Laser-Induced Generation of Hydrogen in Water by Using Graphene Target
Abstract
1. Introduction
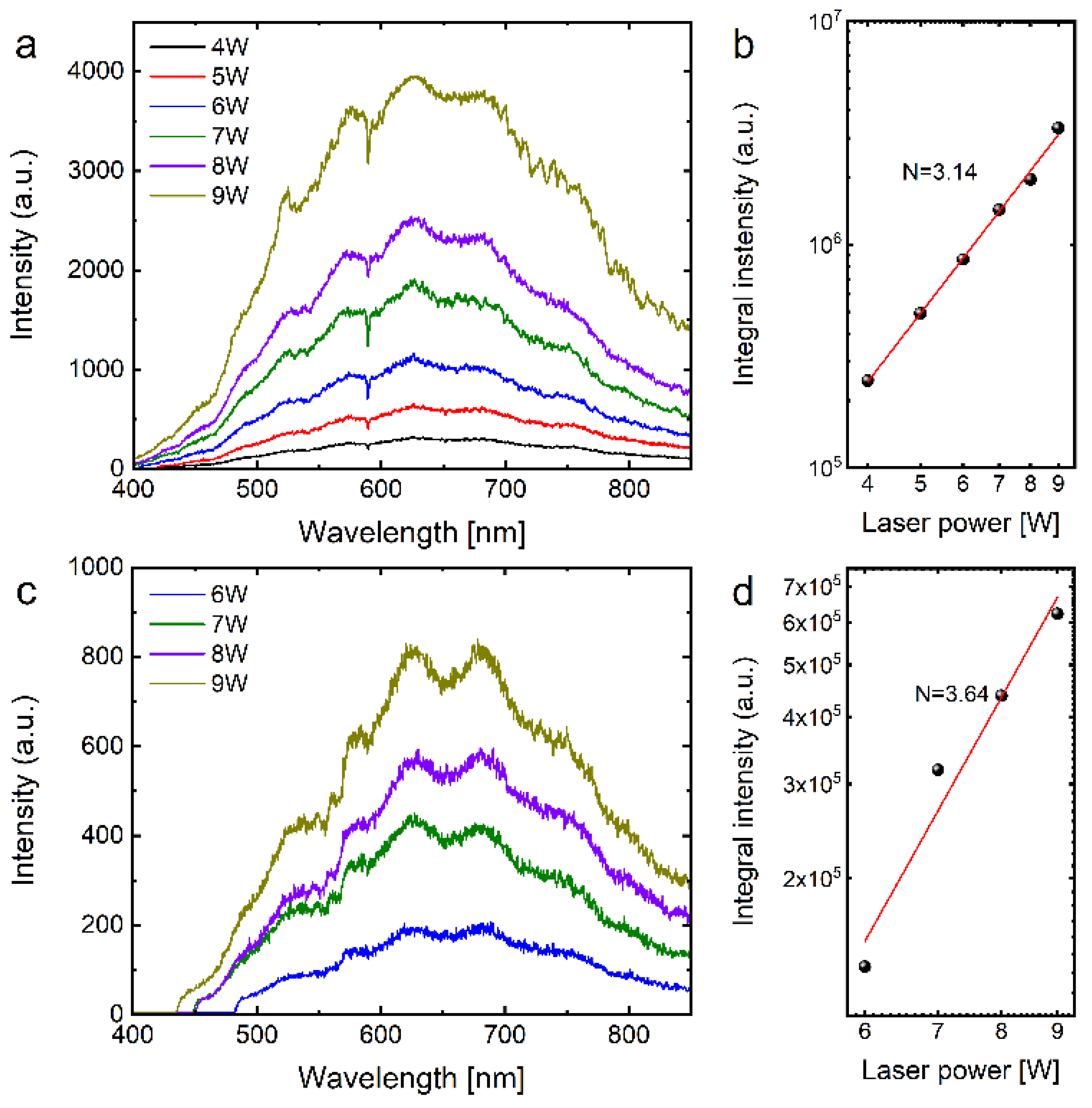
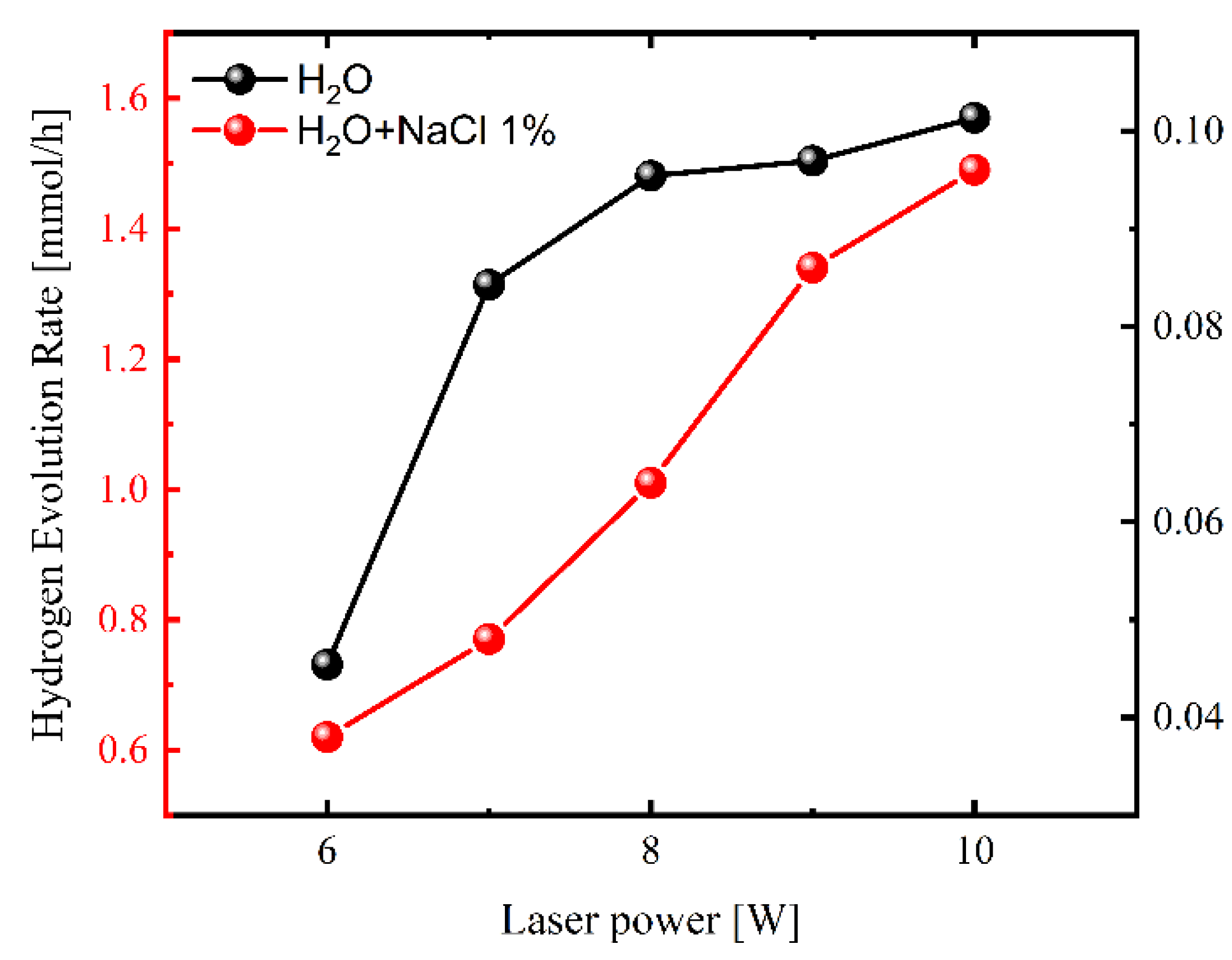
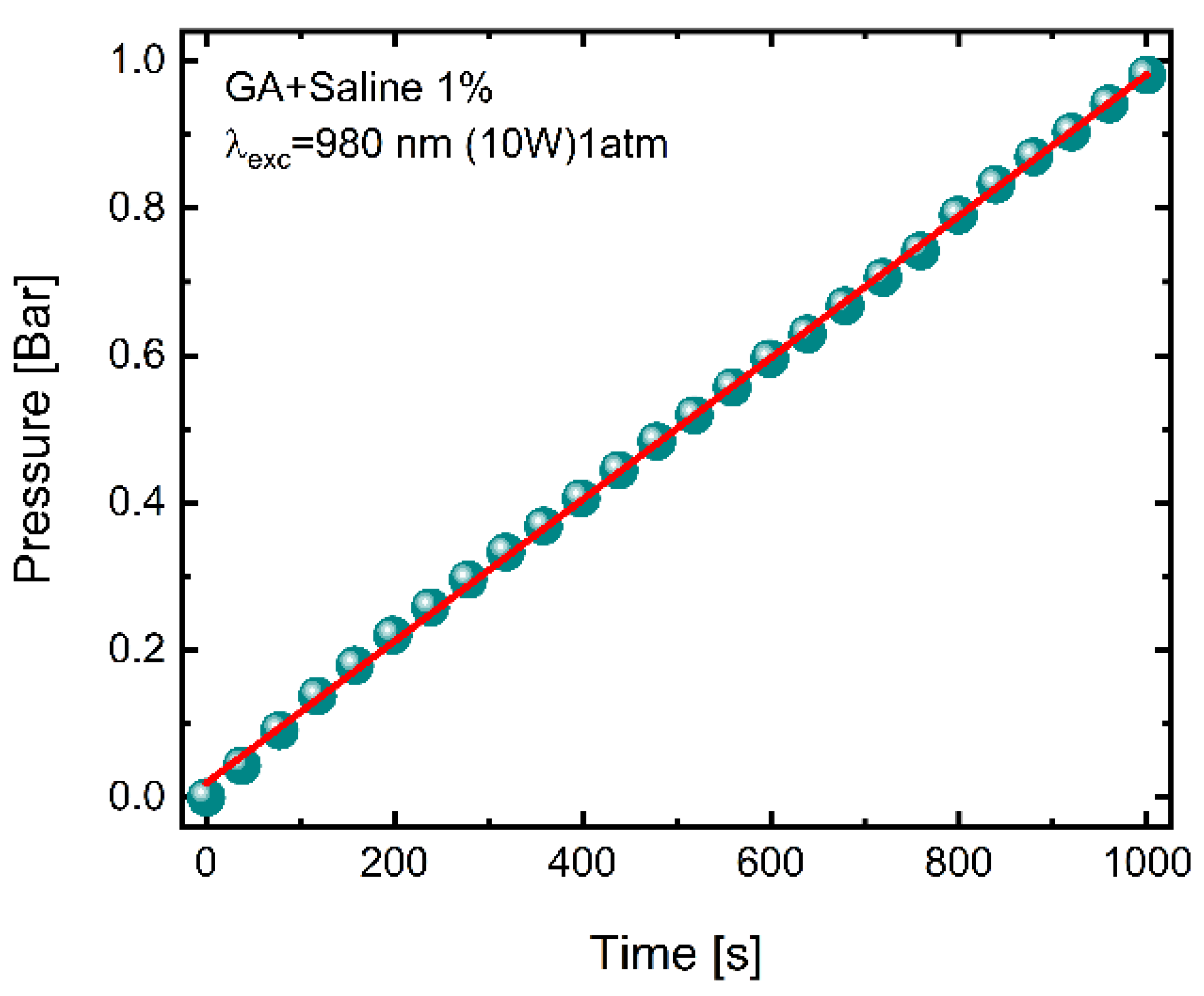
2. Results and Discussion
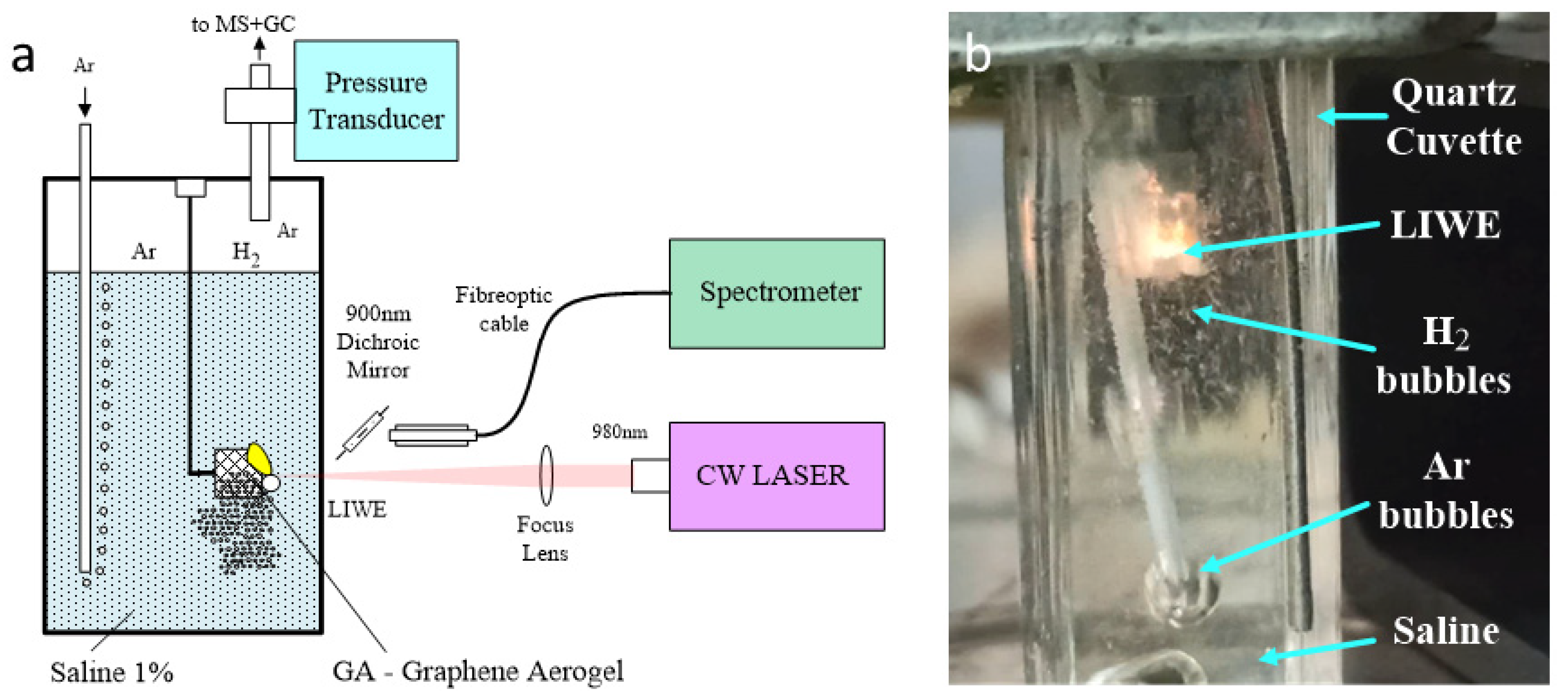
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Abuadala, A.; Dincer, I. A review on biomass-based hydrogen production and potential applications. Int. J. Energy Res. 2012, 36, 415–455. [Google Scholar] [CrossRef]
- Mujeebu, M.A. Hydrogen and syngas production by superadiabatic combustion—A review. Appl. Energy 2016, 173, 210–224. [Google Scholar] [CrossRef]
- Kalinci, Y.; Hepbasli, A.; Dincer, I. Biomass-based hydrogen production: A review and analysis. Int. J. Hydrogen Energy 2009, 34, 8799–8817. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Jafari, M.; Iranshahi, D. Progress in catalytic naphtha reforming process: A review. Appl. Energy 2013, 109, 79–93. [Google Scholar] [CrossRef]
- Trane, R.; Dahl, S.; Skjøth-Rasmussen, M.S.; Jensen, A.D. Catalytic steam reforming of bio-oil. Int. J. Hydrogen Energy 2012, 37, 6447–6472. [Google Scholar] [CrossRef]
- Iranshahi, D.; Pourazadi, E.; Paymooni, K.; Rahimpour, M.R.; Jahanmiri, A.; Moghtaderi, B. A dynamic membrane reactor concept for naphtha reforming, considering radial-flow patterns for both sweeping gas and reacting materials. Chem. Eng. J. 2011, 178, 264–275. [Google Scholar] [CrossRef]
- Ligthart, D.A.J.M.; Van Santen, R.A.; Hensen, E.J.M. Influence of particle size on the activity and stability in steam methane reforming of supported Rh nanoparticles. J. Catal. 2011, 280, 206–220. [Google Scholar] [CrossRef]
- Boyano, A.; Blanco-Marigorta, A.M.; Morosuk, T.; Tsatsaronis, G. Exergoenvironmental analysis of a steam methane reforming process for hydrogen production. Energy 2011, 36, 2202–2214. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Tan, K.F.; Borgna, A.; Saeys, M. Effect of boron on the stability of Ni catalysts during steam methane reforming. J. Catal. 2009, 261, 158–165. [Google Scholar] [CrossRef]
- Elsharnouby, O.; Hafez, H.; Nakhla, G.; El Naggar, M.H. A critical literature review on biohydrogen production by pure cultures. Int. J. Hydrogen Energy 2013, 38, 4945–4966. [Google Scholar] [CrossRef]
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrogen Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Das, D.; Veziroǧlu, T.N. Hydrogen production by biological processes: A survey of literature. Int. J. Hydrogen Energy 2001, 26, 13–28. [Google Scholar] [CrossRef]
- Burmistrz, P.; Chmielniak, T.; Czepirski, L.; Gazda-Grzywacz, M. Carbon footprint of the hydrogen production process utilizing subbituminous coal and lignite gasification. J. Clean. Prod. 2016, 139, 858–865. [Google Scholar] [CrossRef]
- Huang, J.; Dincer, I. Parametric analysis and assessment of a coal gasification plant for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 3294–3303. [Google Scholar] [CrossRef]
- Seyitoglu, S.S.; Dincer, I.; Kilicarslan, A. Energy and exergy analyses of hydrogen production by coal gasification. Int. J. Hydrogen Energy 2017, 42, 2592–2600. [Google Scholar] [CrossRef]
- Slama, R. Ben Production of Hydrogen by Electrolysis of Water: Effects of the Electrolyte Type on the Electrolysis Performances. Comput. Water Energy Environ. Eng. 2013, 2, 54–58. [Google Scholar] [CrossRef][Green Version]
- Atlam, O.; Kolhe, M. Equivalent electrical model for a proton exchange membrane (PEM) electrolyser. Energy Convers. Manag. 2011, 52, 2952–2957. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; Briguglio, N.; Brunaccini, G.; Di Blasi, A.; Stassi, A.; Ornelas, R.; Trifoni, E.; Antonucci, V.; Aricò, A.S. An electrochemical study of a PEM stack for water electrolysis. Int. J. Hydrogen Energy 2012, 37, 1939–1946. [Google Scholar] [CrossRef]
- Barbir, F. PEM electrolysis for production of hydrogen from renewable energy sources. Sol. Energy 2005, 78, 661–669. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Grubb, W.T. Batteries with Solid Ion Exchange Electrolytes.1. Secondary Cells Employing Metal Electrodes. J. Electrochem. Soc. 1959, 106, 275–278. [Google Scholar] [CrossRef]
- Xu, W.; Scott, K. The effects of ionomer content on PEM water electrolyser membrane electrode assembly performance. Int. J. Hydrogen Energy 2010, 35, 12029–12037. [Google Scholar] [CrossRef]
- Dönitz, W.; Erdle, E. High-temperature electrolysis of water vapor-status of development and perspectives for application. Int. J. Hydrogen Energy 1985, 10, 291–295. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A. Recent advances in high temperature electrolysis using solid oxide fuel cells: A review. J. Power Sources 2012, 203, 4–16. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; Kalil, M.S. A comprehensive review of microbial electrolysis cells (MEC) reactor designs and configurations for sustainable hydrogen gas production. Alex. Eng. J. 2016, 55, 427–443. [Google Scholar] [CrossRef]
- Liu, H.; Grot, S.; Logan, B.E. Electrochemically assisted microbial production of hydrogen from acetate. Environ. Sci. Technol. 2005, 39, 4317–4320. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Burnat, D.; Schlupp, M.; Wichser, A.; Lothenbach, B.; Gorbar, M.; Züttel, A.; Vogt, U.F. Composite membranes for alkaline electrolysis based on polysulfone and mineral fillers. J. Power Sources 2015, 291, 163–172. [Google Scholar] [CrossRef]
- Kawai, T.; Sakata, T. Hydrogen evolution from water using solid carbon and light energy. Nature 1979, 282, 283–284. [Google Scholar] [CrossRef]
- Akimoto, I.; Maeda, K.; Ozaki, N. Hydrogen generation by laser irradiation of carbon powder in water. J. Phys. Chem. C 2013, 117, 18281–18285. [Google Scholar] [CrossRef]
- Maeda, K.; Ozaki, N.; Akimoto, I. Alcohol additive effect in hydrogen generation from water with carbon by photochemical reaction. Jpn. J. Appl. Phys. 2014, 53, 05FZ03. [Google Scholar] [CrossRef]
- Seyitliyev, D.; Kholikov, K.; Grant, B.; San, O.; Er, A.O. Laser-induced hydrogen generation from graphite and coal. Int. J. Hydrogen Energy 2017, 42, 26277–26288. [Google Scholar] [CrossRef]
- Coughlin, R.W.; Farooque, M. Hydrogen production from coal, water and electrons. Nature 1979, 279, 301–303. [Google Scholar] [CrossRef]
- Barmina, E.V.; Simakin, A.V.; Shafeev, G.A. Hydrogen emission under laser exposure of colloidal solutions of nanoparticles. Chem. Phys. Lett. 2016, 655–656, 35–38. [Google Scholar] [CrossRef]
- Kierzkowska-Pawlak, H.; Tyczkowski, J.; Jarota, A.; Abramczyk, H. Hydrogen production in liquid water by femtosecond laser-induced plasma. Appl. Energy 2019, 247, 24–31. [Google Scholar] [CrossRef]
- Strek, W.; Mista, W.; Wiewiorski, P.; Tomala, R. Laser induced hydrogen emission from ethanol with dispersed graphene particles. Chem. Phys. Lett. 2021, 775, 138649. [Google Scholar] [CrossRef]
- Strek, W.; Wiewiórski, P.; Mista, W.; Hanulia, T.; Tomala, R. Laser-Induced Hydrogen Generation from Methanol with Graphene Aerogel as the Target. ACS Omega 2021, 6, 3711–3716. [Google Scholar] [CrossRef] [PubMed]
- Strek, W.; Cichy, B.; Radosinski, L.; Gluchowski, P.; Marciniak, L.; Lukaszewicz, M.; Hreniak, D. Laser-induced white-light emission from graphene ceramics–opening a band gap in graphene. Light Sci. Appl. 2015, 4, e237. [Google Scholar] [CrossRef]
- Strek, W.; Tomala, R.; Lukaszewicz, M.; Cichy, B.; Gerasymchuk, Y.; Gluchowski, P.; Marciniak, L.; Bednarkiewicz, A.; Hreniak, D. Laser induced white lighting of graphene foam. Sci. Rep. 2017, 7, 41281. [Google Scholar] [CrossRef] [PubMed]
- Widodo, C.S.; Sela, H.; Santosa, D.R. The effect of NaCl concentration on the ionic NaCl solutions electrical impedance value using electrochemical impedance spectroscopy methods. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; Volume 2021, p. 050003. [Google Scholar]
- Yu, Z.; Xu, J.; Meng, L.; Liu, L. Efficient hydrogen production by saline water electrolysis at high current densities without the interfering chlorine evolution. J. Mater. Chem. A 2021, 9, 22248–22253. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]

| Ar 20 mL/min H2O-Distilled Water | ||||
|---|---|---|---|---|
| Laser Power [W] | Gas Products | |||
| H2 [%] | O2 [%] | CO2 [%] | CO [%] | |
| 10.0 | 47.00 | 10.44 | 11.23 | 31.33 |
| 9.0 | 54.42 | 6.80 | 11.56 | 27.21 |
| 8.0 | 54.30 | 9.05 | 9.50 | 27.15 |
| 7.0 | 53.25 | 11.83 | 11.24 | 23.67 |
| 6.0 | 55.56 | 7.94 | 12.70 | 23.81 |
| Ar 20 mL/min H2O + 1% NaCl | ||||
|---|---|---|---|---|
| Laser Power [W] | Gas Products | |||
| H2 [%] | O2 [%] | CO2 [%] | CO [%] | |
| 10.0 | 79.95 | 8.13 | 3.66 | 8.27 |
| 9.0 | 78.99 | 8.52 | 4.27 | 8.21 |
| 8.0 | 78.48 | 8.43 | 4.85 | 8.23 |
| 7.0 | 78.21 | 8.93 | 5.10 | 7.75 |
| 6.0 | 77.81 | 9.07 | 5.42 | 7.70 |
| 5.5 | 80.91 | 8.17 | 4.84 | 6.08 |
| 5.0 | 59.21 | 14.47 | 6.58 | 19.74 |
| 4.5 | 57.14 | 22.86 | 8.57 | 11.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strek, W.; Wiewiórski, P.; Miśta, W.; Tomala, R.; Stefanski, M. Laser-Induced Generation of Hydrogen in Water by Using Graphene Target. Molecules 2022, 27, 718. https://doi.org/10.3390/molecules27030718
Strek W, Wiewiórski P, Miśta W, Tomala R, Stefanski M. Laser-Induced Generation of Hydrogen in Water by Using Graphene Target. Molecules. 2022; 27(3):718. https://doi.org/10.3390/molecules27030718
Chicago/Turabian StyleStrek, Wieslaw, Przemysław Wiewiórski, Włodzimierz Miśta, Robert Tomala, and Mariusz Stefanski. 2022. "Laser-Induced Generation of Hydrogen in Water by Using Graphene Target" Molecules 27, no. 3: 718. https://doi.org/10.3390/molecules27030718
APA StyleStrek, W., Wiewiórski, P., Miśta, W., Tomala, R., & Stefanski, M. (2022). Laser-Induced Generation of Hydrogen in Water by Using Graphene Target. Molecules, 27(3), 718. https://doi.org/10.3390/molecules27030718







