A Review of the Effects of Olive Oil-Cooking on Phenolic Compounds
Abstract
1. Introduction
2. Phenolic Compounds Classification
3. Origin and Functions of Phenolic Compounds
4. Are the Phenolic Compounds Influenced by Cooking?
4.1. Non-Olive Oils
4.2. Effects of Oil Heating without Food
4.2.1. Microwaving
4.2.2. Pan-Frying
4.2.3. Boiling
4.2.4. Air Baking
4.3. Effects of Oil Heating with Vegetables
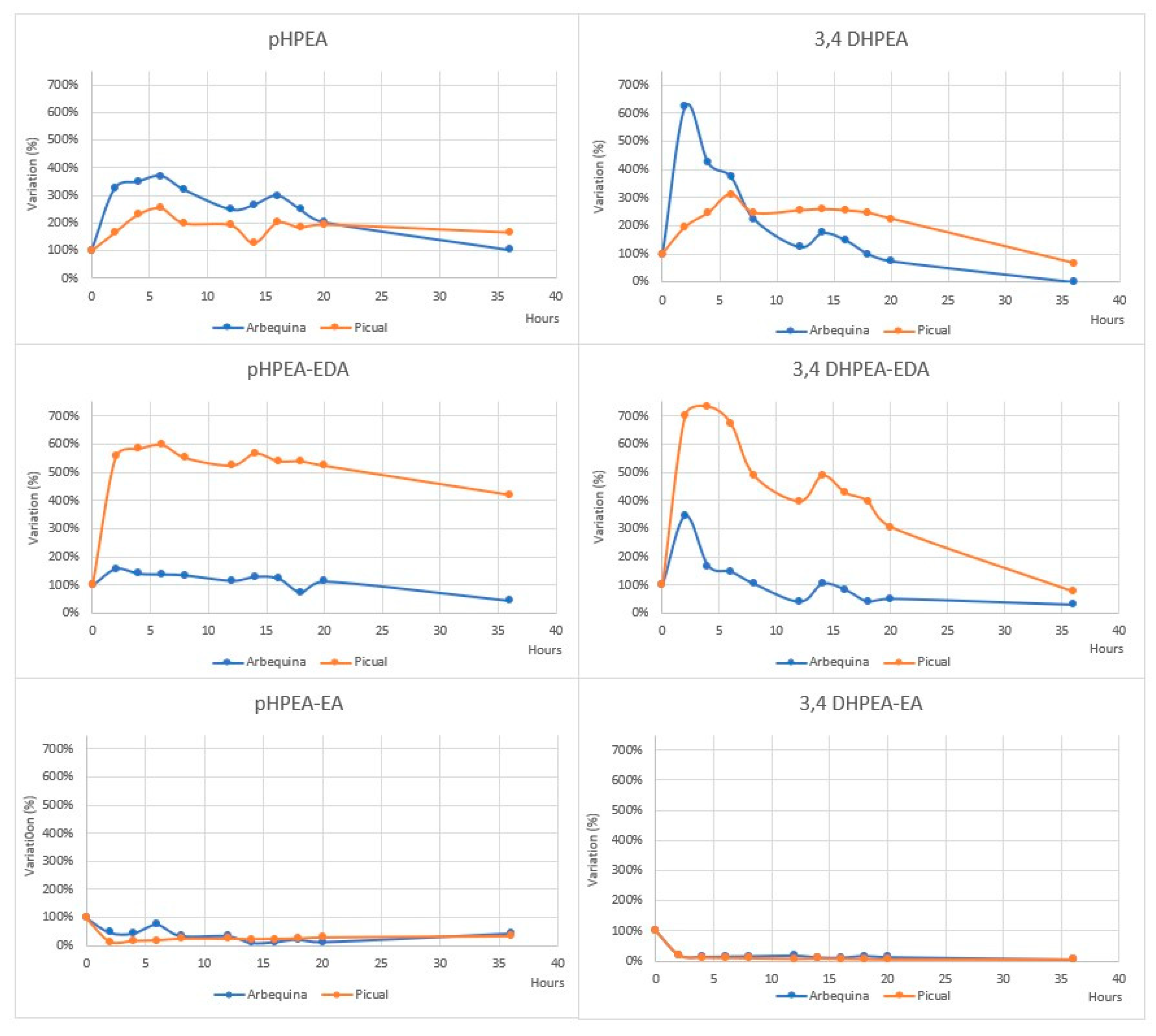
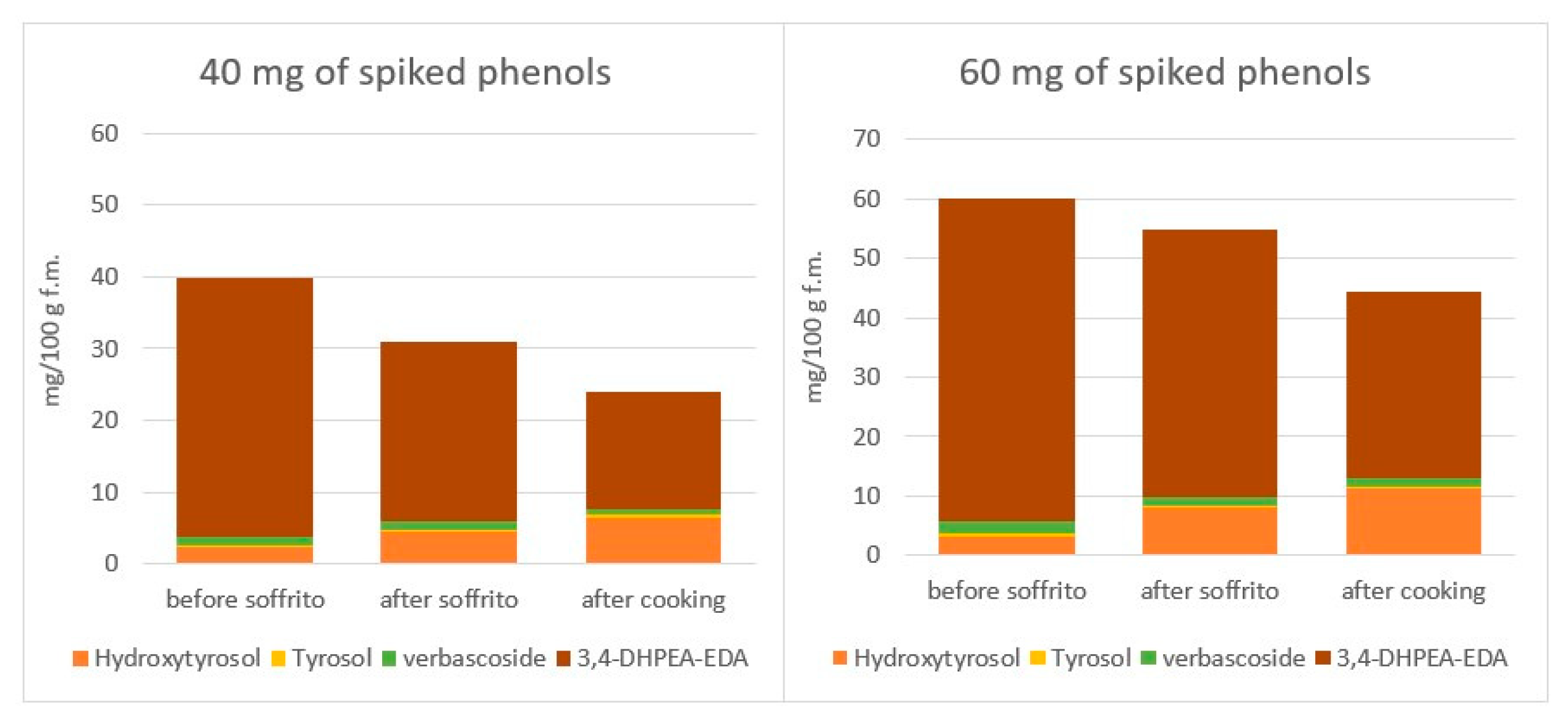
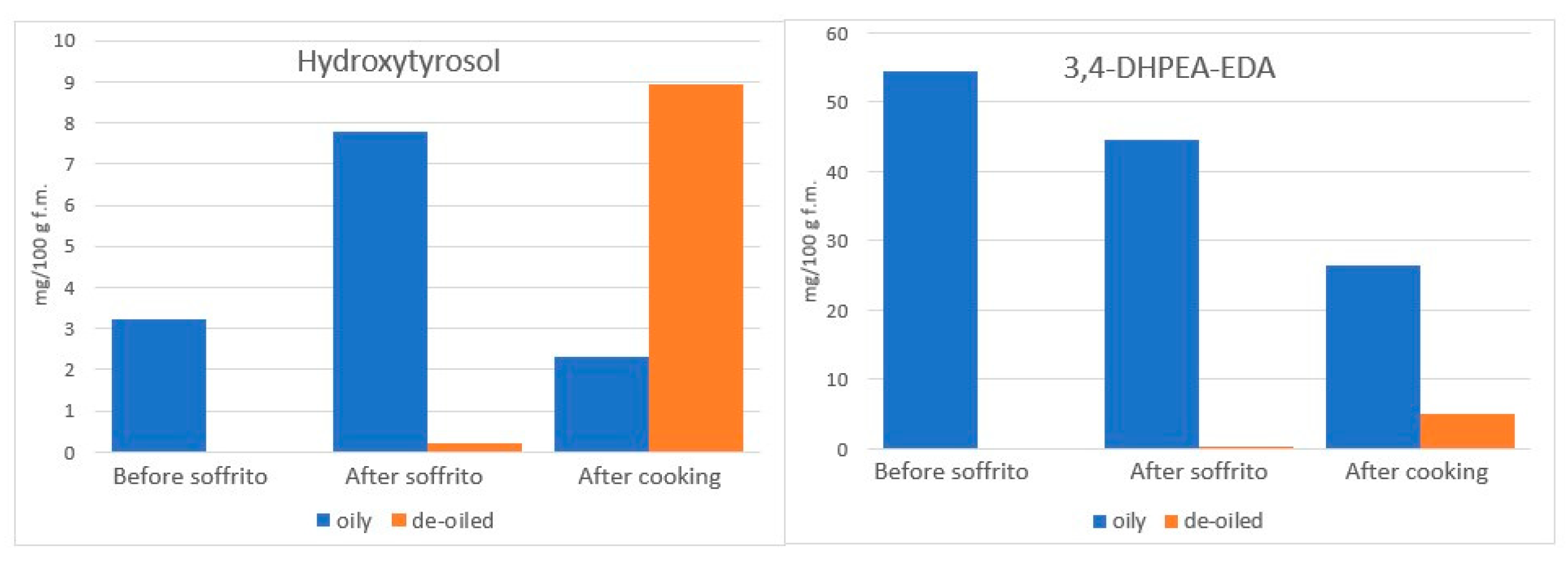
4.3.1. The Fate of Phenolic Compounds of EVOO during Cooking
4.3.2. The Incorporation of Phenolic Compounds of Vegetables in EVOO during Cooking
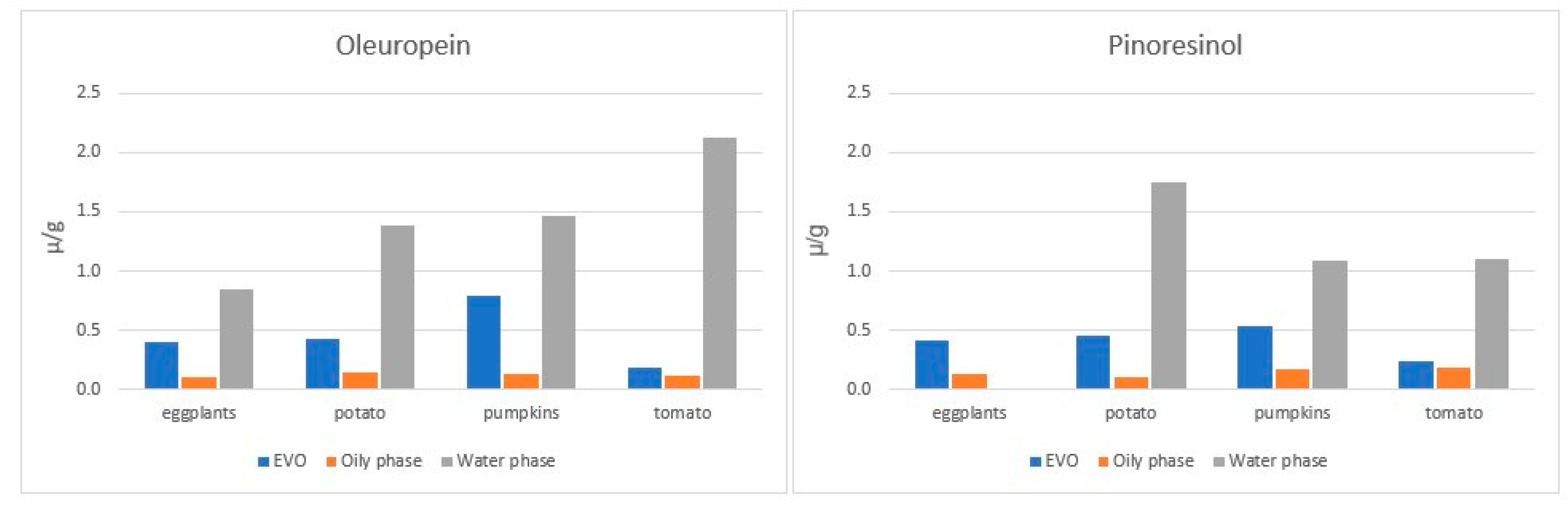
4.3.3. The Incorporation of EVOO Phenolic Compounds in Vegetables during Cooking
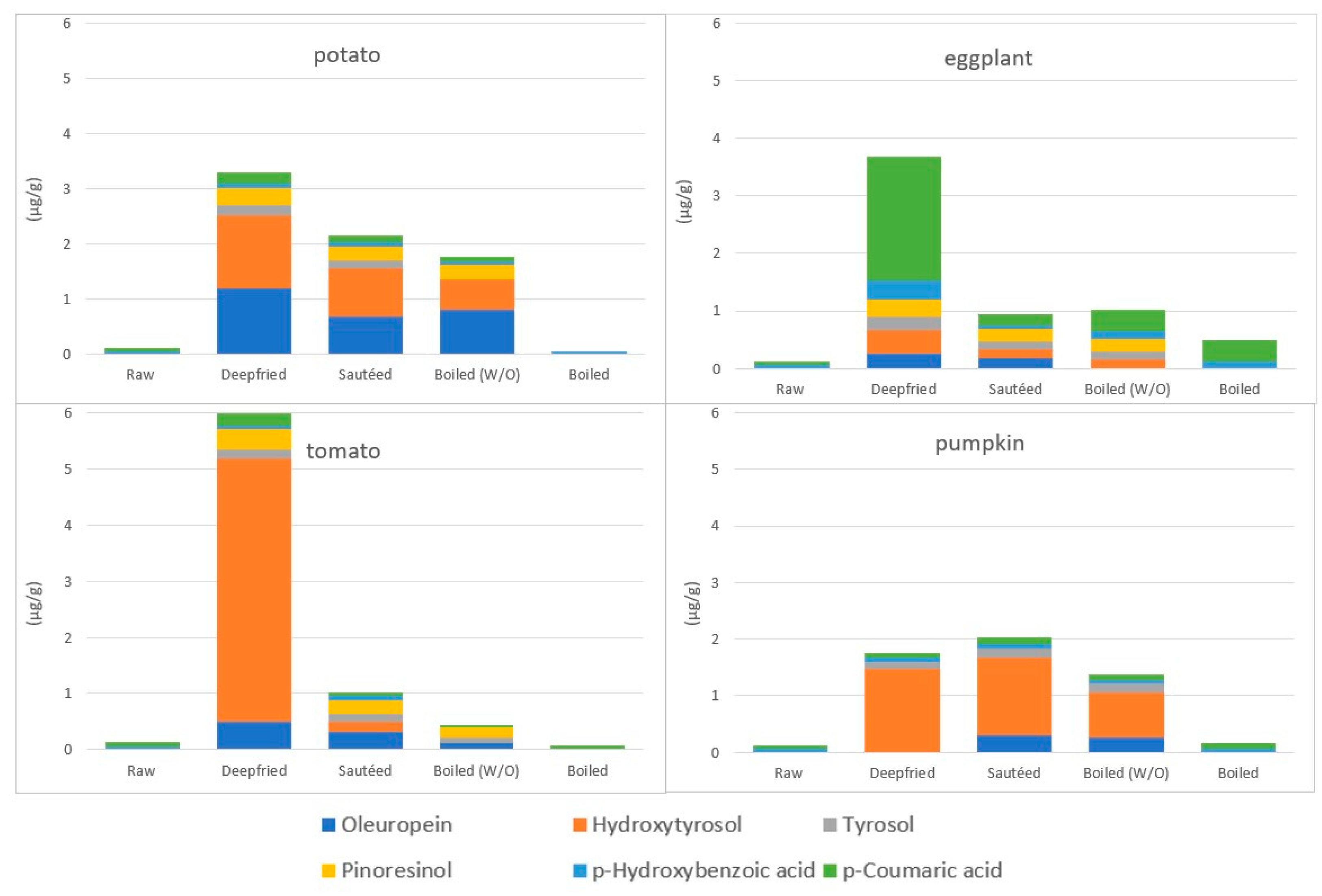
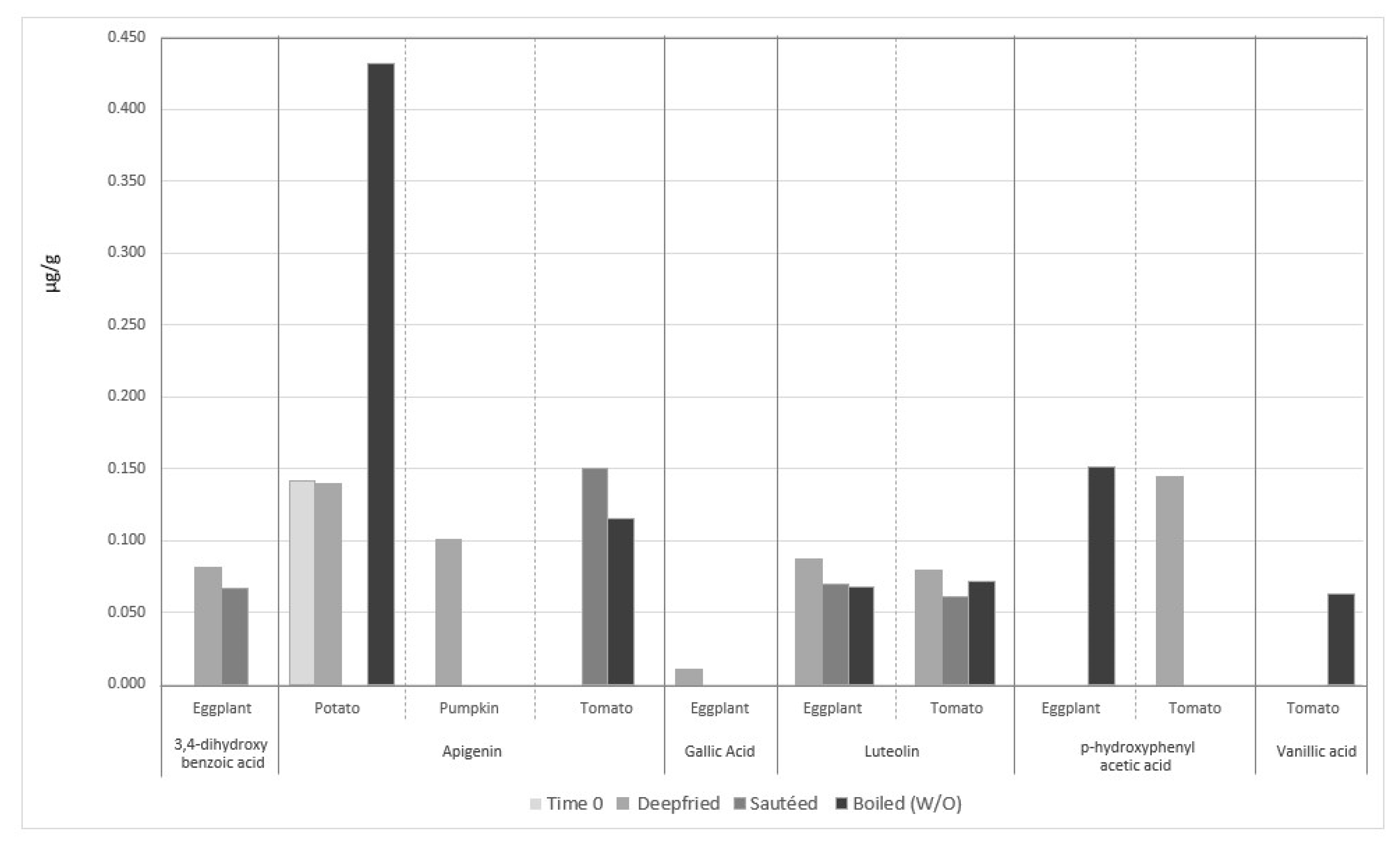
4.3.4. The Fate of Phenolic Compounds of Vegetables during EVOO-Cooking
4.4. Changes in Bioaccessibility and Bioavailability of Vegetable PC Following Cooking with Oil
4.4.1. Bioaccessibility
4.4.2. Bioavailability
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bastida, S.; Sánchez-Muniz, F.J. Chapter 21—Frying: A Cultural Way of Cooking in the Mediterranean Diet. In The Mediterranean Diet; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 217–234. ISBN 978-0-12-407849-9. [Google Scholar]
- Mesias, M.; Delgado-Andrade, C.; Morales, F.J. Evaluation of domestic frying habits and consumer’s preferences in Spanish households. SDRP J. Food Sci. Technol. 2018, 3, 450–459. [Google Scholar] [CrossRef][Green Version]
- Hosseini, H.; Ghorbani, M.; Meshginfar, N.; Mahoonak, A.S. A Review on Frying: Procedure, Fat, Deterioration Progress and Health Hazards. J. Am. Oil Chem. Soc. 2016, 93, 445–466. [Google Scholar] [CrossRef]
- Ng, C.-Y.; Leong, X.-F.; Masbah, N.; Adam, S.K.; Kamisah, Y.; Jaarin, K. Heated vegetable oils and cardiovascular disease risk factors. Vascul. Pharmacol. 2014, 61, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cinquanta, L.; Esti, M.; Di Matteo, M. Oxidative stability of virgin olive oils. J. Am. Oil Chem. Soc. 2001, 78, 1197. [Google Scholar] [CrossRef]
- Napolitano, A.; Morales, F.; Sacchi, R.; Fogliano, V. Relationship between Virgin Olive Oil Phenolic Compounds and Acrylamide Formation in Fried Crisps. J. Agric. Food Chem. 2008, 56, 2034–2040. [Google Scholar] [CrossRef]
- Murador, D.; Braga, A.R.; Da Cunha, D.; De Rosso, V. Alterations in phenolic compound levels and antioxidant activity in response to cooking technique effects: A meta-analytic investigation. Crit. Rev. Food Sci. Nutr. 2018, 58, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Chiou, A.; Kalogeropoulos, N. Virgin Olive Oil as Frying Oil. Compr. Rev. Food Sci. Food Saf. 2017, 16, 632–646. [Google Scholar] [CrossRef]
- Varela, G.; Ruiz-Rosso, B. Frying process in the relation fat/degenerative diseases. Grasas Aceites 1998, 49, 359–365. [Google Scholar] [CrossRef]
- Garcimartín, A.; Macho-González, A.; Caso, G.; Benedí, J.; Bastida, S.; Sánchez-Muniz, F.J. Chapter 19—Frying a cultural way of cooking in the Mediterranean diet and how to obtain improved fried foods. In The Mediterranean Diet, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2020; pp. 191–207. ISBN 978-0-12-818649-7. [Google Scholar]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Chapter 12—Phenolic Compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Carrillo-Lopez, A., Eds.; Woodhead Publishing: Cambridge, England, 2019; pp. 253–271. ISBN 978-0-12-813278-4. [Google Scholar]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2—Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Cambridge, England, 2019; pp. 33–50. ISBN 978-0-12-814774-0. [Google Scholar]
- Swain, T.; Bate-Smith, E.C. Flavonoid compounds. Comp. Biochem. 1962, Volume III, 755–809. [Google Scholar]
- Basli, A.; Belkacem, N.; Amrani, I. Health Benefits of Phenolic Compounds Against Cancers. In Phenolic Compounds-Biological Activity; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Tsimogiannis, D.; Oreopoulou, V. Classification of Phenolic Compounds in Plants. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 263–284. [Google Scholar]
- Romero, C.; Brenes, M. Analysis of Total Contents of Hydroxytyrosol and Tyrosol in Olive Oils. J. Agric. Food Chem. 2012, 60, 9017–9022. [Google Scholar] [CrossRef]
- Birt, D.F.; Jeffery, E. Flavonoids. Adv. Nutr. 2013, 4, 576–577. [Google Scholar] [CrossRef]
- López-Biedma, A.; Sánchez-Quesada, C.; Delgado-Rodríguez, M.; Gaforio, J.J. The biological activities of natural lignans from olives and virgin olive oils: A review. J. Funct. Foods 2016, 26, 36–47. [Google Scholar] [CrossRef]
- Luque-Muñoz, A.; Tapia, R.; Haidour, A.; Justicia, J.; Cuerva, J.M. Direct determination of phenolic secoiridoids in olive oil by ultra-high performance liquid chromatography-triple quadruple mass spectrometry analysis. Sci. Rep. 2019, 9, 15545. [Google Scholar] [CrossRef]
- Knaggs, A.R. The biosynthesis of shikimate metabolites. Nat. Prod. Rep. 2003, 20, 119–136. [Google Scholar] [CrossRef]
- Fraser, C.M.; Chapple, C. The phenylpropanoid pathway in Arabidopsis. Arab. B 2011, 9, e0152. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Ávila-Román, J.; Soliz-Rueda, J.R.; Bravo, F.I.; Aragonès, G.; Suárez, M.; Arola-Arnal, A.; Mulero, M.; Salvadó, M.-J.; Arola, L.; Torres-Fuentes, C.; et al. Phenolic compounds and biological rhythms: Who takes the lead? Trends Food Sci. Technol. 2021, 113, 77–85. [Google Scholar] [CrossRef]
- Fernandez-Panchon, M.S.; Villano, D.; Troncoso, A.M.; Garcia-Parrilla, M.C. Antioxidant Activity of Phenolic Compounds: From In Vitro Results to In Vivo Evidence. Crit. Rev. Food Sci. Nutr. 2008, 48, 649–671. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Martin-Cabrejas, M.A.; González de Mejia, E. Phenolic compounds in fruits and beverages consumed as part of the mediterranean diet: Their role in prevention of chronic diseases. Phytochem. Rev. 2016, 15, 405–423. [Google Scholar] [CrossRef]
- Ambra, R.; Lucchetti, S.; Pastore, G. The health benefits of oleocanthal and other oil phenols. In Handbook of olive Oil: Phenolic Compounds, Production and Helath Benefits; Nova Science Publisher: New York, NY, USA, 2017; pp. 215–236. ISBN 978-1-53612-356-2. [Google Scholar]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed]
- Anter, J.; Tasset, I.; Demyda-Peyrás, S.; Ranchal, I.; Moreno-Millán, M.; Romero-Jimenez, M.; Muntané, J.; Luque de Castro, M.D.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Evaluation of potential antigenotoxic, cytotoxic and proapoptotic effects of the olive oil by-product “alperujo”, hydroxytyrosol, tyrosol and verbascoside. Mutat. Res. Toxicol. Environ. Mutagen. 2014, 772, 25–33. [Google Scholar] [CrossRef]
- Gheena, S.; Ezhilarasan, D. Syringic acid triggers reactive oxygen species–mediated cytotoxicity in HepG2 cells. Hum. Exp. Toxicol. 2019, 38, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Celano, M.; Maggisano, V.; Lepore, S.M.; Russo, D.; Bulotta, S. Secoiridoids of olive and derivatives as potential coadjuvant drugs in cancer: A critical analysis of experimental studies. Pharmacol. Res. 2019, 142, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.K.; Keast, R.S.J.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.-H.; Smith, A.B.; Breslin, P.A.S. Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Sharma, S.H.; Rajamanickam, V.; Nagarajan, S. Antiproliferative effect of p-Coumaric acid targets UPR activation by downregulating Grp78 in colon cancer. Chem. Biol. Interact. 2018, 291, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.W.; Mahesh, R.; Lee, J.G.; Lee, S.H.; Kim, Y.S.; Park, Y.-K. Pinoresinol from the fruits of Forsythia koreana inhibits inflammatory responses in LPS-activated microglia. Neurosci. Lett. 2010, 480, 215–220. [Google Scholar] [CrossRef]
- Lee, J.Y.; Woo, E.-R.; Kang, K.W. Inhibition of lipopolysaccharide-inducible nitric oxide synthase expression by acteoside through blocking of AP-1 activation. J. Ethnopharmacol. 2005, 97, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Gholami, M.H.; Zabolian, A.; Saleki, H.; Farahani, M.V.; Hamzehlou, S.; Far, F.B.; Sharifzadeh, S.O.; Samarghandian, S.; Khan, H.; et al. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: New hope in the fight against cancer. Pharmacol. Res. 2021, 171, 105759. [Google Scholar] [CrossRef]
- López-Biedma, A.; Sánchez-Quesada, C.; Beltrán, G.; Delgado-Rodríguez, M.; Gaforio, J.J. Phytoestrogen (+)-pinoresinol exerts antitumor activity in breast cancer cells with different oestrogen receptor statuses. BMC Complement. Altern. Med. 2016, 16, 350. [Google Scholar] [CrossRef]
- Cheng, Y.; Han, X.; Mo, F.; Zeng, H.; Zhao, Y.; Wang, H.; Zheng, Y.; Ma, X. Apigenin inhibits the growth of colorectal cancer through down-regulation of E2F1/3 by miRNA-215-5p. Phytomedicine 2021, 89, 153603. [Google Scholar] [CrossRef]
- El-Asfar, R.K.; El-Derany, M.O.; Sallam, A.-A.M.; Wahdan, S.A.; El-Demerdash, E.; Sayed, S.A.; El-Mesallamy, H.O. Luteolin mitigates tamoxifen-associated fatty liver and cognitive impairment in rats by modulating beta-catenin. Eur. J. Pharmacol. 2021, 908, 174337. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Nwozo, S.O.; Arunsi, U.O.; Oyelere, A.K.; Odunola, O.A. Co-administration of Luteolin mitigated toxicity in rats’ lungs associated with doxorubicin treatment. Toxicol. Appl. Pharmacol. 2021, 411, 115380. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Wu, S.; Li, B.; Tan, J.; Yan, J.; Wang, Y.; Tang, Z.; Liu, M.; Fu, C.; Zhang, H.; et al. Dietary ferulic acid and vanillic acid on inflammation, gut barrier function and growth performance in lipopolysaccharide-challenged piglets. Anim. Nutr. 2021, 8, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, L.; Wang, X.; Wu, W.; Qin, R. Effect of Syringic acid on antioxidant biomarkers and associated inflammatory markers in mice model of asthma. Drug Dev. Res. 2019, 80, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Lende, A.B.; Kshirsagar, A.D.; Deshpande, A.D.; Muley, M.M.; Patil, R.R.; Bafna, P.A.; Naik, S.R. Anti-inflammatory and analgesic activity of protocatechuic acid in rats and mice. Inflammopharmacology 2011, 19, 255. [Google Scholar] [CrossRef]
- Sánchez-Muniz, F.J.; Bastida, S.; Márquez-Ruiz, G.; Dobarganes, C. Effect of heating and frying on oil and food fatty acids. In Fatty Acids in Foods and Their Health Implications; CRC Press: Boca Raton, FL, USA, 2007; pp. 525–558. ISBN 0429127553. [Google Scholar]
- Nogueira-de-Almeida, C.A.; Castro, G.A. Effects of heat treatment by immersion in household conditions on olive oil as compared to other culinary oils: A descriptive study. Int. J. Food Stud. 2018, 7, 89–99. [Google Scholar] [CrossRef]
- Casal, S.; Malheiro, R.; Sendas, A.; Oliveira, B.P.P.; Pereira, J.A. Olive oil stability under deep-frying conditions. Food Chem. Toxicol. 2010, 48, 2972–2979. [Google Scholar] [CrossRef]
- Chiou, A.; Salta, F.N.; Kalogeropoulos, N.; Mylona, A.; Ntalla, I.; Andrikopoulos, N.K. Retention and Distribution of Polyphenols after Pan-Frying of French Fries in Oils Enriched with Olive Leaf Extract. J. Food Sci. 2007, 72, S574–S584. [Google Scholar] [CrossRef]
- Chiou, A.; Kalogeropoulos, N.; Salta, F.N.; Efstathiou, P.; Andrikopoulos, N.K. Pan-frying of French fries in three different edible oils enriched with olive leaf extract: Oxidative stability and fate of microconstituents. LWT—Food Sci. Technol. 2009, 42, 1090–1097. [Google Scholar] [CrossRef]
- Chiou, A.; Kalogeropoulos, N.; Efstathiou, P.; Papoutsi, M.; Andrikopoulos, N.K. French Fries oleuropein content during the successive deep frying in oils enriched with an olive leaf extract. Int. J. Food Sci. Technol. 2013, 48, 1165–1171. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Mylona, A.; Chiou, A.; Ioannou, M.S.; Andrikopoulos, N.K. Retention and distribution of natural antioxidants (α-tocopherol, polyphenols and terpenic acids) after shallow frying of vegetables in virgin olive oil. LWT—Food Sci. Technol. 2007, 40, 1008–1017. [Google Scholar] [CrossRef]
- Santos, C.S.P.; Cunha, S.C.; Casal, S. Deep or air frying? A comparative study with different vegetable oils. Eur. J. Lipid Sci. Technol. 2017, 119, 1600375. [Google Scholar] [CrossRef]
- Goulas, V.; Orphanides, A.; Pelava, E.; Gekas, V. Impact of Thermal Processing Methods on Polyphenols and Antioxidant Activity of Olive Oil Polar Fraction. J. Food Process. Preserv. 2015, 39, 1919–1924. [Google Scholar] [CrossRef]
- Campanella, L.; Nuccilli, A.; Tomassetti, M.; Vecchio, S. Biosensor analysis for the kinetic study of polyphenols deterioration during the forced thermal oxidation of extra-virgin olive oil. Talanta 2008, 74, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Amati, L.; Campanella, L.; Dragone, R.; Nuccilli, A.; Tomassetti, M.; Vecchio, S. New Investigation of the Isothermal Oxidation of Extra Virgin Olive Oil: Determination of Free Radicals, Total Polyphenols, Total Antioxidant Capacity, and Kinetic Data. J. Agric. Food Chem. 2008, 56, 8287–8295. [Google Scholar] [CrossRef]
- Kishimoto, N. Microwave heating induces oxidative degradation of extra virgin olive oil. Food Sci. Technol. Res. 2019, 25, 75–79. [Google Scholar] [CrossRef]
- Kamvissis, V.N.; Barbounis, E.G.; Megoulas, N.C.; Koupparis, M.A. A Novel Photometric Method for Evaluation of the Oxidative Stability of Virgin Olive Oils. J. AOAC Int. 2008, 91, 794–801. [Google Scholar] [CrossRef]
- Attya, M.; Benabdelkamel, H.; Perri, E.; Russo, A.; Sindona, G. Effects of Conventional Heating on the Stability of Major Olive Oil Phenolic Compounds by Tandem Mass Spectrometry and Isotope Dilution Assay. Molecules 2010, 15, 8734–8746. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Lercker, G.; Fernández-Gutiérrez, A. Evaluation of the Influence of Thermal Oxidation on the Phenolic Composition and on the Antioxidant Activity of Extra-Virgin Olive Oils. J. Agric. Food Chem. 2007, 55, 4771–4780. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, D.; Kefi, G.; Kotsiou, K.; Tasioula-Margari, M. Evaluation of phenolic compounds degradation in virgin olive oil during storage and heating. J. Food Nutr. Res. 2009, 48, 31–41. [Google Scholar]
- Brenes, M.; García, A.; Dobarganes, M.C.; Velasco, J.; Romero, C. Influence of Thermal Treatments Simulating Cooking Processes on the Polyphenol Content in Virgin Olive Oil. J. Agric. Food Chem. 2002, 50, 5962–5967. [Google Scholar] [CrossRef] [PubMed]
- Allouche, Y.; Jiménez, A.; Gaforio, J.J.; Uceda, M.; Beltrán, G. How Heating Affects Extra Virgin Olive Oil Quality Indexes and Chemical Composition. J. Agric. Food Chem. 2007, 55, 9646–9654. [Google Scholar] [CrossRef]
- Gutiérrez, F.; Arnaud, T.; Garrido, A. Contribution of polyphenols to the oxidative stability of virgin olive oil. J. Sci. Food Agric. 2001, 81, 1463–1470. [Google Scholar] [CrossRef]
- Pellegrini, N.; Visioli, F.; Buratti, S.; Brighenti, F. Direct Analysis of Total Antioxidant Activity of Olive Oil and Studies on the Influence of Heating. J. Agric. Food Chem. 2001, 49, 2532–2538. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M.B.T.-M. In E. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Oxidants and Antioxidants Part A; Academic Press: San Diego, CA, USA, 1999; Volume 299, pp. 152–178. ISBN 0076-6879. [Google Scholar]
- Papadopoulos, G.; Boskou, D. Antioxidant effect of natural phenols on olive oil. J. Am. Oil Chem. Soc. 1991, 68, 669–671. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Veneziani, G.; Urbani, S.; Taticchi, A.; Di Maio, I.; Selvaggini, R.; Sordini, B.; Montedoro, G. Improvement of bioactive phenol content in virgin olive oil with an olive-vegetation water concentrate produced by membrane treatment. Food Chem. 2011, 124, 1308–1315. [Google Scholar] [CrossRef]
- Esposto, S.; Taticchi, A.; Di Maio, I.; Urbani, S.; Veneziani, G.; Selvaggini, R.; Sordini, B.; Servili, M. Effect of an olive phenolic extract on the quality of vegetable oils during frying. Food Chem. 2015, 176, 184–192. [Google Scholar] [CrossRef]
- Di Maio, I.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Veneziani, G.; Urbani, S.; Servili, M. HPLC–ESI-MS investigation of tyrosol and hydroxytyrosol oxidation products in virgin olive oil. Food Chem. 2011, 125, 21–28. [Google Scholar] [CrossRef]
- Cerretani, L.; Bendini, A.; Rodriguez-Estrada, M.T.; Vittadini, E.; Chiavaro, E. Microwave heating of different commercial categories of olive oil: Part I. Effect on chemical oxidative stability indices and phenolic compounds. Food Chem. 2009, 115, 1381–1388. [Google Scholar] [CrossRef]
- Lozano-Castellón, J.; Vallverdú-Queralt, A.; Rinaldi de Alvarenga, J.F.; Illán, M.; Torrado-Prat, X.; Lamuela-Raventós, R.M. Domestic sautéing with EVOO: Change in the phenolic profile. Antioxidants 2020, 9, 77. [Google Scholar] [CrossRef]
- Silva, L.; Garcia, B.; Paiva-Martins, F. Oxidative stability of olive oil and its polyphenolic compounds after boiling vegetable process. LWT—Food Sci. Technol. 2010, 43, 1336–1344. [Google Scholar] [CrossRef]
- Brkić Bubola, K.; Klisović, D.; Lukić, I.; Novoselić, A. Vegetable species significantly affects the phenolic composition and oxidative stability of extra virgin olive oil used for roasting. LWT 2020, 129, 109628. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; Fregapane, G.; Salvador, M.D.; Gordon, M.H. Changes in Phenolic Composition and Antioxidant Activity of Virgin Olive Oil during Frying. J. Agric. Food Chem. 2003, 51, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Montedoro, G.; Servili, M.; Baldioli, M.; Miniati, E. Simple and hydrolyzable phenolic compounds in virgin olive oil. 2. Initial characterization of the hydrolyzable fraction. J. Agric. Food Chem. 1992, 40, 1577–1580. [Google Scholar] [CrossRef]
- Baldioli, M.; Servili, M.; Perretti, G.; Montedoro, G.F. Antioxidant activity of tocopherols and phenolic compounds of virgin olive oil. J. Am. Oil Chem. Soc. 1996, 73, 1589–1593. [Google Scholar] [CrossRef]
- Olivero-David, R.; Mena, C.; Pérez-Jimenez, M.A.; Sastre, B.; Bastida, S.; Márquez-Ruiz, G.; Sánchez-Muniz, F.J. Influence of Picual Olive Ripening on Virgin Olive Oil Alteration and Stability during Potato Frying. J. Agric. Food Chem. 2014, 62, 11637–11646. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Gordon, M.H. Interactions of Ferric Ions with Olive Oil Phenolic Compounds. J. Agric. Food Chem. 2005, 53, 2704–2709. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Anaya, J.D.P.; Castañeda-Saucedo, M.C.; Olalla-Herrera, M.; Villalón-Mir, M.; de la Serrana, H.L.-G.; Samaniego-Sánchez, C. Changes in the Antioxidant Properties of Extra Virgin Olive Oil after Cooking Typical Mediterranean Vegetables. Antioxidants 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Zembyla, M.; Murray, B.S.; Radford, S.J.; Sarkar, A. Water-in-oil Pickering emulsions stabilized by an interfacial complex of water-insoluble polyphenol crystals and protein. J. Colloid Interface Sci. 2019, 548, 88–99. [Google Scholar] [CrossRef] [PubMed]
- de Alvarenga, J.F.; Quifer-Rada, P.; Francetto Juliano, F.; Hurtado-Barroso, S.; Illan, M.; Torrado-Prat, X.; Lamuela-Raventós, R.M. Using Extra Virgin Olive Oil to Cook Vegetables Enhances Polyphenol and Carotenoid Extractability: A Study Applying the sofrito Technique. Molecules 2019, 24, 1555. [Google Scholar] [CrossRef] [PubMed]
- Taticchi, A.; Esposto, S.; Urbani, S.; Veneziani, G.; Selvaggini, R.; Sordini, B.; Servili, M. Effect of an olive phenolic extract added to the oily phase of a tomato sauce, on the preservation of phenols and carotenoids during domestic cooking. LWT 2017, 84, 572–578. [Google Scholar] [CrossRef]
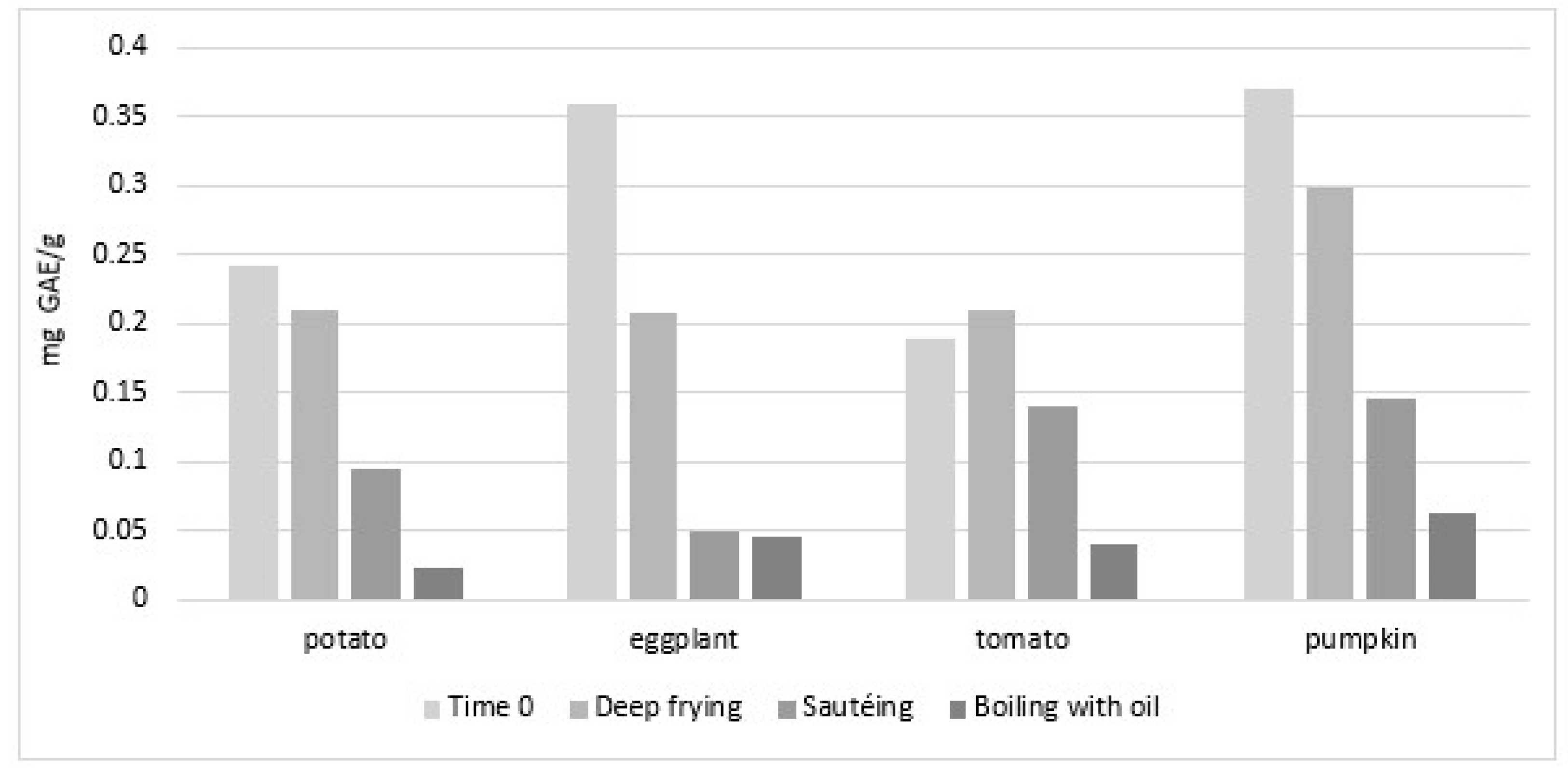
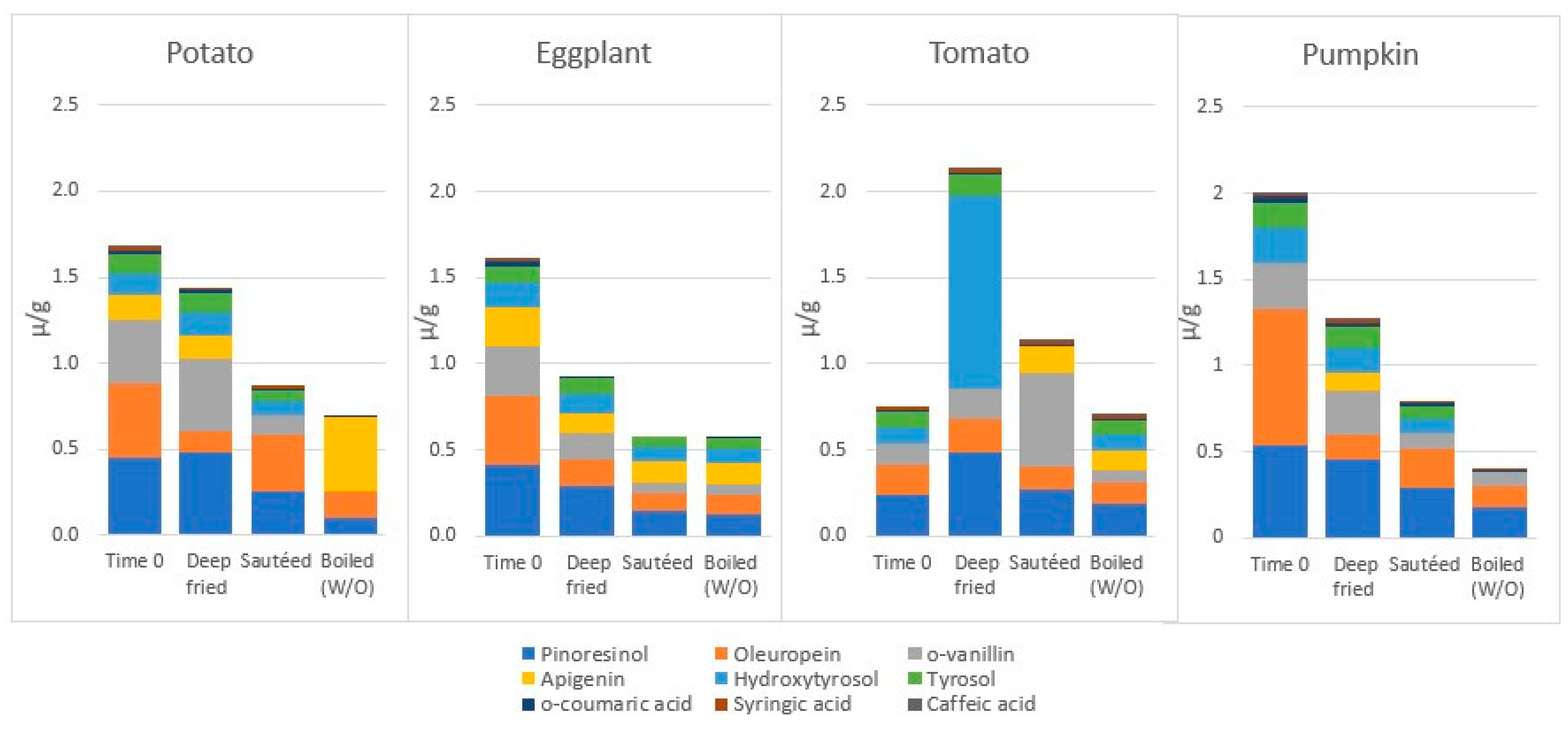
- Samaniego-Sánchez, C.; Castañeda-Saucedo, M.C.; Villalón-Mir, M.; De La Serrana, H.L. Phenols and the antioxidant capacity of Mediterranean vegetables prepared with extra virgin olive oil using different domestic cooking techniques. Food Chem. 2015, 188, 430–438. [Google Scholar] [CrossRef]
- Xu, F.; Zheng, Y.; Yang, Z.; Cao, S.; Shao, X.; Wang, H. Domestic cooking methods affect the nutritional quality of red cabbage. Food Chem. 2014, 161, 162–167. [Google Scholar] [CrossRef]
- Tian, J.; Chen, J.; Lv, F.; Chen, S.; Chen, J.; Liu, D.; Ye, X. Domestic cooking methods affect the phytochemical composition and antioxidant activity of purple-fleshed potatoes. Food Chem. 2016, 197, 1264–1270. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. Effect of Different Cooking Methods on Polyphenols, Carotenoids and Antioxidant Activities of Selected Edible Leaves. Antioxidants 2018, 7, 117. [Google Scholar] [CrossRef]
- Mashiane, P.; Mashitoa, F.M.; Slabbert, R.M.; Sivakumar, D. Impact of household cooking techniques on colour, antioxidant and sensory properties of African pumpkin and pumpkin leaves. Int. J. Gastron. Food Sci. 2021, 23, 100307. [Google Scholar] [CrossRef]
- Ioku, K.; Aoyama, Y.; Tokuno, A.; Terao, J.; Nakatani, N.; Takei, Y. Various cooking methods and the flavonoid content in onion. J. Nutr. Sci. Vitaminol. 2001, 47, 78–83. [Google Scholar] [CrossRef]
- Jung, J.-K.; Lee, S.-U.; Kozukue, N.; Levin, C.E.; Friedman, M. Distribution of phenolic compounds and antioxidative activities in parts of sweet potato (Ipomoea batata L.) plants and in home processed roots. J. Food Compos. Anal. 2011, 24, 29–37. [Google Scholar] [CrossRef]
- Finotti, E.; Bersani, E.; Del Prete, E.; Friedman, M. A functional mathematical index for predicting effects of food processing on eight sweet potato (Ipomoea batatas) cultivars. J. Food Compos. Anal. 2012, 27, 81–86. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Cattivelli, A.; Tagliazucchi, D. Domestic cooking methods affect the stability and bioaccessibility of dark purple eggplant (Solanum melongena) phenolic compounds. Food Chem. 2021, 341, 128298. [Google Scholar] [CrossRef]
- Juániz, I.; Ludwig, I.A.; Huarte, E.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Cid, C.; De Peña, M.-P. Influence of heat treatment on antioxidant capacity and (poly) phenolic compounds of selected vegetables. Food Chem. 2016, 197, 466–473. [Google Scholar] [CrossRef]
- Managa, M.G.; Shai, J.; Phan, A.D.T.; Sultanbawa, Y.; Sivakumar, D. Impact of Household Cooking Techniques on African Nightshade and Chinese Cabbage on Phenolic Compounds, Antinutrients, in vitro Antioxidant, and β-Glucosidase Activity. Front. Nutr. 2020, 7, 292. [Google Scholar] [CrossRef]
- Ferracane, R.; Pellegrini, N.; Visconti, A.; Graziani, G.; Chiavaro, E.; Miglio, C.; Fogliano, V. Effects of Different Cooking Methods on Antioxidant Profile, Antioxidant Capacity, and Physical Characteristics of Artichoke. J. Agric. Food Chem. 2008, 56, 8601–8608. [Google Scholar] [CrossRef]
- Sergio, L.; Boari, F.; Pieralice, M.; Linsalata, V.; Cantore, V.; Di Venere, D. Bioactive phenolics and antioxidant capacity of some wild edible greens as affected by different cooking treatments. Foods 2020, 9, 1320. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Peng, H. Bioaccessibility and bioavailability of phenolic compounds. J. Food Bioact. 2018, 4, 11–68. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef]
- Li, Q.; Li, T.; Liu, C.; Chen, J.; Zhang, R.; Zhang, Z.; Dai, T.; McClements, D.J. Potential physicochemical basis of Mediterranean diet effect: Ability of emulsified olive oil to increase carotenoid bioaccessibility in raw and cooked tomatoes. Food Res. Int. 2016, 89, 320–329. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Juániz, I.; Ludwig, I.A.; Bresciani, L.; Dall’Asta, M.; Mena, P.; Del Rio, D.; Cid, C.; de Peña, M.P. Bioaccessibility of (poly)phenolic compounds of raw and cooked cardoon (Cynara cardunculus L.) after simulated gastrointestinal digestion and fermentation by human colonic microbiota. J. Funct. Foods 2017, 32, 195–207. [Google Scholar] [CrossRef]
- Bugianesi, R.; Salucci, M.; Leonardi, C.; Ferracane, R.; Catasta, G.; Azzini, E.; Maiani, G. Effect of domestic cooking on human bioavailability of naringenin, chlorogenic acid, lycopene and β-carotene in cherry tomatoes. Eur. J. Nutr. 2004, 43, 360–366. [Google Scholar] [CrossRef]
- Tulipani, S.; Martinez Huelamo, M.; Rotches Ribalta, M.; Estruch, R.; Ferrer, E.E.; Andres-Lacueva, C.; Illan, M.; Lamuela-Raventós, R.M. Oil matrix effects on plasma exposure and urinary excretion of phenolic compounds from tomato sauces: Evidence from a human pilot study. Food Chem. 2012, 130, 581–590. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Rinaldi De Alvarenga, J.F.; Torrado, X.; Lamuela-Raventos, R.M. Home cooking and phenolics: Effect of thermal treatment and addition of extra virgin olive oil on the phenolic profile of tomato sauces. J. Agric. Food Chem. 2014, 62, 3314–3320. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huélamo, M.; Tulipani, S.; Estruch, R.; Escribano, E.; Illán, M.; Corella, D.; Lamuela-Raventós, R.M. The tomato sauce making process affects the bioaccessibility and bioavailability of tomato phenolics: A pharmacokinetic study. Food Chem. 2015, 173, 864–872. [Google Scholar] [CrossRef]
- Martínez-Huélamo, M.; Vallverdú-Queralt, A.; Di Lecce, G.; Valderas-Martínez, P.; Tulipani, S.; Jáuregui, O.; Escribano-Ferrer, E.; Estruch, R.; Illan, M.; Lamuela-Raventós, R.M. Bioavailability of tomato polyphenols is enhanced by processing and fat addition: Evidence from a randomized feeding trial. Mol. Nutr. Food Res. 2016, 60, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, R.; Jiménez-Altayó, F.; Alsina, L.; Onetti, Y.; Rinaldi de Alvarenga, J.F.; Claro, C.; Ogalla, E.; Casals, N.; Lamuela-Raventos, R.M. Mediterranean tomato-based sofrito protects against vascular alterations in obese Zucker rats by preserving NO bioavailability. Mol. Nutr. Food Res. 2017, 61, 1601010. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, V.; Rodríguez-Rodríguez, R.; Martínez-Garza, Ú.; Rosell-Cardona, C.; Lamuela-Raventós, R.M.; Marrero, P.F.; Haro, D.; Relat, J. Mediterranean Tomato-Based Sofrito Sauce Improves Fibroblast Growth Factor 21 (FGF21) Signaling in White Adipose Tissue of Obese ZUCKER Rats. Mol. Nutr. Food Res. 2018, 62, 1700606. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-Item Mediterranean Diet Assessment Tool and Obesity Indexes among High-Risk Subjects: The PREDIMED Trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, N. and A. (NDA) Scientific Opinion on the substantiation of a health claim related to 3 g/day plant sterols/stanols and lowering blood LDL-cholesterol and reduced risk of (coronary) heart disease pursuant to Article 19 of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2693. [Google Scholar] [CrossRef]
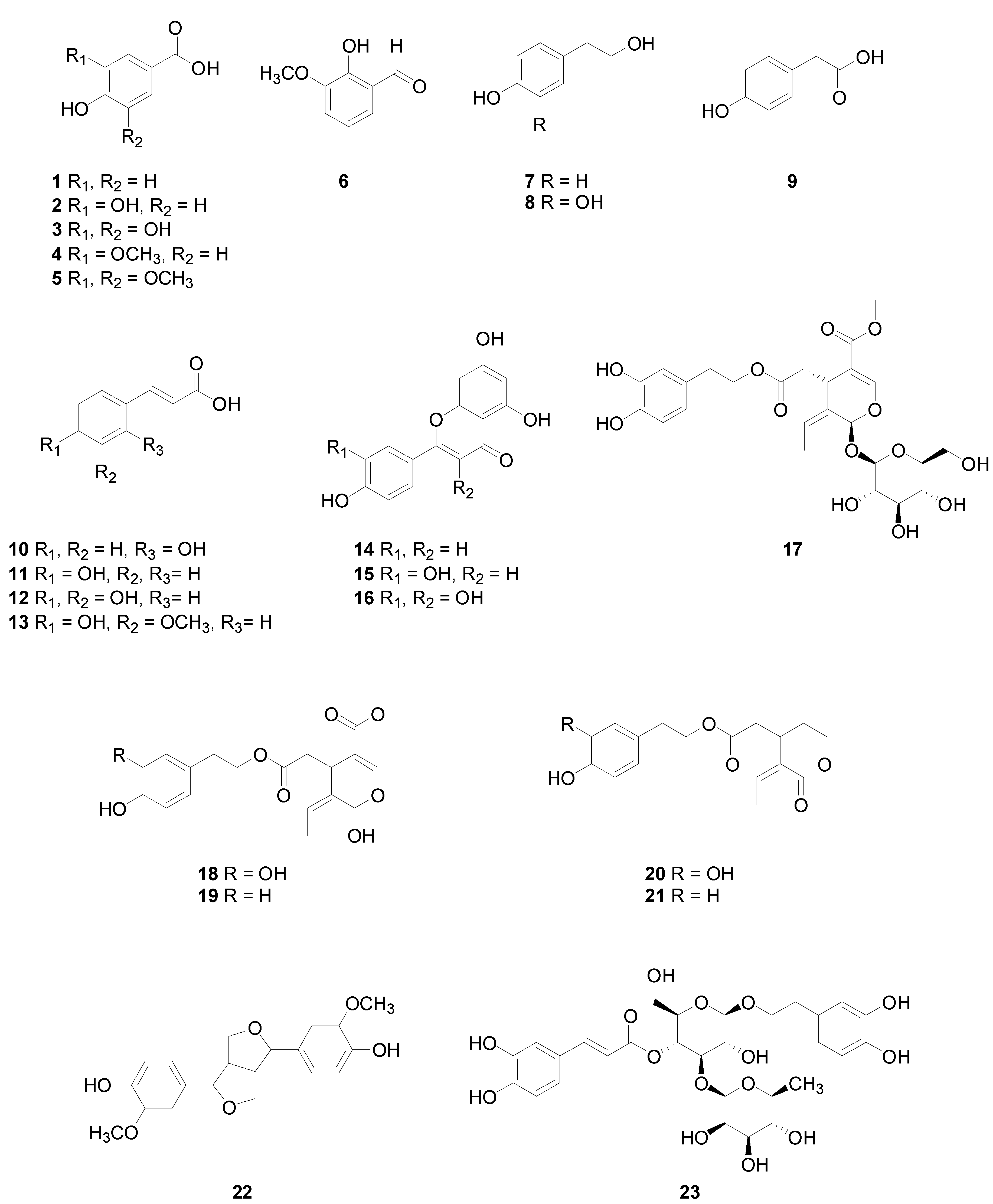


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambra, R.; Lucchetti, S.; Pastore, G. A Review of the Effects of Olive Oil-Cooking on Phenolic Compounds. Molecules 2022, 27, 661. https://doi.org/10.3390/molecules27030661
Ambra R, Lucchetti S, Pastore G. A Review of the Effects of Olive Oil-Cooking on Phenolic Compounds. Molecules. 2022; 27(3):661. https://doi.org/10.3390/molecules27030661
Chicago/Turabian StyleAmbra, Roberto, Sabrina Lucchetti, and Gianni Pastore. 2022. "A Review of the Effects of Olive Oil-Cooking on Phenolic Compounds" Molecules 27, no. 3: 661. https://doi.org/10.3390/molecules27030661
APA StyleAmbra, R., Lucchetti, S., & Pastore, G. (2022). A Review of the Effects of Olive Oil-Cooking on Phenolic Compounds. Molecules, 27(3), 661. https://doi.org/10.3390/molecules27030661





