Anti-Cancer Effects of α-Cubebenoate Derived from Schisandra chinensis in CT26 Colon Cancer Cells
Abstract
:1. Introduction
2. Results
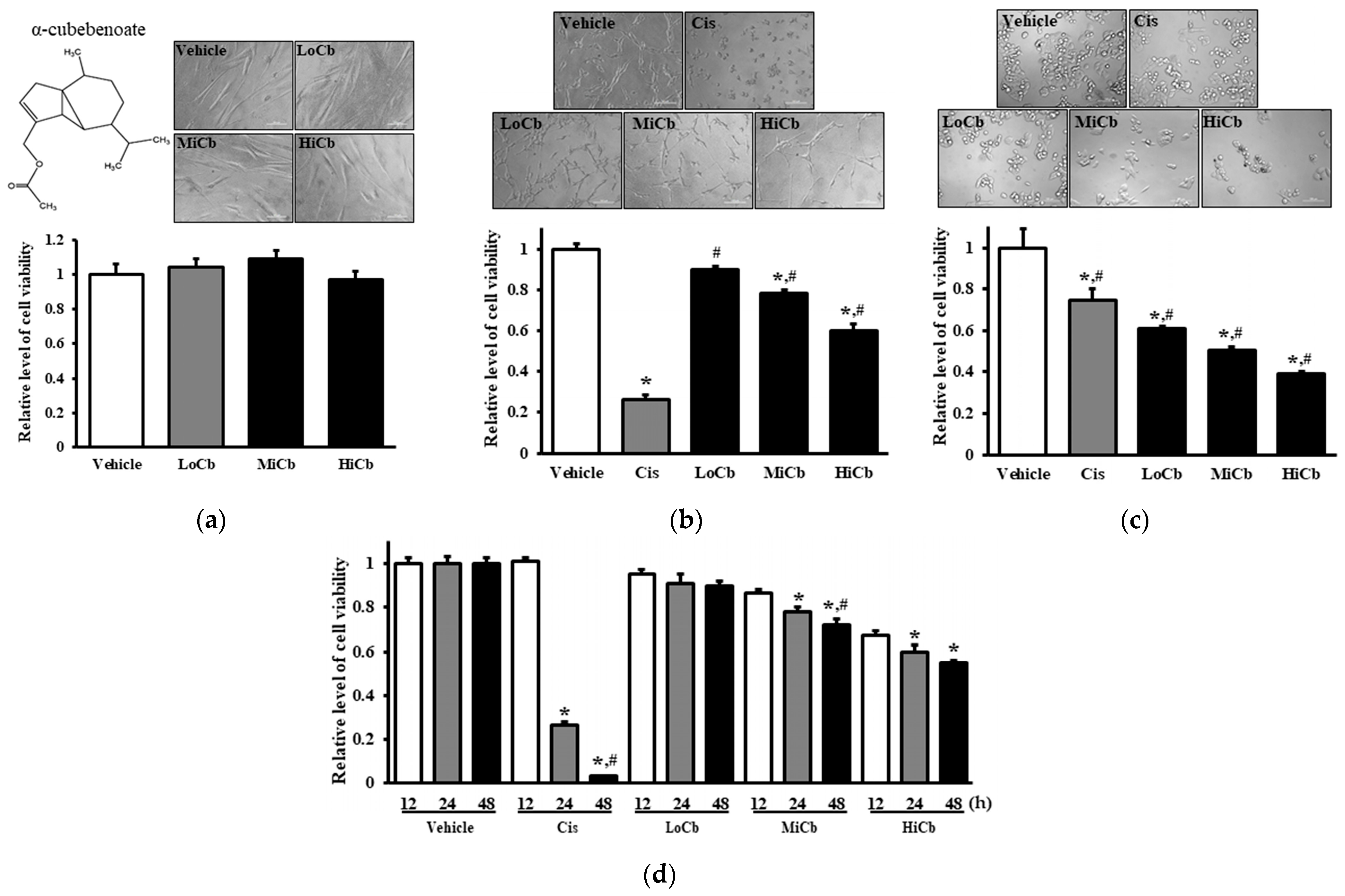
2.1. Effects of α-Cubebenoate on the Viabilities of CCD-18Co and CT26 Cells
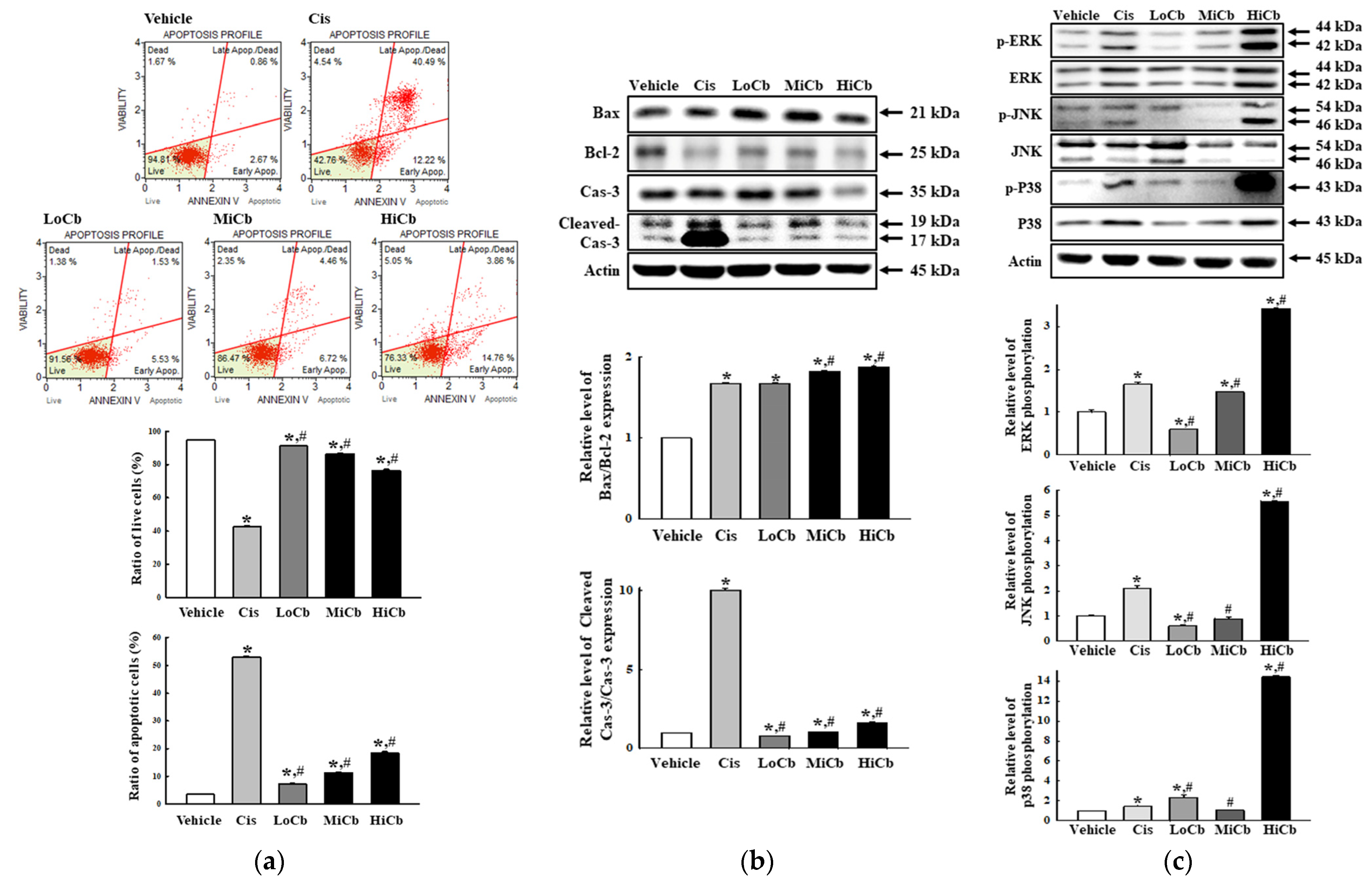
2.2. Effects of α-Cubebenoate on Apoptosis-Associated Processes in CT26 Cells
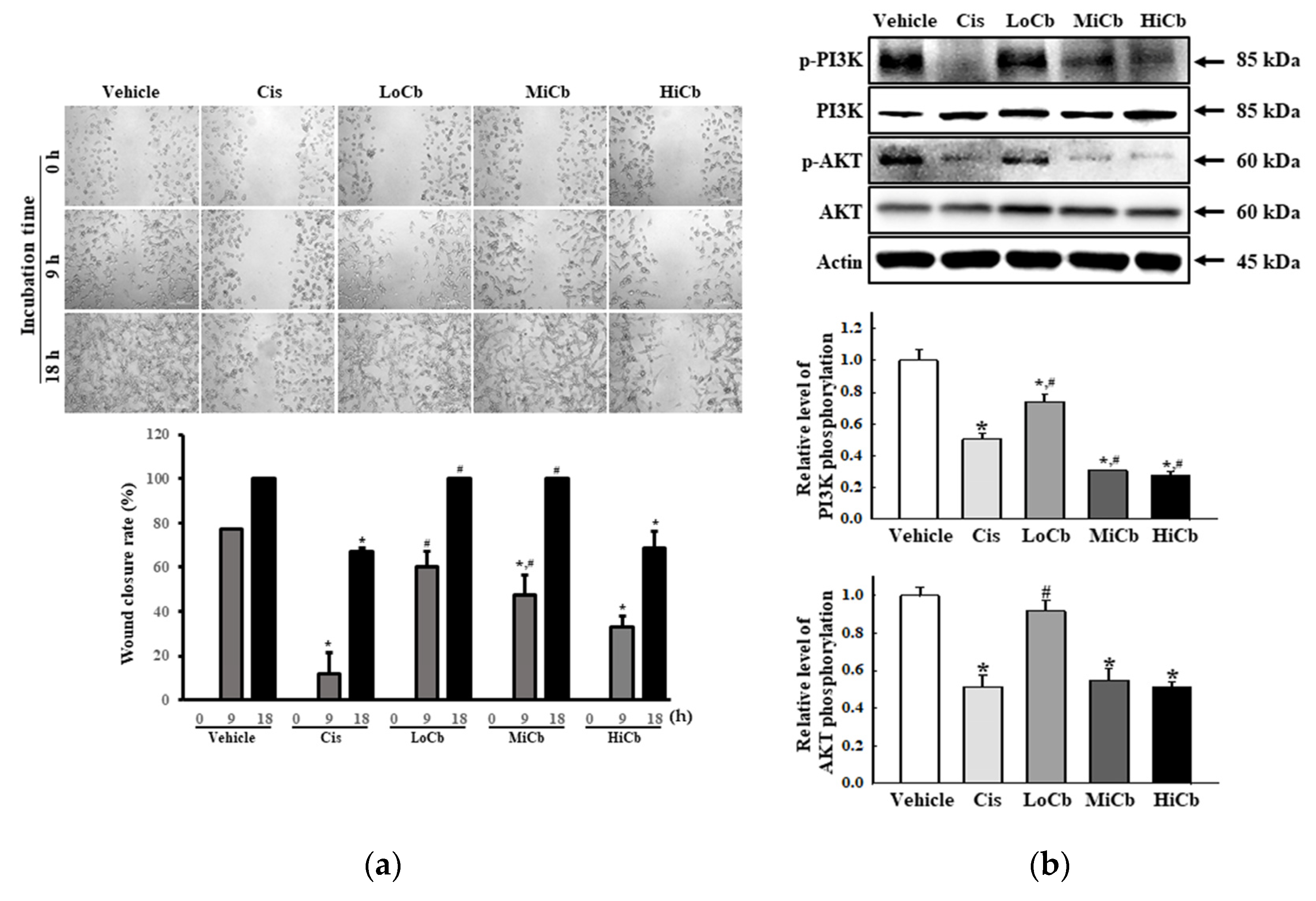
2.3. Effects of α-Cubebenoate on the Migration Ability and Its Associated Signals in CT26 Cells
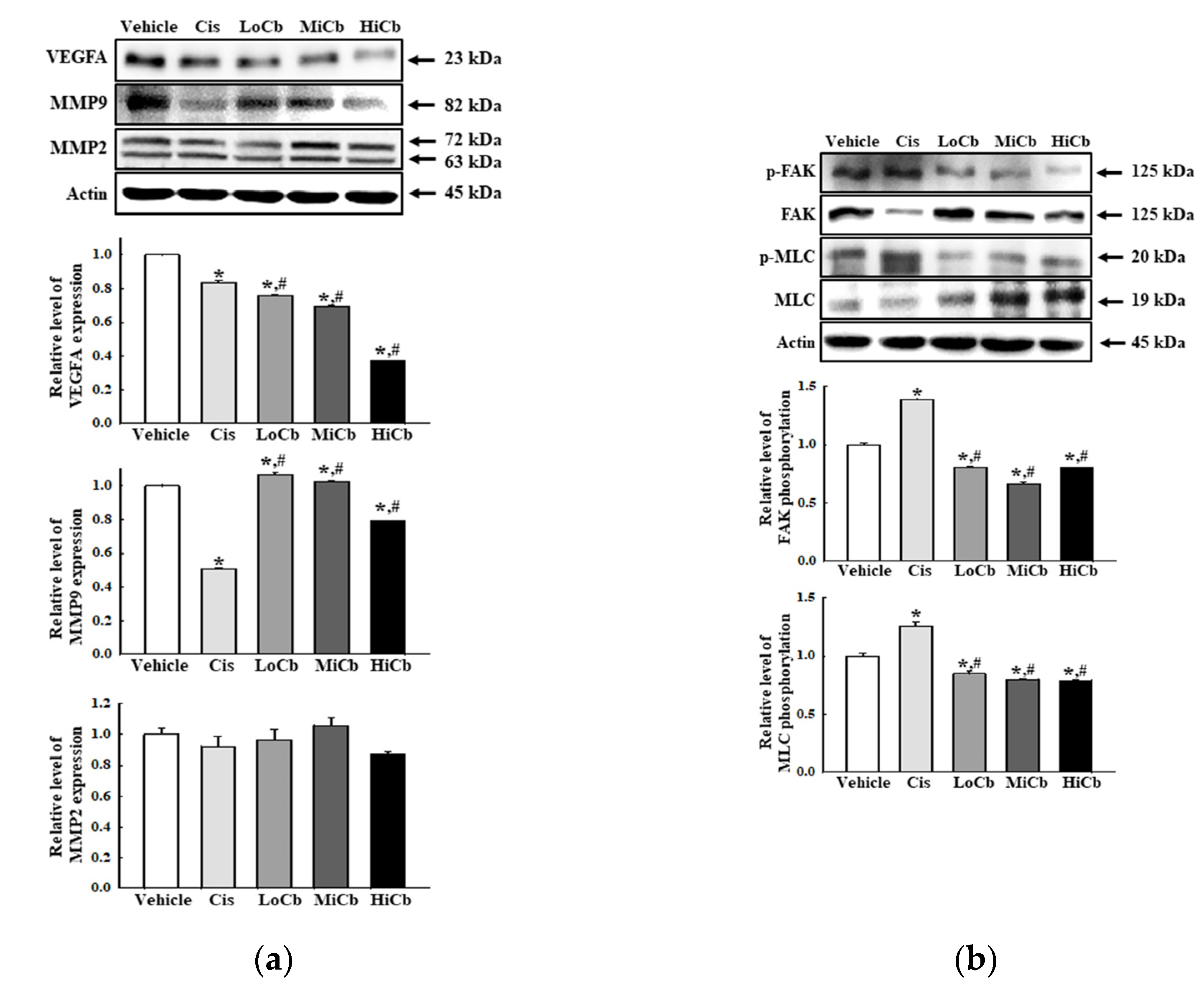
2.4. Effects of α-Cubebenoate on the Adhesive Ability of CT26 Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of α-Cubebenoate
4.2. Cell Culture
4.3. Cytotoxicity Assay
4.4. Analysis of Apoptotic Cells by Fluorescence-Activated Cell Sorting (FACS)
4.5. Wound-Healing Assay
4.6. Western Blot
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kinghorn, A.D. Drug discovery from natural products. In Foye’s Principles of Medicinal Chemistry, 6th ed.; Lemke, T.L., Williams, D.A., Eds.; Wolters Kluwer/Williams & Wilkins: Philadelphia, PA, USA, 2008; Volume 1, pp. 12–25. [Google Scholar]
- Feher, M.; Schmidt, J.M. Property distributions: Differences between drugs, natural products, and molecules from combinatorial chemistry. J. Chem. Inf. Comput. Sc.I 2003, 43, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, P.; Erehman, J.; Gohlke, B.O.; Wilhelm, T.; Preissner, R.; Dunkel, M. Super natural II—A database of natural products. Nucleic Acids Res. 2015, 43, D935–D939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fridlender, M.; Kapulnik, Y.; Koltai, H.; Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant derived substances with anti-cancer activity: From folklore to practice. Front Plant Sci. 2015, 6, 799. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. An overview of neuroprotective and cognitive enhancement properties of lignans from Schisandra Chinensis. Biomed. Pharm. 2018, 97, 958–968. [Google Scholar] [CrossRef]
- Opletal, L.; Sovova, H.; Bartlova, M.; Opletal, L.; Sovová, H.; Bártlová, M. Dibenzo[a,c]cyclooctadiene lignans of the genus Schisandra: Importance, isolation and determination. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 812, 357–471. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Pharmacology of Schisandra chinensis Bail.: An overview of Russian research and uses in medicine. J. Ethnopharmacol. 2008, 118, 183–212. [Google Scholar] [CrossRef]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Fructus Schisandrae. In WHO Monographs on Selected Medicinal Plants, 3rd ed.; Al-Said, M.S., Aung, K.H., Eds.; World Health Organization: Geneva, Switzerland, 2007; Volume 3, pp. 296–313. [Google Scholar]
- Xu, X.; Rajamanicham, V.; Xu, S.; Liu, Z.; Yan, T.; Liang, G.; Guo, G.; Zhou, H.; Wang, Y. Schisandrin A inhibits triple negative breast cancer cells by regulating Wnt/ER stress signaling pathway. Biomed. Pharmacother. 2019, 115, 108922. [Google Scholar] [CrossRef]
- Hwang, D.; Shin, S.Y.; Lee, Y.; Hyun, J.; Yong, Y.; Park, J.C.; Lee, Y.H.; Lim, Y. A compound isolated from Schisandra chinensis induces apoptosis. Bioorg. Med. Chem. Lett. 2011, 21, 6054–6057. [Google Scholar] [CrossRef]
- Wang, X.; Liao, X.; Zhang, Y.; Wei, L.; Pang, Y. Schisandrin B regulates MC3T3-E1 subclone 14 cells proliferation and differentiation through BMP2-SMADs-RUNX2-SP7 signaling axis. Sci. Rep. 2020, 10, 14476. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ahn, J.H.; Lee, K.T.; Jang, D.S.; Choi, J.H. Deoxyschizandrin, isolated from Schisandra Berries, induces cell cycle arrest in ovarian cancer cells and inhibits the protumoural activation of tumour-associated macrophages. Nutrients 2018, 10, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yim, S.Y.; Lee, Y.J.; Lee, Y.K.; Jung, S.E.; Kim, J.H.; Kim, H.J.; Son, B.G.; Park, Y.H.; Lee, Y.G.; Choi, Y.W.; et al. Gomisin N isolated from Schisandra chinensis significantly induces anti-proliferative and pro-apoptotic effects in hepatic carcinoma. Mol. Med. Rep. 2009, 2, 725–732. [Google Scholar] [PubMed] [Green Version]
- Park, C.; Choi, Y.W.; Hyun, S.K.; Kwon, H.J.; Hwang, H.J.; Kim, G.Y.; Choi, B.T.; Kim, B.W.; Choi, I.W.; Moon, S.K.; et al. Induction of G1 arrest and apoptosis by schisandrin C isolated from Schizandra chinensis Baill in human leukemia U937 cells. Int. J. Mol. Med. 2009, 24, 495–502. [Google Scholar]
- Waiwut, P.; Shin, M.S.; Yokoyama, S.; Saiki, I.; Sakurai, H. Gomisin A enhances tumor necrosis factor-α-induced G1 cell cycle arrest via signal transducer and activator of transcription 1-mediated phosphorylation of retinoblastoma protein. Biol. Pharm. Bull. 2012, 35, 1997–2003. [Google Scholar] [CrossRef] [Green Version]
- Poornima, B.; Siva, B.; Venkanna, A.; Shankaraiah, G.; Jain, N.; Yadav, D.K.; Misra, S.; Babu, K.S. Novel Gomisin B analogues as potential cytotoxic agents: Design, synthesis, biological evaluation and docking studies. Eur. J. Med. Chem. 2017, 139, 441–453. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, S.G.; Goo, J.S.; Park, D.J.; Lee, Y.J.; Hwang, I.S.; Lee, H.R.; Choi, S.I.; Lee, Y.J.; Oh, C.H.; et al. The α-iso-cubebenol compound isolated from Schisandra chinensis induces p53-independent pathway-mediated apoptosis in hepatocellular carcinoma cells. Oncol. Rep. 2012, 28, 1103–1109. [Google Scholar]
- Kang, S.; Lee, K.P.; Park, S.J.; Noh, D.Y.; Kim, J.M.; Moon, H.R.; Lee, Y.G.; Choi, Y.W.; Im, D.S. Identification of a novel anti-inflammatory compound, α-cubebenoate from Schisandra chinensis. J. Ethnopharmacol. 2014, 153, 242–249. [Google Scholar] [CrossRef]
- Lee, K.P.; Kang, S.; Park, S.J.; Kim, J.M.; Lee, J.M.; Lee, A.Y.; Chung, H.Y.; Choi, Y.W.; Lee, Y.G.; Im, D.S. Anti-allergic effect of α-cubebenoate isolated from Schisandra chinensis using in vivo and in vitro experiments. J. Ethnopharmacol. 2015, 173, 361–369. [Google Scholar] [CrossRef]
- Kook, M.; Lee, S.K.; Kim, S.D.; Lee, H.Y.; Hwang, J.S.; Choi, Y.W.; Bae, Y.S. Anti-septic activity of α-cubebenoate isolated from Schisandra chinensis. BMB Rep. 2015, 48, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.J.; Kim, J.E.; Choi, Y.J.; Lee, S.J.; Gong, J.E.; Choi, Y.W.; Hwang, D.Y. Novel function of α-cubebenoate derived from Schisandra chinensis as lipogenesis inhibitor, lipolysis stimulator and inflammasome suppressor. Molecules 2020, 25, 4995. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.K.; Zhu, G.Y.; Shen, X.L.; Chattopadhyay, A.; Dey, S.; Fong, W.F. Gomisin A alters substrate interaction and reverses P-glycoprotein-mediated multidrug resistance in HepG2-DR cells. Biochem. Pharm. 2006, 72, 824–837. [Google Scholar] [CrossRef]
- Jung, S.; Moon, H.I.; Kim, S.; Quynh, N.T.N.; Yu, J.; Sandag, Z.; Le, D.T.; Lee, H.; Lee, H.; Lee, M.S. Anticancer activity of gomisin J from Schisandra chinensis fruit. Oncol. Rep. 2019, 41, 711–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Wang, X.; Shi, X.; Zhang, Y.; Laster, K.V.; Liu, K.; Dong, Z.; Kim, D.J. Anwulignan is a novel JAK1 inhibitor that suppresses non-small cell lung cancer growth. J. Cell Mol. Med. 2021, 25, 2645–2654. [Google Scholar] [CrossRef]
- Florento, L.; Matias, R.; Tuaño, E.; Santiago, K.; Dela Cruz, F.; Tuazon, A. Comparison of cytotoxic activity of anticancer drugs against various human tumor cell lines using in vitro cell-based approach. Int. J. Biomed. Sci. 2012, 8, 76–80. [Google Scholar] [PubMed]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef] [Green Version]
- Baig, S.; Seevasant, I.; Mohamad, J.; Mukheem, A.; Huri, H.Z.; Kamarul, T. Potential of apoptotic pathway-targeted cancer therapeutic research: Where do we stand? Cell Death Dis. 2016, 7, e2058. [Google Scholar] [CrossRef] [Green Version]
- Vitagliano, O.; Addeo, R.; D’Angelo, V.; Indolfi, C.; Indolfi, P.; Casale, F. The Bcl-2/Bax and Ras/Raf/MEK/ERK signaling pathways: Implications in pediatric leukemia pathogenesis and new prospects for therapeutic approaches. Expert Rev. Hematol. 2013, 6, 587–597. [Google Scholar] [CrossRef]
- Inoue, S.; Salah-Eldin, A.E.; Omoteyama, K. Apoptosis and anticancer drug resistance. Hum. Cell 2001, 14, 211–221. [Google Scholar]
- Hickman, J.A. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992, 11, 121–139. [Google Scholar] [CrossRef]
- Ricci, M.S.; Zong, W.X. Chemotherapeutic approaches for targeting cell death pathways. Oncologist 2006, 11, 342–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, M.; Suzuki, R.; Sakaguchi, N.; Li, Z.; Takeda, T.; Ogihara, Y.; Jiang, B.Y.; Chen, Y. Selective induction of cell death in cancer cells by gallic acid. Biol. Pharm. Bull. 1995, 18, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Veselinović, J.B.; Kocić, G.M.; Pavic, A.; Nikodinovic-Runic, J.; Senerovic, L.; Nikolić, G.M.; Veselinović, A.M. Selected 4-phenyl hydroxycoumarins: In vitro cytotoxicity, teratogenic effect on zebrafish (Danio rerio) embryos and molecular docking study. Chem. Biol. Interact. 2015, 231, 10–17. [Google Scholar] [CrossRef]
- Lee, C.L.; Lin, Y.T.; Chang, F.R.; Chen, G.Y.; Backlund, A.; Yang, J.C.; Chen, S.L.; Wu, Y.C. Synthesis and biological evaluation of phenanthrenes as cytotoxic agents with pharmacophore modeling and ChemGPS-NP prediction as topo II inhibitors. PLoS ONE 2012, 7, e37897. [Google Scholar] [CrossRef] [Green Version]
- Hunter, K.W.; Crawford, N.P.; Alsarraj, J. Mechanisms of metastasis. Breast Cancer Res. 2008, 10 (Suppl. 1), S2. [Google Scholar] [CrossRef] [Green Version]
- Al-Mehdi, A.B.; Tozawa, K.; Fisher, A.B.; Shientag, L.; Lee, A.; Muschel, R.J. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: A new model for metastasis. Nat. Med. 2000, 6, 100–102. [Google Scholar] [CrossRef]
- Folkman, J.; Shing, Y. Angiogenesis. J. Biol. Chem. 1992, 267, 10931–10934. [Google Scholar] [CrossRef]
- Martin, T.A.; Jiang, W.G. Loss of tight junction barrier function and its role in cancer metastasis. Biochim. Biophys. Acta 2009, 1788, 872–891. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Kim, S.; Koh, H.; Yoon, S.O.; Chung, A.S.; Cho, K.S.; Chung, J. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 2001, 15, 1953–1962. [Google Scholar]
- Yang, S.X.; Polley, E.; Lipkowitz, S. New insights on PI3K/AKT pathway alterations and clinical outcomes in breast cancer. Cancer Treat. Rev. 2016, 45, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoca, M.; Becer, E.; Kabadayı, H.; Yücecan, S.; Vatansever, H.S. The effect of resveratrol and quercetin on epithelial-mesenchymal transition in pancreatic cancer stem cell. Nutr. Cancer 2020, 72, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.C.; Huang, C.C.; Lu, Y.T.; Yeh, C.M.; Ho, Y.T.; Yang, S.F.; Hsin, C.H.; Lin, C.W. Epigallocatechin-3-gallate inhibits migration of human nasopharyngeal carcinoma cells by repressing MMP-2 expression. J. Cell Physiol. 2019, 234, 20915–20924. [Google Scholar] [CrossRef] [PubMed]
- Mukund, V.; Mukund, D.; Sharma, V.; Mannarapu, M.; Alam, A. Genistein: Its role in metabolic diseases and cancer. Crit. Rev. Oncol. Hematol. 2017, 119, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Farina, H.G.; Pomies, M.; Alonso, D.F.; Gomez, D.E. Antitumor and antiangiogenic activity of soy isoflavone genistein in mouse models of melanoma and breast cancer. Oncol. Rep. 2006, 16, 885–891. [Google Scholar] [CrossRef] [Green Version]
- Shafiee, G.; Saidijam, M.; Tayebinia, H.; Khodadadi, I. Beneficial effects of genistein in suppression of proliferation, inhibition of metastasis, and induction of apoptosis in PC3 prostate cancer cells. Arch. Physiol. Biochem. 2020, 1–9. [Google Scholar] [CrossRef]
- Pang, X.; Yi, Z.; Zhang, X.; Sung, B.; Qu, W.; Lian, X.; Aggarwal, B.B.; Liu, M. Acetyl-11-keto-β-boswellic acid inhibits prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. Cancer Res. 2009, 69, 5893–5900. [Google Scholar] [CrossRef] [Green Version]
- Maluegha, D.P.; Widodo, M.A.; Pardjianto, B.; Widjajanto, E. The effects of bromelain on angiogenesis, nitric oxide, and matrix metalloproteinase-3 and-9 in rats exposed to electrical burn injury. Wound Med. 2015, 9, 5–9. [Google Scholar] [CrossRef]
- Mohr, T.; Desser, L. Plant proteolytic enzyme papain abrogates angiogenic activation of human umbilical vein endothelial cells (HUVEC) in vitro. BMC Complement. Altern Med. 2013, 13, 231. [Google Scholar] [CrossRef] [Green Version]
- Tesniere, A.; Schlemmer, F.; Boige, V.; Kepp, O.; Martins, I.; Ghiringhelli, F.; Aymeric, L.; Michaud, M.; Apetoh, L.; Barault, L.; et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2010, 29, 482–491. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.L.; Chang, J.C.; Fang, L.W.; Hsu, H.F.; Lee, L.C.; Yang, J.F.; Liang, M.T.; Hsiao, P.C.; Wang, C.P.; Wang, S.W.; et al. Bulnesia sarmientoi supercritical fluid extract exhibits necroptotic effects and anti-metastatic activity on lung cancer cells. Molecules 2018, 23, 3304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, J.E.; Kim, J.E.; Lee, S.J.; Choi, Y.J.; Jin, Y.J.; Choi, Y.W.; Choi, S.I.; Hwang, D.Y. Anti-Cancer Effects of α-Cubebenoate Derived from Schisandra chinensis in CT26 Colon Cancer Cells. Molecules 2022, 27, 737. https://doi.org/10.3390/molecules27030737
Gong JE, Kim JE, Lee SJ, Choi YJ, Jin YJ, Choi YW, Choi SI, Hwang DY. Anti-Cancer Effects of α-Cubebenoate Derived from Schisandra chinensis in CT26 Colon Cancer Cells. Molecules. 2022; 27(3):737. https://doi.org/10.3390/molecules27030737
Chicago/Turabian StyleGong, Jeong Eun, Ji Eun Kim, Su Jin Lee, Yun Ju Choi, You Jeong Jin, Young Whan Choi, Sun Il Choi, and Dae Youn Hwang. 2022. "Anti-Cancer Effects of α-Cubebenoate Derived from Schisandra chinensis in CT26 Colon Cancer Cells" Molecules 27, no. 3: 737. https://doi.org/10.3390/molecules27030737
APA StyleGong, J. E., Kim, J. E., Lee, S. J., Choi, Y. J., Jin, Y. J., Choi, Y. W., Choi, S. I., & Hwang, D. Y. (2022). Anti-Cancer Effects of α-Cubebenoate Derived from Schisandra chinensis in CT26 Colon Cancer Cells. Molecules, 27(3), 737. https://doi.org/10.3390/molecules27030737





