Quantitative Analysis of Abamectin, Albendazole, Levamisole HCl and Closantel in Q-DRENCH Oral Suspension Using a Stability-Indicating HPLC-DAD Method
Abstract
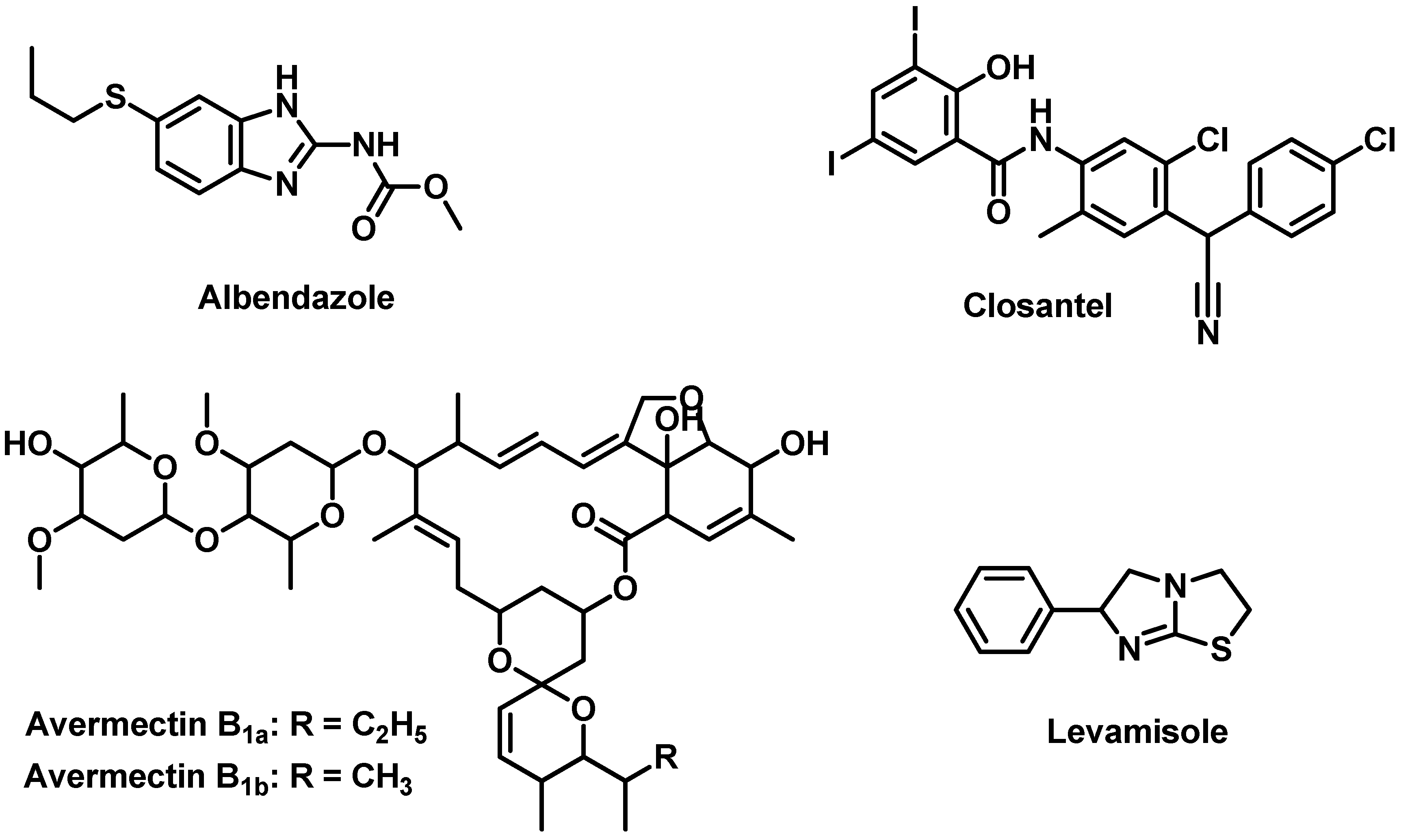
:1. Introduction
2. Results and Discussion
2.1. Method Optimization
2.2. Method Validation
2.2.1. Estimation of Linearity Ranges
2.2.2. Accuracy
2.2.3. Precision
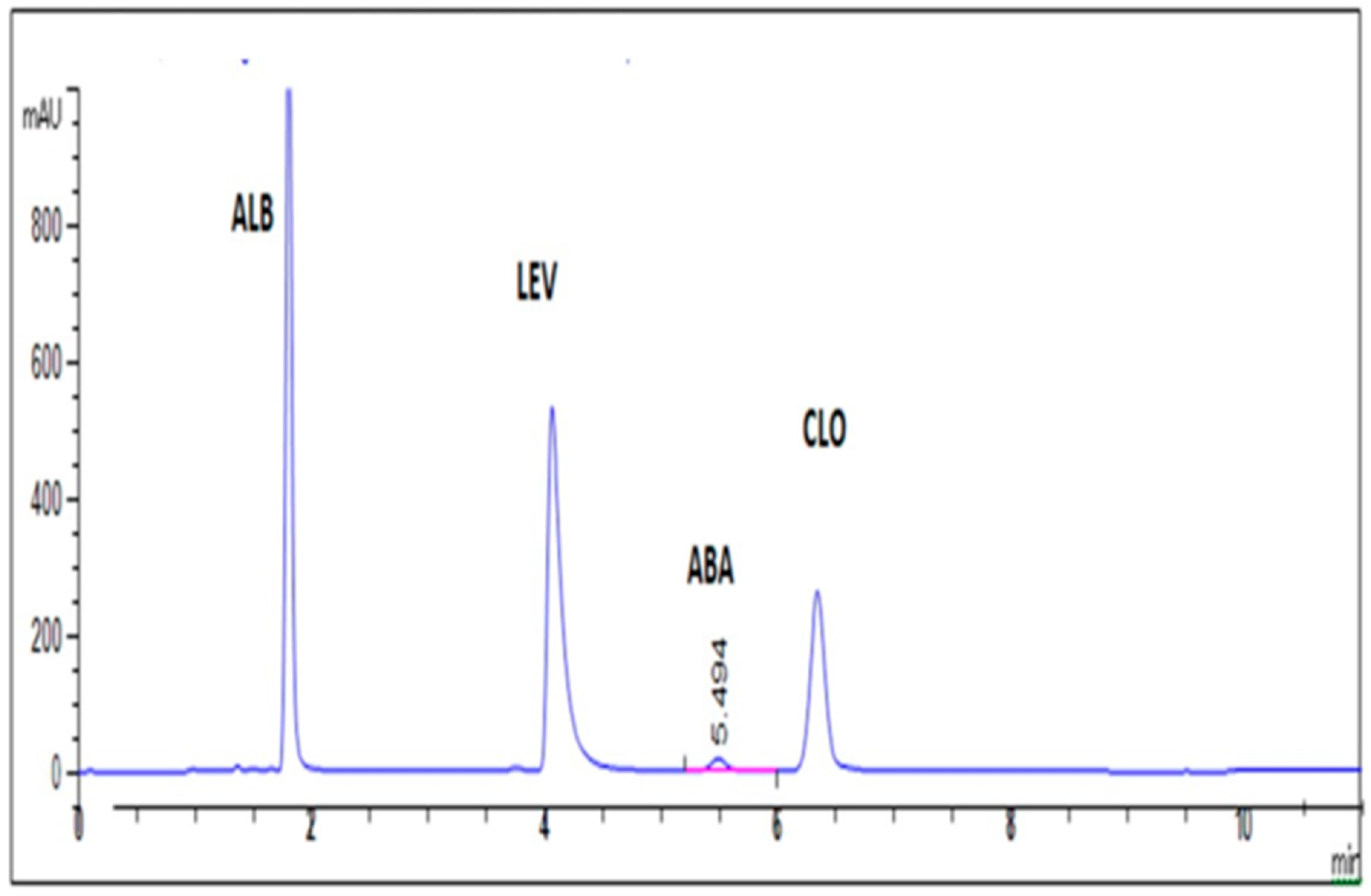
2.2.4. Selectivity
2.2.5. Detection and Quantitation Limits (LOD and LOQ)
2.2.6. System Suitability Parameters
2.2.7. Robustness
2.3. Confirmation of the Purity of the Four Drugs
2.4. Application to the Q-DRENCH Oral Suspension for Sheep
2.5. Results for the Forced Degradation Study
2.6. Recommendations Based on the Outcomes of Stability Studies
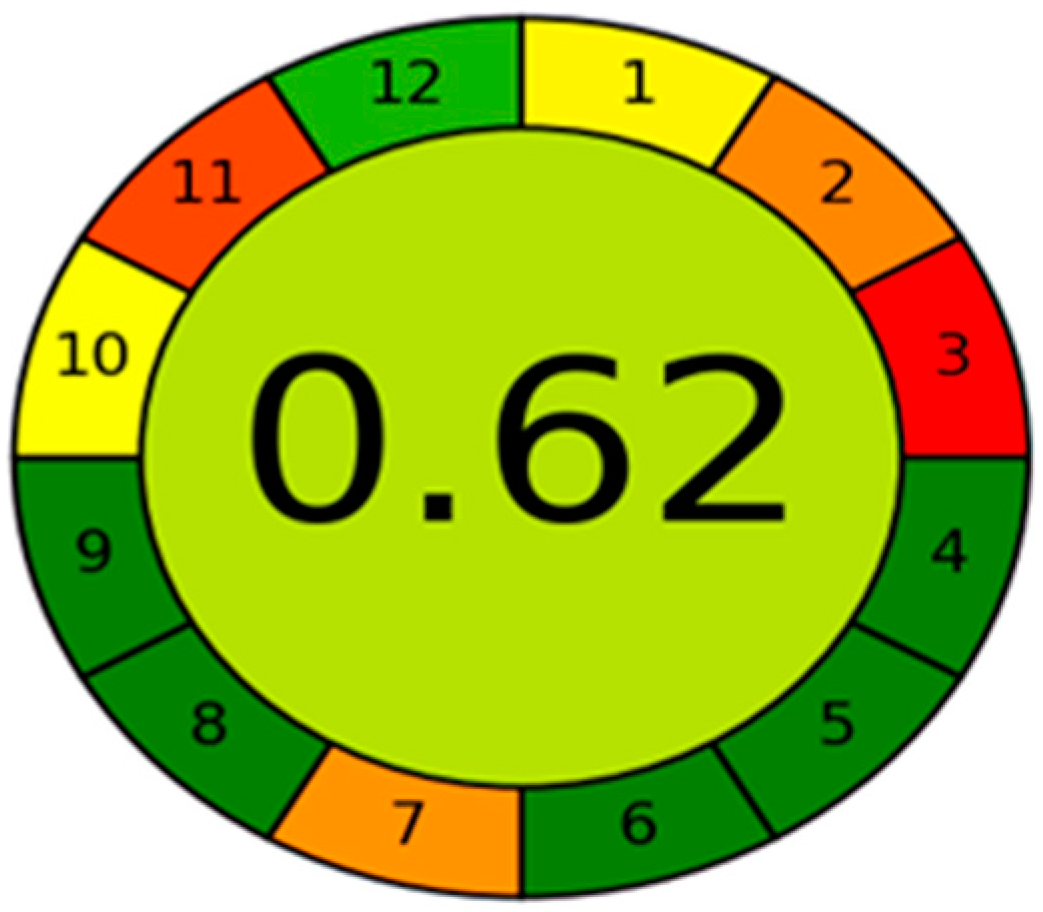
2.7. Eco-Friendly Nature Estimation for the New HPLC Method
2.8. Future Research and Limitations of the Study
3. Materials and Methods
3.1. Apparatus
3.2. Materials
3.2.1. Chemicals and Pure Drug Samples
3.2.2. Pharmaceutical Formulations
3.3. Preparation of Pure Standard’s Working and Stock Organic Solutions
3.4. Preparation of Q-DRENCH Oral Suspension Solution
3.5. General Chromatographic Procedures
3.6. Processes for Stability Studies for the Four Drugs
3.7. Assessment of Eco-Friendly Qualities of the Novel HPLC Method via AGREE Tool
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rashid, M.H.; Vaughan, J.L.; Stevenson, M.A.; Stevenson, A.; Campbell, J.D.; Beveridge, I.; Jabbar, A. Anthelmintic resistance in gastrointestinal nematodes of alpacas (Vicugna pacos) in Australia. Parasit. Vectors 2018, 11, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falzon, L.C.; O’Neill, T.J.; Menzies, P.I.; Peregrine, A.S.; Jones-Bitton, A.; vanLeeuwen, J.; Mederos, A. A systematic review and meta-analysis of factors associated with anthelmintic resistance in sheep. Prev. Vet. Med. 2014, 117, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Le Jambre, L.F.; Martin, P.J.; Johnston, A. Efficacy of combination anthelmintics against multiple resistant strains of sheep nematodes. Anim. Prod. Sci. 2010, 50, 946–952. [Google Scholar] [CrossRef]
- Gamal, M.; Elhalim, L.M.A. Novel Eco-friendly HPLC Methods Using Refractive Index Detector for Analysis of Three Veterinary Antibiotics in Pharmaceutical Formulations and Rat Plasma. J. Chromatogr. Sci. 2020, 58, 940–950. [Google Scholar] [CrossRef]
- Gamal, M.; Ali, H.M.; Fraihat, S.M.; Elnasr, T.A.S. Simultaneous determination of piroxicam and norfloxacin in biological fluids by high-performance liquid chromatography with fluorescence detection at zero-order emission mode. Luminescence 2019, 34, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Gamal, M. Analytical review: Analytical techniques for hyoscine N butyl bromide. Analyst 2020, 145, 2025–2037. [Google Scholar] [CrossRef]
- Gamal, M.; Ali, H.M.; Abdelfatah, R.M.; Magdy, M.A. A green approach for simultaneous analysis of two natural hepatoprotective drugs in pure forms, capsules and human plasma using HPLC-UV method. Microchem. J. 2019, 151, 104258. [Google Scholar] [CrossRef]
- Ali, N.W.; Gamal, M.; Abdelkawy, M. Chromatographic methods for simultaneous determination of diiodohydroxyquinoline and Metronidazole in their binary mixture. Pak. J. Pharm. Sci. 2013, 26, 865–871. [Google Scholar]
- Ali, N.W.; Gamal, M.; Abdelkawy, M. Simultaneous determination of hyoscine N-butyl bromide and paracetamol in their binary mixture by RP-HPLC method. Arab. J. Chem. 2017, 10, S1868–S1874. [Google Scholar] [CrossRef] [Green Version]
- Gamal, M.; Naguib, I.A.; Abdelfatah, R.M. Simultaneous analysis of oxytetracycline hydrochloride, lidocaine, and bromhexine hydrochloride in the presence of many interfering excipients. Arch. Pharm. 2021, e2100131. [Google Scholar] [CrossRef]
- Mandour, A.A.; Nabil, N.; Zaazaa, H.E.; Abdelkawy, M. Review on analytical studies of some pharmaceutical compounds containing heterocyclic rings: Brinzolamide, timolol maleate, flumethasone pivalate, and clioquinol. Futur. J. Pharm. Sci. 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Mahaparale, S.; Banju, D. Recent Analytical Methods of Anti-Helmintic Agents. Asian J. Pharm. Res. 2019, 9, 209. [Google Scholar] [CrossRef]
- Abongwa, M.; Martin, R.J.; Robertson, A.P. A brief review on the mode of action of antinematodal drugs. Acta Vet. Brno. 2017, 67, 137–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caffrey, C.R. Parasitic Helminths; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Batiha, G.E.S.; Alqahtani, A.; Ilesanmi, O.B.; Saati, A.A.; El-Mleeh, A.; Hetta, H.F.; Beshbishy, A.M. Avermectin Derivatives, Pharmacokinetics, Therapeutic and Toxic Dosages, Mechanism of Action, and Their Biological Effects. Pharmaceuticals 2020, 13, 1–37. [Google Scholar]
- Tabatabaei, S.A.; Soleimani, M.; Mansouri, M.R.; Mirshahi, A.; Inanlou, B.; Abrishami, M.; Pakrah, A.R.; Masarat, H. Closantel: A veterinary drug with potential severe morbidity in humans. BMC Ophthalmol. 2016, 16, 207. [Google Scholar] [CrossRef] [Green Version]
- Mooney, D.; Coxon, C.; Richards, K.G.; Gill, L.; Mellander, P.E.; Danaher, M. Development and optimisation of a multiresidue method for the determination of 40 anthelmintic compounds in environmental water samples by solid phase extraction (SPE) with LC-MS/MS detection. Molecules 2019, 24, 1978. [Google Scholar] [CrossRef] [Green Version]
- Horvat, A.J.M.; Babić, S.; Pavlović, D.M.; Ašperger, D.; Pelko, S.; Kaštelan-Macan, M.; Petrović, M.; Mance, A.D. TrAC—Trends. Anal. Chem. 2012, 31, 61–84. [Google Scholar]
- Vandamme, T.F.; Demoustier, M.; Rollmann, B. Quantitation of levamisole in plasma using high performance liquid chromatography. Eur. J. Drug Metab. Pharmacokinet. 1995, 20, 145–149. [Google Scholar] [CrossRef]
- Abdalraheem, A.; Shantier, S.; Abureid, I.; Gadkariem, E.A. Stability-Indicating High-Performance Liquid Chromatographic Determination of Levamisole Hydrochloride in Bulk and Dosage Forms. J. Chem. 2020, 2020. [Google Scholar] [CrossRef]
- Dreassi, E.; Corbini, G.; la Rosa, C.; Politi, N.; Corti, P. Determination of levamisole in animal tissues using liquid chromatography with ultraviolet detection. J. Agric. Food Chem. 2001, 49, 5702–5705. [Google Scholar] [CrossRef]
- Rezaee, M.; Mashayekhi, H.A.; Saleh, A.; Abdollahzadeh, Y.; Naeeni, M.H.; Fattahi, N. Determination of abamectin in citrus fruits using SPE combined with dispersive liquid-liquid microextraction and HPLC-UV detection. J. Sep. Sci. 2013, 36, 2629–2634. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.H.; Ravelo-Pérez, L.M.; Hernández-Suárez, E.M.; Carnero, A.; Rodríguez-Delgado, M.Á. Determination of abamectin residues in avocados by microwave-assisted extraction and HPLC with fluorescence detection. Chromatographia 2008, 67, 69–75. [Google Scholar] [CrossRef]
- Valois, M.E.C.; Takayanagui, O.M.; Bonato, P.S.; Lanchote, V.L.; Carvalho, D. Determination of albendazole metabolites in plasma by HPLC. J. Anal. Toxicol. 1994, 18, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.; Cheng, K.J.; Fleckenstein, L. HPLC assay for albendazole and metabolites in human plasma for clinical pharmacokinetic studies. J. Pharm. Biomed. Anal. 2002, 30, 801–813. [Google Scholar] [CrossRef]
- Sari, P.; Sun, J.; Razzak, M.; Tucker, I. HPLC assay of levamisole and abamectin in sheep plasma for application to pharmacokinetic studies. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 2277–2290. [Google Scholar] [CrossRef]
- Sari, P.; Sun, J.; Razzak, M.; Tucker, I.G. Pharmacokinetics of Abamectin/Levamisole Combination in a Medium Chain Mono and Diglyceride-Based Vehicle and an In Vitro Release and In Vitro In Vivo Correlation Study for Levamisole. AAPS PharmSciTech. 2017, 18, 1254–1260. [Google Scholar] [CrossRef]
- Sowjanya, S.; Devadasu, C. Development of RP-HPLC Method for the Simultaneous Quantitation of Levamisole and Albendazole: Application to Assay Validation. Int. J. Anal. Chem. 2018, 2018. [Google Scholar] [CrossRef]
- Yeung, H.S.; Lee, W.O.; Wong, Y.T. Screening of closantel and rafoxanide in animal muscles by HPLC with fluorescence detection and confirmation using MS. J. Sep. Sci. 2010, 33, 206–211. [Google Scholar] [CrossRef]
- Malan, C.E.P.; de Villiers, M.M.; Lötter, A.P. Evaluation of compatibility of tablet excipients with albendazole and closantel using DSC and HPLC. Drug Dev. Ind. Pharm. 1997, 23, 533–537. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Lightfield, A.R. Simultaneous analysis of aminoglycosides with many other classes of drug residues in bovine tissues by ultrahigh-performance liquid chromatography–tandem mass spectrometry using an ion-pairing reagent added to final extracts. Anal. Bioanal. Chem. 2018, 410, 1095–1109. [Google Scholar] [CrossRef]
- Gamal, M.; Naguib, I.A.; Panda, D.S.; Abdallah, F.F. Comparative study of four greenness assessment tools for selection of greenest analytical method for assay of hyoscine N -butyl bromide. Anal. Methods 2021, 13, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; Fabjanowicz, M.; Kalinowska, K.; Namieśnik, J. History and milestones of green analytical chemistry. In Green Analytical Chemistry; Springer: Singapore, 2019; pp. 1–17. [Google Scholar]
- Kannaiah, K.P.; Sugumaran, A.; Chanduluru, H.K.; Rathinam, S. Environmental impact of greenness assessment tools in liquid chromatography–A review. Microchem. J. 2021, 170, 106685. [Google Scholar] [CrossRef]
- Dunge, A.; Sharda, N.; Singh, B.; Singh, S. Validated specific HPLC method for determination of zidovudine during stability studies. J. Pharm. Biomed. Anal. 2005, 37, 1109–1114. [Google Scholar] [CrossRef]
- Gamal, M. Development of a green stability-indicating hplc-dad method for the analysis of tildipirosin in pharmaceutical formulation. Acta Pol. Pharm. Drug Res. 2020, 77, 443–452. [Google Scholar] [CrossRef]
- Bajaj, S.; Sakhuja, N.; Singla, D. Stability Testing of Pharmaceutical Products. J. Appl. Pharm. Sci. 2012, 2, 129–138. [Google Scholar]
- Swartz, M.E.; Krull, I.S. Handbook of Analytical Validation; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]

| Parameters | ALB | LEV | ABA | CLO |
|---|---|---|---|---|
| Range in (µg/mL) | 15.15–93.75 | 25–150 | 30–150 | 11.7–140.63 |
| Slope (mAU*s mL/µg) | 32.252 | 23.239 | 4.174 | 18.312 |
| Intercept (mAU*s) | −9.008 | −26.128 | 2.028 | 4.568 |
| R2 | 0.99984 | 0.99980 | 0.99997 | 0.99990 |
| Accuracy (mean ± SD) | 101.06 ± 0.489 | 101.15 ± 0.579 | 99.61 ± 0.588 | 100.58 ± 0.723 |
| Precision (%RSD) Repeatability * RSD ≤ 2% Day to day precision ** | 0.47 | 0.33 | 0.35 | 1.25 |
| Pooled RSD ≤ 3% | 1.47 | 1.62 | 0.29 | 2.03 |
| LOD in (µg/mL) *** | 1.669 | 2.989 | 1.167 | 1.991 |
| LOQ in (µg/mL) *** | 5.056 | 9.057 | 3.537 | 6.033 |
| Drug Name | ALB | LEV | ||
| Conc (%) | Average Peak Area (mAU*s) | Recovery (%) | Average Peak Area (mAU*s) | Recovery (%) |
| 50% | 1003.0 | 101.14% | 1134.3 | 100.49% |
| 100% | 2015.3 | 101.61% | 2300.5 | 101.90% |
| 150% | 2987.6 | 100.42% | 3422.6 | 101.07% |
| Drug name | ABA | CLO | ||
| Conc (%) | Average peak area (mAU*s) | Recovery (%) | Average peak area (mAU*s) | Recovery (%) |
| 50% | 207.2 | 100.34% | 869.0 | 101.49% |
| 100% | 408.5 | 98.90% | 1721.6 | 100.53% |
| 150% | 617.0 | 99.58% | 2561.7 | 99.72% |
| Parameters | Values | Reference Value | |||
|---|---|---|---|---|---|
| ALB | LEV | CLO | ABA * | ||
| Rt (min) | 1.88 | 4.33 | 6.76 | 5.53 | – |
| Resolution (R) | 16.2 | 11.10 | R > 1.5 | ||
| Selectivity factor (α) | 2.27 | 1.58 | >1 | ||
| Symmetry factor “Tailing factor” (T) | 1.25 | 1.61 | 1.08 | 1.05 | ≈1 |
| Column efficiency (N) | 7228 | 6962 | 12,443 | 8501 | Increase with efficiency of the separation |
| HETP b | 0.0035 | 0.0036 | 0.002 | 0.0029 | The lower the value, the higher the efficiency of the analytical column |
| Replicate | ALB | LEV | CLO | ABA | ||||
|---|---|---|---|---|---|---|---|---|
| Condition 1 | Condition 2 | Condition 1 | Condition 2 | Condition 1 | Condition 2 | Condition 1 | Condition 2 | |
| 1 | 1984.16 | 2018.49 | 2286.96 | 2308.80 | 1696.93 | 1754.22 | 426.648 | 417.386 |
| 2 | 2003.96 | 1998.72 | 2286.48 | 2313.65 | 1703.78 | 1706.78 | 425.807 | 417.432 |
| 3 | 2003.50 | 1990.95 | 2275.82 | 2301.01 | 1695.26 | 1710.67 | 428.765 | 416.438 |
| Pooled mean Peak areas (mAU*s) | 2000.0 | 2295.5 | 1711.3 | 422.1 | ||||
| Pooled SD | 11.9 | 14.7 | 21.8 | 5.6 | ||||
| Pooled RSD Accepted criteria ≤ 3% | 0.59 | 0.64 | 1.28 | 1.32 | ||||
| ALB | LEV | |||||
| Test Name | Conc (µg/mL) | Peak RT (min) | Peak Area (mAU*s) | Conc (µg/mL) | Peak RT (min) | Peak Area (mAU*s) |
| Standard | 62.5 | 1.894 | 2004.78 | 100.0 | 4.311 | 2307.77 |
| Q-DRENCH suspension test | 62.5 | 1.892 | 1988.94 | 100.0 | 4.304 | 2255.12 |
| Placebo | 0 | No peak observed | No peak observed | 0 | No peak observed | No peak observed |
| CLO | ABA | |||||
| Standard | 93.8 | 6.757 | 1695.07 | 100.0 | 5.494 | 414.11 |
| Q-DRENCH suspension test | 93.8 | 6.765 | 1684.66 | 100.0 | 5.498 | 409.28 |
| Placebo | 0 | No peak observed | No peak observed | 0 | No peak observed | No peak observed |
| Degradation Type | Conditions | ABA | ALB | LEV | CLO | ||||
|---|---|---|---|---|---|---|---|---|---|
| Assay% | Degradation% | Assay% | Degradation% | Assay% | Degradation% | Assay% | Degradation% | ||
| Light | Light (48 h)/UV (12 h) | 74.50 | 25.50 | 79.04 | 20.96 | 43.65 | 56.35 | 85.72 | 14.28 |
| Heat | 80 °C (8 h.) | 82.92 | 17.08 | 75.45 | 24.55 | 65.32 | 34.68 | 80.59 | 19.41 |
| Acid | 1N HCl/80 °C (1 h) | 69.55 | 30.45 | 70.36 | 29.64 | 65.19 | 34.81 | 85.90 | 14.10 |
| Base | 1N NaOH/80 °C (1 h) | 92.57 | 7.43 | 75.75 | 24.25 | 76.07 | 23.93 | 74.63 | 25.37 |
| H2O2 | 0.5% H2O2/80 °C (1 h) | 88.86 | 11.14 | 98.55 | 1.45 | 87.04 | 12.96 | 81.24 | 18.76 |
| Instrument: | Agilent HP1200 or equivalent |
| System Type: | Reverse Phase |
| Column Type: | Zorbax C18 |
| Length and Diameter: | 4.6 × 250 mm, 5 µm |
| Conditioning | With mobile phase for 45 min prior to operating the injection sequence |
| Column Temperature: | Ambient |
| Injection Type and Volume: | Auto Sampler, 10 µm |
| Detector Type: | UV Detector |
| Wavelength: | 210 nm |
| Mobile Phase Composition: | 1 mL of Triethylamine/L water, adjusting pH to 3.5 by glacial acetic acid: acetonitrile (20:80), v/v |
| Flow Rate: | 2.0 mL/min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, H.M.; Gamal, M.; Ghoneim, M.M.; Mohammed Abd Elhalim, L. Quantitative Analysis of Abamectin, Albendazole, Levamisole HCl and Closantel in Q-DRENCH Oral Suspension Using a Stability-Indicating HPLC-DAD Method. Molecules 2022, 27, 764. https://doi.org/10.3390/molecules27030764
Ali HM, Gamal M, Ghoneim MM, Mohammed Abd Elhalim L. Quantitative Analysis of Abamectin, Albendazole, Levamisole HCl and Closantel in Q-DRENCH Oral Suspension Using a Stability-Indicating HPLC-DAD Method. Molecules. 2022; 27(3):764. https://doi.org/10.3390/molecules27030764
Chicago/Turabian StyleAli, Hazim M., Mohammed Gamal, Mohammed M. Ghoneim, and Lobna Mohammed Abd Elhalim. 2022. "Quantitative Analysis of Abamectin, Albendazole, Levamisole HCl and Closantel in Q-DRENCH Oral Suspension Using a Stability-Indicating HPLC-DAD Method" Molecules 27, no. 3: 764. https://doi.org/10.3390/molecules27030764
APA StyleAli, H. M., Gamal, M., Ghoneim, M. M., & Mohammed Abd Elhalim, L. (2022). Quantitative Analysis of Abamectin, Albendazole, Levamisole HCl and Closantel in Q-DRENCH Oral Suspension Using a Stability-Indicating HPLC-DAD Method. Molecules, 27(3), 764. https://doi.org/10.3390/molecules27030764








