Plasmonic Enhancement of Two-Photon Excited Luminescence of Gold Nanoclusters
Abstract
:1. Introduction
2. Results
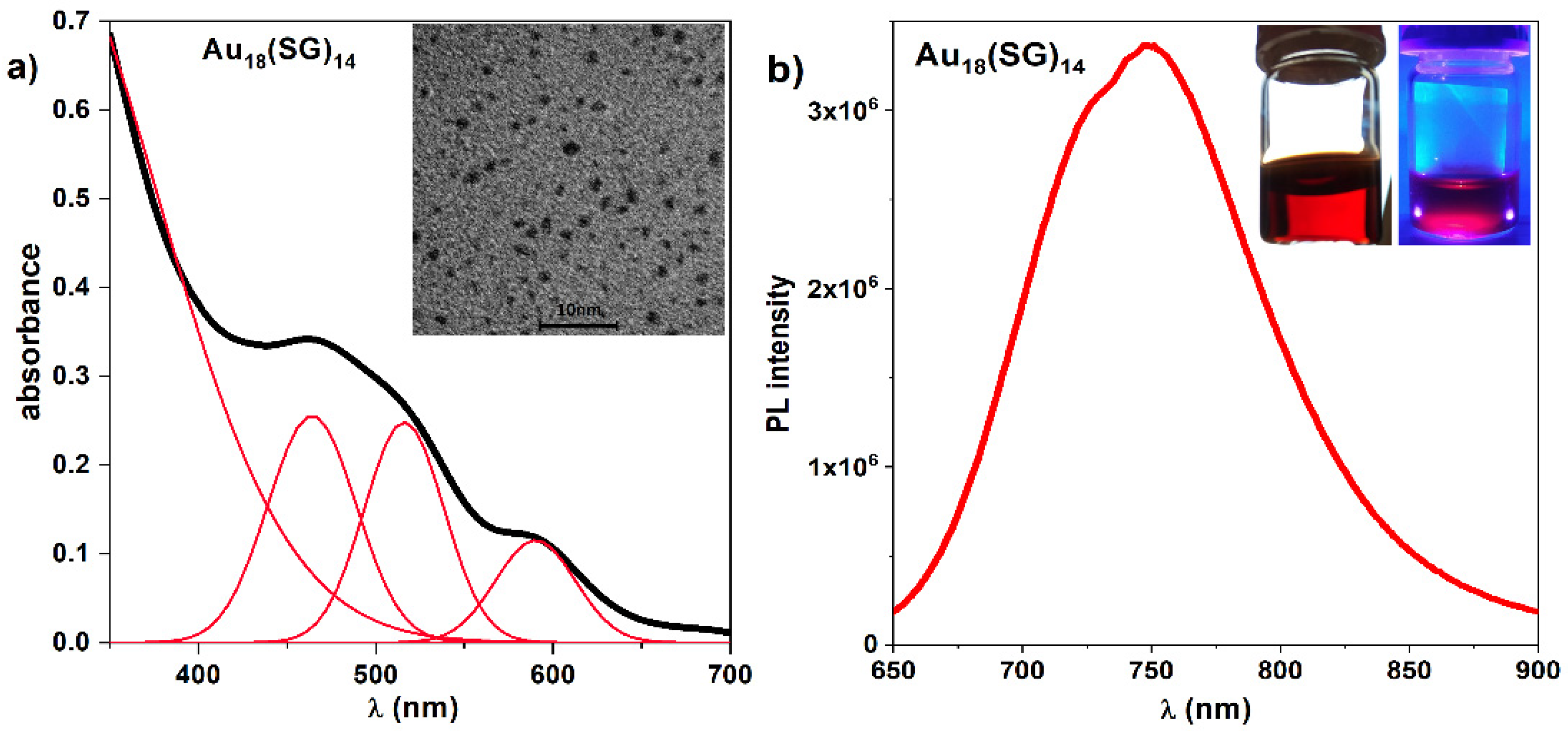
2.1. Synthesis and Characterisation of Gold Nanoclusters
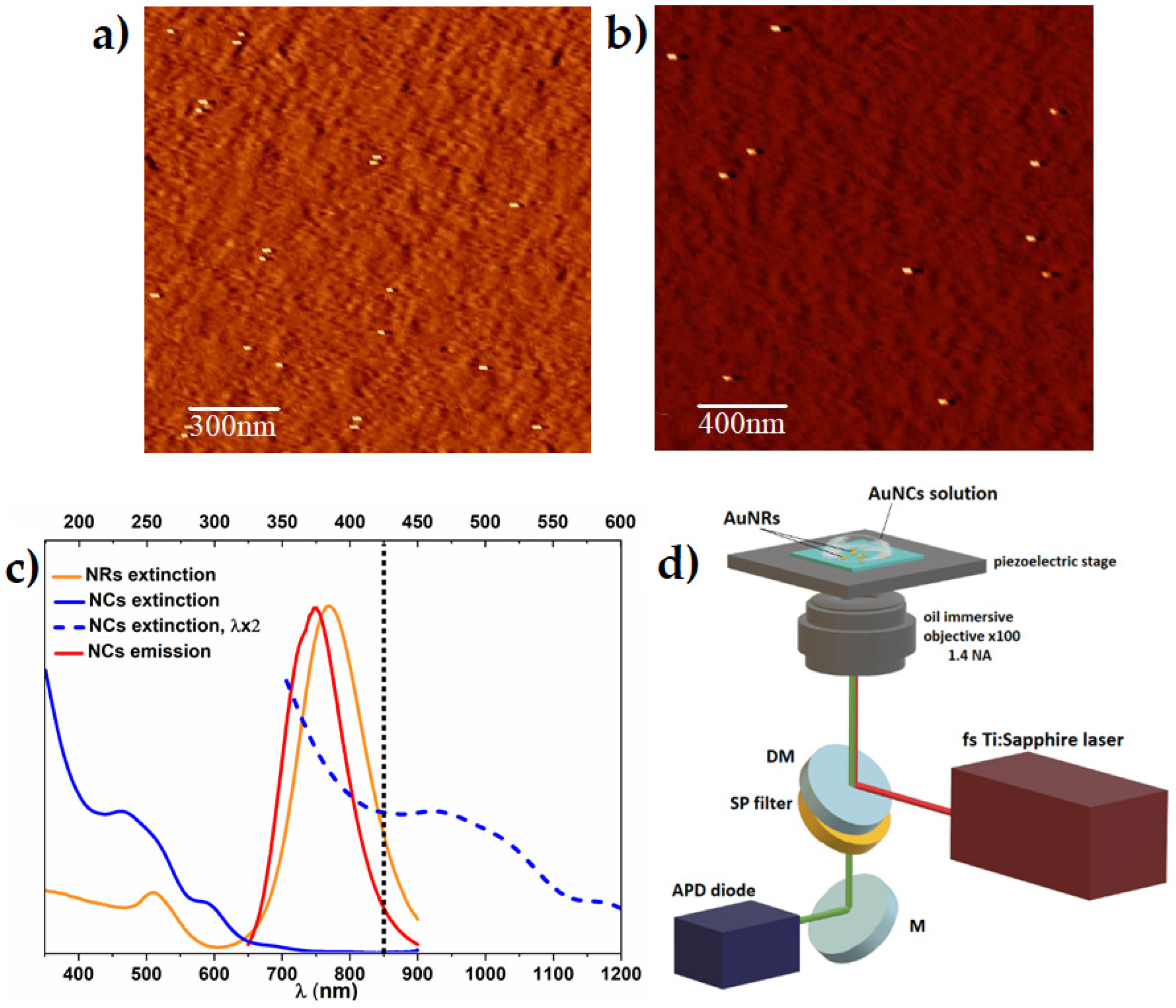
2.2. Single Nanorods Sample Preparation for Multiphoton Study
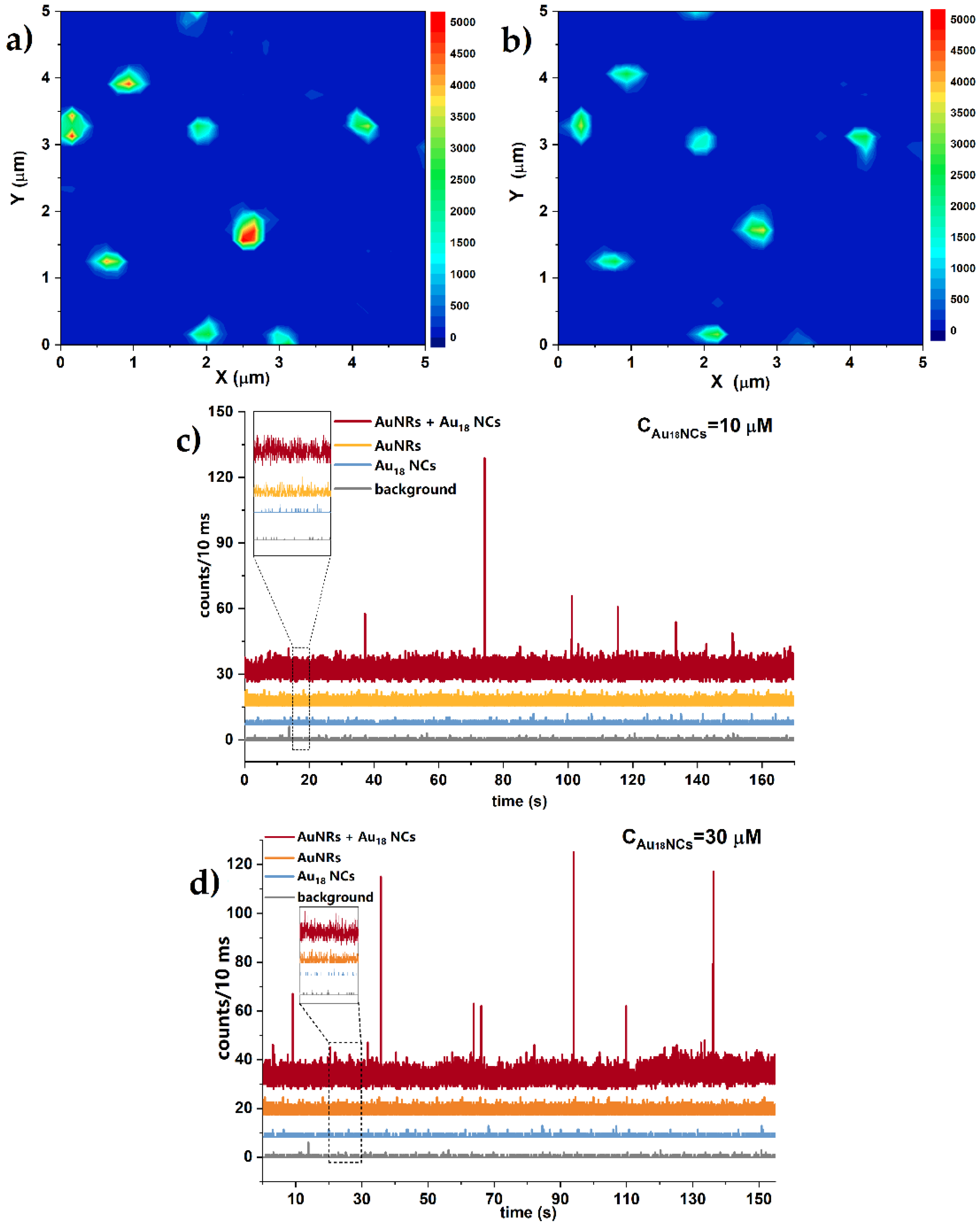
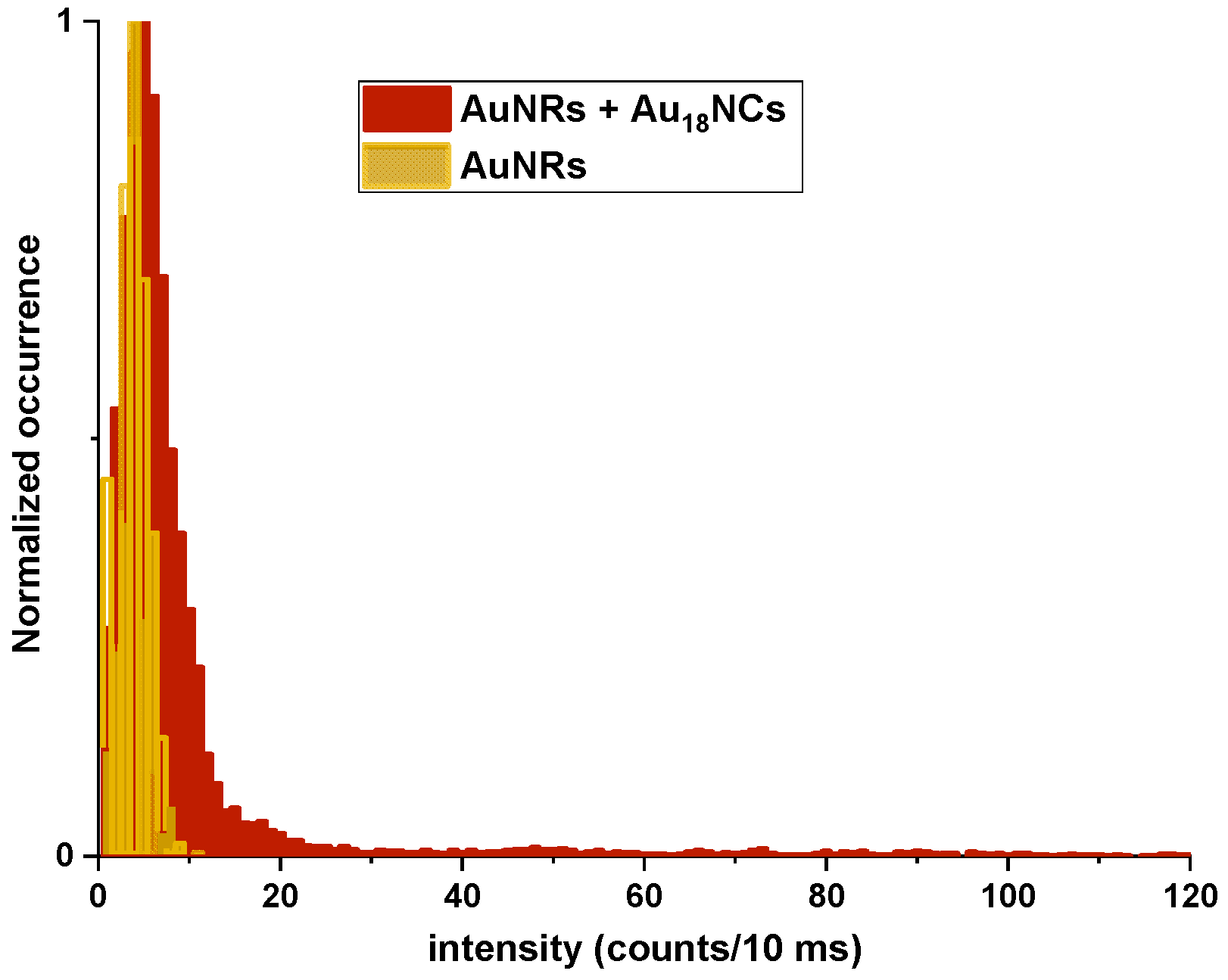
2.3. Multiphoton Study of Single Nanoclusters Luminescence Enhancement on Single Nanorods
3. Discussion
4. Materials and Methods
4.1. Chemical
4.2. Synthesis of Gold Au18(SG)14 Nanoclusters
4.3. Synthesis of Gold Au:SG Nanoclusters
4.4. PAGE Electrophoresis
4.5. Preparation of Gold Nanorods Sample and Imaging of Gold Nanoparticles
4.6. Characterization of Linear and Nonlinear Optical Properties of Nanoparticles
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Gruenke, N.L.; Cardinal, M.F.; McAnally, M.O.; Frontiera, R.R.; Schatz, G.C.; Van Duyne, R.P. Ultrafast and nonlinear surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2016, 45, 2263–2290. [Google Scholar] [CrossRef] [PubMed]
- Geddes, C.D.; Lakowicz, J.R. Editorial: Metal-Enhanced Fluorescence. J. Fluoresc. 2002, 12, 121–129. [Google Scholar] [CrossRef]
- Khatua, S.; Paulo, P.; Yuan, H.; Gupta, A.; Zijlstra, P.P.; Orrit, M. Resonant Plasmonic Enhancement of Single-Molecule Fluorescence by Individual Gold Nanorods. ACS Nano 2014, 8, 4440–4449. [Google Scholar] [CrossRef] [Green Version]
- Olesiak-Banska, J.; Waszkielewicz, M.; Obstarczyk, P.; Samoc, M. Two-photon absorption and photoluminescence of colloidal gold nanoparticles and nanoclusters. Chem. Soc. Rev. 2019, 48, 4087–4117. [Google Scholar] [CrossRef]
- Yuan, H.; Khatua, S.; Zijlstra, P.; Yorulmaz, M.; Orrit, M. Thousand-fold Enhancement of Single-Molecule Fluorescence Near a Single Gold Nanorod. Angew. Chem. Int. Ed. 2013, 52, 1217–1221. [Google Scholar] [CrossRef]
- Mulpur, P.; Kurdekar, A.; Podila, R.; Rao, A.M.; Kamisetti, V. Surface plasmon coupled emission as a novel analytical platform for the sensitive detection of cysteine. Nanotechnol. Rev. 2015, 4, 393–400. [Google Scholar] [CrossRef]
- Fu, B.; Flynn, J.D.; Isaacoff, B.P.; Rowland, D.J.; Biteen, J.S. Super-Resolving the Distance-Dependent Plasmon-Enhanced Fluorescence of Single Dye and Fluorescent Protein Molecules. J. Phys. Chem. C 2015, 119, 19350–19358. [Google Scholar] [CrossRef]
- Tam, F.; Goodrich, G.P.; Johnson, B.R.; Halas, N.J. Plasmonic Enhancement of Molecular Fluorescence. Nano Lett. 2007, 7, 496–501. [Google Scholar] [CrossRef]
- Zhang, W.; Caldarola, M.; Lu, X.; Orrit, M. Plasmonic Enhancement of Two-Photon-Excited Luminescence of Single Quantum Dots by Individual Gold Nanorods. ACS Photon 2018, 5, 2960–2968. [Google Scholar] [CrossRef]
- Chen, Y.; Munechika, K.; Ginger, D.S. Dependence of Fluorescence Intensity on the Spectral Overlap between Fluorophores and Plasmon Resonant Single Silver Nanoparticles. Nano Lett. 2007, 7, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Yu, K.; Li, L.; Zhang, T.; Guan, Z.; Gao, N.; Yuan, P.; Li, S.; Yao, S.Q.; Xu, Q.-H.; et al. Gold Nanorod Enhanced Two-Photon Excitation Fluorescence of Photosensitizers for Two-Photon Imaging and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2014, 6, 2700–2708. [Google Scholar] [CrossRef] [PubMed]
- Abadeer, N.S.; Brennan, M.R.; Wilson, W.L.; Murphy, C.J. Distance and Plasmon Wavelength Dependent Fluorescence of Molecules Bound to Silica-Coated Gold Nanorods. ACS Nano 2014, 8, 8392–8406. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Xu, Q.-H. Separation distance dependent fluorescence enhancement of fluorescein isothiocyanate by silver nanoparticles. Chem. Commun. 2007, 3, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.B.; Siddiquee, A.M.; Chon, J.W.M. Below Melting Point Photothermal Reshaping of Single Gold Nanorods Driven by Surface Diffusion. ACS Nano 2014, 8, 12071–12079. [Google Scholar] [CrossRef] [PubMed]
- Gordel, M.; Olesiak-Banska, J.; Matczyszyn, K.; Nogues, C.; Buckle, M.; Samoc, M. Post-synthesis reshaping of gold nanorods using a femtosecond laser. Phys. Chem. Chem. Phys. 2014, 16, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kao, F.-J.; Chuang, C.-C.; He, S. Enhancing fluorescence of quantum dots by silica-coated gold nanorods under one- and two-photon excitation. Opt. Express 2010, 18, 11335–11346. [Google Scholar] [CrossRef]
- Craciun, A.; Focsan, M.; Gaina, L.; Astilean, S. Enhanced one- and two-photon excited fluorescence of cationic (phenothiazinyl)vinyl-pyridinium chromophore attached to polyelectrolyte-coated gold nanorods. Dye. Pigment. 2017, 136, 24–30. [Google Scholar] [CrossRef]
- Shokova, M.A.; Bochenkov, V.E. Efficiency of Plasmon-Induced Dual-Mode Fluorescence Enhancement upon Two-Photon Excitation. Nanomaterials 2021, 11, 3334. [Google Scholar] [CrossRef]
- Sobska, J.; Waszkielewicz, M.; Podleśny-Drabiniok, A.; Olesiak-Banska, J.; Krężel, W.; Matczyszyn, K. Gold Nanoclusters Display Low Immunogenic Effect in Microglia Cells. Nanomaterials 2021, 11, 1066. [Google Scholar] [CrossRef]
- Zheng, Y.; Lai, L.; Liu, W.; Jiang, H.; Wang, X. Recent advances in biomedical applications of fluorescent gold nanoclusters. Adv. Colloid Interface Sci. 2017, 242, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Wang, C.-W.; Yuan, Z.; Chang, H.-T. Fluorescent Gold Nanoclusters: Recent Advances in Sensing and Imaging. Anal. Chem. 2015, 87, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Yousefalizadeh, G.; Ahmadi, S.; Mosey, N.J.; Stamplecoskie, K.G. Exciting clusters, what does off-resonance actually mean? Nanoscale 2020, 13, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Brach, K.; Waszkielewicz, M.; Olesiak-Banska, J.; Samoc, M.; Matczyszyn, K. Two-Photon Imaging of 3D Organization of Bimetallic AuAg Nanoclusters in DNA Matrix. Langmuir 2017, 33, 8993–8999. [Google Scholar] [CrossRef] [PubMed]
- Waszkielewicz, M.; Olesiak–Banska, J.; Grzelczak, M.; Sánchez–Iglesias, A.; Pniakowska, A.; Samoc, M. Enhanced one-photon and two-photon excited luminescence of polymer-stabilized AuAg nanoclusters aggregates. J. Lumin. 2020, 221, 116994. [Google Scholar] [CrossRef]
- Olesiak-Banska, J.; Waszkielewicz, M.; Matczyszyn, K.; Samoc, M. A closer look at two-photon absorption, absorption saturation and nonlinear refraction in gold nanoclusters. RSC Adv. 2016, 6, 98748–98752. [Google Scholar] [CrossRef] [Green Version]
- Antoine, R.; Bonačić-Koutecký, V. Liganded Silver and Gold Quantum Clusters. Towards a New Class of Nonlinear Optical Nanomaterials; Springer: Singapore, 2018. [Google Scholar]
- Ghosh, A.; Udayabhaskararao, T.; Pradeep, T. One-Step Route to Luminescent Au18SG14 in the Condensed Phase and Its Closed Shell Molecular Ions in the Gas Phase. J. Phys. Chem. Lett. 2012, 3, 1997–2002. [Google Scholar] [CrossRef]
- Stamplecoskie, K.G.; Chen, Y.-S.; Kamat, P.V. Excited-State Behavior of Luminescent Glutathione-Protected Gold Clusters. J. Phys. Chem. C 2014, 118, 1370–1376. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Chen, S.; Shen, Y.; Zhu, M. Switching the subcellular organelle targeting of atomically precise gold nanoclusters by modifying the capping ligand. Chem. Commun. 2018, 54, 9222–9225. [Google Scholar] [CrossRef]
- Negishi, Y.; Nobusada, K.; Tsukuda, T. Glutathione-Protected Gold Clusters Revisited: Bridging the Gap between Gold(I)−Thiolate Complexes and Thiolate-Protected Gold Nanocrystals. J. Am. Chem. Soc. 2005, 127, 5261–5270. [Google Scholar] [CrossRef]
- Sreeprasad, T.S.; Samal, A.K.; Pradeep, T. Body- or Tip-Controlled Reactivity of Gold Nanorods and Their Conversion to Particles through Other Anisotropic Structures. Langmuir 2007, 23, 9463–9471. [Google Scholar] [CrossRef] [PubMed]
- Meena, S.K.; Celiksoy, S.; Schäfer, P.; Henkel, A.; Sönnichsen, C.; Sulpizi, M. The role of halide ions in the anisotropic growth of gold nanoparticles: A microscopic, atomistic perspective. Phys. Chem. Chem. Phys. 2016, 18, 13246–13254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosharay, S.; Talebi, M.; Saeed, T.A.; Talaghani, S.S. Experimental and modeling study of the surface tension and interface of aqueous solutions of alcohols, cetyltrimethylammonium bromide (CTAB) and their mixtures. J. Mol. Liq. 2018, 249, 245–253. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef]
- He, J.; Unser, S.; Bruzas, I.; Cary, R.; Shi, Z.; Mehra, R.; Aron, K.; Sagle, L. The facile removal of CTAB from the surface of gold nanorods. Colloids Surfaces B Biointerfaces 2018, 163, 140–145. [Google Scholar] [CrossRef]
- Zhang, W.; Caldarola, M.; Lu, X.; Pradhan, B.; Orrit, M. Single-molecule fluorescence enhancement of a near-infrared dye by gold nanorods using DNA transient binding. Phys. Chem. Chem. Phys. 2018, 20, 20468–20475. [Google Scholar] [CrossRef]
- Wientjes, E.; Renger, J.; Curto, A.; Cogdell, R.; Van Hulst, N.F. Strong antenna-enhanced fluorescence of a single light-harvesting complex shows photon antibunching. Nat. Commun. 2014, 5, 4236. [Google Scholar] [CrossRef] [Green Version]
- Ming, T.; Chen, H.; Jiang, R.; Li, Q.; Wang, J. Plasmon-Controlled Fluorescence: Beyond the Intensity Enhancement. J. Phys. Chem. Lett. 2012, 3, 191–202. [Google Scholar] [CrossRef]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef]
- Donehue, J.E.; Wertz, E.; Talicska, C.N.; Biteen, J. Plasmon-Enhanced Brightness and Photostability from Single Fluorescent Proteins Coupled to Gold Nanorods. J. Phys. Chem. C 2014, 118, 15027–15035. [Google Scholar] [CrossRef]
- Lu, X.; Ye, G.; Punj, D.; Chiechi, R.; Orrit, M. Quantum Yield Limits for the Detection of Single-Molecule Fluorescence Enhanced by a Gold Nanorod. ACS Photon 2020, 7, 2498–2505. [Google Scholar] [CrossRef]
- Mahoney, C.; Park, K.; Jawaid, A.; Kowalski, B.; Gillman, A.; Tondiglia, V.; Treml, B.; White, T.; Vaia, R.A. Low-energy, nanoparticle reshaping for large-area, patterned, plasmonic nanocomposites. J. Mater. Chem. C 2018, 6, 7157–7169. [Google Scholar] [CrossRef]
- Pradhan, B.; Khatua, S.; Gupta, A.; Aartsma, T.; Canters, G.; Orrit, M.J. Gold-nanorod-enhanced fluorescence correlation spectroscopy of fluorophores with high quantum yield in lipid bilayers. Phys. Chem. C 2016, 120, 25996–26003. [Google Scholar] [CrossRef]
- Schaaff, T.G.; Whetten, R.L. Giant Gold−Glutathione Cluster Compounds: Intense Optical Activity in Metal-Based Transitions. J. Phys. Chem. B 2000, 104, 2630–2641. [Google Scholar] [CrossRef]
- Waszkielewicz, M.; Olesiak-Banska, J.; Comby-Zerbino, C.; Bertorelle, F.; Dagany, X.; Bansal, A.K.; Sajjad, M.T.; Samuel, I.D.W.; Sanader, Z.; Rozycka, M.; et al. pH-Induced transformation of ligated Au25 to brighter Au23 nanoclusters. Nanoscale 2018, 10, 11335–11341. [Google Scholar] [CrossRef] [Green Version]
- Jin, R. Atomically precise metal nanoclusters: Stable sizes and optical properties. Nanoscale 2015, 7, 1549–1565. [Google Scholar] [CrossRef]
- Yu, Y.; Yao, Q.; Chen, T.; Lim, G.X.; Xie, J. The Innermost Three Gold Atoms Are Indispensable To Maintain the Structure of the Au18(SR)14 Cluster. J. Phys. Chem. C 2016, 120, 22096–22102. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pniakowska, A.; Olesiak-Banska, J. Plasmonic Enhancement of Two-Photon Excited Luminescence of Gold Nanoclusters. Molecules 2022, 27, 807. https://doi.org/10.3390/molecules27030807
Pniakowska A, Olesiak-Banska J. Plasmonic Enhancement of Two-Photon Excited Luminescence of Gold Nanoclusters. Molecules. 2022; 27(3):807. https://doi.org/10.3390/molecules27030807
Chicago/Turabian StylePniakowska, Anna, and Joanna Olesiak-Banska. 2022. "Plasmonic Enhancement of Two-Photon Excited Luminescence of Gold Nanoclusters" Molecules 27, no. 3: 807. https://doi.org/10.3390/molecules27030807
APA StylePniakowska, A., & Olesiak-Banska, J. (2022). Plasmonic Enhancement of Two-Photon Excited Luminescence of Gold Nanoclusters. Molecules, 27(3), 807. https://doi.org/10.3390/molecules27030807






