368. [Google Scholar] [CrossRef]
- Yu, J.; Dandekar, D.V.; Toledo, R.T.; Singh, R.K.; Patil, B.S. Supercritical fluid extraction of limonoids and naringin from grapefruit (Citrus paradisi Macf.) seeds. Food Chem. 2007, 105, 1026–1031. [Google Scholar] [CrossRef]
- Proestos, C. The Benefits of Plant Extracts for Human Health. Foods 2020, 9, 1653. [Google Scholar] [CrossRef] [PubMed]
- Padilla de la Rosa, J.D.; Ruiz-Palomino, P.; Arriola-Guevara, E.; García-Fajardo, J.; Sandoval, G.; Guatemala-Morales, G.M. A Green Process for the Extraction and Purification of Hesperidin from Mexican Lime Peel (Citrus aurantifolia Swingle) that is Extendible to the Citrus Genus. Processes 2018, 6, 266. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Liu, X.; Xiong, B.; Qiu, X.; Sun, G.; Wang, X.; Zhang, X.; Dong, Z.; Wang, Z. Variation in limonin and nomilin content in citrus fruits of eight varieties determined by modified HPLC. Food Sci. Biotechnol. 2019, 28, 641–647. [Google Scholar] [CrossRef]
- Alam, P.; Alam, A.; Anwer, M.K.; Alqasoumi, S.I. Quantitative estimation of hesperidin by HPTLC in different varieties of citrus peels. Asian Pac. J. Trop Biomed. 2014, 4, 262–266. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Sánchez, J.; Borrás-Linares, I.; Sass-Kiss, A.; Segura-Carretero, A. Chapter 13-Chromatographic Technique: High-Performance Liquid Chromatography (HPLC). In Modern Techniques for Food Authentication, 2nd ed.; Sun, D.-W., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 459–526. [Google Scholar]
- Pitt, J.J. Principles and applications of liquid chromatography-mass spectrometry in clinical biochemistry. Clin. Biochem. Rev. 2009, 30, 19–34. [Google Scholar]
- Ishii, T.; Ohta, H.; Nogata, Y.; Yano, M.; Hasegawa, S. Limonoids in seeds of iyo tangor (Citrus iyo hort. ex Tanaka). Food Sci. Technol. Res. 2003, 9, 162–164. [Google Scholar] [CrossRef] [Green Version]
- Merino, M.T.; Humanes, L.; Roldán, J.M.; Díez, J.; Lopez ruiz, A. Production of limonoate A-ring lactone by immobilized limonin D-ring lactone hydrolase. Biotechnology Letters 1996, 18, 1175–1180. [Google Scholar] [CrossRef]
- Majumdar, S.; Srirangam, R. Solubility, stability, physicochemical characteristics and in vitro ocular tissue permeability of hesperidin: A natural bioflavonoid. Pharm Res. 2009, 26, 1217–1225. [Google Scholar] [CrossRef] [Green Version]
- Gertenbach, D.D. Solid–Liquid Extraction Technologies for Manufacturing Nutraceuticals. In Functional Foods, 1st ed.; John Shi, G.M., Maguer, M.L., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 331–366. [Google Scholar]
- Ma, Y.; Ye, X.; Hao, Y.; Xu, G.; Xu, G.; Liu, D. Ultrasound-assisted extraction of hesperidin from Penggan (Citrus reticulata) peel. Ultrason Sonochem 2008, 15, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.; Kaur, L.; Marwaha, S.-S. Partial Purification and Characterization of Limonoate Dehydrogenase from Rhodococcus fascians for the Degradation of Limonin. J. Microbio. Biotechnol. 2002, 12, 669–673. [Google Scholar]
- Binkowska, I. Hesperidin: Synthesis and characterization of bioflavonoid complex. SN Appl. Sci. 2020, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.; Gharachorloo, M.; Ghiassi-Tarzi, B.; Ghavami, M. Evaluation of the Organic Acids Ability for Extraction of Anthocyanins and Phenolic Compounds from Different Sources and Their Degradation Kinetics during Cold Storage. Polish J. Food Nutr. Sci. 2016, 66, 261–269. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Gualdani, R.; Cavalluzzi, M.M.; Lentini, G.; Habtemariam, S. The Chemistry and Pharmacology of Citrus Limonoids. Molecules 2016, 21, 1530. [Google Scholar] [CrossRef]
- Tekin, K.; Hao, N.; Karagoz, S.; Ragauskas, A.J. Ethanol: A Promising Green Solvent for the Deconstruction of Lignocellulose. Chem. Sus. Chem. 2018, 11, 3559–3575. [Google Scholar] [CrossRef]
- Zheng, G.; Yang, X.; Chen, B.; Chao, Y.; Hu, P.; Cai, Y.; Wu, B.; Wei, M. Identification and determination of chemical constituents of Citrus reticulata semen through ultra high performance liquid chromatography combined with Q Exactive Orbitrap tandem mass spectrometry. J. Sep. Sci. 2020, 43, 438–451. [Google Scholar] [CrossRef]
- Kinsella, B. Analysis of limonin in citrus juice using QuEChERS and LC-MS/MS. LC-GC N. Am. 2017, 35, 20. [Google Scholar]



| Extraction Condition | pH of Extract (before Adjusting) | Limonin Yield (mg/g Dry Peel) | Hesperidin Yield (mg/g Dry Peel) | ||
|---|---|---|---|---|---|
| %ETOH | pH | Temp. | |||
| 60 | 7 | 50 | 4.15 | 2.086 ± 0.015 abc | 2.373 ± 0.023 ef |
| 60 | 7 | 60 | 4.12 | 1.838 ± 0.140 fghi | 1.327 ± 0.379 hi |
| 60 | 7 | 70 | 4.12 | 1.143 ± 0.167 nop | 1.108 ± 0.172 ijk |
| 60 | 9 | 50 | 3.98 | 1.731 ± 0.022 ij | 2.142 ± 0.015 fg |
| 60 | 9 | 60 | 4.08 | 1.749 ± 0.033 hij | 1.196 ± 0.023 ij |
| 60 | 9 | 70 | 4.01 | 1.540 ± 0.011 lm | 0.605 ± 0.011 nop |
| 60 | 11 | 50 | 4.25 | 2.095 ± 0.051 abc | 0.330 ± 0.153 qrs |
| 60 | 11 | 60 | 4.43 | 1.548 ± 0.031 lm | 0.876 ± 0.010 klmn |
| 60 | 11 | 70 | 4.20 | 1.577 ± 0.025 klm | 0.665 ± 0.010 mnop |
| 70 | 7 | 50 | 4.22 | 2.125 ± 0.036 ab | 2.523 ± 0.058 de |
| 70 | 7 | 60 | 4.27 | 2.191 ± 0.024 a | 2.054 ± 0.243 g |
| 70 | 7 | 70 | 4.29 | 1.984 ± 0.094 bcdef | 0.958 ± 0.135 jkl |
| 70 | 9 | 50 | 4.29 | 2.043 ± 0.049 abcd | 2.191 ± 0.027 fg |
| 70 | 9 | 60 | 4.28 | 1.796 ± 0.053 ghi | 1.230 ± 0.085 ij |
| 70 | 9 | 70 | 4.43 | 1.264 ± 0.002 n | 0.588 ± 0.026 opq |
| 70 | 11 | 50 | 4.45 | 1.996 ± 0.056 bcde | 2.131 ± 0.032 fg |
| 70 | 11 | 60 | 4.46 | 1.909 ± 0.072 defgh | 0.801 ± 0.014 lmno |
| 70 | 11 | 70 | 4.49 | 1.260 ± 0.013 n | 0.590 ± 0.013 op |
| 80 | 7 | 50 | 4.50 | 2.072 ± 0.173 abcd | 3.353 ± 0.121 a |
| 80 | 7 | 60 | 4.46 | 0.653 ± 0.013 r | 0.370 ± 0.013 qr |
| 80 | 7 | 70 | 3.60 | 0.716 ± 0.058 r | 1.544 ± 0.055 h |
| 80 | 9 | 50 | 5.44 | 1.944 ± 0.019 cdefg | 2.748 ± 0.057 bcd |
| 80 | 9 | 60 | 4.63 | 0.894 ± 0.002 q | 0.824 ± 0.006 klmno |
| 80 | 9 | 70 | 3.64 | 1.537 ± 0.041 lm | 2.707 ± 0.111 cd |
| 80 | 11 | 50 | 8.61 | 1.048 ± 0.046 p | 2.906 ± 0.078 bc |
| 80 | 11 | 60 | 10.48 | 0.173 ± 0.004 s | 0.770 ± 0.011 lmno |
| 80 | 11 | 70 | 9.42 | 1.607 ± 0.026 jkl | 3.003 ± 0.038 b |
| 100 | 7 | 50 | 5.03 | 1.417 ± 0.376 m | 0.705 ± 0.041 lmno |
| 100 | 7 | 60 | 5.10 | 1.052 ± 0.066 op | 0.681 ± 0.048 lmno |
| 100 | 7 | 70 | 4.66 | 1.208 ± 0.025 no | 0.708 ± 0.037 lmno |
| 100 | 9 | 50 | 5.28 | 1.720 ± 0.059 ijk | 2.000 ± 0.130 g |
| 100 | 9 | 60 | 5.97 | 0.738 ± 0.056 r | 0.341 ± 0.007 qrs |
| 100 | 9 | 70 | 6.07 | 0.692 ± 0.027 r | 0.371 ± 0.004 pqr |
| 100 | 11 | 50 | 10.43 | 0.170 ± 0.004 s | 0.081 ± 0.084 st |
| 100 | 11 | 60 | 10.86 | 0.174 ± 0.003 s | 0.218 ± 0.109 rst |
| 100 | 11 | 70 | 10.84 | 0.173 ± 0.004 s | 0.003 + 0.001 t |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phucharoenrak, P.; Muangnoi, C.; Trachootham, D. A Green Extraction Method to Achieve the Highest Yield of Limonin and Hesperidin from Lime Peel Powder (Citrus aurantifolia). Molecules 2022, 27, 820. https://doi.org/10.3390/molecules27030820
Phucharoenrak P, Muangnoi C, Trachootham D. A Green Extraction Method to Achieve the Highest Yield of Limonin and Hesperidin from Lime Peel Powder (Citrus aurantifolia). Molecules. 2022; 27(3):820. https://doi.org/10.3390/molecules27030820
Chicago/Turabian StylePhucharoenrak, Pakkapong, Chawanphat Muangnoi, and Dunyaporn Trachootham. 2022. "A Green Extraction Method to Achieve the Highest Yield of Limonin and Hesperidin from Lime Peel Powder (Citrus aurantifolia)" Molecules 27, no. 3: 820. https://doi.org/10.3390/molecules27030820
APA StylePhucharoenrak, P., Muangnoi, C., & Trachootham, D. (2022). A Green Extraction Method to Achieve the Highest Yield of Limonin and Hesperidin from Lime Peel Powder (Citrus aurantifolia). Molecules, 27(3), 820. https://doi.org/10.3390/molecules27030820






