Identification of Antibacterial Components in the Methanol-Phase Extract from Edible Herbaceous Plant Rumex madaio Makino and Their Antibacterial Action Modes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antibacterial Activity of Crude Extracts from R. madaio Makino
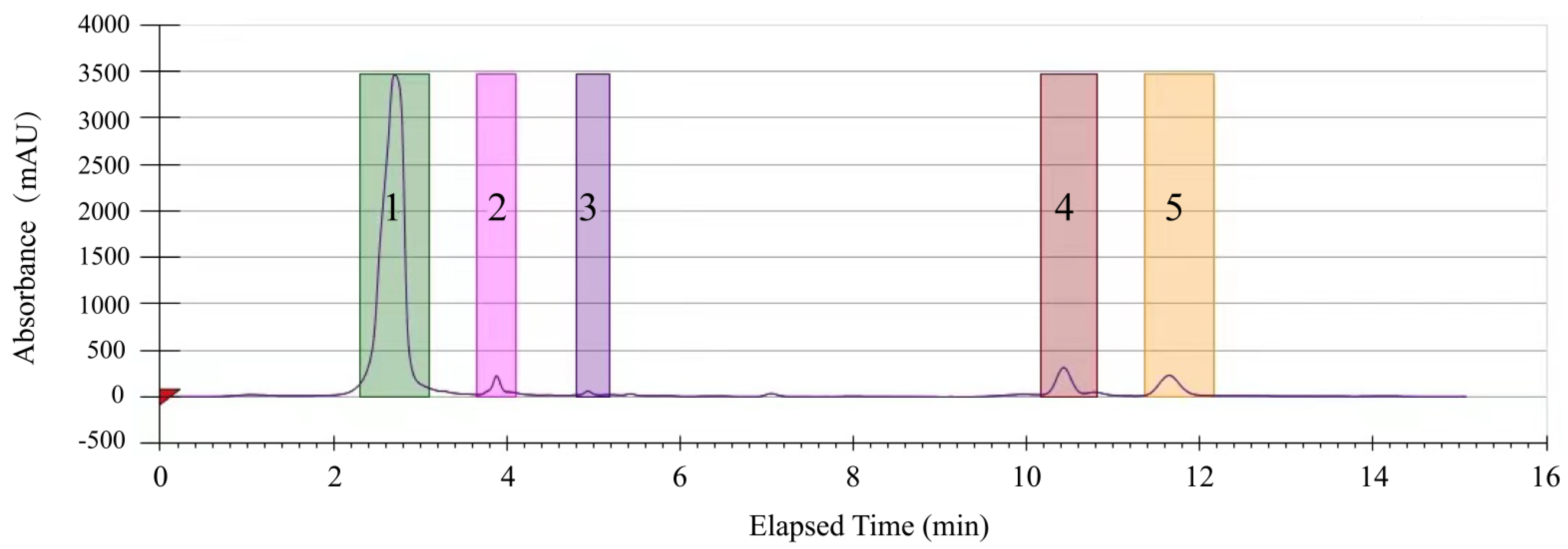
2.2. Purification of the Methanol-Phase Crude Extract from R. madaio Makino
2.3. Changed Bacterial Cell Surface Structure by the CC 1 Extract
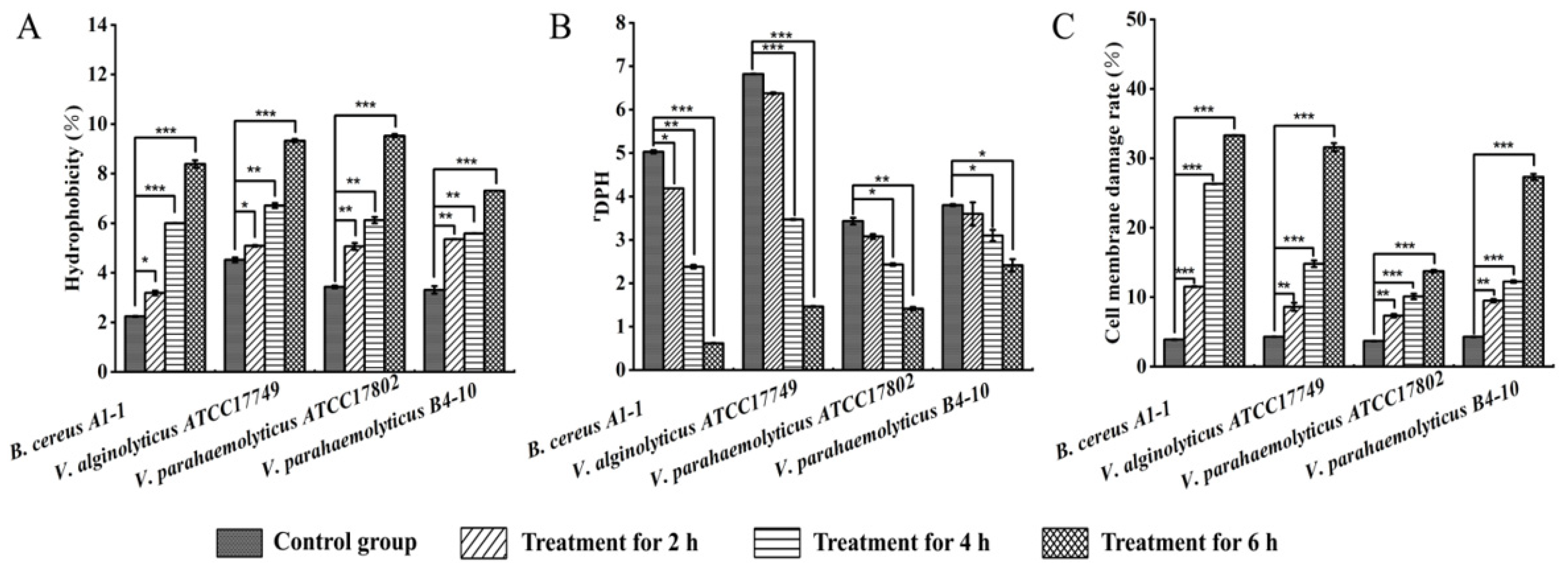
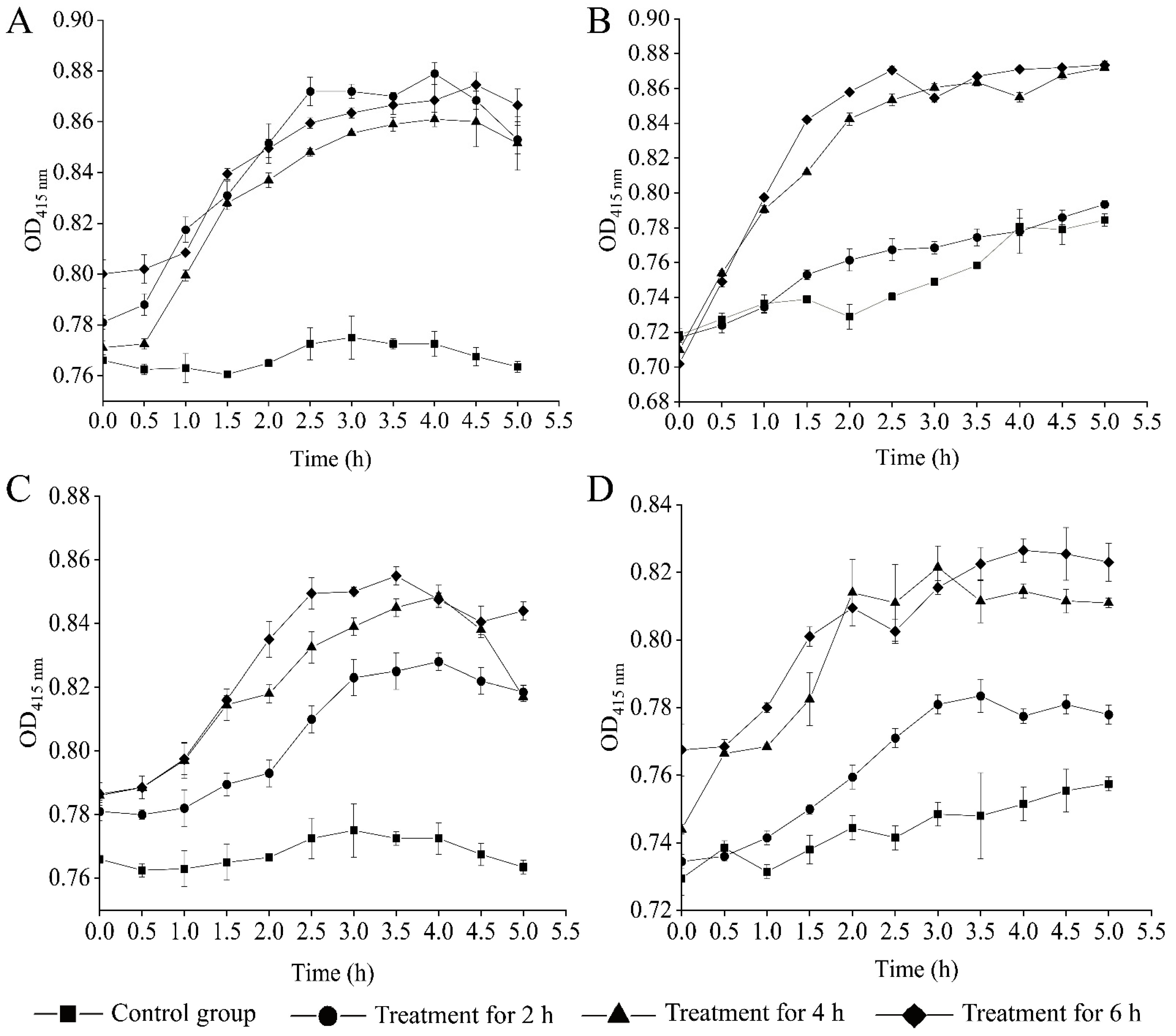
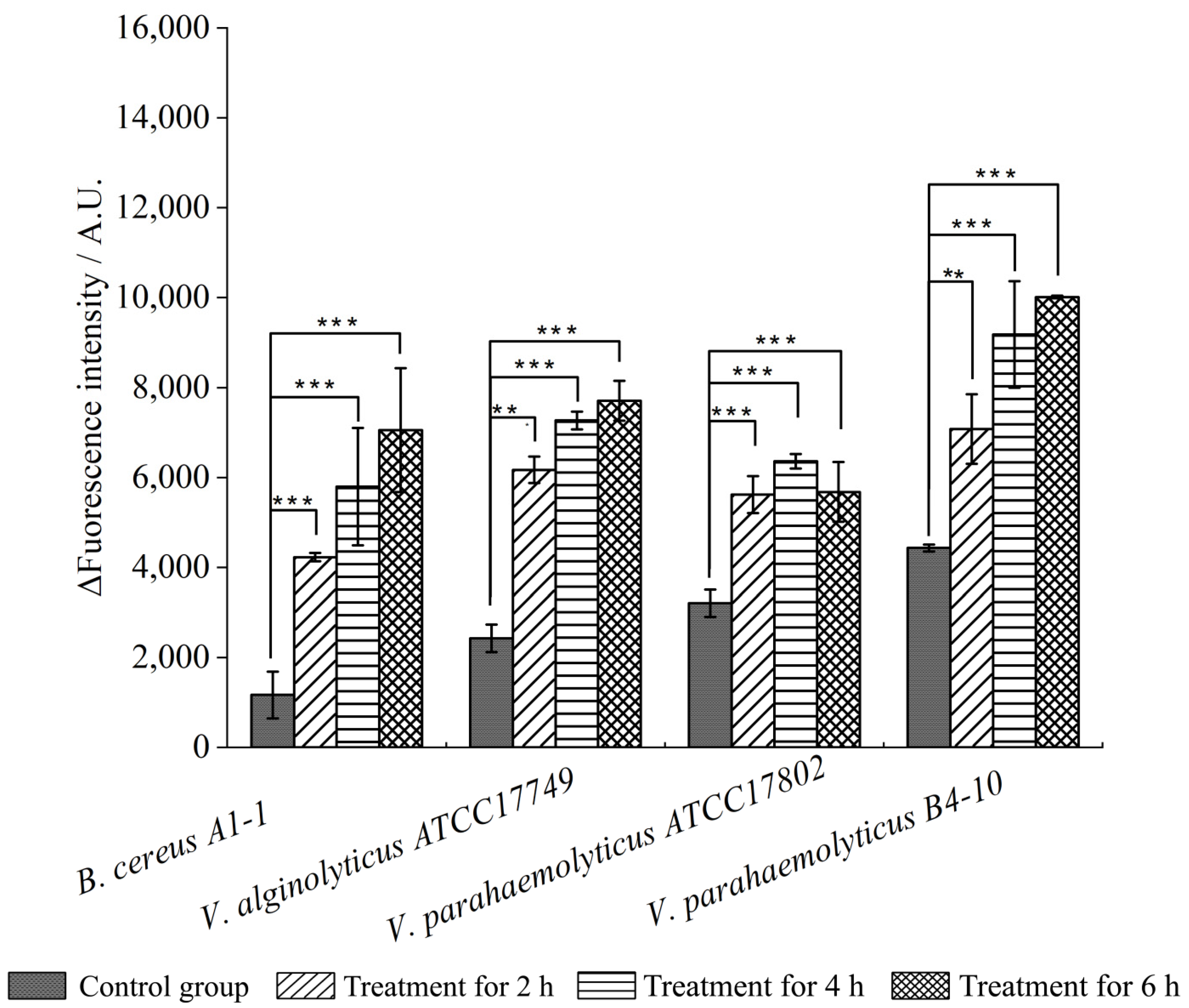
2.4. Changed Bacterial Cell Surface Hydrophobicity, Cell Membrane Fluidity, Permeability, and Damage by the CC 1 from R. madaio Makino
2.5. Identification of Potential Antibacterial Compounds in the CC 1 from R. madaio Makino
2.6. Differential Transcriptomes Mediated by the CC 1 from R. madaio Makino
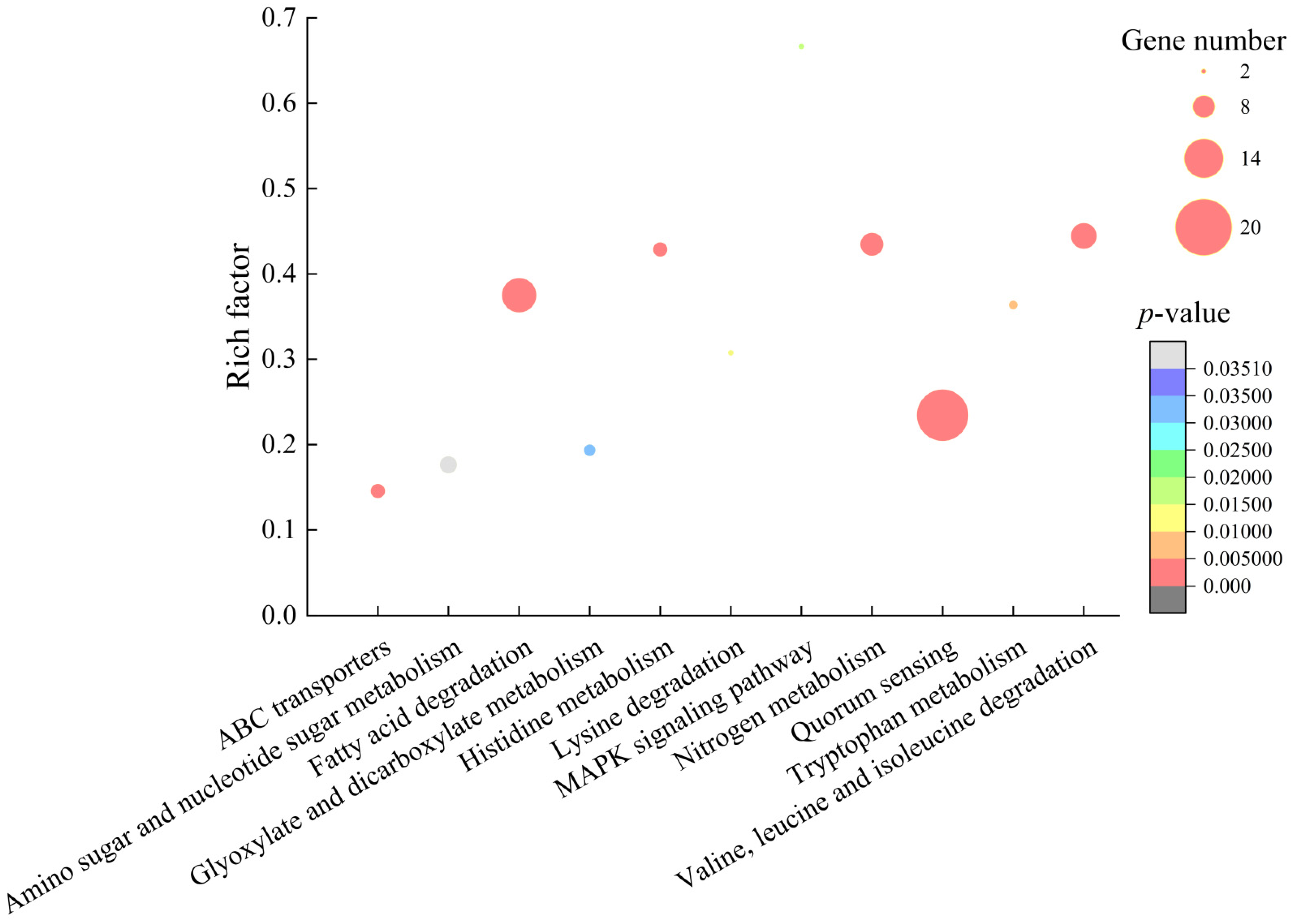
2.6.1. The Major Altered Metabolic Pathways in V. alginolyticus ATCC17749
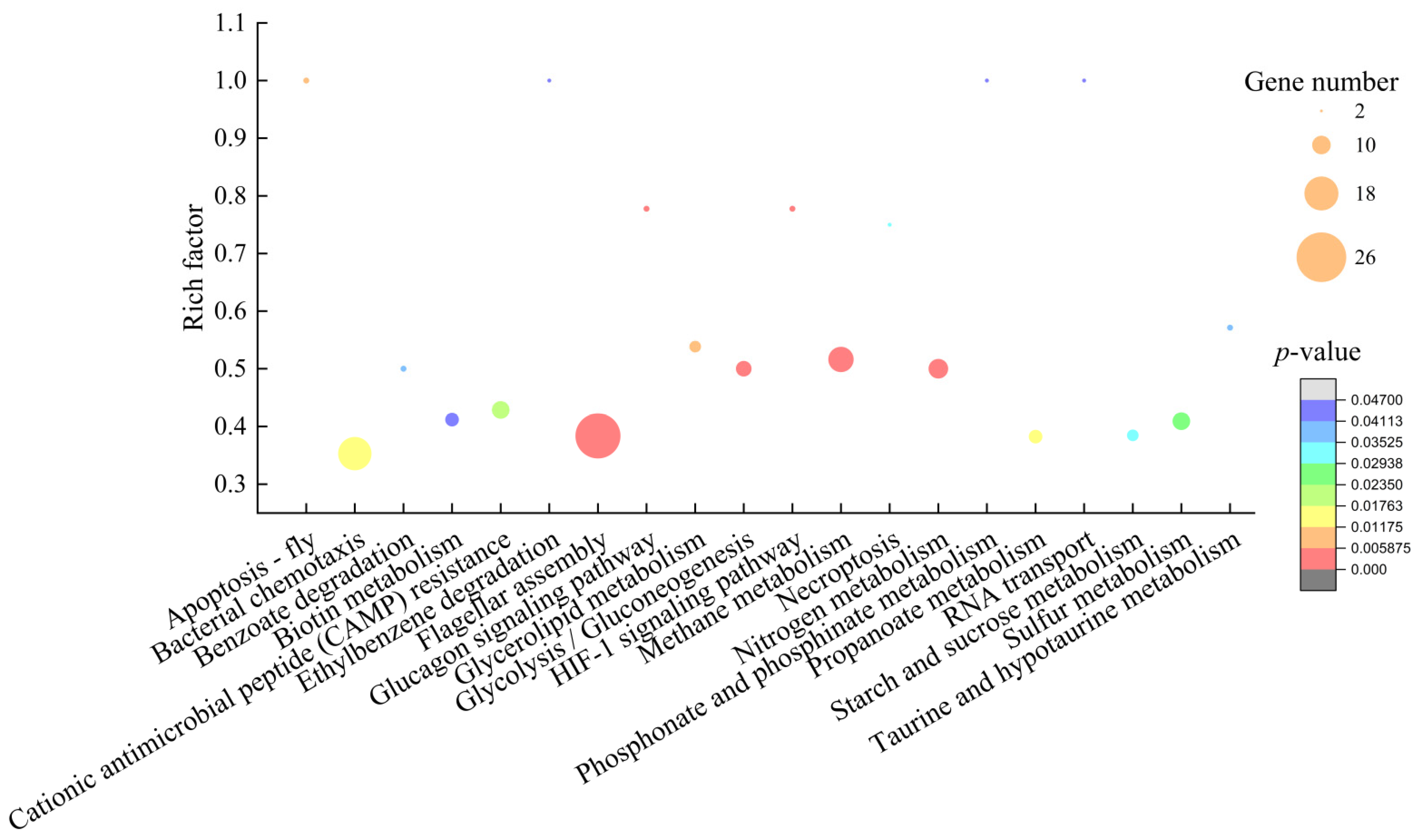
2.6.2. The Major Altered Metabolic Pathways in V. parahaemolyticus ATCC17802
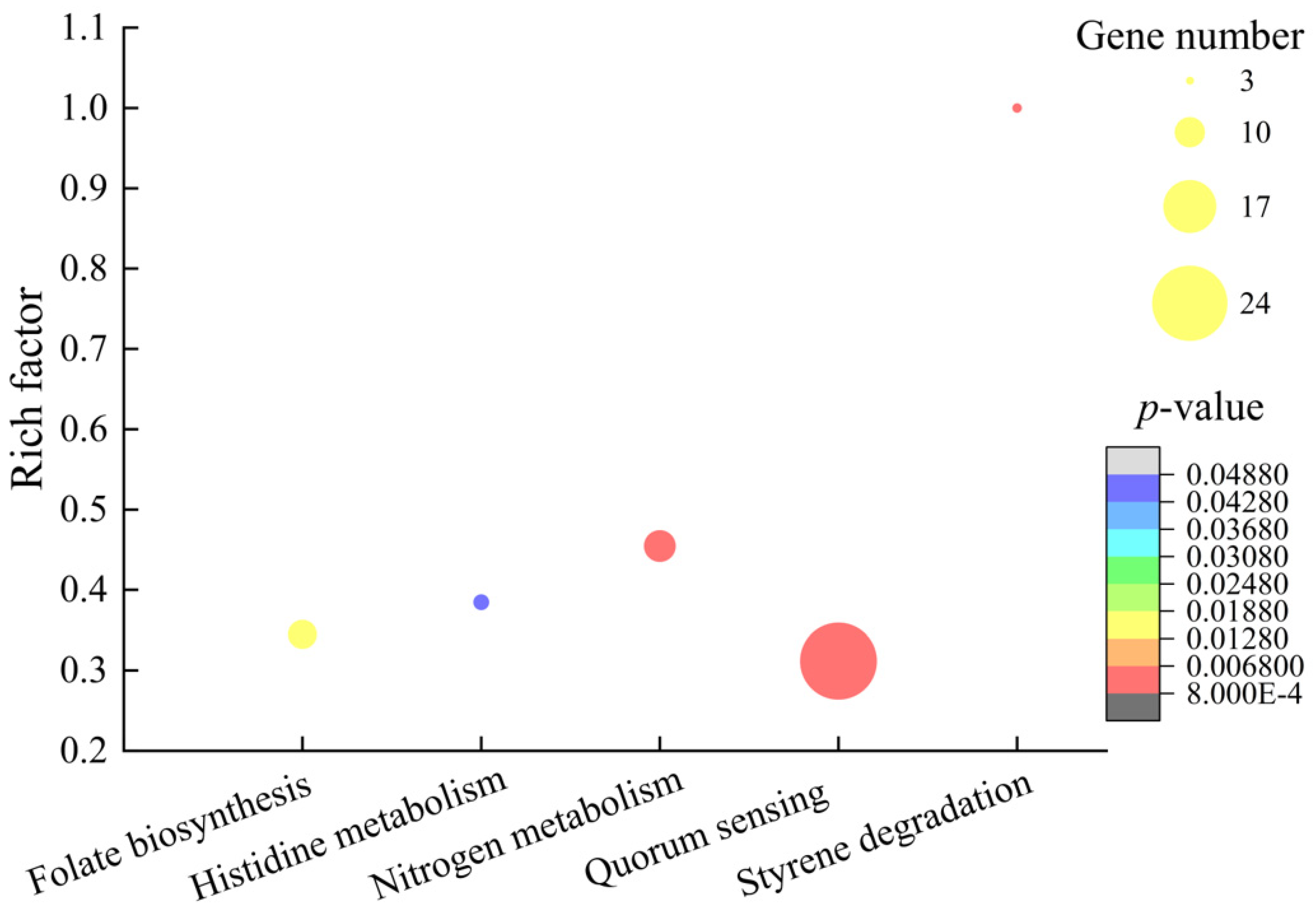
2.6.3. The Major Altered Metabolic Pathways in V. parahaemolyticus B4-10
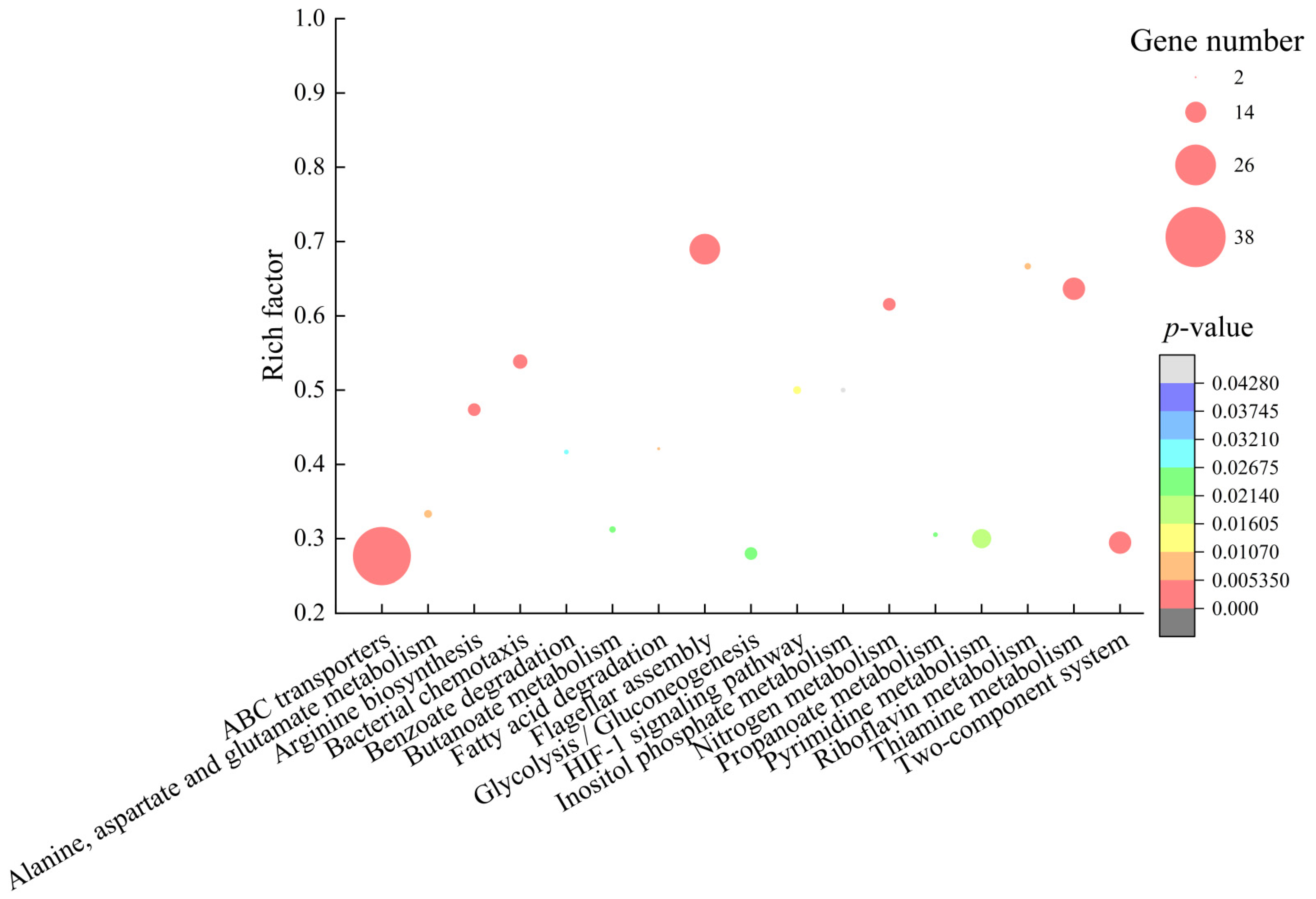
2.6.4. The Major Altered Metabolic Pathways in B. cereus A1-1
3. Materials and Methods
3.1. Bacterial Strains and Culture Conditions
3.2. Extraction of Bioactive Substances from R. madaio Makino
3.3. Antimicrobial Susceptibility Assay
3.4. Prep-HPLC Analysis
3.5. UHPLC–MS Analysis
3.6. Transmission Electron Microscope (TEM) Assay
3.7. Bacterial Cell Surface Hydrophobicity, Membrane Fluidity and Damage Assays
3.8. Cell Membrane Permeability Analysis
3.9. Illumina RNA Sequencing
3.10. Reverse Transcription Real Time-Quantitative PCR (RT-qPCR) Assay
3.11. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Huang, J.; Huang, J.; Lu, X.; Ma, K. Diversity distribution patterns of Chinese endemic seed plant species and their implications for conservation planning. Sci. Rep. 2016, 6, 33913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Xu, H.; Shang, Y.; Zhu, R.; Hong, X.; Song, Z.; Yang, Z. Development of the general chapters of the Chinese Pharmacopoeia 2020 edition: A review. J. Pharm. Anal. 2021, 11, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Xia, M.; Mu, C.; Li, R.; Wang, C. Acute metabolic response of Portunus trituberculatus to Vibrio alginolyticus infection. Aquaculture 2016, 463, 201–208. [Google Scholar] [CrossRef]
- Lv, T.; Song, T.; Liu, H.; Peng, R.; Jiang, X.; Zhang, W.; Han, Q. Isolation and characterization of a virulence related Vibrio alginolyticus strain Wz11 pathogenic to Cuttlefish, Sepia pharaonis. Microb. Pathog. 2019, 126, 165–171. [Google Scholar] [CrossRef]
- Li, L.; Meng, H.; Gu, D.; Li, Y.; Jia, M. Molecular mechanisms of Vibrio parahaemolyticus pathogenesis. Microbiol. Res. 2019, 222, 43–51. [Google Scholar] [CrossRef]
- Bottone, E.J. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef] [Green Version]
- Danchik, C.; Casadevall, A. Role of cell surface hydrophobicity in the pathogenesis of medically-significant fungi. Front. Cell. Infect. Microbiol. 2020, 10, 594973. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Ding, L.; Hu, H.; Ma, H.; Xu, K.; Huang, H.; Geng, J.; Ren, H. Cell membrane characteristics and microbial population distribution of MBBR and IFAS with different dissolved oxygen concentration. Bioresour. Technol. 2018, 265, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-bacterial properties of cannabigerol toward Streptococcus mutans. Front. Microbiol. 2021, 12, 656471. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Sharma, A.; Baek, K.-H. Antibacterial mode of action of Ginkgo biloba leaf essential oil: Effect on morphology and membrane permeability. Bangl. J. Pharmacol. 2015, 10, 337. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Nie, S.; Xie, M.; Hu, J. Antioxidant and antibacterial capabilities of phenolic compounds and organic acids from Camellia oleifera cake. Food Sci. Biotechnol. 2020, 29, 17–25. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Singh, R.K. Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives. Bioorg. Chem. 2019, 89, 103021. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, J.; Wu, D. Cloning and expression of the sucrose phosphorylase gene in Bacillus subtilis and synthesis of kojibiose using the recombinant enzyme. Microb. Cell Fact. 2018, 17, 23. [Google Scholar] [CrossRef] [Green Version]
- Garcia, C.A.; Gardner, J.G. Bacterial α-diglucoside metabolism: Perspectives and potential for biotechnology and biomedicine. Appl. Microbiol. Biotechnol. 2021, 105, 4033–4052. [Google Scholar] [CrossRef] [PubMed]
- Jarzyniak, K.; Banasiak, J.; Jamruszka, T.; Pawela, A.; Di Donato, M.; Novák, O.; Geisler, M.; Jasiński, M. Early stages of legume-rhizobia symbiosis are controlled by ABCG-mediated transport of active cytokinins. Nat. Plants 2021, 7, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.; Rahkhoodaee, F.; Raymond, M. Relative contributions of the Candida albicans ABC transporters Cdr1p and Cdr2p to clinical azole resistance. Antimicrob. Agents Chemother. 2009, 53, 1344–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarling, E.J.; de Aguiar Vallim, T.Q.; Edwards, P.A. Role of ABC transporters in lipid transport and human disease. Trends Endocrinol. Metab. 2013, 24, 342–350. [Google Scholar] [CrossRef] [Green Version]
- Whitley, M.J.; Arjunan, P.; Nemeria, N.S.; Korotchkina, L.G.; Park, Y.H.; Patel, M.S.; Jordan, F.; Furey, W. Pyruvate dehydrogenase complex deficiency is linked to regulatory loop disorder in the αV138M variant of human pyruvate dehydrogenase. J. Biol. Chem. 2018, 293, 13204–13213. [Google Scholar] [CrossRef] [Green Version]
- Aquilano, K.; Vigilanza, P.; Rotilio, G.; Ciriolo, M.R. Mitochondrial damage due to SOD1 deficiency in SH-SY5Y neuroblastoma cells: A rationale for the redundancy of SOD1. FASEB J. 2006, 20, 1683–1685. [Google Scholar] [CrossRef] [Green Version]
- Hajam, I.A.; Dar, P.A.; Shahnawaz, I.; Jaume, J.C.; Lee, J.H. Bacterial flagellin-a potent immunomodulatory agent. Exp. Mol. Med. 2017, 49, e373. [Google Scholar] [CrossRef]
- Nedeljković, M.; Sastre, D.E.; Sundberg, E.J. Bacterial flagellar filament: A supramolecular multifunctional nanostructure. Int. J. Mol. Sci. 2021, 22, 7521. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.L.; Nishikino, T.; Guo, W.; Zhu, S.; Kojima, S.; Homma, M.; Liu, J. The flagellar motor of Vibrio alginolyticus undergoes major structural remodeling during rotational switching. Elife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Zhu, D.; Sun, J. Bacterial chemotaxis: A way forward to aromatic compounds biodegradation. Environ. Sci. Eur. 2020, 32, 52. [Google Scholar] [CrossRef]
- LeBlanc, M.A.; Fink, M.R.; Perkins, T.T.; Sousa, M.C. Type III secretion system effector proteins are mechanically labile. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, W.A.; Hagen, W.R.; van Dongen, W.M. The hybrid-cluster protein (‘prismane protein’) from Escherichia coli. Characterization of the hybrid-cluster protein, redox properties of the [2Fe-2S] and [4Fe-2S-2O] clusters and identification of an associated NADH oxidoreductase containing FAD and [2Fe-2S]. Eur. J. Biochem. 2000, 267, 666–676. [Google Scholar] [CrossRef]
- Xue, M.; Raheem, M.A.; Gu, Y.; Lu, H.; Song, X.; Tu, J.; Xue, T.; Qi, K. The KdpD/KdpE two-component system contributes to the motility and virulence of avian pathogenic Escherichia coli. Res. Vet. Sci. 2020, 131, 24–30. [Google Scholar] [CrossRef]
- Xu, M.; Fu, H.; Chen, D.; Shao, Z.; Zhu, J.; Alali, W.Q.; Chen, L. Simple visualized detection method of virulence-associated genes of Vibrio cholerae by loop-mediated isothermal amplification. Front. Microbiol. 2019, 10, 2899. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Pandak, W.M.; Heuman, D.; Hylemon, P.B.; Ren, S. High glucose Induces lipid accumulation via 25-Hydroxycholesterol DNA-CpG methylation. iScience 2020, 23, 101102. [Google Scholar] [CrossRef]
- Chen, D.; Li, X.; Ni, L.; Xu, D.; Xu, Y.; Ding, Y.; Xie, L.; Chen, L. First experimental evidence for the presence of potentially toxic Vibrio cholerae in Snails, and virulence, cross-resistance and genetic diversity of the bacterium in 36 species of aquatic food animals. J. Antibiot. 2021, 10, 412. [Google Scholar] [CrossRef]
- Shan, X.; Fu, J.; Li, X.; Peng, X.; Chen, L. Comparative proteomics and secretomics revealed virulence, and coresistance-related factors in non O1/O139 Vibrio cholerae recovered from 16 species of consumable aquatic animals. J. Proteom. 2021, 251, 104408. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Y.; Yu, P.; Ren, S.; Zhu, Z.; Jin, Y.; Yan, J.; Peng, X.; Chen, L. Prophage-related gene VpaChn25_0724 contributes to cell membrane Integrity and growth of Vibrio parahaemolyticus CHN25. Front. Cell. Infect. Microbiol. 2020, 10, 595709. [Google Scholar] [CrossRef] [PubMed]
- Krausova, G.; Hyrslova, I.; Hynstova, I. In vitro evaluation of adhesion capacity, hydrophobicity, and auto-aggregation of newly isolated potential probiotic strains. Fermentation 2019, 5, 100. [Google Scholar] [CrossRef] [Green Version]
- Kuhry, J.G.; Duportail, G.; Bronner, C.; Laustriat, G. Plasma membrane fluidity measurements on whole living cells by fluorescence anisotropy of trimethylammoniumdiphenylhexatriene. Biochim. Biophys Acta. 1985, 845, 60–67. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, Q.; Zheng, Y.; Li, F.; Zhao, Y.; Chen, G.-Q. Engineering the permeability of Halomonas bluephagenesis enhanced its chassis properties. Metab. Eng. 2021, 67, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Liu, X.; Li, Z.; Zheng, Y.; Wai Kwok Yeung, K.; Cui, Z.; Liang, Y.; Zhu, S.; Wu, S. Rapid bacteria capturing and killing by AgNPs/N-CD@ZnO hybrids strengthened photo-responsive xerogel for rapid healing of bacteria-infected wounds. Chem. Eng. J. 2021, 414, 128805. [Google Scholar] [CrossRef]



| pStrain | Inhibition Zone (Diameter, mm) | MIC (μg/mL) | ||
|---|---|---|---|---|
| CPE | MPE | CPE | MPE | |
| Aeromonas hydrophila ATCC35654 | — | 11.30 ± 0.47 | — | 126 |
| Bacillus cereus A1-1 | — | 14.70 ± 1.25 | — | 32 |
| Enterobacter cloacae ATCC13047 | 7.90 ± 0.05 | 13.00 ± 0.86 | 512 | 64 |
| Enterobacter cloacae | — | 8.30 ± 0.24 | — | 512 |
| Escherichia coli ATCC8739 | — | — | — | — |
| Escherichia coli ATCC25922 | — | — | — | — |
| Escherichia coli K12 | — | 9.30 ± 1.25 | — | 128 |
| Enterobacter sakazakii CMCC45401 | 8.90 ± 0.14 | 8.70 ± 0.47 | 256 | 512 |
| Listeria monocytogenes ATCC19115 | 9.80 ± 0.17 | — | 256 | — |
| Pseudomonas aeruginosa ATCC9027 | — | 9.30 ± 0.94 | — | 256 |
| Pseudomonas aeruginosa ATCC27853 | — | 9.00 ± 0.21 | — | 256 |
| Salmonella choleraesuis ATCC13312 | — | 9.70 ± 0.94 | — | 256 |
| Salmonella paratyphi-A CMCC50093 | 8.70 ± 0.94 | 9.40 ± 0.43 | 512 | 256 |
| Salmonella typhimurium ATCC15611 | 8.90 ± 0.17 | 14.00 ± 0.82 | 256 | 32 |
| Salmonella | 8.20 ± 0.17 | 20.30 ± 0.47 | 512 | 8 |
| Shigella dysenteriae CMCC51252 | — | — | — | — |
| Shigella flexneri CMCC51572 | — | 10.00 ± 0.00 | — | 128 |
| Shigella flexneri ATCC12022 | — | — | — | — |
| Shigella flexneri CMCC51574 | — | — | — | — |
| Shigella sonnei ATCC25931 | — | — | — | — |
| Shigella sonnet CMCC51592 | 9.40 ± 0.29 | 8.10 ± 0.05 | 256 | 512 |
| Staphylococcus aureus ATCC25923 | 10.60 ± 0.42 | 8.10 ± 0.29 | 128 | 512 |
| Staphylococcus aureus ATCC8095 | 8.00 ± 0.05 | 7.30 ± 0.21 | 512 | 1024 |
| Staphylococcus aureus ATCC29213 | — | 7.20 ± 0.08 | — | 1024 |
| Staphylococcus aureus ATCC6538 | 10.00 ± 0.82 | 10.00 ± 2.16 | 256 | 256 |
| Staphylococcus aureus ATCC6538P | — | 10.50 ± 0.41 | — | 128 |
| Staphylococcus aureus | 7.00 ± 0.00 | 8.50 ± 0.41 | 1024 | 512 |
| Vibrio alginolyticus ATCC17749 | — | 24.30 ± 1.25 | — | 4 |
| Vibrio alginolyticus ATCC33787 | — | — | — | — |
| Vibrio cholerae Q10-54 | — | — | — | — |
| Vibrio cholerae b10-49 | — | 9.00 ± 0.24 | — | 256 |
| Vibrio cholerae GIM1.449 | 10.30 ± 0.36 | 10.50 ± 0.41 | 256 | 128 |
| Vibrio fluvialis ATCC33809 | 11.30 ± 0.47 | 7.90 ± 0.09 | 128 | 512 |
| Vibrio harvey ATCC BAA-1117 | — | 8.00 ± 0.05 | — | 512 |
| Vibrio harveyi ATCC33842 | — | — | — | — |
| Vibrio metschnikovii ATCC700040 | 8.40 ± 0.42 | — | 512 | — |
| Vibrio mimicus bio-56759 | 9.20 ± 0.12 | 13.00 ± 0.82 | 512 | 64 |
| Vibrio parahaemolyticus B3-13 | 10.50 ± 0.41 | 9.10 ± 0.12 | 128 | 256 |
| Vibrio parahaemolyticus B4-10 | — | 10.30 ± 0.47 | — | 128 |
| Vibrio parahaemolyticus B5-29 | — | 12.30 ± 0.94 | — | 64 |
| Vibrio parahaemolyticus B9-35 | — | 8.30 ± 0.21 | — | 512 |
| Vibrio parahaemolyticus ATCC17802 | — | 13.70 ± 0.94 | — | 128 |
| Vibrio parahaemolyticus ATCC33847 | — | 13.00 ± 0.00 | — | 64 |
| Vibrio vulnificus ATCC27562 | 11.70 ± 1.25 | 8.70 ± 0.47 | 128 | 256 |
| Strain | Inhibition Zone (Diameter, mm) | MIC (μg/mL) |
|---|---|---|
| B. cereus A1-1 | 10.30 ± 0.24 | 128 |
| S. typhimurium ATCC15611 | 7.90 ± 0.22 | 512 |
| S. aureus ATCC6538 | 7.00 ± 0.05 | 1024 |
| V. alginolyticus ATCC17749 | 11.20 ± 0.21 | 64 |
| V. parahaemolyticus ATCC17802 | 11.10 ± 0.08 | 64 |
| V. parahaemolyticus ATCC33847 | 7.90 ± 0.25 | 256 |
| V. parahaemolyticus B3-13 | 7.10 ± 0.09 | 512 |
| V. parahaemolyticus B4-10 | 9.40 ± 0.26 | 256 |
| V. parahaemolyticus B5-29 | 8.10 ± 0.12 | 512 |
| Peak No. | Identified Compound | Compound Nature | Rt (min) | Formula | Exact Mass | Peak Area (%) |
|---|---|---|---|---|---|---|
| 1 | p-Octopamine | Biogenic amine | 3.84 | C8H11NO2 | 153.08 | 18.62 |
| 2 | D-alpha-Aminobutyric acid | Amino acids and derivatives | 0.65 | C4H9NO2 | 103.06 | 9.46 |
| 3 | Sucrose | Carbohydrates | 0.89 | C12H22O11 | 342.12 | 7.01 |
| 4 | Turanose | Carbohydrates | 0.79 | C12H22O11 | 342.12 | 7.01 |
| 5 | Lactulose | Organooxygen compounds | 0.77 | C12H22O11 | 342.12 | 7.01 |
| 6 | L-Arginine | Amino acids and derivatives | 0.60 | C6H14N4O2 | 174.11 | 4.98 |
| 7 | L-Lysine; L-Glutamine | Amino acids and derivatives | 0.64 | C6H14N2O2 | 146.11 | 4.68 |
| 8 | D-Glutamine | Amino acids and derivatives | 0.66 | C5H10N2O3 | 146.07 | 4.68 |
| 9 | (2E)-Decenoyl-ACP | Carboxylic acids and derivatives | 1.47 | C6H11NO2 | 129.08 | 3.14 |
| 10 | O-Acetylethanolamine | Alkaloids | 0.67 | C4H9NO2 | 103.06 | 3.00 |
| 11 | L-Pipecolic acid | Amino acids and derivatives | 0.69 | C6H11NO2 | 129.08 | 2.48 |
| 12 | Pyrrolidonecarboxylic acid | Amino acids and derivatives | 0.67 | C5H7NO3 | 129.04 | 2.48 |
| 13 | D-Maltose | Carbohydrates | 0.76 | C12H22O11 | 342.12 | 1.86 |
| 14 | Trigonelline | Alkaloids | 0.82 | C7H7NO2 | 137.05 | 1.74 |
| 15 | Indole | Alkaloids | 3.82 | C8H7N | 117.06 | 1.66 |
| 16 | Uridine 5’-diphospho-d-glucose | Carbohydrates | 0.71 | C15H24N2O17P2 | 566.06 | 1.65 |
| 17 | Proline; L-Proline | Amino acids and derivatives; | 0.73 | C5H9NO2 | 115.06 | 1.53 |
| 18 | D-Proline | Amino acids and derivatives | 0.76 | C5H9NO2 | 115.06 | 1.53 |
| 19 | Lubiprostone | Fatty acyls | 12.75 | C20H32F2O5 | 390.22 | 1.40 |
| 20 | Phosphoric acid | Inganic acids | 0.65 | H3O4P | 97.98 | 1.29 |
| 21 | Sarracine | Alkaloids | 13.14 | C18H27NO5 | 337.19 | 0.83 |
| 22 | Galactose 1-phosphate | Organooxygen compounds | 0.65 | C6H13O9P | 260.03 | 0.75 |
| 23 | L-Glutamic acid | Amino acids and derivatives | 0.66 | C5H9NO4 | 147.05 | 0.67 |
| 24 | Kojibiose | Carbohydrates | 0.72 | C12H22O11 | 342.12 | 0.50 |
| 25 | Glucose 6-phosphate | Carbohydrates | 0.65 | C6H13O9P | 260.03 | 0.49 |
| 26 | p-Aminobenzoate | Benzoic acid derivatives | 0.74 | C7H7NO2 | 137.05 | 0.47 |
| 27 | Betaine | Alkaloids | 1.06 | C5H11NO2 | 117.08 | 0.47 |
| 28 | L-Histidine | Amino acids and derivatives | 0.59 | C6H9N3O2 | 155.07 | 0.44 |
| 29 | 8,9-DiHETrE | Fatty Acyls | 13.03 | C20H34O4 | 338.25 | 0.43 |
| 30 | Gluconic acid | Organic acids | 0.69 | C6H12O7 | 196.06 | 0.43 |
| 31 | N,N-Dimethylglycine | Amino acids and derivatives | 1.04 | C4H9NO2 | 103.05 | 0.40 |
| 32 | 2-Aminoisobutyric acid | Amino acids and derivatives | 0.98 | C4H9NO2 | 103.06 | 0.37 |
| 33 | Diallyl disulfide | Organic disulfide | 0.68 | C6H10S2 | 146.02 | 0.37 |
| 34 | 2-Hydroxybutanoic acid | Organic acids | 0.64 | C4H8O3 | 104.05 | 0.35 |
| 35 | Beta-Sitosterol | Steroids | 12.93 | C29H50O | 414.39 | 0.33 |
| 36 | Phosphorylcholine | Cholines | 0.67 | C5H14NO4P | 183.07 | 0.31 |
| 37 | Campesterol | Steroids and steroid derivatives | 12.18 | C28H48O | 400.37 | 0.31 |
| 38 | Gemcitabine | Pyrimidine nucleosides | 0.75 | C9H11F2N3O4 | 263.07 | 0.30 |
| 39 | L-Threonine | Amino acids and derivatives | 0.64 | C4H9NO3 | 119.06 | 0.29 |
| 40 | L-Homoserine | Amino acids and derivatives | 0.67 | C4H9NO3 | 119.05 | 0.29 |
| 41 | 3-Ethyl-1,2-benzenediol | Phenols | 0.74 | C8H10O2 | 138.07 | 0.29 |
| 42 | Diacylglycerol | Glycerolipids | 13.42 | C37H70O5 | 568.51 | 0.28 |
| 43 | Rutin | Flavonoids | 5.85 | C27H30O16 | 610.15 | 0.27 |
| 44 | cis-Aconitic acid | Organic acids and derivatives | 1.46 | C6H6O6 | 174.02 | 0.25 |
| 45 | L-Citruline | Amino acids and derivatives | 0.66 | C6H13N3O3 | 175.09 | 0.25 |
| 46 | Wighteone | Flavonoids | 13.01 | C20H18O5 | 338.11 | 0.24 |
| 47 | Beta-d-Fructose 2-phosphate | Carbohydrates | 0.75 | C6H13O9P | 260.03 | 0.22 |
| 48 | Maltol | Flavonoids | 0.90 | C6H6O3 | 126.03 | 0.21 |
| 49 | Itaconic acid | Organic acids | 0.52 | C5H6O4 | 130.03 | 0.21 |
| 50 | Safrole | Benzodioxoles | 12.26 | C10H10O2 | 162.07 | 0.20 |
| 51 | 22-Dehydroclerosterol | Steroids | 12.59 | C29H46O | 410.35 | 0.18 |
| 52 | 8-Hydroxybergapten | Coumarins | 10.56 | C12H8O5 | 232.04 | 0.17 |
| 53 | Isoquercitrin | Flavonoids | 6.06 | C21H20O12 | 464.10 | 0.14 |
| 54 | Miltirone | Diterpenoids | 12.98 | C19H22O2 | 282.16 | 0.11 |
| 55 | Puerarin | Flavonoids | 4.89 | C21H20O9 | 416.11 | 0.11 |
| 56 | Cinchonine | Alkaloids | 11.99 | C19H22N2O | 294.17 | 0.09 |
| 57 | 3-Ethoxy-4-hydroxybenzaldehyde | Phenols | 5.72 | C9H10O3 | 166.06 | 0.07 |
| 58 | Lumichrome | Alkaloids | 6.69 | C12H10N4O2 | 242.08 | 0.07 |
| Metabolic Pathway | Gene ID | Fold Change | Gene Description |
|---|---|---|---|
| Valine, leucine and isoleucine degradation | N646_4585 | 2.117 | Acetoacetyl-coenzyme A synthetase |
| N646_4506 | 2.127 | Putative 3-hydroxyisobutyrate dehydrogenase | |
| N646_4019 | 2.293 | Acetoacetyl-coenzyme A synthetase | |
| N646_4049 | 2.793 | Putative acyl-CoA carboxyltransferase beta chain | |
| N646_4047 | 3.123 | Putative acyl-CoA carboxylase alpha chain | |
| N646_4057 | 3.302 | 3-hydroxyisobutyrate dehydrogenase | |
| N646_4048 | 4.128 | Putative enoyl-CoA hydratase/isomerase | |
| N646_4053 | 4.602 | Putative aldehyde dehydrogenase | |
| N646_4050 | 4.619 | Putative acyl-CoA dehydrogenase | |
| Nitrogen metabolism | N646_3727 | 2.193 | Putative oxidoreductase protein |
| N646_4426 | 2.656 | Hypothetical protein | |
| N646_3915 | 5.506 | Periplasmic nitrate reductase | |
| N646_4365 | 5.657 | Hypothetical protein | |
| N646_3914 | 6.137 | Periplasmic nitrate reductase%2C cytochrome c-type protein | |
| N646_4364 | 11.868 | Nitrite reductase [NAD(P)H]%2C small subunit | |
| N646_1010 | 29.988 | Nitrite reductase periplasmic cytochrome c552 | |
| N646_0236 | 87.807 | Hydroxylamine reductase | |
| Quorum sensing | N646_0372 | 2.104 | ABC-type spermidine/putrescine transport system%2C permease component II |
| N646_2230 | 2.108 | Peptide ABC transporter%2C permease protein | |
| N646_4026 | 2.258 | Putative ABC transporter%2C membrane spanning protein | |
| N646_1576 | 2.315 | Peptide ABC transporter%2C periplasmic peptide-binding protein | |
| N646_0379 | 2.493 | Oligopeptide ABC transporter%2C permease protein | |
| N646_2228 | 2.531 | Peptide ABC transporter%2C periplasmic peptide-binding protein | |
| N646_4027 | 2.666 | Putative high-affinity branched-chain amino acid transport permease protein | |
| N646_0377 | 2.688 | Oligopeptide ABC transporter%2C ATP-binding protein | |
| N646_1580 | 2.821 | Peptide ABC transporter%2C ATP-binding protein | |
| N646_0378 | 2.836 | Oligopeptide ABC transporter%2C ATP-binding protein | |
| N646_4024 | 2.850 | Putative high-affinity branched-chain amino acid transport ATP-binding protein | |
| N646_0380 | 2.854 | Oligopeptide ABC transporter%2C permease protein | |
| N646_4025 | 2.951 | Putative long-chain-fatty-acid-CoA ligase | |
| N646_0381 | 3.075 | Oligopeptide ABC transporter%2C periplasmic oligopeptide-binding protein | |
| N646_0370 | 3.909 | Putative ATP-binding component of ABC transporter | |
| N646_4029 | 4.034 | Putative high-affinity branched-chain amino acid transport ATP-binding protein | |
| N646_0371 | 4.049 | Putative permease of ABC transporter | |
| N646_0367 | 4.112 | Putative binding protein component of ABC transporter | |
| Histidine metabolism | N646_0312 | 2.001 | Formimidoylglutamase |
| N646_0189 | 2.072 | Imidazoleglycerol-phosphate dehydratase/histidinol-phosphatase | |
| N646_0190 | 2.090 | Imidazole glycerol phosphate synthase subunit HisH | |
| N646_0313 | 3.141 | Imidazolonepropionase | |
| N646_0311 | 3.168 | Urocanate hydratase | |
| N646_0310 | 3.187 | Histidine ammonia-lyase | |
| Fatty acid degradation | N646_1753 | 0.344 | Hypothetical protein |
| N646_0066 | 2.033 | Amino acid ABC transporter%2C permease protein | |
| N646_3145 | 2.064 | Rubredoxin/rubredoxin reductase | |
| N646_2209 | 2.122 | Acetyl-CoA C-acyltransferase FadA | |
| N646_3116 | 2.163 | Maltose ABC transporter periplasmic protein | |
| N646_3117 | 2.319 | Maltose/maltodextrin ABC transporter%2C ATP-binding protein | |
| N646_3389 | 2.793 | Putative ferrichrome ABC transporter (permease) | |
| N646_1395 | 2.879 | Acyl-CoA dehydrogenase | |
| N646_4429 | 3.400 | Nitrate ABC transporter nitrate-binding protein | |
| N646_4028 | 5.585 | Hypothetical protein | |
| N646_4427 | 6.398 | Hypothetical protein | |
| N646_3568 | 14.448 | Putative ABC transporter%2C ATP-binding protein | |
| ABC transporters | N646_4485 | 2.173 | Arginine ABC transporter%2C permease protein |
| N646_4527 | 3.899 | Putative inner-membrane permease | |
| N646_4487 | 4.958 | Arginine ABC transporter%2C periplasmic arginine-binding protein | |
| N646_4488 | 5.676 | Arginine ABC transporter%2C ATP-binding protein | |
| N646_4486 | 7.585 | ABC-type arginine transport system%2C permease component | |
| Tryptophan metabolism | N646_2210 | 2.123 | Fatty oxidation complex%2C alpha subunit |
| N646_3629 | 2.155 | Tryptophanase | |
| N646_4052 | 5.154 | Putative acyl-CoA thiolase | |
| Lysine degradation | N646_3623 | 2.972 | Transcriptional regulator |
| N646_1979 | 3.332 | Arginine/lysine/ornithine decarboxylase | |
| MAPK signaling pathway | N646_2909 | 0.123 | Cation transport ATPase%2C E1-E2 family protein |
| N646_3134 | 0.369 | Catalase | |
| Glyoxylate and dicarboxylate metabolism | N646_1965 | 2.122 | Acetyl-coenzyme A synthetase |
| N646_2741 | 2.135 | Isocitrate lyase | |
| N646_2740 | 2.88 | Malate synthase | |
| N646_3637 | 3.006 | Malate synthase | |
| Amino sugar and nucleotide sugar metabolism | N646_4226 | 0.400 | Glucose-1-phosphate adenylyltransferase |
| N646_1583 | 2.322 | Beta-N-hexosaminidase | |
| N646_3834 | 2.610 | Hypothetical protein | |
| N646_1582 | 3.440 | Ptative N-acetylglucosamine kinase | |
| N646_4346 | 4.386 | Ptative mannose-6-phosphate isomerase | |
| N646_3455 | 5.366 | Hpothetical protein |
| Metabolic Pathway | Gene ID | Fold Change | Gene Description |
|---|---|---|---|
| Methane metabolism | VP_RS15865 | 0.091 | NapC/NirT family cytochrome c |
| VP_RS15860 | 0.067 | Trimethylamine-N-oxide reductase 2 | |
| VP_RS07325 | 0.224 | Acetate kinase | |
| VP_RS13930 | 0.206 | 2%2C3-bisphosphoglycerate-independent phosphoglycerate mutase | |
| VP_RS18135 | 0.104 | Formate dehydrogenase subunit gamma | |
| VP_RS12615 | 0.320 | Phosphate acetyltransferase | |
| VP_RS07335 | 0.227 | Trimethylamine-N-oxide reductase TorA | |
| VP_RS15585 | 0.304 | S-(hydroxymethyl)glutathione dehydrogenase/class III alcohol dehydrogenase | |
| VP_RS05645 | 0.302 | Phosphoglycerate dehydrogenase | |
| VP_RS07330 | 0.338 | Pentaheme c-type cytochrome TorC | |
| VP_RS05030 | 0.381 | Molecular chaperone TorD | |
| VP_RS15580 | 0.412 | S-formylglutathione hydrolase | |
| VP_RS05640 | 0.342 | 6-phosphofructokinase | |
| Glycolysis/Gluconeogenesis | VP_RS23260 | 0.087 | 6-phospho-beta-glucosidase |
| VP_RS12915 | 0.272 | 6-phospho-beta-glucosidase | |
| VP_RS12215 | 0.310 | Pyruvate dehydrogenase (acetyl-transferring) | |
| VP_RS12210 | 0.331 | Pyruvate dehydrogenase complex dihydrolipoyllysine-residue acetyltransferase | |
| VP_RS13410 | 0.406 | Glucose-6-phosphate isomerase | |
| VP_RS10485 | 0.416 | D-hexose-6-phosphate mutarotase | |
| VP_RS09910 | 0.433 | Pyruvate kinase | |
| VP_RS18295 | 2.558 | 2-oxo acid dehydrogenase subunit E2 | |
| Flagellar assembly | VP_RS22540 | 0.055 | Flagellar biosynthesis protein FliQ |
| VP_RS16540 | 0.064 | Flagellar basal body rod protein FlgB | |
| VP_RS16565 | 0.086 | Flagellar basal-body rod protein FlgG | |
| VP_RS22520 | 0.091 | OmpA family protein | |
| VP_RS16550 | 0.129 | Flagellar hook assembly protein FlgD | |
| VP_RS22605 | 0.193 | Flagellar motor stator protein MotA | |
| VP_RS22545 | 0.210 | Flagellar biosynthetic protein FliR | |
| VP_RS22575 | 0.225 | Flagellar filament capping protein FliD | |
| VP_RS22535 | 0.237 | Flagellar type III secretion system pore protein FliP | |
| VP_RS22490 | 0.265 | Flagellar protein export ATPase FliI | |
| VP_RS16555 | 0.272 | Flagellar basal body protein FlgE | |
| VP_RS22590 | 0.281 | Flagellar hook-length control protein FliK | |
| VP_RS16575 | 0.327 | Flagellar basal body P-ring protein FlgI | |
| VP_RS10920 | 0.363 | Flagellar M-ring protein FliF | |
| VP_RS22495 | 0.366 | Flagellar assembly protein H | |
| VP_RS10900 | 0.386 | Flagella biosynthesis chaperone FliJ | |
| VP_RS16585 | 0.396 | Flagellar hook-associated protein FlgK | |
| VP_RS16590 | 0.412 | Flagellar hook-associated protein FlgL | |
| VP_RS13775 | 0.416 | Sel1 repeat family protein | |
| VP_RS10835 | 0.429 | RNA polymerase sigma factor FliA | |
| VP_RS10895 | 0.452 | Flagellar hook-length control protein FliK | |
| VP_RS03835 | 0.462 | Flagellar hook protein FlgE | |
| VP_RS03855 | 0.490 | Flagellar basal body P-ring protein FlgI | |
| Glucagon signaling pathway | VP_RS01720 | 0.369 | Pyruvate kinase PykF |
| VP_RS18300 | 3.294 | Alpha-ketoacid dehydrogenase subunit beta | |
| VP_RS22915 | 5.913 | Glycogen/starch/alpha-glucan phosphorylase | |
| HIF-1 signaling pathway | VP_RS10480 | 0.168 | Type I glyceraldehyde-3-phosphate dehydrogenase |
| VP_RS14700 | 0.301 | ArsJ-associated glyceraldehyde-3-phosphate dehydrogenase | |
| VP_RS12650 | 0.479 | Phosphoglycerate kinase | |
| Nitrogen metabolism | VP_RS20240 | 0.126 | Nitrite reductase large subunit NirB |
| VP_RS02310 | 0.158 | Glutamate synthase subunit beta | |
| VP_RS20280 | 0.226 | Nitrate reductase | |
| VP_RS02315 | 0.236 | Glutamate synthase large subunit | |
| VP_RS20255 | 0.270 | ABC transporter substrate-binding protein | |
| VP_RS12190 | 0.418 | Carbonate dehydratase | |
| VP_RS20915 | 2.061 | Nitrate reductase cytochrome c-type subunit | |
| VP_RS20910 | 2.197 | Periplasmic nitrate reductase subunit alpha | |
| VP_RS05780 | 14.974 | Hydroxylamine reductase | |
| VP_RS09370 | 19.809 | Ammonia-forming nitrite reductase cytochrome c552 subunit | |
| Glycerolipid metabolism | VP_RS01760 | 0.040 | Dihydroxyacetone kinase ADP-binding subunit DhaL |
| VP_RS01755 | 0.067 | Dihydroxyacetone kinase subunit DhaK | |
| VP_RS21295 | 0.193 | Diacylglycerol kinase | |
| VP_RS11580 | 0.239 | Glycerol kinase GlpK | |
| VP_RS15810 | 0.431 | Glycerate kinase | |
| VP_RS05740 | 2.015 | Triacylglycerol lipase | |
| Apoptosis | VP_RS23210 | 0.086 | Alkyl hydroperoxide reductase subunit C |
| VP_RS20650 | 0.282 | C-type cytochrome | |
| VP_RS02795 | 0.415 | Peroxiredoxin C | |
| Bacterial chemotaxis | VP_RS22610 | 0.101 | OmpA family protein |
| VP_RS22160 | 0.243 | Methyl-accepting chemotaxis protein | |
| VP_RS03815 | 0.255 | Protein-glutamate O-methyltransferase | |
| VP_RS17585 | 0.267 | Methyl-accepting chemotaxis protein | |
| VP_RS22500 | 0.294 | Flagellar motor switch protein FliG | |
| VP_RS22100 | 0.337 | Methyl-accepting chemotaxis protein | |
| VP_RS10915 | 0.356 | Flagellar motor switch protein FliG | |
| VP_RS05760 | 0.374 | Methyl-accepting chemotaxis protein | |
| VP_RS10820 | 0.386 | Chemotaxis protein CheA | |
| VP_RS10825 | 0.389 | Protein phosphatase CheZ | |
| VP_RS10880 | 0.411 | Flagellar motor switch protein FliN | |
| VP_RS03810 | 0.415 | Chemotaxis protein CheV | |
| VP_RS03305 | 0.433 | Flagellar motor protein PomA | |
| VP_RS10815 | 0.471 | Chemotaxis response regulator protein-glutamate methylesterase | |
| VP_RS10830 | 0.473 | Chemotaxis response regulator CheY | |
| VP_RS05310 | 0.486 | Methyl-accepting chemotaxis protein | |
| VP_RS10800 | 0.491 | Chemotaxis protein CheW | |
| Propanoate metabolism | VP_RS01750 | 0.051 | Glycerol dehydrogenase |
| VP_RS04855 | 0.072 | Formate C-acetyltransferase | |
| VP_RS18985 | 0.119 | Acetyl-CoA carboxylase%2C carboxyltransferase subunit beta | |
| VP_RS16405 | 0.240 | Aspartate aminotransferase family protein | |
| VP_RS07930 | 2.084 | 2-methylcitrate synthase | |
| VP_RS07925 | 2.094 | Fe/S-dependent 2-methylisocitrate dehydratase AcnD | |
| VP_RS20545 | 2.450 | CoA-acylating methylmalonate-semialdehyde dehydrogenase | |
| Cationic antimicrobial peptide (CAMP) resistance | VP_RS00200 | 0.120 | Multidrug efflux RND transporter permease subunit VmeD |
| VP_RS00205 | 0.159 | Multidrug efflux RND transporter periplasmic adaptor subunit VmeC | |
| VP_RS21260 | 0.344 | Thiol: disuLfide interchange protein DsbA/DsbL | |
| VP_RS05670 | 0.456 | ATP-binding cassette domain-containing protein | |
| VP_RS21300 | 0.489 | Phosphoethanolamine-lipid A transferase | |
| VP_RS05315 | 2.030 | Multidrug efflux RND transporter periplasmic adaptor subunit VmeA | |
| VP_RS20865 | 2.560 | Multidrug efflux RND transporter periplasmic adaptor subunit VmeY | |
| VP_RS14065 | 4.124 | Envelope stress sensor histidine kinase CpxA | |
| VP_RS14060 | 4.705 | Response regulator | |
| Sulfur metabolism | VP_RS07020 | 0.050 | Dimethyl sulfoxide reductase subunit A |
| VP_RS07030 | 0.052 | Dimethyl sulfoxide reductase anchor subunit | |
| VP_RS07025 | 0.058 | Dimethyl sulfoxide reductase subunit B | |
| VP_RS05930 | 0.110 | Cytochrome subunit of suLfide dehydrogenase | |
| VP_RS03905 | 0.337 | Cysteine synthase A | |
| VP_RS13370 | 0.417 | Assimilatory suLfite reductase (NADPH) hemoprotein subunit | |
| VP_RS13375 | 0.440 | Assimilatory sulfite reductase (NADPH) flavoprotein subunit | |
| VP_RS01435 | 0.442 | Sulfate adenylyltransferase subunit CysN | |
| VP_RS01430 | 0.450 | Sulfate adenylyltransferase subunit CysD | |
| Starch and sucrose metabolism | VP_RS12920 | 0.206 | PTS lactose/cellobiose transporter subunit IIA |
| VP_RS19165 | 0.393 | Glucose-1-phosphate adenylyltransferase | |
| VP_RS03410 | 0.474 | Alpha%2Calpha-phosphotrehalase | |
| VP_RS23025 | 0.498 | Glycogen debranching protein GlgX | |
| VP_RS03405 | 0.499 | PTS trehalose transporter subunit IIBC | |
| VP_RS22910 | 4.693 | 4-alpha-glucanotransferase | |
| Necroptosis | VP_RS04005 | 0.261 | Molecular chaperone HtpG |
| VP_RS00595 | 0.363 | Glutamate-ammonia ligase | |
| Taurine and hypotaurine metabolism | VP_RS10125 | 0.167 | Acetate kinase |
| VP_RS05370 | 0.219 | Alanine dehydrogenase | |
| VP_RS10130 | 0.244 | Phosphate acetyltransferase | |
| Benzoate degradation | VP_RS20635 | 0.295 | Carboxymuconolactone decarboxylase family protein |
| VP_RS20550 | 2.679 | Thiolase family protein | |
| VP_RS00135 | 2.713 | Fatty acid oxidation complex subunit alpha FadB | |
| RNA transport | VP_RS19430 | 0.440 | Stress response translation initiation inhibitor YciH |
| VP_RS01980 | 0.485 | Multifunctional CCA addition/repair protein | |
| Phosphonate and phosphinate metabolism | VP_RS16410 | 0.206 | 2-aminoethylphosphonate--pyruvate Transaminase |
| VP_RS16400 | 0.491 | Phosphonoacetaldehyde hydrolase | |
| Ethylbenzene degradation | VP_RS10720 | 2.111 | Acetyl-CoA C-acyltransferase FadI |
| VP_RS00130 | 2.465 | Acetyl-CoA C-acyltransferase FadA | |
| Biotin metabolism | VP_RS05435 | 0.057 | Dethiobiotin synthase |
| VP_RS21415 | 0.265 | Beta-ketoacyl-ACP reductase | |
| VP_RS05415 | 0.376 | Adenosylmethionine-8-amino-7-oxononanoate transaminase | |
| VP_RS05425 | 0.454 | 8-amino-7-oxononanoate synthase | |
| VP_RS05420 | 0.479 | Biotin synthase BioB | |
| VP_RS05430 | 0.492 | Malonyl-ACP O-methyltransferase BioC | |
| VP_RS20520 | 2.061 | SDR family oxidoreductase |
| Metabolic Pathway | Gene ID | Fold Change | Gene Description |
|---|---|---|---|
| Styrene degradation | VP_RS06550 | 0.394 | Homogentisate 1%2C2-dioxygenase |
| VP_RS06560 | 0.408 | Maleylacetoacetate isomerase | |
| VP_RS06555 | 0.471 | Fumarylacetoacetate hydrolase family protein | |
| Nitrogen metabolism | VP_RS20240 | 2.129 | Nitrite reductase large subunit NirB |
| VP_RS19890 | 2.518 | Nitrite reductase small subunit NirD | |
| VP_RS20235 | 2.823 | Nitrite reductase small subunit NirD | |
| VP_RS20280 | 3.753 | Nitrate reductase | |
| VP_RS20915 | 3.759 | Nitrate reductase cytochrome c-type subunit | |
| VP_RS19895 | 3.988 | Nitrite reductase large subunit NirB | |
| VP_RS20910 | 4.186 | Periplasmic nitrate reductase subunit alpha | |
| VP_RS20250 | 10.250 | ABC transporter permease | |
| VP_RS09370 | 29.586 | Ammonia-forming nitrite reductase cytochrome c552 subunit | |
| VP_RS05780 | 107.754 | Hydroxylamine reductase | |
| Quorum sensing | VP_RS06530 | 0.241 | Oligopeptide ABC transporter permease OppB |
| VP_RS06520 | 0.256 | ATP-binding cassette domain-containing protein | |
| VP_RS06525 | 0.265 | ABC transporter permease subunit | |
| VP_RS06515 | 0.297 | ATP-binding cassette domain-containing protein | |
| VP_RS06485 | 0.310 | ABC transporter ATP-binding protein | |
| VP_RS06495 | 0.346 | ABC transporter permease | |
| VP_RS06535 | 0.362 | Peptide ABC transporter substrate-binding protein | |
| VP_RS20670 | 0.368 | ABC transporter ATP-binding protein | |
| VP_RS06490 | 0.370 | ABC transporter permease | |
| VP_RS20680 | 0.381 | Branched-chain amino acid ABC transporter permease | |
| VP_RS06470 | 0.388 | Polyamine ABC transporter substrate-binding protein | |
| VP_RS21025 | 0.416 | Autoinducer 2-binding periplasmic protein LuxP | |
| VP_RS20695 | 0.455 | ABC transporter ATP-binding protein | |
| VP_RS01695 | 0.468 | Long-chain fatty acid--CoA ligase | |
| VP_RS20675 | 0.475 | ABC transporter substrate-binding protein | |
| VP_RS00850 | 0.495 | ABC transporter ATP-binding protein | |
| VP_RS12050 | 2.098 | ABC transporter ATP-binding protein | |
| VP_RS15305 | 2.117 | GTP cyclohydrolase II | |
| VP_RS22315 | 2.159 | ABC transporter ATP-binding protein | |
| VP_RS12040 | 2.232 | ABC transporter permease | |
| VP_RS08360 | 2.551 | Two-component sensor histidine kinase | |
| VP_RS22015 | 2.976 | Response regulator transcription factor | |
| VP_RS08355 | 3.014 | Response regulator | |
| VP_RS16930 | 3.141 | Permease | |
| Folate biosynthesis | VP_RS17975 | 0.476 | Phenylalanine 4-monooxygenase |
| VP_RS09130 | 0.494 | Aminodeoxychorismate synthase component I | |
| VP_RS03365 | 0.491 | NADPH-dependent 7-cyano-7-deazaguanine reductase QueF | |
| VP_RS07885 | 0.497 | 7-cyano-7-deazaguanine synthase QueC | |
| VP_RS09170 | 0.389 | 6-carboxytetrahydropterin synthase QueD | |
| VP_RS13730 | 0.433 | Aminodeoxychorismate/anthranilate synthase component II | |
| VP_RS07890 | 0.484 | 7-carboxy-7-deazaguanine synthase QueE | |
| VP_RS17980 | 0.432 | 4a-hydroxytetrahydrobiopterin dehydratase | |
| VP_RS01970 | 0.431 | 2-amino-4-hydroxy-6-hydroxymethyldihydropteridine diphosphokinase | |
| Histidine metabolism | VP_RS06185 | 10.231 | Urocanate hydratase |
| VP_RS06180 | 6.284 | Histidine ammonia-lyase | |
| VP_RS06195 | 6.998 | Imidazolonepropionase | |
| VP_RS06190 | 5.106 | Formimidoylglutamase | |
| VP_RS05565 | 0.496 | Bifunctional phosphoribosyl-AMP cyclohydrolase/phosphoribosyl-ATP diphosphatase HisIE |
| Metabolic Pathway | Gene ID | Fold Change | Gene Description |
|---|---|---|---|
| Flagellar assembly | BCN_RS08555 | 0.038 | Flagellar assembly protein FliH |
| BCN_RS08605 | 0.045 | Flagellin | |
| BCN_RS08610 | 0.072 | Flagellin | |
| BCN_RS08640 | 0.108 | Flagellar type III secretion system pore protein FliP | |
| BCN_RS08550 | 0.113 | Flagellar motor switch protein FliG | |
| BCN_RS22265 | 0.115 | Flagellar motor stator protein MotA | |
| BCN_RS22260 | 0.143 | Flagellar motor protein MotB | |
| BCN_RS08545 | 0.154 | Flagellar M-ring protein FliF | |
| BCN_RS08470 | 0.158 | Flagellar motor switch protein | |
| BCN_RS08560 | 0.158 | Flagellar protein export ATPase FliI | |
| BCN_RS08535 | 0.173 | Flagellar basal body rod protein FlgC | |
| BCN_RS08670 | 0.188 | Flagellar basal-body rod protein FlgG | |
| BCN_RS08520 | 0.196 | Flagellar protein FliS | |
| BCN_RS08530 | 0.197 | Flagellar basal body rod protein FlgB | |
| BCN_RS08625 | 0.200 | Flagellar motor switch protein FliM | |
| BCN_RS08660 | 0.230 | Flagellar biosynthesis protein FlhA | |
| BCN_RS08510 | 0.241 | Flagellar hook-associated protein 3 | |
| BCN_RS08655 | 0.392 | Flagellar type III secretion system protein FlhB | |
| BCN_RS08650 | 0.438 | Flagellar type III secretion system protein FliR | |
| Bacterial chemotaxis | BCN_RS10010 | 0.063 | Methyl-accepting chemotaxis protein |
| BCN_RS03675 | 0.088 | Methyl-accepting chemotaxis protein | |
| BCN_RS02280 | 0.185 | Methyl-accepting chemotaxis protein | |
| BCN_RS08460 | 0.186 | Response regulator | |
| BCN_RS08625 | 0.200 | Flagellar motor switch protein FliM | |
| BCN_RS25160 | 0.265 | DUF4077 domain-containing protein | |
| BCN_RS24975 | 0.321 | Methyl-accepting chemotaxis protein | |
| BCN_RS08595 | 0.357 | Chemotaxis protein | |
| BCN_RS08455 | 0.474 | OmpA family protein | |
| Two-component system | BCN_RS27005 | 0.136 | Respiratory nitrate reductase subunit gamma |
| BCN_RS26190 | 0.152 | Cytochrome d ubiquinol oxidase subunit II | |
| BCN_RS23710 | 0.219 | Potassium-transporting ATPase subunit KdpA | |
| BCN_RS27000 | 0.231 | Acetyl-CoA C-acyltransferase | |
| BCN_RS23715 | 0.258 | Methyl-accepting chemotaxis protein | |
| BCN_RS04080 | 0.385 | Nitrate reductase molybdenum cofactor assembly chaperone | |
| BCN_RS15080 | 0.401 | Response regulator | |
| BCN_RS04090 | 0.419 | Methyl-accepting chemotaxis protein | |
| BCN_RS07505 | 2.006 | Phosphate ABC transporter substrate-binding protein PstS | |
| BCN_RS26540 | 2.297 | Cytochrome ubiquinol oxidase subunit I | |
| BCN_RS17290 | 2.348 | Chemotaxis protein CheA | |
| BCN_RS02700 | 3.703 | Antiholin-like murein hydrolase modulator LrgA | |
| BCN_RS10795 | 4.600 | Acetyl-CoA C-acetyltransferase | |
| BCN_RS07495 | 5.804 | Hypothetical protein | |
| Thiamine metabolism | BCN_RS29465 | 0.031 | TenA family transcriptional regulator |
| BCN_RS02365 | 0.205 | Thiamine phosphate synthase | |
| BCN_RS04005 | 0.224 | Thiaminase II | |
| BCN_RS04040 | 0.274 | Thiazole synthase | |
| BCN_RS04030 | 0.282 | Glycine oxidase ThiO | |
| BCN_RS04050 | 0.304 | Bifunctional hydroxymethylpyrimidine kinase/phosphomethylpyrimidine kinase | |
| BCN_RS04025 | 0.310 | Thiazole tautomerase TenI | |
| BCN_RS25935 | 0.320 | Phosphomethylpyrimidine synthase ThiC | |
| BCN_RS21485 | 0.342 | Alkaline phosphatase | |
| BCN_RS12695 | 0.397 | Thiaminase II | |
| BCN_RS02360 | 0.407 | Hydroxyethylthiazole kinase | |
| BCN_RS10005 | 0.407 | Ribosome small subunit-dependent GTPase A | |
| BCN_RS22955 | 0.433 | Cysteine desulfurase | |
| BCN_RS02660 | 0.457 | Acetylornithine deacetylase | |
| ABC transporters | BCN_RS03130 | 0.051 | Amino acid ABC transporter permease |
| BCN_RS14125 | 0.051 | Glycine betaine ABC transporter substrate-binding protein | |
| BCN_RS15895 | 0.056 | Substrate-binding domain-containing protein | |
| BCN_RS06920 | 0.179 | ABC transporter ATP-binding protein | |
| BCN_RS17880 | 0.205 | Ribose ABC transporter ATP-binding protein RbsA | |
| BCN_RS01110 | 0.221 | Amino acid ABC transporter ATP-binding protein | |
| BCN_RS06915 | 0.225 | Peptide ABC transporter substrate-binding protein | |
| BCN_RS01100 | 0.258 | Amino acid ABC transporter ATP-binding protein | |
| BCN_RS04010 | 0.263 | Phosphate ABC transporter permease PstA | |
| BCN_RS08770 | 0.268 | Peptide ABC transporter substrate-binding protein | |
| BCN_RS14120 | 0.268 | BMP family protein | |
| BCN_RS20515 | 0.272 | ABC transporter ATP-binding protein | |
| BCN_RS03855 | 0.278 | Phosphonate ABC transporter ATP-binding protein | |
| BCN_RS01165 | 0.282 | Molybdate ABC transporter permease subunit | |
| BCN_RS20525 | 0.283 | ABC transporter ATP-binding protein | |
| BCN_RS21100 | 0.320 | Metal ABC transporter substrate-binding protein | |
| BCN_RS04020 | 0.322 | ABC transporter substrate-binding protein | |
| BCN_RS04015 | 0.326 | Phosphate ABC transporter permease subunit PstC | |
| BCN_RS03845 | 0.330 | ATP-binding cassette domain-containing protein | |
| BCN_RS03850 | 0.347 | Phosphate ABC transporter ATP-binding protein | |
| BCN_RS24655 | 0.347 | Transporter substrate-binding domain-containing protein | |
| BCN_RS01125 | 0.351 | Putative 2-aminoethylphosphonate ABC transporter ATP-binding protein | |
| BCN_RS20520 | 0.355 | Aliphatic sulfonate ABC transporter substrate-binding protein | |
| BCN_RS18335 | 0.379 | Iron ABC transporter permease | |
| BCN_RS09350 | 0.405 | Energy-coupling factor transporter transmembrane protein EcfT | |
| BCN_RS24665 | 0.405 | Putative 2-aminoethylphosphonate ABC transporter substrate-binding protein | |
| BCN_RS01160 | 0.413 | Molybdate ABC transporter substrate-binding protein | |
| BCN_RS04750 | 0.458 | ABC transporter permease | |
| BCN_RS01870 | 0.465 | ABC transporter permease | |
| BCN_RS17755 | 0.470 | Methionine ABC transporter substrate-binding lipoprotein MetQ | |
| BCN_RS03600 | 0.487 | Phosphate ABC transporter substrate-binding protein PstS | |
| BCN_RS09570 | 0.487 | Peptide ABC transporter substrate-binding protein | |
| BCN_RS10085 | 0.487 | Sugar ABC transporter permease | |
| BCN_RS09640 | 4.508 | Thiol reductant ABC exporter subunit CydC | |
| BCN_RS26090 | 14.65 | ABC transporter substrate-binding protein | |
| BCN_RS13495 | 20.285 | MetQ/NlpA family ABC transporter substrate-binding protein | |
| Arginine biosynthesis | BCN_RS20420 | 0.070 | N-acetyl-gamma-glutamyl-phosphate reductase |
| BCN_RS20400 | 0.117 | Ornithine carbamoyltransferase | |
| BCN_RS20410 | 0.159 | Acetylglutamate kinase | |
| BCN_RS20405 | 0.171 | Acetylornithine transaminase | |
| BCN_RS20415 | 0.271 | Bifunctional glutamate N-acetyltransferase/amino-acid acetyltransferase ArgJ | |
| BCN_RS00945 | 0.281 | Arginase | |
| BCN_RS22860 | 0.292 | Argininosuccinate lyase | |
| BCN_RS22865 | 0.486 | Argininosuccinate synthase | |
| Nitrogen metabolism | BCN_RS07150 | 0.365 | Nitronate monooxygenase |
| BCN_RS10835 | 5.001 | Nitrate transporter NarK | |
| BCN_RS10790 | 6.281 | Nitrate reductase subunit beta | |
| BCN_RS10800 | 7.880 | Respiratory nitrate reductase subunit gamma | |
| BCN_RS10785 | 8.675 | Nitrate reductase subunit alpha | |
| BCN_RS10870 | 8.912 | Nitrite reductase small subunit NirD | |
| BCN_RS10875 | 15.156 | NADPH-nitrite reductase large subunit | |
| BCN_RS16540 | 150.780 | Hydroxylamine reductase | |
| Riboflavin metabolism | BCN_RS20310 | 3.325 | Bifunctional diaminohydroxyphosphoribosylaminopyrimidine deaminase/5-Amino-6-(5-phosphoribosylamino) uracil reductase RibD |
| BCN_RS20320 | 4.247 | Bifunctional 3%2C4-dihydroxy-2-butanone 4-phosphate synthase/GTP Cyclohydrolase II | |
| BCN_RS20325 | 4.361 | 6%2C7-dimethyl-8-ribityllumazine synthase | |
| BCN_RS20315 | 4.769 | Riboflavin synthase subunit alpha | |
| Pyrimidine metabolism | BCN_RS15125 | 0.304 | 5’-nucleotidase C-terminal domain-containing protein |
| BCN_RS24625 | 0.355 | Bifunctional metallophosphatase/5’-nucleotidase | |
| BCN_RS18815 | 0.381 | Carbamoyl-phosphate synthase large subunit | |
| BCN_RS18820 | 0.406 | Carbamoyl phosphate synthase small subunit | |
| BCN_RS18795 | 0.419 | Orotate phosphoribosyltransferase | |
| BCN_RS18805 | 0.430 | Dihydroorotate oxidase B catalytic subunit | |
| BCN_RS18800 | 0.438 | Orotidine-5’-phosphate decarboxylase | |
| BCN_RS18810 | 0.441 | Dihydroorotate oxidase B electron transfer subunit | |
| BCN_RS20265 | 0.445 | 5’-nucleotidase C-terminal domain-containing protein | |
| BCN_RS18825 | 0.449 | Dihydroorotase | |
| BCN_RS07895 | 0.462 | Nucleoside-diphosphate kinase | |
| BCN_RS09440 | 0.473 | Pyrimidine-nucleoside phosphorylase | |
| HIF-1 signaling pathway | BCN_RS24725 | 0.191 | L-lactate dehydrogenase |
| BCN_RS25405 | 2.598 | Phosphoglycerate kinase | |
| BCN_RS25410 | 2.736 | Type I glyceraldehyde-3-phosphate dehydrogenase | |
| BCN_RS25390 | 3.143 | phosphopyruvate hydratase | |
| BCN_RS24095 | 5.531 | L-lactate dehydrogenase | |
| Fatty acid degradation | BCN_RS17445 | 0.340 | Acetyl-CoA C-acetyltransferase |
| BCN_RS17450 | 0.456 | Acyl-CoA synthetase | |
| Alanine, aspartate and glutamate metabolism | BCN_RS08845 | 0.353 | Glutaminase A |
| BCN_RS08855 | 0.361 | Hypothetical protein | |
| BCN_RS19905 | 0.420 | Carbon-nitrogen family hydrolase | |
| BCN_RS15030 | 0.486 | Asparaginase | |
| BCN_RS03305 | 0.498 | Aspartate ammonia-lyase | |
| BCN_RS00970 | 2.986 | Glutamine--fructose-6-phosphate transaminase (isomerizing) | |
| BCN_RS03230 | 7.200 | Alanine dehydrogenase | |
| Benzoate degradation | BCN_RS26535 | 2.191 | 3-hydroxybutyryl-CoA dehydrogenase |
| BCN_RS24780 | 2.199 | Acetyl-CoA C-acetyltransferase | |
| BCN_RS24785 | 2.285 | 3-hydroxyacyl-CoA dehydrogenase/enoyl-CoA hydratase family protein | |
| Glycolysis/Gluconeogenesis | BCN_RS08815 | 0.225 | Histidine phosphatase family protein |
| BCN_RS21600 | 0.299 | Bifunctional acetaldehyde-CoA/alcohol dehydrogenase | |
| BCN_RS11285 | 0.411 | Alcohol dehydrogenase AdhP | |
| BCN_RS28275 | 0.413 | S-(hydroxymethyl)glutathione dehydrogenase/class III alcohol dehydrogenase | |
| BCN_RS22940 | 0.489 | Acyl-CoA ligase | |
| BCN_RS26420 | 2.666 | PTS glucose transporter subunit IIA | |
| BCN_RS25395 | 2.901 | 2%2C3-bisphosphoglycerate-independent phosphoglycerate mutase | |
| BCN_RS25815 | 5.561 | 6-phospho-beta-glucosidase | |
| Inositol phosphate metabolism | BCN_RS18155 | 0.186 | Phosphatidylinositol diacylglycerol-lyase |
| BCN_RS03640 | 0.245 | Phospholipase C | |
| BCN_RS25400 | 2.616 | Triose-phosphate isomerase | |
| Butanoate metabolism | BCN_RS02750 | 0.158 | Formate C-acetyltransferase |
| BCN_RS07305 | 0.199 | Acetolactate synthase large subunit | |
| BCN_RS11410 | 0.359 | Acetate CoA-transferase subunit alpha | |
| BCN_RS11415 | 0.382 | CoA transferase subunit B | |
| BCN_RS04800 | 2.474 | Alpha-acetolactate decarboxylase | |
| Propanoate metabolism | BCN_RS18555 | 0.407 | ADP-forming succinate--CoA ligase subunit beta |
| BCN_RS07995 | 0.451 | Methylglyoxal synthase | |
| BCN_RS18550 | 0.467 | Succinate-CoA ligase subunit alpha |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yang, L.; Liu, P.; Jin, Y.; Qin, S.; Chen, L. Identification of Antibacterial Components in the Methanol-Phase Extract from Edible Herbaceous Plant Rumex madaio Makino and Their Antibacterial Action Modes. Molecules 2022, 27, 660. https://doi.org/10.3390/molecules27030660
Liu Y, Yang L, Liu P, Jin Y, Qin S, Chen L. Identification of Antibacterial Components in the Methanol-Phase Extract from Edible Herbaceous Plant Rumex madaio Makino and Their Antibacterial Action Modes. Molecules. 2022; 27(3):660. https://doi.org/10.3390/molecules27030660
Chicago/Turabian StyleLiu, Yue, Lianzhi Yang, Pingping Liu, Yinzhe Jin, Si Qin, and Lanming Chen. 2022. "Identification of Antibacterial Components in the Methanol-Phase Extract from Edible Herbaceous Plant Rumex madaio Makino and Their Antibacterial Action Modes" Molecules 27, no. 3: 660. https://doi.org/10.3390/molecules27030660
APA StyleLiu, Y., Yang, L., Liu, P., Jin, Y., Qin, S., & Chen, L. (2022). Identification of Antibacterial Components in the Methanol-Phase Extract from Edible Herbaceous Plant Rumex madaio Makino and Their Antibacterial Action Modes. Molecules, 27(3), 660. https://doi.org/10.3390/molecules27030660







