Sesquiterpenes and Monoterpenes from the Leaves and Stems of Illicium simonsii and Their Antibacterial Activity
Abstract
:1. Introduction
2. Results
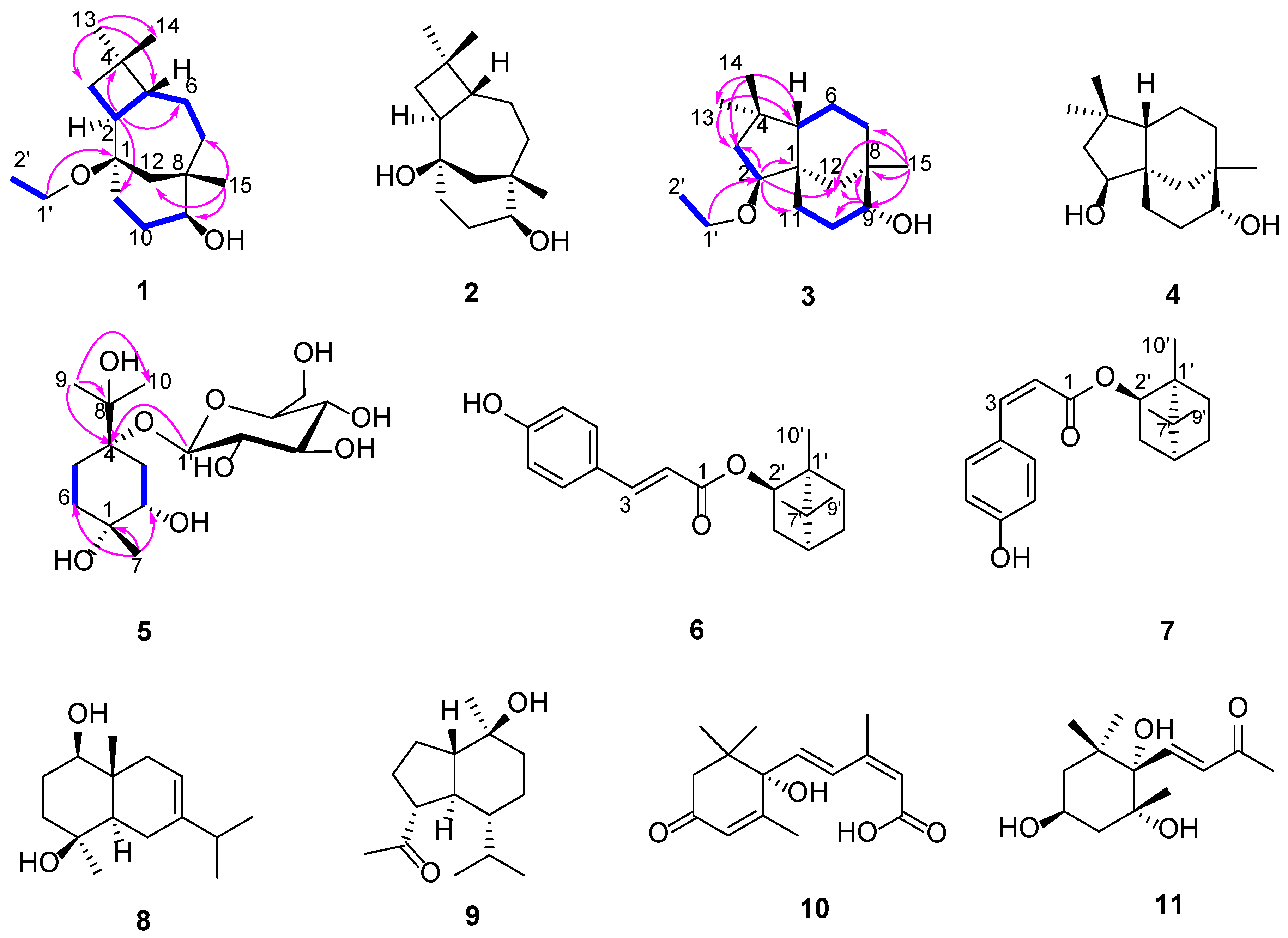
2.1. Chemistry
2.2. Antibacterial Activity
2.2.1. Antimicrobial Activity Test of the I. simonsii Extract
2.2.2. Determination of Minimum Inhibitory Concentration (MIC)
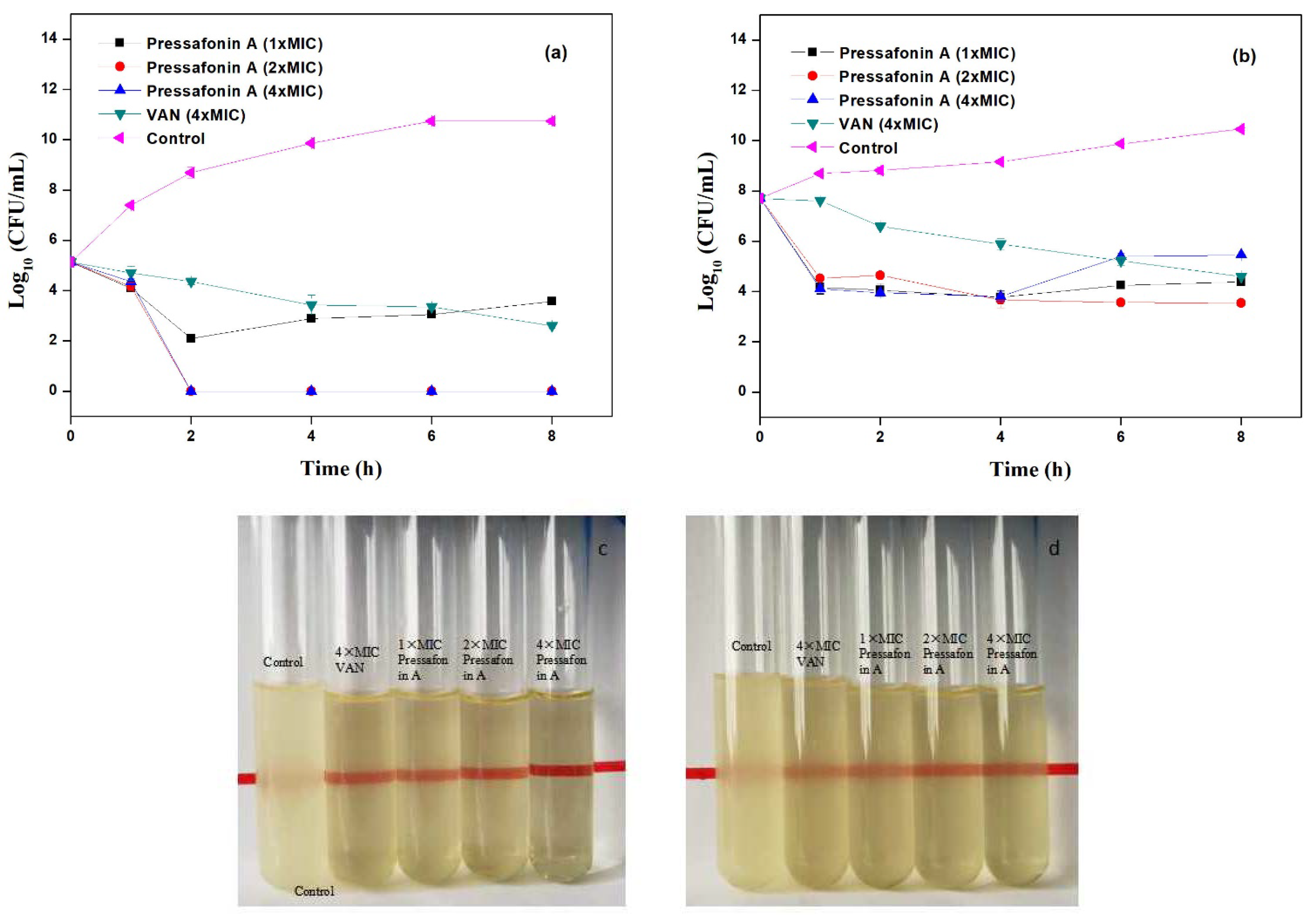
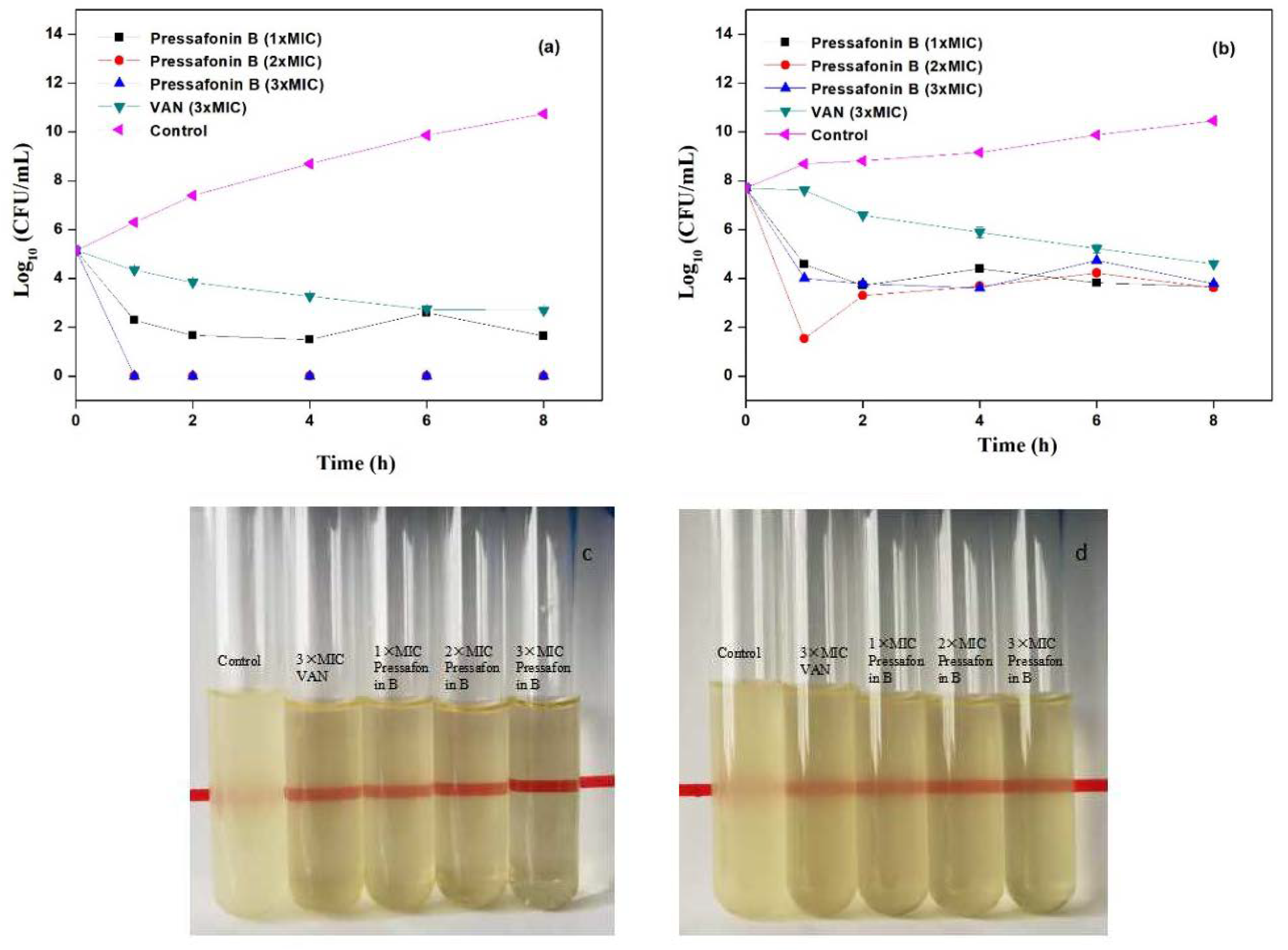
2.2.3. Time−Kill Kinetics Assay
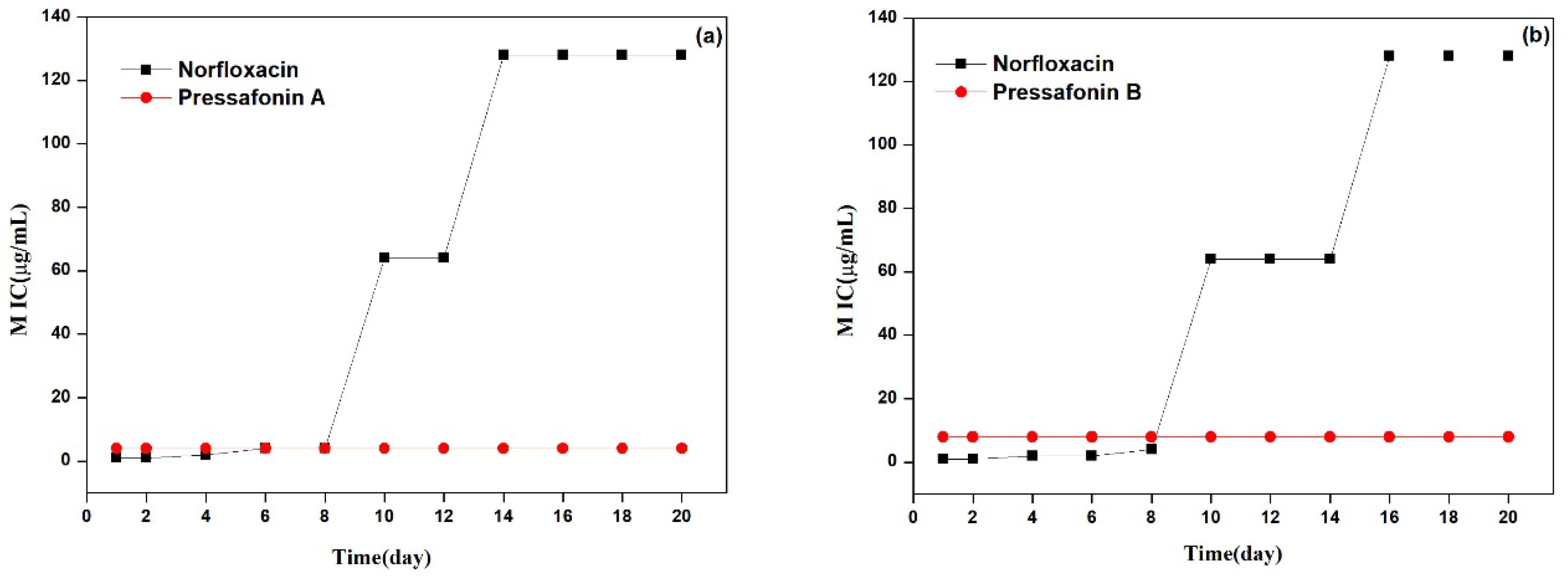
2.2.4. Resistance Development Study
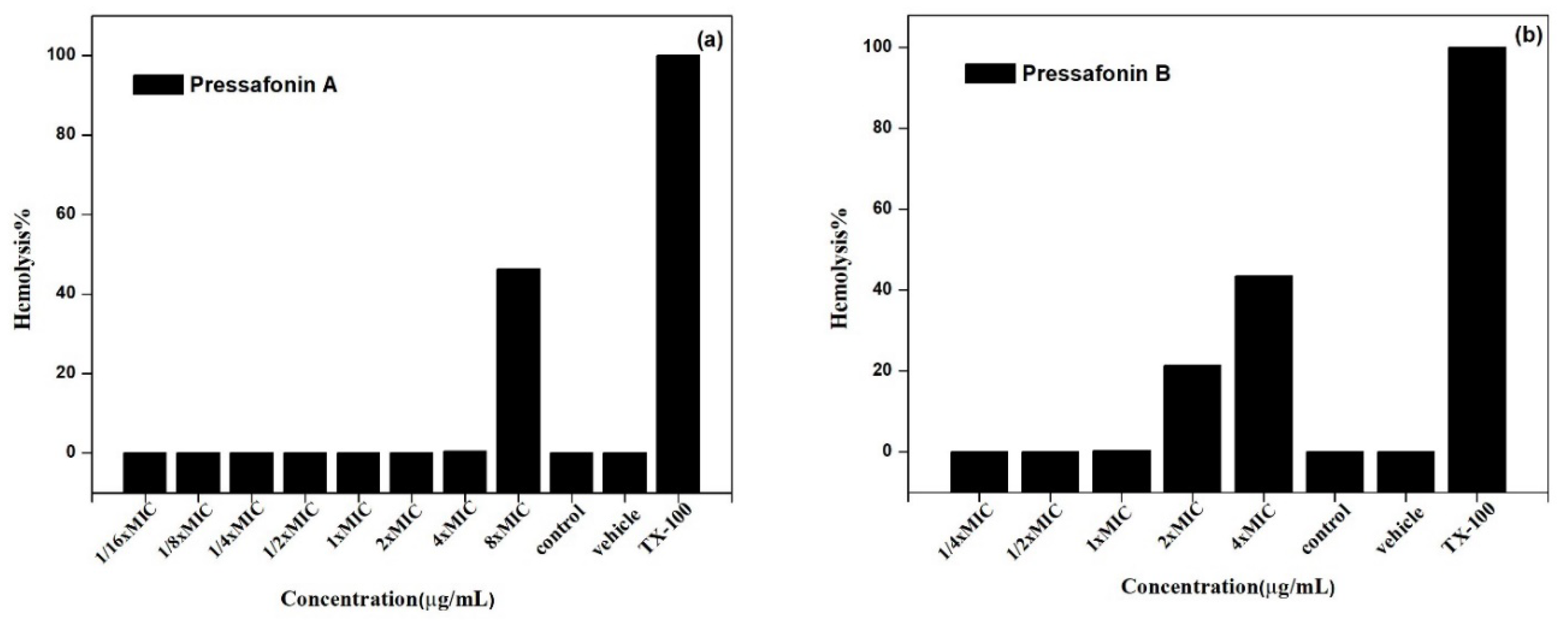
2.2.5. Hemolytic Activity
2.3. Mode of Antimicrobial Action
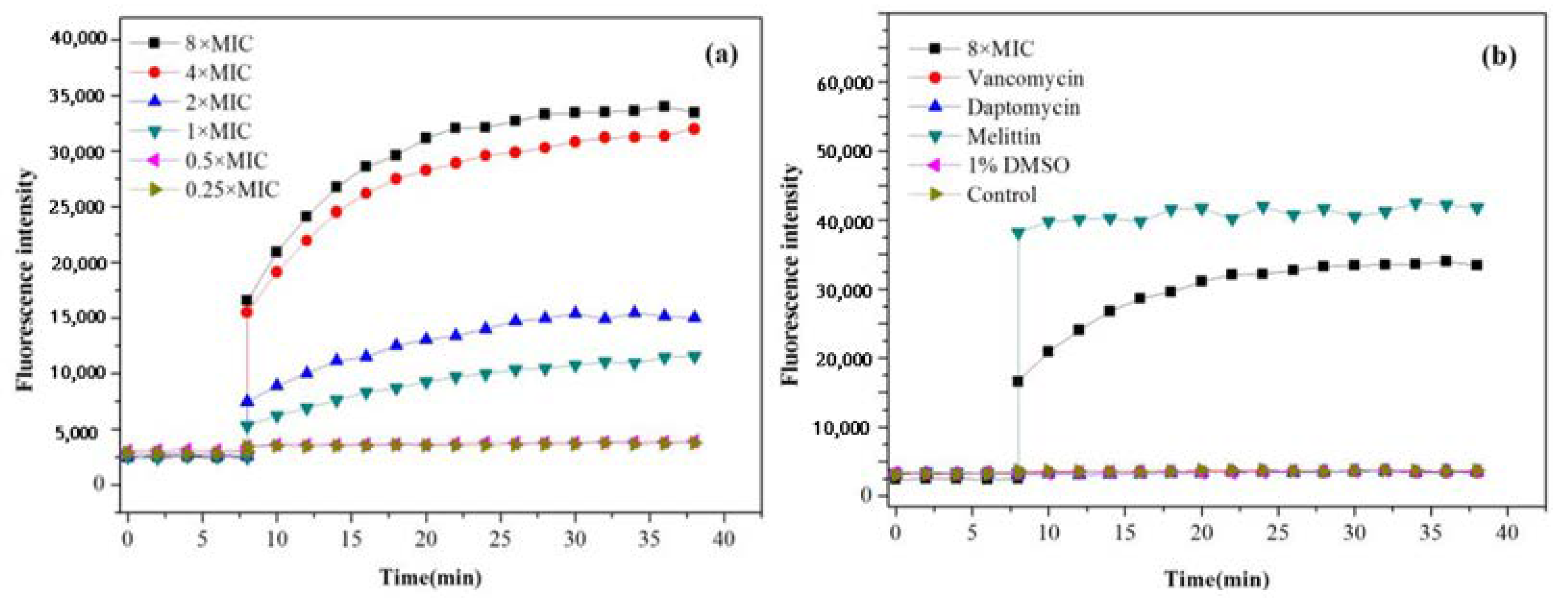
2.3.1. SYTOX Green Assay
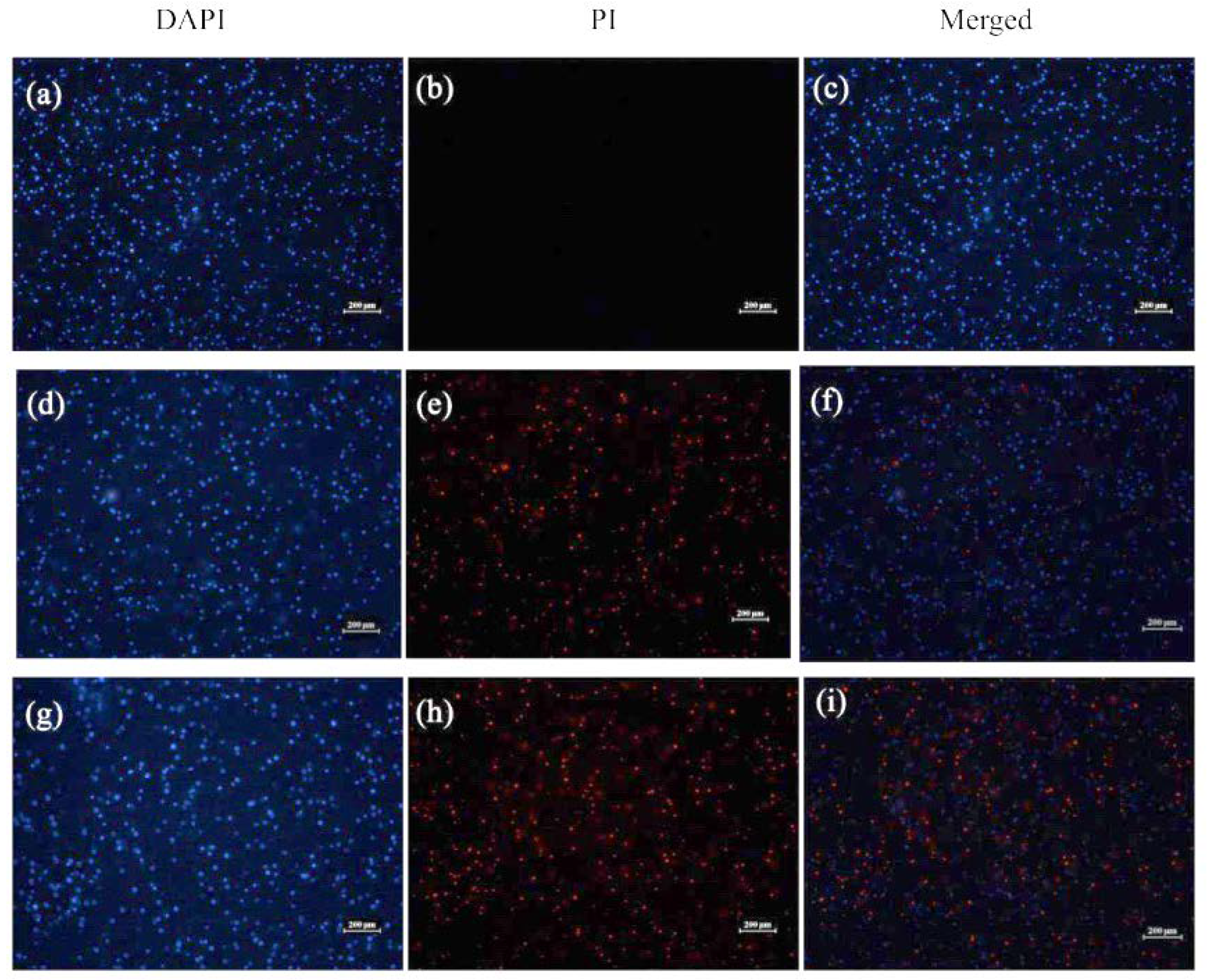
2.3.2. Visualization of Bacterial Membrane Permeability
3. Discussion
4. Material and Methods
4.1. General Experimental Procedures
4.2. Plant Material, Separation and Purification of Compounds 1–11
4.3. Antibacterial Assays
4.3.1. Bacterial Strains and Growth Condition
4.3.2. Antimicrobial Activity by K-B Disk Diffusion Test
4.3.3. Minimum Inhibitory Concentration (MIC) Assay
4.3.4. Time–Kill Kinetics Assay
4.3.5. Bacteria Resistance Study
4.3.6. Hemolytic Activity
4.4. Investigation of Antibacterial Mechanism
4.4.1. SYTOX Green Staining Assay
4.4.2. Visualization of Bacterial Membrane Permeability
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Richter, M.F.; Drown, B.S.; Riley, A.P.; Garcia, A.; Shirai, T.; Svec, R.L.; Hergenrother, P.J. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 2017, 545, 299–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connell, K.M.G.; Hodgkinson, J.T.; Sore, H.F.; Welch, M.; Salmond, G.P.C.; Spring, D.R. Combating multidrug-resistant bacteria: Current strategies for the discovery of novel antibacterials. Angew. Chem. Int. Ed. 2013, 52, 10706–10733. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Therapeutic challenges in the era of antibiotic resistance. Int. J. Antimicrob. Ag. 2008, 32, S197–S199. [Google Scholar] [CrossRef]
- Howden, B.P.; Grayson, M.L. Dumb and dumber-the potential waste of a useful antistaphylococcal agent: Emerging fusidic acid resistance in Staphylococcus aureus. Clin. Infect. Dis. 2006, 42, 394–400. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.Y.; Wang, X.X.; Zhuang, P.Y.; Feng, Y.J.; Jin, X.Y. Sesquiterpenoids and lignans from the fruits of Illicium simonsii Maxim. Biochem. Syst. Ecol. 2019, 83, 47–50. [Google Scholar] [CrossRef]
- Chu, S.S.; Liu, S.L.; Jiang, G.H.; Liu, Z.L. Composition and toxicity of essential oil of Illicium simonsii Maxim (Illiciaceae) fruit against the maize weevils. Rec. Nat. Prod. 2010, 4, 205–210. [Google Scholar]
- Wei, D.D.; Wang, J.S.; Kong, L.Y. Reversal effects of components from the fruits of Illicium simonsii on human adriamycin-resistant MCF-7 and 5-fluorouracil-resistant Bel7402 cells. Phytother. Res. 2012, 26, 562–567. [Google Scholar] [CrossRef]
- Guo, Y.; Bao, C.N.; Li, F.; Hou, E.H.; Qin, S.S.; Zhang, Q.R.; Liu, J.F. Discovery, synthesis, and biological evaluation of dunnianol-based mannich bases against methicillin-resistant Staphylococcus Aureus (MRSA). ACS Infect. Dis. 2020, 6, 2478–2489. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, R.; Chen, F.; Yan, T.; Wen, T.; Li, F.; Su, X.; Wang, L.; Du, J.; Liu, J. Triphenyl-sesquineolignan analogues derived from Illicium simonsii Maxim exhibit potent antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA) by disrupting bacterial membranes. Bioorganic. Chem. 2021, 110, 104824. [Google Scholar] [CrossRef]
- Liu, J.F.; Zhang, X.M.; Shi, Y.; Zhang, Q.; Ma, Y.B.; Chen, J.J. Chemical constituents in stems and leaves of Illicium simonsii. Chin. Tradit. Herbal Drugs 2012, 43, 51–54. [Google Scholar]
- Heymann, H.; Tezuka, Y.; Kikuchi, T.; Supriyatna, S. Constituents of Sindora sumatrana MIQ.I. isolation and NMR spectral analysis of sesquiterpenes from the dried pods. Chem. Pharm. Bull. 1994, 42, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.F.; Li, H.J.; Yang, L.J.; Liu, M.Q.; Bi, Y.F.; Zhang, Y.B. Two new monoterpenes from the fruits of Illicium lanceolatum. Molecules 2013, 18, 11866–11872. [Google Scholar] [CrossRef]
- Cheng, M.J.; Lo, W.L.; Huang, J.C.; Yeh, Y.T.; Hong, Z.L.; Lu, Y.C.; Chang, M.S.; Chen, C.Y. Isolation of a new monoterpenic ester from the leaves of Michelia compressa (Maxim.) Sargent var. formosana Kanehira (Magnoliaceae). Nat. Prod. Res. 2010, 24, 682–686. [Google Scholar] [CrossRef]
- Hu, Y.M.; Yang, Z.L.; Ye, W.C.; Cheng, Q.H. Studies on the constituents in rhizome of Homalomena occuta. Chin. J. Chin. Mater. Med. 2003, 28, 342–344. [Google Scholar]
- Liu, C.M.; Zhou, H.B.; Zhang, W.D. Terpenoids from stems and leaves of Cupressus gigantea. Chin. J. Nat. Med. 2010, 8, 0405–0410. [Google Scholar] [CrossRef]
- Liu, X.; Xu, W.; Yang, X.; Zhang, P.; Zhao, W.; Gong, Y.; Liu, N. Study on non-flavonoids chemical constituents from Spatholobi Caulis. Chin. J. Chin. Mater. Med. 2020, 45, 1120–1127. [Google Scholar]
- Park, J.H.; Lee, D.G.; Yeon, S.W.; Kwon, H.S.; Ko, J.H.; Shin, D.J.; Park, H.S.; Kim, Y.S.; Bang, M.H.; Baek, N.I. Isolation of megastigmane sesquiterpenes from the Silkworm (Bombyx mori L.) droppings and their promotion activity on HO-1 and SIRT1. Arch. Pharm. Res. 2011, 34, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Berney, M.; Hammes, F.; Bosshard, F.; Weilenmann, H.U.; Egli, T. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight kit in combination with flow cytometry. Appl. Environ. Microbiol. 2007, 73, 3283–3290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzamic, A.; Sokovic, M.; Ristic, M.S.; Grijic-Jovanovic, S.; Vukojevic, J.; Marin, P.D. Chemical composition and antifungal activity of Illicium verum and Eugenia caryophyllata essential oils. Chem. Nat. Compd. 2009, 45, 259–261. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Duraipandiyan, V.; Jeyaraj, B.; Agastian, P.; Raj, M.K.; Ignacimuthu, S. Phytochemical analysis and in vitro antimicrobial activity of Illicium griffithii Hook. f. & Thoms extracts. Asian Pac. J. Trop. Dis. 2012, 2, 190–199. [Google Scholar]
- Min, Y.; Zhang, W.; Yang, J.; Liu, W. Chemical composition and antimicrobial activity of volatile components from wild Illicium wardii in China. Adv. Mat. Res. 2011, 239–242, 3367–3370. [Google Scholar] [CrossRef]
- Szymulanska-Ramamurthy, K.M.; Zhao, M.; Che, C.T. Phytochemical study of Illicium angustisepalum and its biological activities. Acta Pharm. Sin. B 2017, 7, 485–490. [Google Scholar] [CrossRef]
- Liu, J.F.; Jiang, Z.Y.; Geng, C.A.; Zou, X.B.; Shi, Y.; Ma, Y.B.; Zhang, X.M.; Chen, J.J. Two new phenylpropanoid derivatives and other constituents from Illicium simonsii active against oral microbial organisms. Planta Med. 2010, 76, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Setzer, W.N.; Setzer, M.C.; Bates, R.B.; Nakkiew, P.; Jackes, B.R.; Chen, L.; McFerrin, M.B.; Meehan, E.J. Antibacterial hydroxycinnamic esters from Piper caninum from Paluma, North Queensland, Australia. The crystal and molecular structure of (+)-bornyl coumarate. Planta Med. 1999, 65, 747–749. [Google Scholar] [CrossRef]
- e Silva, A.T.M.; Pereira, V.V.; Takahashi, J.A.; Silva, R.R.; Duarte, L.P. Microwave-assisted synthesis of borneol esters and their antimicrobial activity. Nat. Prod. Res. 2018, 32, 1714–1720. [Google Scholar] [CrossRef]
- Konuk, H.B.; Ergüden, B. Phenolic–OH group is crucial for the antifungal activity of terpenoids via disruption of cell membrane integrity. Folia Microbiol. 2020, 65, 775–783. [Google Scholar] [CrossRef]
- Epand, R.M.; Vogel, H.J. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1999, 1462, 11–28. [Google Scholar] [CrossRef] [Green Version]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 980–987. [Google Scholar] [CrossRef]
- Kim, W.; Zhu, W.; Hendricks, G.L.; Tyne, T.D.; Steele, A.D.; Keohane, C.E.; Fricke, N.; Conery, A.L.; Shen, S.; Pan, W.; et al. A new class of synthetic retinoid antibiotics effective against bacterial persisters. Nature 2018, 556, 103–107. [Google Scholar] [CrossRef]
- Drew, W.L.; Barry, A.L.; Toole, R.; Sherris, J.C. Reliability of the Kirby-Bauer disc diffusion method for detecting methicillin-resistant strains of Staphylococcus aureus. Appl. Microbiol. 1972, 24, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.B.; Weinstein, M.P.; Eliopoulos, G.M.; Jenkins, S.G.; Lewis, J.S.; Limbago, B.; Mathers, A.J.; Mazzulli, T.; Patel, R.S.; Richter, S.; et al. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Seventh Informational Supplement M100-S27; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Guo, Y.; Xu, T.; Bao, C.; Liu, Z.; Fan, J.; Yang, R.; Qin, S. Design and synthesis of new norfloxacin-1, 3, 4-oxadiazole hybrids as antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Pharm. Sci. 2019, 136, 104966. [Google Scholar] [CrossRef] [PubMed]
- Konai, M.M.; Haldar, J. Fatty acid comprising lysine conjugates: Anti-MRSA agents that display in vivo efficacy by disrupting biofilms with no resistance development. Bioconjug. Chem. 2017, 28, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, D.; Wang, L.; Lin, C.; Ma, C.; Xi, X.; Chen, T.; Shaw, C.; Zhou, M. Dermaseptin-PH: A novel peptide with antimicrobial and anticancer activities from the skin secretion of the South American orange-legged leaf frog, Pithecopus (phyllomedusa) hypochondrialis. Molecules 2017, 22, 1805. [Google Scholar] [CrossRef] [PubMed]
| Position | 1 | 3 | ||
|---|---|---|---|---|
| δH (Multi, J in Hz) | δC (Multi) | δH (Multi, J in Hz) | δC (Multi) | |
| 1 | – | 75.3 (s) | – | 44.2 (s) |
| 2 | 2.09–2.14 (m) | 38.8 (d) | 3.42 (dd, 10.6, 5.5) | 88.1 (d) |
| 3α | 1.61 (d, 9.3) | 36.3 (t) | 1.66 (dd, 11.8, 5.6) | 45.0 (t) |
| 3β | 1.48–1.51 (overlap) | 1.49 (dd, 11.4, 11.0) | ||
| 4 | – | 35.2 (s) | – | 36.9 (s) |
| 5 | 1.90–1.93 (m) | 44.9 (d) | 1.40–1.42 (overlap) | 50.6 (d) |
| 6α | 1.51–1.53 (m) | 20.9 (t) | 1.40–1.42 (overlap) | 20.6 (t) |
| 6β | 1.31–1.36 (m) | 1.29–1.32 (m) | ||
| 7α | 1.43–1.45 (m) | 35.9 (t) | 1.36–1.39 (m) | 33.1 (t) |
| 7β | 1.12–1.16 (m) | 1.09–1.11 (m) | ||
| 8 | – | 39.0 (s) | – | 34.8 (s) |
| 9 | 3.44–3.46 (m) | 72.6 (d) | 3.31 (br s) | 75.3 (d) |
| 10α | 1.76–1.80 (m) | 28.0 (t) | 1.58–1.61 (overlap) | 26.0 (t) |
| 10β | 1.96–2.01 (m) | 1.95–2.01 (m) | ||
| 11α | 1.73 (dd, 12.2, 5.5) | 29.2 (t) | 1.69 (dd, 13.7, 4.7) | 26.7 (t) |
| 11β | 1.61–1.63 (m) | 1.11–1.13 (m) | ||
| 12α | 1.46–1.51 (overlap) | 41.2 (t) | 0.98–1.00 (m) | 36.6 (t) |
| 12β | 1.46–1.51 (overlap) | 1.58–1.61 (overlap) | ||
| 13 | 0.98 (s) | 20.7 (q) | 0.85 (s) | 25.4 (q) |
| 14 | 0.10 (s) | 30.4 (q) | 1.02 (s) | 31.3 (q) |
| 15 | 0.91 (s) | 26.8 (q) | 0.96 (s) | 28.4 (q) |
| 1′ | 3.46–3.49 (m) | 57.3 (t) | 3.47–3.55 (m) | 65.8 (t) |
| 2′ | 1.14 (t, 7.0) | 16.0 (q) | 1.71 (t, 7.0) | 15.7 (q) |
| Position | 5 | ||||
|---|---|---|---|---|---|
| δH (Multi, J in Hz) | δC (Multi) | Position | δH (Multi, J in Hz) | δC (Multi) | |
| 1 | – | 72.7 (s) | 9 | 1.12 (s) | 24.3 (q) |
| 2 | 3.61–3.64 (m) | 71.6 (d) | 10 | 1.20 (s) | 24.4 (q) |
| 3α | 1.64–1.67 (dd, 13.3, 3.7) | 37.0 (t) | 1′ | 4.37 (d, 7.7) | 97.2 (d) |
| 3β | 2.36–2.40 (m) | ||||
| 4 | – | 76.3 (s) | 2′ | 3.01 (m) | 73.8 (d) |
| 5α | 1.93–2.03 (overlap) | 27.1 (t) | 3′ | 3.21–3.24 (m) | 76.9 (d) |
| 5β | 1.50–1.56 (m) | ||||
| 6α | 1.75–1.80 (m) | 27.2 (t) | 4′ | 3.15–3.18 (m) | 70.2 (d) |
| 6β | 1.93–2.03 (overlap) | ||||
| 7 | 0.94 (s) | 22.4 (q) | 5′ | 3.12–3.15 (m) | 76.3 (d) |
| 8 | – | 76.5 (s) | 6′a | 3.72–3.74 (dd, 12.0, 2.3) | 61.4 (t) |
| 6′b | 3.53–3.56 (dd, 11.9, 5.6) | ||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | VAN c | MER d | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aa | >128 | >128 | 128 | >128 | >128 | 4 | 8 | >128 | >128 | >128 | >128 | 2 | ND e |
| E. cb | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | ND e | 0.0625 |
| Compound | Clinical Isolates of MRSA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M-1 | M-2 | M-3 | M-4 | M-5 | M-6 | M-7 | M-8 | M-9 | M-10 | |
| 6 | 2 | 4 | 4 | 2 | 4 | 2 | 4 | 4 | 4 | 4 |
| 7 | 4 | 8 | 8 | 4 | 8 | 4 | 8 | 8 | 4 | 8 |
| Van a | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Song, X.; Li, H.; Zhu, L.; Cao, S.; Liu, J. Sesquiterpenes and Monoterpenes from the Leaves and Stems of Illicium simonsii and Their Antibacterial Activity. Molecules 2022, 27, 1115. https://doi.org/10.3390/molecules27031115
Li H, Song X, Li H, Zhu L, Cao S, Liu J. Sesquiterpenes and Monoterpenes from the Leaves and Stems of Illicium simonsii and Their Antibacterial Activity. Molecules. 2022; 27(3):1115. https://doi.org/10.3390/molecules27031115
Chicago/Turabian StyleLi, Huijuan, Xinghui Song, Huiru Li, Lifei Zhu, Shengbo Cao, and Jifeng Liu. 2022. "Sesquiterpenes and Monoterpenes from the Leaves and Stems of Illicium simonsii and Their Antibacterial Activity" Molecules 27, no. 3: 1115. https://doi.org/10.3390/molecules27031115
APA StyleLi, H., Song, X., Li, H., Zhu, L., Cao, S., & Liu, J. (2022). Sesquiterpenes and Monoterpenes from the Leaves and Stems of Illicium simonsii and Their Antibacterial Activity. Molecules, 27(3), 1115. https://doi.org/10.3390/molecules27031115






