Surface Functionalization of Magnetite Nanoparticles with Multipotent Antioxidant as Potential Magnetic Nanoantioxidants and Antimicrobial Agents
Abstract
:1. Introduction
2. Results
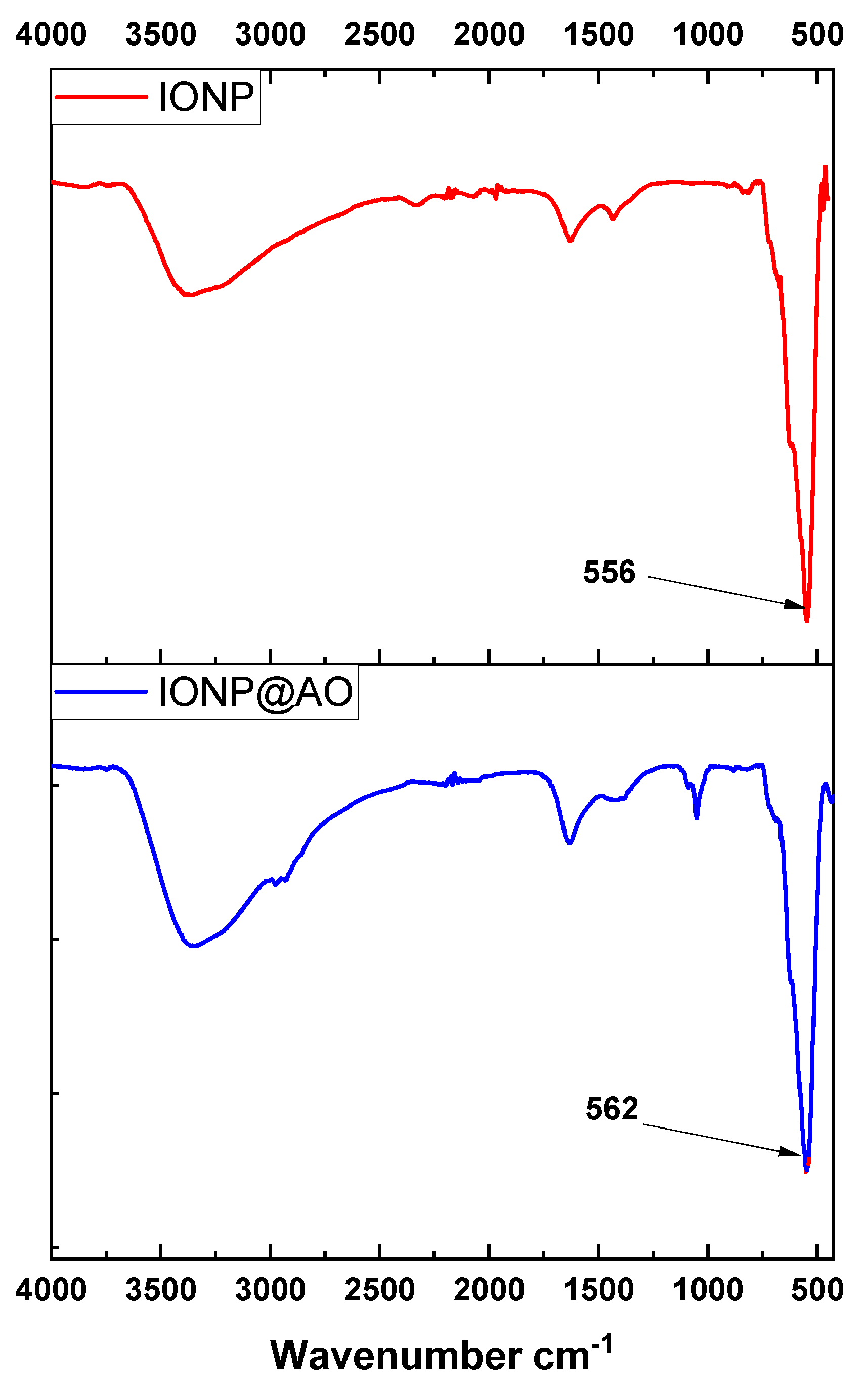
2.1. FTIR Analysis
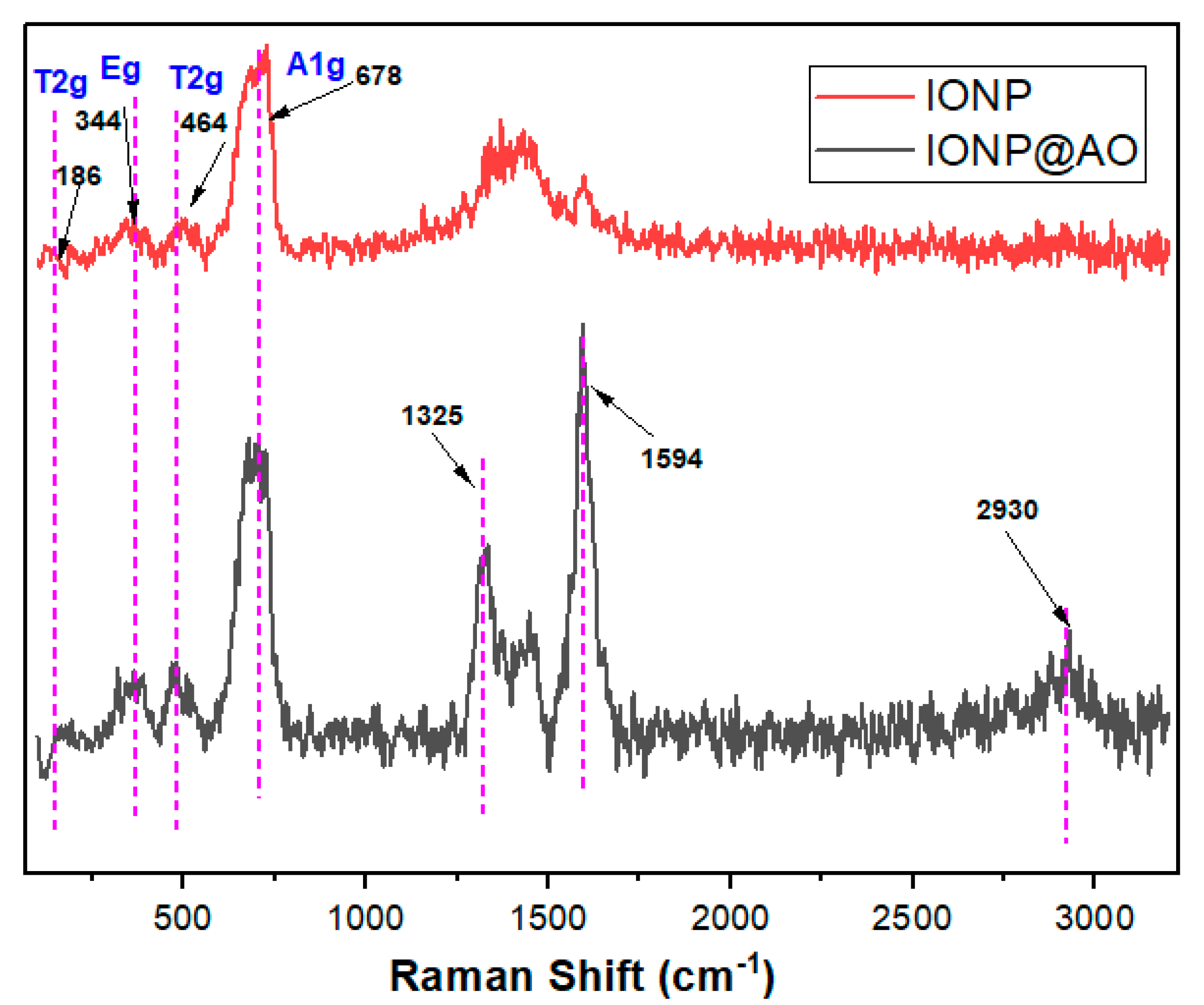
2.2. Raman Spectra
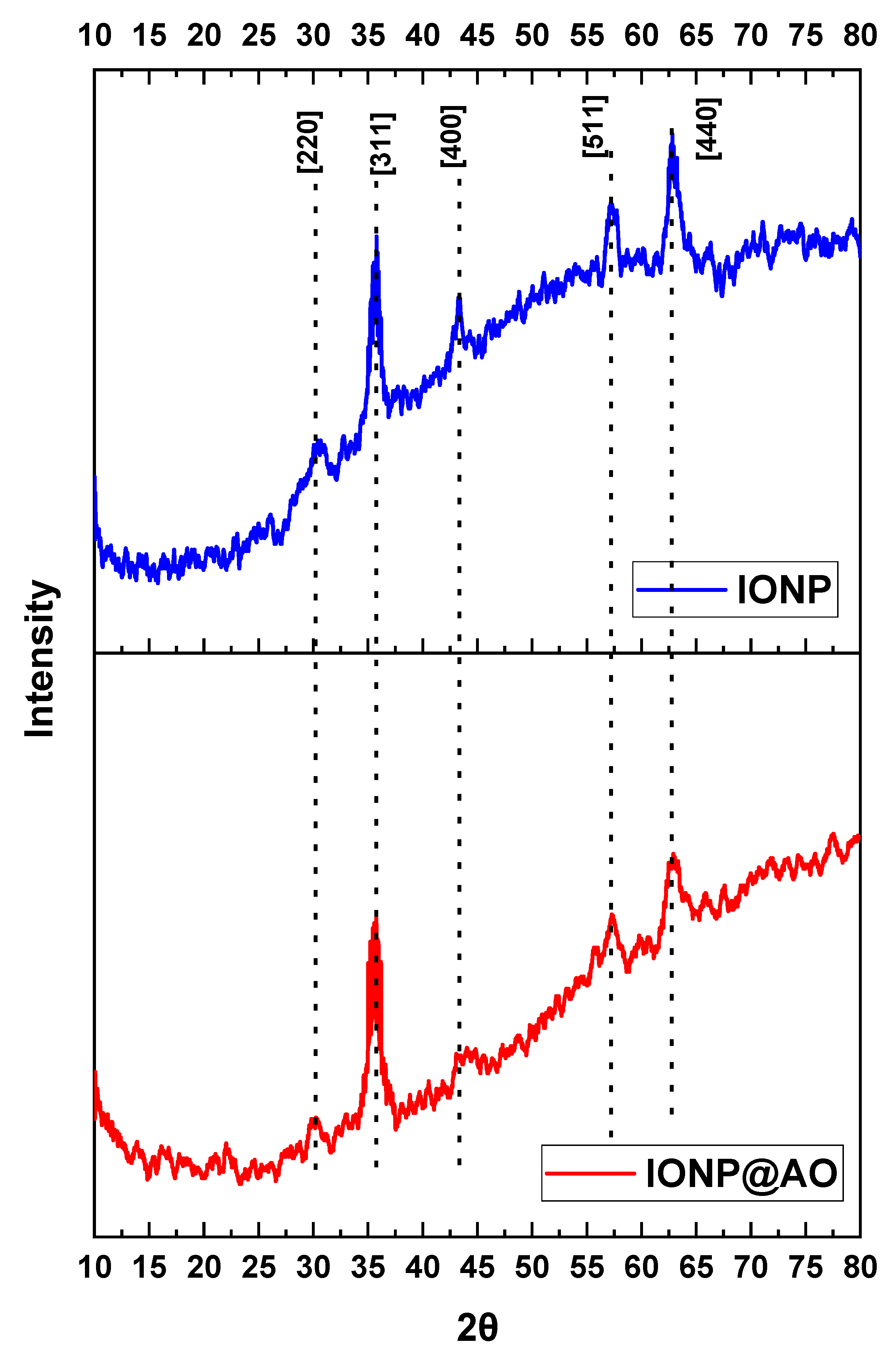
2.3. XRD Analysis
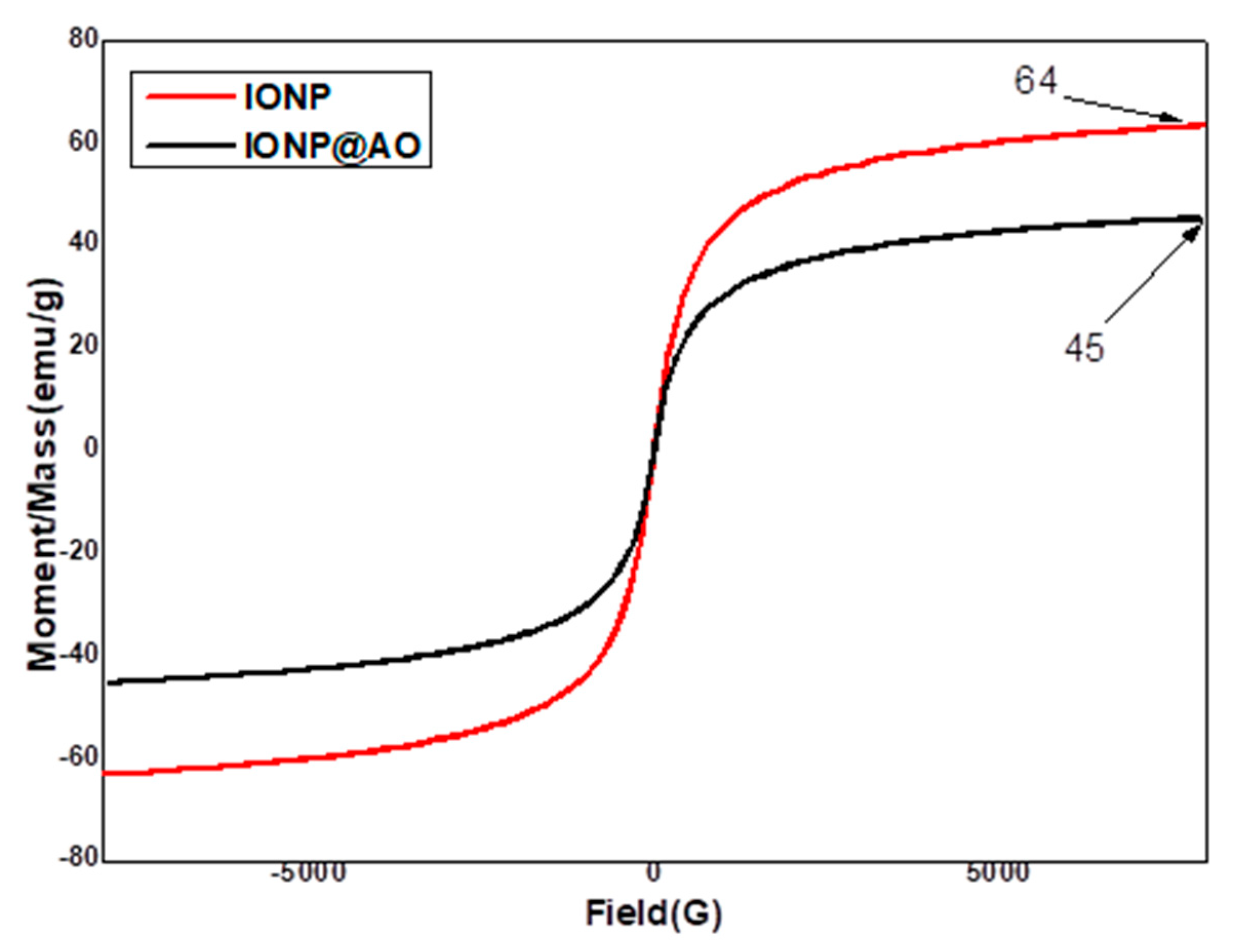
2.4. Magnetic Properties
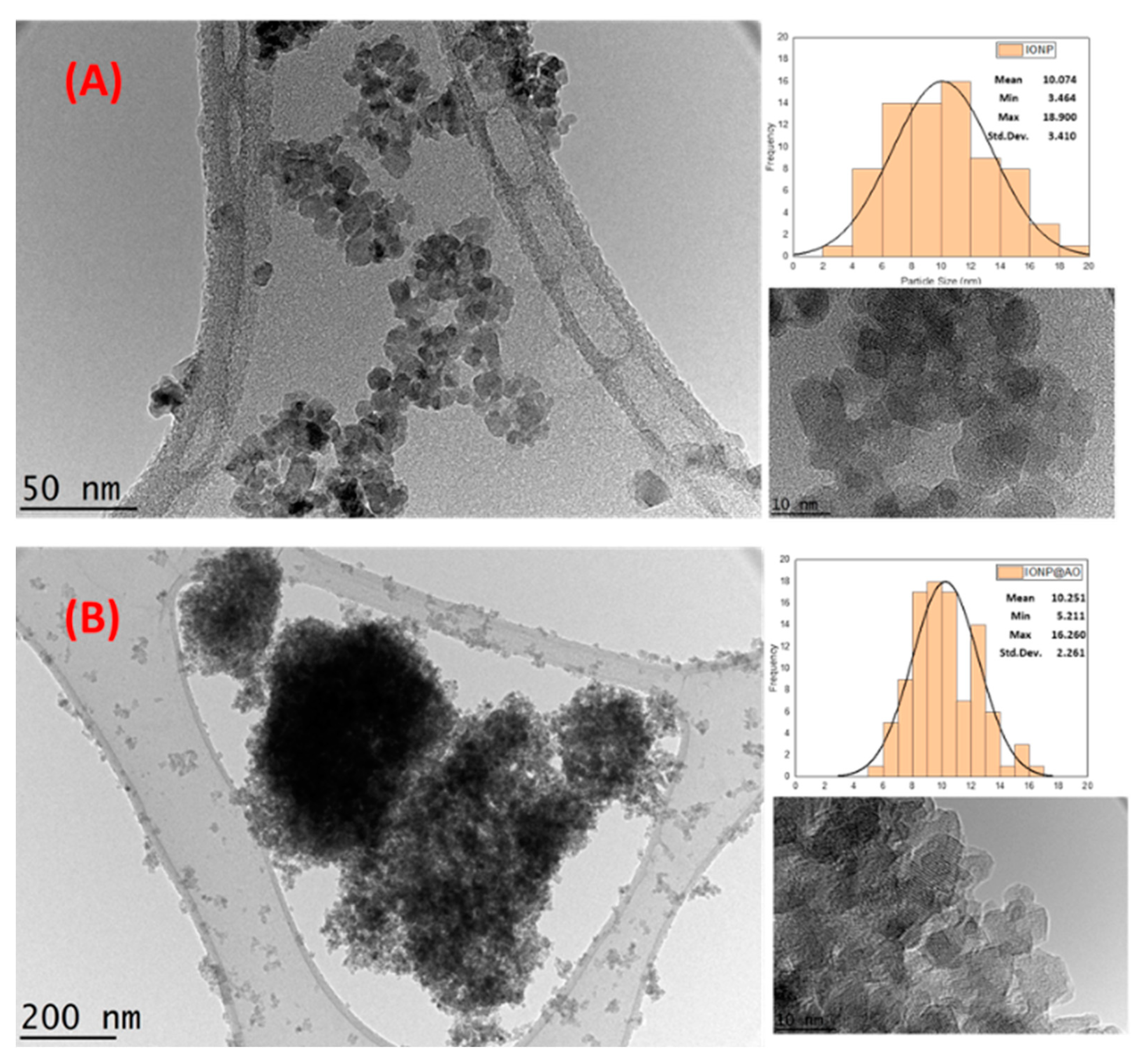
2.5. Morphological and Structural Studies
2.6. EDX Analysis
2.7. Computational Analysis
2.7.1. ADMET Studies
2.7.2. PASS Analysis
2.8. Antioxidant Activity
2.9. Antibacterial Activity
2.10. Antifungal Activity
3. Materials and Methods
3.1. Materials
3.2. Chracterizations
3.3. Methods
3.3.1. Computational Studies
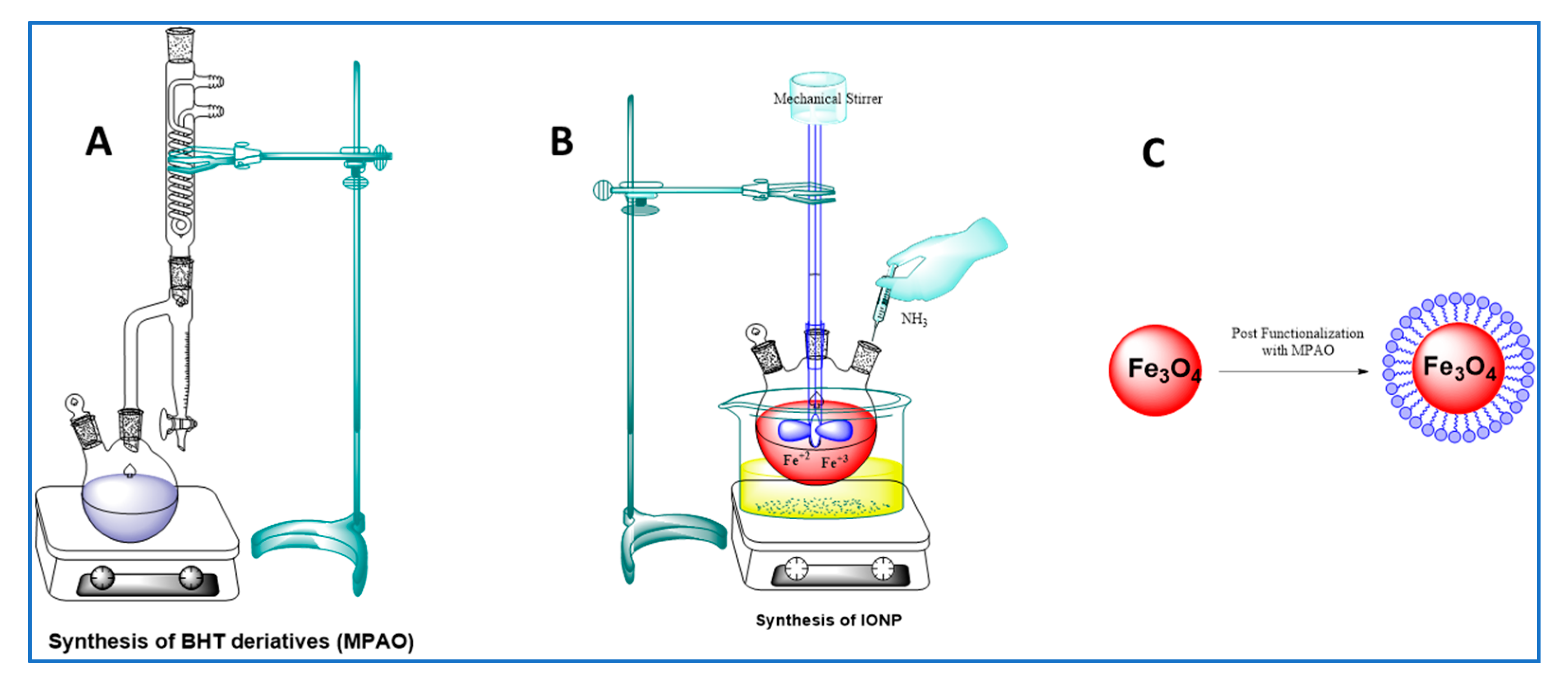
3.3.2. Synthesis of MPAO
3.3.3. Synthesis of IONP
3.3.4. Functionalization
Synthesis of IONP@AO
3.3.5. Antioxidant Activity
3.3.6. Antimicrobial Activity
Determination of Antibacterial Activity
Determination of Antifungal Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| ADME | Absorption, Distribution, Metabolism and Excretion. |
| ADMET | Absorption, Distribution, Metabolism, Excretion and Toxicity |
| AO | Antioxidant |
| BBB | Blood–Brain Barrier |
| BHT | Butylated Hydroxytoluene |
| DI | Deionized Water |
| DNA | Deoxyribonucleic Acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EDX | Energy Dispersive X-ray Analysis |
| FESEM | Field Emission Scanning Electron Microscope |
| FTIR | Fourier-transform infrared spectroscopy |
| HRTEM | High-Resolution Transmission Electron Microscopy |
| IC50 | Half-maximal inhibitory concentration |
| IONP | Iron Oxide Nanoparticle |
| MLP | Molecular Lipophilicity Potential |
| MNA | Multi-level Neighbour of Atoms |
| MPAO | Multipotent Anioxidant |
| NMR | Nuclear Magnetic Resonance Spectroscopy |
| NP | Nanoparticles |
| PASS | Prediction of Activity Spectra for Biologically Active Substances |
| PDA | Potato Dextrose Aagar |
| PEG | Polyethylene Glycol |
| PIGD | Percentage Inhibition of Diameter Growth |
| POI | Percentage of Inhibition |
| PSA | Polar Surface Area |
| PTSA | p-Toluenesulfonic acid |
| ROS | Reactive Oxygen Species |
| SAR | Structure Activity Relation |
| TEM | Transmission Electron Microscopy |
| TPSA | TotalPolar Surface Area |
| UV | Ultraviolet |
| VSM | Vibrating-Sample Magnetometer |
| XRD | X-ray Crystallography |
References
- Sandhir, R.; Yadav, A.; Sunkaria, A.; Singhal, N. Nano-antioxidants: An emerging strategy for intervention against neurodegenerative conditions. Neurochem. Int. 2015, 89, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [Green Version]
- Masoudkabir, F.; Sarrafzadegan, N.; Gotay, C.; Ignaszewski, A.; Krahn, A.D.; Davis, M.K.; Franco, C.; Mani, A. Cardiovascular disease and cancer: Evidence for shared disease pathways and pharmacologic prevention. Atherosclerosis 2017, 263, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valgimigli, L.; Baschieri, A.; Amorati, R. Antioxidant activity of nanomaterials. J. Mater. Chem. B 2018, 6, 2036–2051. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Severino, P.; Basso, R.; Santana, M.H.A. Encapsulation of antioxidants in gastrointestinal-resistant nanoparticulate carriers. Oxidative Stress Nanotechnol. 2013, 1028, 37–46. [Google Scholar]
- Milinčić, D.D.; Popović, D.A.; Lević, S.M.; Kostić, A.Ž.; Tešić, Ž.L.; Nedović, V.A.; Pešić, M.B. Application of polyphenol-loaded nanoparticles in food industry. Nanomaterials 2019, 9, 1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eftekhari, A.; Ahmadian, E.; Panahi-Azar, V.; Hosseini, H.; Tabibiazar, M.; Maleki Dizaj, S. Hepatoprotective and free radical scavenging actions of quercetin nanoparticles on aflatoxin b1-induced liver damage: In vitro/in vivo studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 411–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent trends in antioxidant delivery applications. Antioxidants 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deligiannakis, Y.; Sotiriou, G.A.; Pratsinis, S.E. Antioxidant and antiradical sio2 nanoparticles covalently functionalized with gallic acid. ACS Appl. Mater. Interfaces 2012, 4, 6609–6617. [Google Scholar] [CrossRef]
- Marrazzo, P.; O’Leary, C. Repositioning natural antioxidants for therapeutic applications in tissue engineering. Bioengineering 2020, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Riela, S.; Guernelli, S.; Parisi, F.; Lazzara, G.; Baschieri, A.; Valgimigli, L.; Amorati, R. A synergic nanoantioxidant based on covalently modified halloysite–trolox nanotubes with intra-lumen loaded quercetin. J. Mater. Chem. B 2016, 4, 2229–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebabe Elle, R.; Rahmani, S.; Lauret, C.; Morena, M.; Bidel, L.P.R.; Boulahtouf, A.; Balaguer, P.; Cristol, J.-P.; Durand, J.-O.; Charnay, C. Functionalized mesoporous silica nanoparticle with antioxidants as a new carrier that generates lower oxidative stress impact on cells. Mol. Pharm. 2016, 13, 2647–2660. [Google Scholar] [CrossRef] [PubMed]
- Bumbudsanpharoke, N.; Choi, J.; Park, I.; Ko, S. Facile biosynthesis and antioxidant property of nanogold-cellulose fiber composite. J. Nanomater. 2015, 16, 195. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Suo, S.; Wang, G.; Jia, H.; Liu, K.J.; Zhao, B.; Liu, Y. Mechanism and cellular kinetic studies of the enhancement of antioxidant activity by using surface-functionalized gold nanoparticles. Chem.-Eur. J. 2013, 19, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.H.; Yehye, W.A.; Rahman, N.A.; Al-Ani, L.A.; Johan, M.R.; Lu, J.; Hashim, N.M. Antioxidant and cytotoxicity activities of butylated hydroxytoluene ligands capped gold nanoparticles. Chiang Mai J. Sci. 2021, 48, 405–419. [Google Scholar]
- Alves, A.d.C.S.; Mainardes, R.M.; Khalil, N.M. Nanoencapsulation of gallic acid and evaluation of its cytotoxicity and antioxidant activity. Mater. Sci. Eng. C 2016, 60, 126–134. [Google Scholar] [CrossRef]
- Salvador, M.; Gutiérrez, G.; Noriega, S.; Moyano, A.; Blanco-López, M.C.; Matos, M. Microemulsion synthesis of superparamagnetic nanoparticles for bioapplications. Int. J. Mol. Sci. 2021, 22, 427. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.T.; A Yehya, W.; Saad, O.; Simarani, K.; Chowdhury, Z.; A Alhadi, A.; Al-Ani, L.A. Surface functionalization of iron oxide nanoparticles with gallic acid as potential antioxidant and antimicrobial agents. Nanomaterials 2017, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.T.; Yehye, W.A.; Chowdhury, Z.Z.; Simarani, K. Magnetically directed antioxidant and antimicrobial agent: Synthesis and surface functionalization of magnetite with quercetin. PeerJ 2019, 7, e7651. [Google Scholar] [CrossRef] [Green Version]
- Ariffin, A.; Rahman, N.A.; Yehye, W.A.; Alhadi, A.A.; Kadir, F.A. Pass-assisted design, synthesis and antioxidant evaluation of new butylated hydroxytoluene derivatives. Eur. J. Med. Chem. 2014, 87, 564–577. [Google Scholar] [CrossRef]
- Elmadfa, I.; Meyer, A.L. Body composition, changing physiological functions and nutrient requirements of the elderly. Ann. Nutr. Metab. 2008, 52, 2–5. [Google Scholar] [CrossRef]
- Sosa-Acosta, J.R.; Silva, J.; Fernández-Izquierdo, L.; Díaz-Castañón, S.; Ortiz, M.; Zuaznabar-Gardona, J.C.; Díaz-García, A. Iron oxide nanoparticles (ionps) with potential applications in plasmid DNA isolation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 167–178. [Google Scholar] [CrossRef]
- Kumar, S.R.; Priyatharshni, S.; Babu, V.N.; Mangalaraj, D.; Viswanathan, C.; Kannan, S.; Ponpandian, N. Quercetin conjugated superparamagnetic magnetite nanoparticles for in-vitro analysis of breast cancer cell lines for chemotherapy applications. J. Colloid Interface Sci. 2014, 436, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Shebanova, O.N.; Lazor, P. Raman study of magnetite (fe3O4): Laser-induced thermal effects and oxidation. J. Raman Spectrosc. 2003, 34, 845–852. [Google Scholar] [CrossRef]
- Francisco, M.; Teresa, C.; María, C.; Ramón, P.; Rolando, R.; Pedro, F.; José María, S.; Eduardo, E.; Carmen, M. Synthesis and characterization of monodisperse magnetite hollow microspheres. Soft Nanosci. Lett. 2011, 1, 25–32. [Google Scholar]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Periakaruppan, R.; Chen, X.; Thangaraj, K.; Jeyaraj, A.; Nguyen, H.H.; Yu, Y.; Hu, S.; Lu, L.; Li, X. Utilization of tea resources with the production of superparamagnetic biogenic iron oxide nanoparticles and an assessment of their antioxidant activities. J. Clean. Prod. 2021, 278, 123962. [Google Scholar] [CrossRef]
- Dorniani, D.; Hussein, M.Z.B.; Kura, A.U.; Fakurazi, S.; Shaari, A.H.; Ahmad, Z. Preparation of fe3O4 magnetic nanoparticles coated with gallic acid for drug delivery. Int. J. Nanomed. 2012, 7, 5745–5756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.-L.; Qi, X.-R.; Maitani, Y.; Nagai, T. Preparation and characterization of superparamagnetic iron oxide nanoparticles stabilized by alginate. Int. J. Pharm. 2007, 333, 177–186. [Google Scholar] [CrossRef]
- Dorniani, D.; Bin, H.M.Z.; Kura, A.U.; Fakurazi, S.; Hussein-Al-Ali, S.H.; Shaari, A.H.; Ahmad, Z. In vitro sustained release study of gallic acid coated with magnetite-peg and magnetite-pva for drug delivery system. Sci. World J. 2014, 2014, 416354. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, S.J.; Joy, M.; Ghosh, C.K.; Dey, S.; Kotnala, R.K.; Ghosh, S. Magnetic, X-ray and mossbauer studies on magnetite/maghemite core-shell nanostructures fabricated through an aqueous route. RSC Adv. 2014, 4, 64919–64929. [Google Scholar] [CrossRef] [Green Version]
- Zoete, V.; Daina, A.; Bovigny, C.; Michielin, O. Swisssimilarity: A web tool for low to ultra high throughput ligand-based virtual screening. J. Chem. Inf. Model. 2016, 56, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A boiled-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [Green Version]
- Gangwar, M.; Gautam, M.K.; Sharma, A.K.; Tripathi, Y.B.; Goel, R.K.; Nath, G. Antioxidant capacity and radical scavenging effect of polyphenol rich Mallotus philippenensis fruit extract on human erythrocytes: An in vitro study. Sci. World J. 2014, 2014, 279451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatami, M.; Alijani, H.Q.; Fakheri, B.; Mobasseri, M.M.; Heydarpour, M.; Farahani, Z.K.; Khan, A.U. Super-paramagnetic iron oxide nanoparticles (spions): Greener synthesis using stevia plant and evaluation of its antioxidant properties. J. Clean. Prod. 2019, 208, 1171–1177. [Google Scholar] [CrossRef]
- Gaidhani, K.A.; Harwalkar, M.; Bhambere, D.; Nirgude, P.S. Lyophilization/freeze drying—A review. World J. Pharm. Res. 2015, 4, 516–543. [Google Scholar]
- Sotiriou, G.A.; Blattmann, C.O.; Deligiannakis, Y. Nanoantioxidant-driven plasmon enhanced proton-coupled electron transfer. Nanoscale 2016, 8, 796–803. [Google Scholar] [CrossRef]
- Sahu, N.; Soni, D.; Chandrashekhar, B.; Sarangi, B.K.; Satpute, D.; Pandey, R.A. Synthesis and characterization of silver nanoparticles using cynodon dactylon leaves and assessment of their antibacterial activity. Bioprocess Biosyst. Eng. 2013, 36, 999–1004. [Google Scholar] [CrossRef]
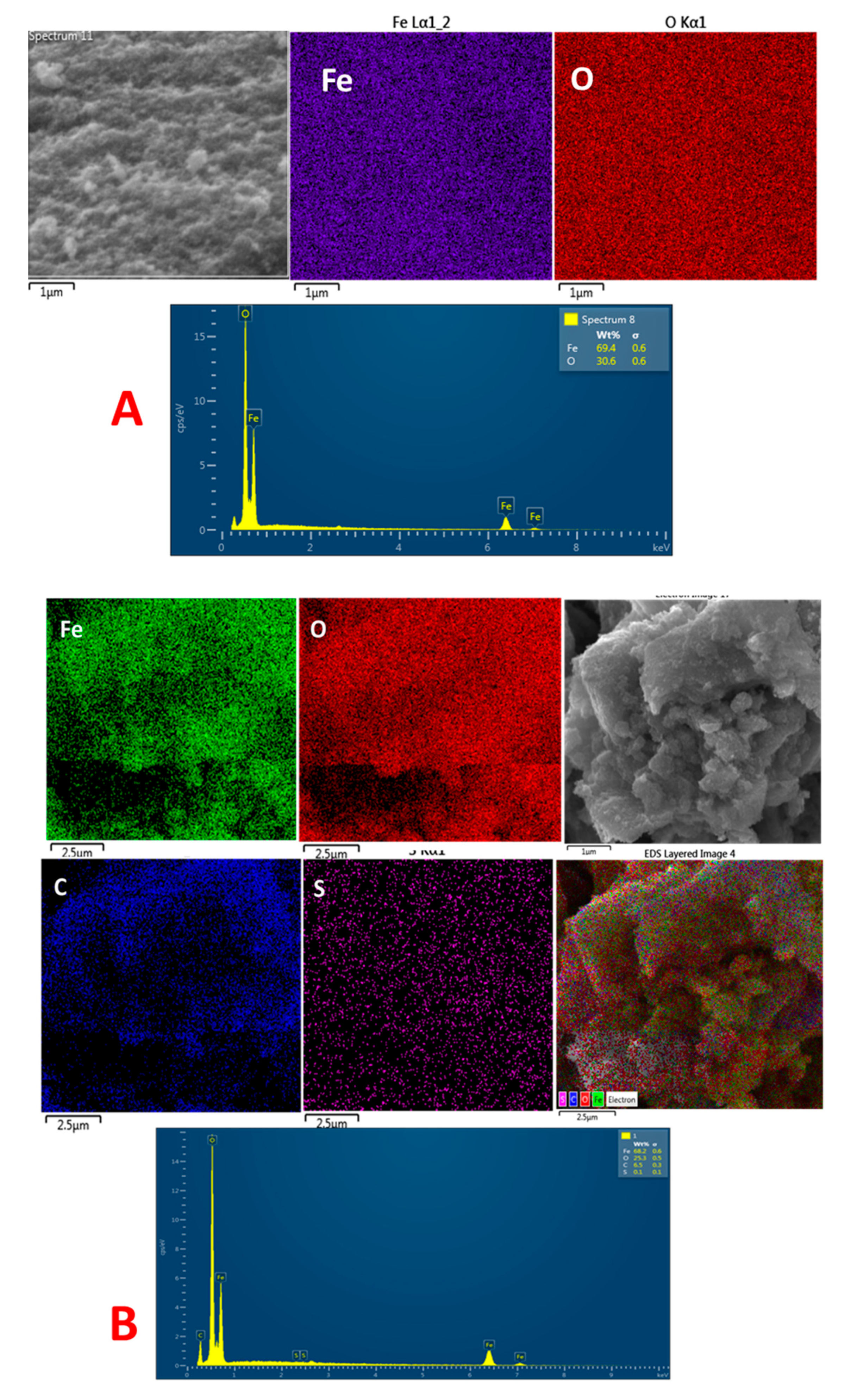



| Sample | Fe | O | C | S |
|---|---|---|---|---|
| IONP | 69.4 | 30.6 | - | - |
| IONP@AO | 68.2 | 25.3 | 6.5 | 0.1 |
| Physicochemical Properties | |
| Number of rotatable bonds | 13 |
| Number of H-bond acceptors | 5 |
| Number of H-bond donors | 1 |
| MR | 116.67 |
| TPSA | 90.29 |
| Lipophilicity | |
| iLOGP | 4.58 |
| XLOGP3 | 4.98 |
| WLOGP | 4.27 |
| MLOGP | 2.99 |
| Silicos-IT LogP | 5.62 |
| Consensus LogP | 4.49 |
| Water Solubility | |
| ESOL Log S | −4.84 |
| ESOL Solubility (mg/mL) | 6.02 × 10−3 |
| ESOL Solubility (mol/l) | 1.46 × 10−5 |
| ESOL Class | Moderately soluble |
| Pharmacokinetics | |
| GI absorption | High |
| BBB permeant | No |
| Pgp substrate | No |
| CYP1A2 inhibitor | No |
| CYP2C19 inhibitor | No |
| CYP2C9 inhibitor | No |
| CYP2D6 inhibitor | Yes |
| CYP3A4 inhibitor | Yes |
| log Kp (cm/s) | −5.28 |
| Druglikeness | |
| Lipinski number of violations | 0 |
| Ghose number of violations | 0 |
| Veber number of violations | 1 |
| Egan number of violations | 0 |
| Muegge number of violations | 0 |
| Bioavailability Score | 0.55 |
| Medicinal Chemistry | |
| PAINS number of alerts | 0 |
| Brenk number of alerts | 0 |
| Leadlikeness number of violations | 3 |
| Synthetic Accessibility | 3.89 |
| a Pa | b Pi | Biological Activity |
|---|---|---|
| 0.456 | 0.013 | Free radical scavenger |
| 0.351 | 0.049 | Lipid peroxidase inhibitor |
| 0.285 | 0.026 | Antioxidant |
| 0.268 | 0.097 | Antifungal |
| 0.224 | 0.098 | Antibacterial |
| IC50 a Values (mg) ± S.E.M b and Max. Inhibition % | |||
|---|---|---|---|
| Sample | IC50 mg/mL | % Inhibition | |
| IONP | 5 mg | 4.7 ± 0.002 | 50 |
| IONP@AO | 5 mg | 1 ± 0.002 | 83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.T.; Chowdhury, Z.Z.; Johan, M.R.B.; Badruddin, I.A.; Khaleed, H.M.T.; Kamangar, S.; Alrobei, H. Surface Functionalization of Magnetite Nanoparticles with Multipotent Antioxidant as Potential Magnetic Nanoantioxidants and Antimicrobial Agents. Molecules 2022, 27, 789. https://doi.org/10.3390/molecules27030789
Shah ST, Chowdhury ZZ, Johan MRB, Badruddin IA, Khaleed HMT, Kamangar S, Alrobei H. Surface Functionalization of Magnetite Nanoparticles with Multipotent Antioxidant as Potential Magnetic Nanoantioxidants and Antimicrobial Agents. Molecules. 2022; 27(3):789. https://doi.org/10.3390/molecules27030789
Chicago/Turabian StyleShah, Syed Tawab, Zaira Zaman Chowdhury, Mohd. Rafie Bin Johan, Irfan Anjum Badruddin, H. M. T. Khaleed, Sarfaraz Kamangar, and Hussein Alrobei. 2022. "Surface Functionalization of Magnetite Nanoparticles with Multipotent Antioxidant as Potential Magnetic Nanoantioxidants and Antimicrobial Agents" Molecules 27, no. 3: 789. https://doi.org/10.3390/molecules27030789
APA StyleShah, S. T., Chowdhury, Z. Z., Johan, M. R. B., Badruddin, I. A., Khaleed, H. M. T., Kamangar, S., & Alrobei, H. (2022). Surface Functionalization of Magnetite Nanoparticles with Multipotent Antioxidant as Potential Magnetic Nanoantioxidants and Antimicrobial Agents. Molecules, 27(3), 789. https://doi.org/10.3390/molecules27030789







