Abstract
The porous and biomimetic cobalt silicate@diatomite (Co2SiO4@diatomite) was successfully synthesized by a two-step method, including the hydrothermal method and calcination to improve the electromagnetic wave absorption property. Different hydrothermal times were well-tuned for Co2SiO4@diatomite composites with different loadings of Co2SiO4. Interestingly, the Co2SiO4@diatomite composites (6 h, 25 wt%) had a smaller minimum reflection loss. Moreover, the minimum reflection loss (RLmin) could reach −12.03 dB at 16.64 GHz and the matched absorber thickness was 10 mm, while the effective absorption bandwidth (EAB, RL ≤ −10 dB) could be 1.92 GHz. In principle, such findings indicate that Co2SiO4@diatomite nanocomposites could be a promising candidate for high-efficiency microwave absorption capability.
1. Introduction
In recent year, with the rapid development of electronic technology, electromagnetic (EM) pollution has become a non-negligible problem. EM pollution will interfere with the normal operation of electronic equipment [1] (such as the electronic equipment of hospitals and airports), which greatly impacts human production and life. Meanwhile, the damage of EM pollution to the human body must be, likewise, taken seriously [2]. Similarly, in the military field, the influence of electromagnetic waves (EMW) on the survival and attack of weapons also needs attention [3]. To solve these problems, EMW-absorbing materials (EMWAMs) are of great concern [3,4,5,6,7,8,9,10,11,12,13].
Carbon materials, such as carbon nanotubes, carbon fibers, porous carbon, and graphene [5,14,15,16], are one type of EMWAMs studied relatively early. Carbon materials are characterized by a low density, light weight, and good electroconductibility. Conductive polymers, such as polypyrrole [17] and polypyrrole@PANI [18], are of good conductivity, which induces the strong permittivity response by dielectric interaction to absorb EMW [19]. Metallic oxides, such as NiCo2O4 [20], Mn3O4 [21], CuCo2O4-CuO composites [22], and Co1.29Ni1.71O4 [8], have good magnetic loss characteristics, thus considered as excellent candidates for EMWAMs. However, carbon materials and conductive polymers have the disadvantages of impedance mismatching and narrow effective absorption bandwidth, and metal oxides suffer from gravimetric density and poor corrosion resistance [23]. To overcome these problems and broaden the application range of EMWAMs, diatomite, a biomimetic material with a unique 3D structure, has been noticed.
Diatoms are of unique siliceous cell walls that are micro-porous to nano-porous [24]. Diatomite is a bio-silica mineral obtained by geological deposition of the remains of single-celled diatoms [25]. Diatomite inherits this good characteristic of diatom and obtains extremely complex pore patterns. Therefore, diatomite with the intricacy and ordered pore pattern, the high surface area, and hollow and double-shell structure is considered to have good application potential in the field of EMWAMs.
Silicate nanomaterials are expected to be developed as absorbing materials due to their advantages of low cost, simple preparation, high thermal stability, and non-toxicity. Some transition metal silicates have both porous and layered structures, which makes them excellent candidates for EMWAMs. It is worth noting that in many transition metal silicates, cobalt silicate (Co2SiO4), as a magnetic material and in its theoretical capacitance, is of consideration [26,27,28]. Therefore, it has great application potential in EMWAM.
In this work, the diatomite-coated Co2SiO4 was synthesized successfully for the first time. Co-precursor@diatomite materials were obtained by the one-step hydrothermal method. Then, under high-temperature calcination at 900 °C, the Co-precursor@diatomite materials were gradually decomposed and reacted with diatomite to obtain Co2SiO4@diatomite materials. When the hydrothermal time was 6 h, the sample obtained by calcination had better EMW absorption performance than the other two samples. At 16.64 GHz, the sample of the reflection loss can reach −12.03 dB. The frequency falls within the Ku band used for satellite communications [29]. In a word, Co2SiO4@diatomite is a promising EMWAM.
2. Materials and Methods
2.1. Materials
Cobalt nitrate hexahydrate (Co(NO3)2·6H2O) and diatomite were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) Ammonium fluoride (NH4F) and urea were purchased from ChengDu Chron Chemicals Co., Ltd. (Chengdu, China). All reagents were of analytical grade and used without any further purification.
2.2. Synthesis
The synthetic process is shown in Figure 1. Co-precursor@diatomite was prepared via a hydrothermal method. Initially, 0.2325 g Co(NO3)2·6H2O, 0.08 g NH4F, and 0.275 g urea were dissolved into 50 mL of deionized water. The mixed solution was stirred magnetically for 30 min. Then, the solution and 0.1 g diatomite (DE) were transferred into a 100 mL Teflon-lined autoclave and heat-treated at 120 °C for 3 h, 6 h, and 12 h in a rotating oven. After the autoclave was cooled to room temperature, the solid product was centrifuged and washed by deionized water and ethyl alcohol. After drying at 60 °C, the Co-precursor@DE (−3, −6, −12) was obtained. Finally, Co-precursor@DE (−3, −6, −12) was calcined in a muffle furnace at 900 °C for 3 h to obtain Co2SiO4@DE (−3, −6, −12).
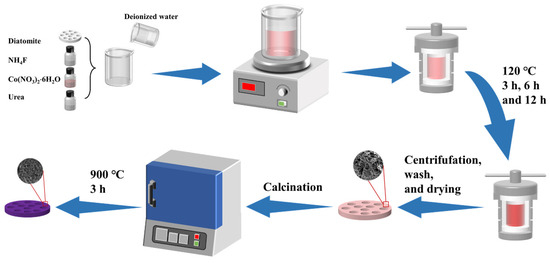
Figure 1.
Preparation flow of Co2SiO4@DE.2.3. Characterization.
The crystal structure and phase composition were characterized by an X-ray diffractometer (XRD, PANalytical X’ Pert Powder, Spectris Pte. Ltd., Shanghai, China). The composition and valence states of elements were characterized by an X-ray photoelectron spectrometer (XPS, ESCALAB250Xi, Thermo Scientific, Waltham, MA, USA). The microstructure, distribution, and composition of elements were characterized by field emission scanning electron microscopy (FESEM, Helios5-FIB, Thermo Scientific, Waltham, MA, USA), field emission transmission electron microscopy (FETEM, Talos F200S, Thermo Scientific, Waltham, MA, USA), and energy disperse spectroscopy (EDS). The changes in the sample quality during calcination were characterized by a thermal gravimetric analyzer (TGA, TGA2, METTLER TOLEDO, Greifensee, Switzerland). Co2SiO4@DE (−3, −6, and −12) was uniformly mixed with paraffin wax and pressed into a concentric ring with an outer diameter of 7.0 mm, an inner diameter of 3.04 mm, and a thickness of 3.04 mm. Thus, the electromagnetism parameters of Co2SiO4@DE (−3, −6, and −12), in the frequency range of 2–18 GHz, were obtained by a vector network analyzer (Agilent N5234A, Santa Clara, CA, USA) using the coaxial method.
3. Results and Discussion
The X-ray diffraction pattern of Co-precursor@DE-6 is presented in Figure S1. The XRD pattern indicates that the diffraction peaks of Co-precursor@DE-6 are consistent with the standard diffraction pattern of cobalt carbonate hydroxide hydrate (JCPDS card no. 48-0083). Thus, Co2SiO4@DE-6 was obtained by subsequent calcine. The amount of absorbent is important for the wave-absorbing performance of the sample. Therefore, different loads of Co2SiO4@DE (corresponding to Co2SiO4@DE-3, Co2SiO4@DE-6, and Co2SiO4@DE-12) were obtained by controlling the hydrothermal time to control the load of Co-precursor.
The X-ray diffraction patterns of Co2SiO4@DE-3, Co2SiO4@DE-6, and Co2SiO4@DE-12 are shown in Figure 2a. The diffraction peaks located at 25.310°, 32.053°, 34.549°, 35.451°, 36.357°, and 52.004° may be assigned to the (111), (031), (220), (131), (211), and (222) crystal planes of Co2SiO4 (JCPDS card no. 15-0865). Other diffraction peaks located at 21.891°, 31.265°, and 35.995° may be assigned to the (101), (102), and (200) of diatomite (JCPDS card no. 76-0937). Apart from the diffraction peaks of Co2SiO4 and diatomite, no other impurity diffraction peaks were found in the XRD pattern of Co2SiO4@DE-3, Co2SiO4@DE-6, and Co2SiO4@DE-12. This indicates that the way of the hydrothermal method combined with calcination can obtain purer Co2SiO4@DE.
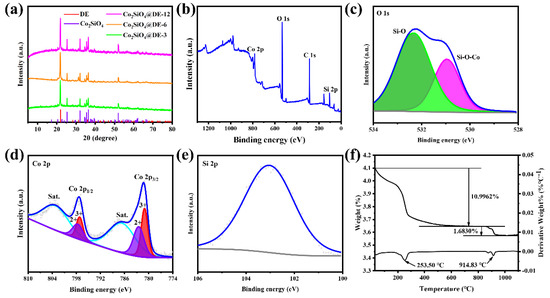
Figure 2.
The XRD patterns (a) of Co2SiO4@DE-3, Co2SiO4@DE-6, and Co2SiO4@DE-12. The XPS (b–e) of Co2SiO4@DE-6: (b) full spectrum, (c) O 1s, (d) Co 2p, and (e) Si 2p. The TGA (f) of Co-precursor@DE-6.
Furthermore, the species and valence distribution of the elements of Co2SiO4@DE-6 were verified by the XPS spectrum. Figure 2b shows the full spectrum of the sample. The sample only contains Co, Si, and O elements. The carbon peak located at 284.6 eV usually is derived from the surroundings [30]. Meanwhile, it proves no other impurity in the sample. The O atom has two states that are only connected with a Si atom and connected together with both a Si atom and Co atom. Therefore, the O element peaks mainly come from the Si–O bond (532.34 eV) in diatomite and Si–O–Co bond (530.95 eV) in Co2SiO4 (Figure 2c). The transition metal element Co is of more complex XPS peaks due to its more complicated outer electron configuration. The Co element only exists in Co2SiO4, which has two valences of divalent and trivalent [30]. The Co 2p1/2 and Co 2p2/3 peaks are composed of Co2+–O bonds (797.6 eV and 782.4 eV) and Co3+–O bonds (797.1 eV and 781.0 eV) (Figure 2d). The Si atom is only connected with the O atom and therefore the Si element peak is from the Si–O bond (103.0 eV; Figure 2e).
Figure 2f presents the mass loss steps of the Co-precursor@DE-6 in the process of increasing the temperature from 30 °C to 1100 °C. Two mass loss steps are observed at 253.50 °C and 914.83 °C with mass losses of 10.9962% and 1.6830%. The phenomena may indicate that the formation of Co2SiO4@DE includes three processes of dehydroxylation, decarbonation, and reaction. Therefore, the chemical reactions inferred are presented in Equations (1) and (2):
The SEM images of the micro-morphology of Co2SiO4@DE-3, Co2SiO4@DE-6, and Co2SiO4@DE-12 are presented Figure 3a–f. Diatomite is of a porous structure, which can increase the multiple reflection and absorption of incident electromagnetic waves to improve the wave-absorbing performance. As shown in Figure 3a–f, Co2SiO4 loaded on diatomite significantly increases the surface roughness of diatomite, which is beneficial to diffuse the reflection of the incident electromagnetic wave. Furthermore, the load of Co2SiO4 increasing with the hydrothermal time increasing from 3 h to 12 h was observed. The optimum hydrothermal time was determined by the subsequent measure of the wave-absorbing performance. The TEM image (Figure 3g) indicates that the morphology of calcined Co2SiO4 may be strip-shaped The high-resolution TEM image (Figure 3h) shows the lattice stripes of the material, which indicates that the material is crystal. The result is consistent with the XRD image. Furthermore, the high-resolution TEM image and EDS mapping (Figure 3i–l) further prove that the sample preparation is successful and Co2SiO4 is uniformly as well as completely coated with diatomite.
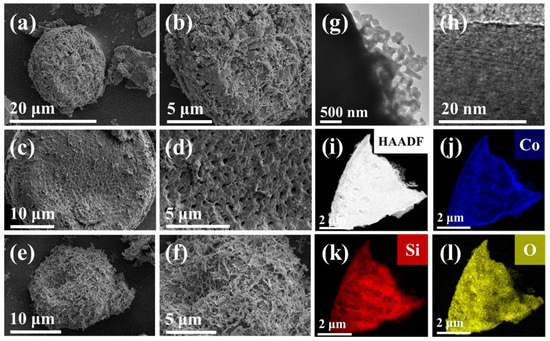
Figure 3.
The SEM images of (a,b) Co2SiO4@DE-3, (c,d) Co2SiO4@DE-6, and (e,f) Co2SiO4@DE-12. The TEM images (g–l) of Co2SiO4@DE-6: (g,h) TEM mode, (i) HADDF mode, and (j–l) EDS mapping mode.
In this experiment, a vector network analyzer was used to obtain the electromagnetic parameters of the material, including the real and imaginary parts of the complex permittivity and permeability. These data can directly indicate whether the material is suitable as EMWAM. The results of the characteristics are shown in Figure 4. Due to the unique hollow and porous structure of pure diatomite, it exhibits a good dielectric property. As shown in Figure 4, whether the material is calcined or not, the real part of its complex permittivity is around 2.5 and the imaginary part is around 1. This shows that the loading of Co2SiO4 will not affect the dielectric properties of diatomite. On the other hand, the imaginary part of the magnetic permeability (μ″) represents the loss modulus of magnetic energy. The μ″ value of the diatomite loaded with Co2SiO4 is higher than the material without calcination and this shows that the EMW absorption ability of calcined diatomite is enhanced. This change may be due to the increase in the specific surface area of the material, resulting in more interface polarization.
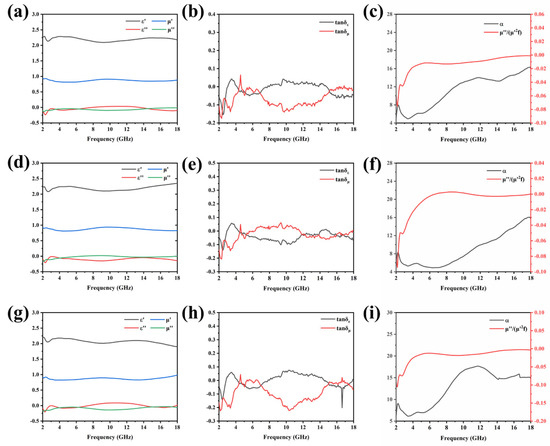
Figure 4.
Relevant EM parameters of Co2SiO4@DE-3 (a–c), Co2SiO4@DE-6 (d–f), and Co2SiO4@DE-12 (g–i). Frequency dependence of , and (a,d,g); dielectric and loss tangent (b,e,h); and attenuation constant and values (c,f,i).
On the basis of electromagnetic parameters, the dielectric loss tangent (ε″/ε′) value and the magnetic loss tangent (μ″/μ′) value (Figure 4) were calculated further. These two values can indicate the absorption performance of the material from the other point of view. In addition to the loss tangent, the attention constant (α) is also an important parameter. α indicates the material’s ability to attenuate EMW. The larger the value, the stronger the EMW dissipate ability of the material. The specific calculation formula is as follows [31]:
where is the frequency of the incident electromagnetic wave and is the speed of the EMW in vacuum (considered as 3 × 108). It can be seen from the gray data line in Figure 4c–i that as the frequency increases from 2 GHz to 18 GHz, the values of the three samples show the same trend. The three reach around 16 at 18 GHz. Therefore, it can be inferred that the three samples obtain a better EMW absorption effect at the maximum frequency of 18 GHz.
On the basis of analyzing the changes of the electromagnetic parameters with frequency, the actual influence of materials on EMW absorption was mainly studied. In order to show the absorption effect more intuitively, the concept of the reflection loss (RL) value was introduced. The RL value is usually a negative value and the lower the RL value, the better the EMW absorption property of the material. Generally speaking, when RL is lower than −10 dB, it means that 90% of incident EMW will be converted into heat or other forms of energy. The frequency range that meets the above conditions is usually defined as the effective absorption bandwidth (EAB). The RL calculation formulas are as follows [32,33]:
where is the characteristic impedance of free space; is the input impedance at the free space and material interface; and f, d, and c are the frequency of microwaves in free space, the thickness of the absorber, and the velocity of light, respectively.
In Figure 5, the first-to-third rows’ figures represent the RL values of Co2SiO4@DE (−3, 6, and 12) and the three columns from left to right are the 1D, 2D, and 3D graphs of the RL value of the three samples as a function of frequency and thickness. By the hydrothermal and calcination process, diatomite and Co2SiO4 combine to form a double-shell structure, which is conducive to the EMW absorption. For the Co2SiO4@DE-3, in the range of 2–18 GHz, even if the thickness is adjusted, the RL value cannot reach −10 dB (Figure 5a–c), which indicates that Co2SiO4@DE-3 has no EMW absorption ability and can only be used to adjust the impedance matching of the composite. Considering Co2SiO4 has the potential to become an EMWAM, when the load is increased, it shows a certain EMW absorption performance. When the thickness of Co2SiO4@DE-6 is 10 mm, its total effective absorption bandwidth in the high frequency range (15.3–17.22 GHz) is 1.92 GHz and the minimum reflection loss is −12.03 dB. When the thickness of Co2SiO4@DE-12 is 10mm, the effective absorption bandwidth reaches 1.76 GHz and the minimum reflection loss can reach −10.7 dB. It can be seen that when the hydrothermal time is 6 h, the EMW absorption performance is the best. As the hydrothermal time increases, the EMW absorption performance tends to deteriorate. This may be the reason why when the loading of Co2SiO4 increases to a certain extent, the Co2SiO4 fully covers the porous structure of diatomite. This leads to an obstruction of the transmission of dielectric signals and to a certain negative influence on the reflection and refraction of EMW.
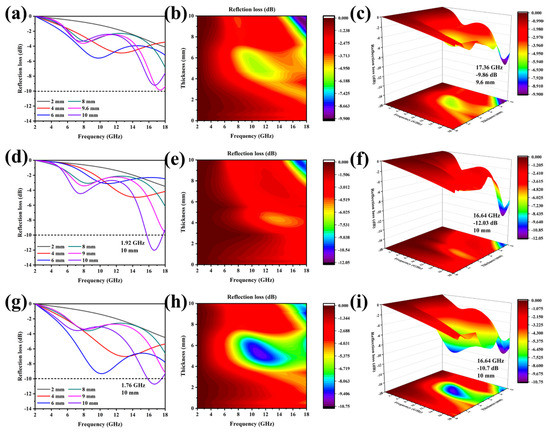
Figure 5.
One-dimensional, 2D, and 3D diagram of the RL value changing with frequency and thickness: (a–c) Co2SiO4@DE-3, (d–f) Co2SiO4@DE-6, and (g–i) Co2SiO4@DE-12.
The Eddy current loss is an important source of magnetic loss and in this experiment, the magnetic Co2SiO4 played the role of the magnetic loss component. According to Figure 4, the influence of the Eddy current loss on the EMW absorption property was analyzed through calculation preliminarily. Equation (6) was used to judge the effect [34]:
The right side of the equation is a constant. If the magnetic loss is only the Eddy current loss, the magnetic permeability parameter is within the frequency range and the material will satisfy the formula. It can be seen that in the range of 2–18 GHz, the value of fluctuates around 0 rather than being a horizontal line. Therefore, it can be inferred that the Eddy current loss is not the only cause of magnetic loss. Other important reasons such as hysteresis loss, natural resonance, and exchange resonance can also cause the magnetic loss of materials.
In addition to magnetic loss, dielectric loss is also an important reason for good EMW absorption property. When EMW is an incident on a dielectric material, the first microscopic mechanism encountered is the polarization of the medium, which is very different from the polarization of a conductor with free electrons. For diatomite, the electrons are in a bound state under the action of an alternating electric field. The silicate molecules produce dielectric polarization and the central position of the positive and negative charges changes from superposition to separation. This phenomenon will generate a rotational torque when an electric dipole and a weak electric field is formed. Therefore, the dielectric loss of the material mainly comes from the rotation and orientation of the dipole during the polarization process. Secondly, it comes from the resonance when the frequency of the external electric field is consistent with the molecular thermal vibration frequency. Consequently, the absorbing principles of Co2SiO4@DE were analyzed in detail in Figure 6. First of all, when EMW is an incident on the EMWAM, the Co2SiO4@DE makes most of the electromagnetic energy smoothly enter the absorbing material by a porous and layered absorber with impedance matching adjustment instead of reflecting on the surface. Then, after loading Co2SiO4, the surface roughness of diatomite increases, which will increase the scattering and refraction effects of EMW. Most importantly, the large cavity and porous structure of diatomite can make the incident wave produce multiple reflections and refractions, prolong the path, and increase the loss of incident waves to absorb most of the incident wave. The structure is not available in many materials, especially in 2D materials. Meanwhile, the interface of the composite also promotes the attenuation of the incident wave. Finally, the dense nano-sheet structure on the inner and outer surfaces of diatomite earth can effectively scatter electromagnetic waves. Therefore, it will cause an EMW incident in the same direction to change the EMW original directions to result in superimposition and cancellation. Meanwhile, the EMW that do not enter the absorbent or escape from the absorbent will be consumed by multiple reflections between the absorbent particles.

Figure 6.
Wave absorption mechanism of Co2SiO4@DE composite material.
Combining the above characterization results and analysis conclusions, it is not difficult to conclude that Co2SiO4@DE has a good application prospect for EMW absorption.
4. Conclusions
In summary, the Co2SiO4@DE composite material has been successfully synthesized through the one-step hydrothermal method and calcination. Through XRD, SEM, TEM, and other characterization results combined with theoretical analysis, the EMW-absorbing properties of Co2SiO4@DE obtained by calcination of a precursor with the hydrothermal time of 6 h are best. The minimum reflection loss can reach −12.03 dB at 16.64 GHz and its effective bandwidth is 1.92 GHz. The magnetic hysteresis loss of Co2SiO4 can make up for the lack of magnetic diatomite. At the same time, the cavity and porous structure of diatomite also promote the EMW absorption performance of the diatomite composite. Furthermore, we will conduct more fine structural regulations on Co2SiO4@DE to improve the EMW-absorbing performance and make it a diatomite composite material with good application prospects as a novel and lightweight EMWAM.
Supplementary Materials
The following supporting information are available online, Figure S1:The XRD patterns of Co-precursor@DE-6 and Co2SiO4@DE-6.
Author Contributions
Conceptualization, Y.Z. (Yuxin Zhang) and P.Y.; data curation, Y.Z. (Yifan Zhang), D.W. and K.L.; formal analysis, R.C.; funding acquisition, Y.Z. (Yuxin Zhang); investigation, Y.Z. (Yifan Zhang), R.C., K.L. and Q.S.; methodology, Y.X., H.T., T.S., Z.L. and Y.Z. (Yuxin Zhang); project administration, Y.Z. (Yuxin Zhang); software, X.H.; validation, K.L. and Q.S.; visualization, D.W.; writing—original draft preparation, Y.Z. (Yifan Zhang) and R.C.; writing—review and editing, P.Y., Y.Z. (Yuxin Zhang) and K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (grant no. 51908092); projects (no. 2020CDJXZ001, 2021CDJJMRH-005, and SKLMT-ZZKT-2021M04) supported by the Fundamental Research Funds for the Central Universities; the Joint Funds of the National Natural Science Foundation of China-Guangdong (grant no. U1801254); the project funded by the Chongqing Special Postdoctoral Science Foundation (XmT2018043); the Chongqing Research Program of Basic Research and Frontier Technology (cstc2017jcyjBX0080); the Natural Science Foundation Project of Chongqing for Post-doctor (cstc2019jcyjbsh0079 and cstc2019jcyjbshX0085); technological projects of the Chongqing Municipal Education Commission (KJZDK201800801); the Innovative Research Team of Chongqing (CXTDG201602014); Innovative Technology of New Materials and Metallurgy (2019CDXYCL0031); and the Technology Innovation and Application Development Special Project of Chongqing (Z20211350 and Z20211351). The authors also thank the Electron Microscopy Center of Chongqing University for the materials’ characterizations.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data of the compounds are available from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Iqbal, A.; Shahzad, F.; Hantanasirisakul, K.; Kim, M.K.; Kwon, J.; Hong, J.; Kim, H.; Kim, D.; Gogotsi, Y.; Koo, C.M. Anomalous absorption of electromagnetic waves by 2D transition metal carbonitride Ti3CNTx (MXene). Science 2020, 369, 446. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.T.; Chen, F.F.; Zhu, Y.J.; Zhang, Y.G.; Jiang, Y.Y.; Ma, M.G.; Chen, F. Binary strengthening and toughening of MXene/cellulose nanofiber composite Paper with nacre-inspired structure and superior electromagnetic interference shielding properties. ACS Nano 2018, 12, 4583–4593. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Zhang, J. Silicate-CoNi-carbon triple shell sandwich structured composite hollow microspheres with low density boosted microwave absorption and high mechanical strength. J. Mater. Chem. C 2021, 9, 702–713. [Google Scholar] [CrossRef]
- Lan, D.; Zhao, Z.; Gao, Z.; Kou, K.; Wu, H. Novel magnetic silicate composite for lightweight and efficient electromagnetic wave absorption. J. Mater. Sci. Technol. 2021, 92, 51–59. [Google Scholar] [CrossRef]
- Feng, Y.; Xia, L.; Ding, C.; Yang, H.; Xu, G.; Zhang, T.; Xiong, L.; Qin, C.; Wen, G. Boosted multi-polarization from silicate-glass@rGO doped with modifier cations for superior microwave absorption. J. Colloid Interface Sci. 2021, 593, 96–104. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, Z.; Feng, A.; Zhou, Z.; Zhang, C.; Wang, K.; Liu, N.; Wu, G. Enhanced microwave absorption performance of sulfur-doped hollow carbon microspheres with mesoporous shell as a broadband absorber. Compos. Commun. 2020, 19, 42–50. [Google Scholar] [CrossRef]
- Zhang, D.; Xiong, Y.; Cheng, J.; Chai, J.; Liu, T.; Ba, X.; Ullah, S.; Zheng, G.; Yan, M.; Cao, M. Synergetic dielectric loss and magnetic loss towards superior microwave absorption through hybridization of few-layer WS2 nanosheets with NiO nanoparticles. Sci. Bull. 2020, 65, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Lan, D.; Qin, M.; Liu, J.; Wu, G.; Zhang, Y.; Wu, H. Novel binary cobalt nickel oxide hollowed-out spheres for electromagnetic absorption applications. Chem. Eng. J. 2020, 382, 122797. [Google Scholar] [CrossRef]
- Yang, P.-A.; Huang, Y.; Li, R.; Huang, X.; Ruan, H.; Shou, M.; Li, W.; Zhang, Y.; Li, N.; Dong, L. Optimization of Fe@Ag core-shell nanowires with improved impedance matching and microwave absorption properties. Chem. Eng. J. 2022, 430, 132878. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, Y.; Yang, P.; Zheng, W.; Li, N.; Bai, J.; Zhang, Y.; Li, K.; Wang, D.; Liu, Z.; et al. Enhanced electromagnetic wave absorption properties of ultrathin MnO2 nanosheet-decorated spherical flower-shaped carbonyl iron powder. Molecules 2021, 27, 135. [Google Scholar] [CrossRef]
- Yang, P.A.; Ruan, H.; Sun, Y.; Li, R.; Lu, Y.; Xiang, C. Excellent microwave absorption performances of high length-diameter ratio iron nanowires with low filling ratio. Nanotechnology 2020, 31, 395708. [Google Scholar] [CrossRef]
- Najafi, A.; Ghasemi, S. A study of APC surfactant role on the surface characteristics, size and morphology improvements of synthesized mesoporous silica nanopowder through a sol-gel process. J. Alloys Compd. 2017, 720, 423–431. [Google Scholar] [CrossRef]
- Najafi, A.; Nematipour, K. Synthesis and Magnetic Properties Evaluation of Monosized FeCo Alloy Nanoparticles Through Microemulsion Method. J. Supercond. Nov. Magn. 2017, 30, 2647–2653. [Google Scholar] [CrossRef]
- Zhou, X.; Jia, Z.; Feng, A.; Qu, S.; Wang, X.; Liu, X.; Wang, B.; Wu, G. Synthesis of porous carbon embedded with NiCo/CoNiO2 hybrids composites for excellent electromagnetic wave absorption performance. J. Colloid Interface Sci. 2020, 575, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Kou, K.; Wu, H. 2-Methylimidazole-mediated hierarchical Co3O4/N-doped carbon/short-carbon-fiber composite as high-performance electromagnetic wave absorber. J. Colloid Interface Sci. 2020, 574, 1–10. [Google Scholar] [CrossRef]
- Liang, H.; Xing, H.; Qin, M.; Wu, H. Bamboo-like short carbon fibers@Fe3O4@phenolic resin and honeycomb-like short carbon fibers@Fe3O4@FeO composites as high-performance electromagnetic wave absorbing materials. Compos. Part A Appl. Sci. Manuf. 2020, 135, 105959. [Google Scholar] [CrossRef]
- Olmedo, L.; Hourquebie, P.; Jousse, F. Microwave absorbing materials based on conducting polymers. Adv. Mater. 1993, 5, 373–377. [Google Scholar] [CrossRef]
- Tian, C.; Du, Y.; Xu, P.; Qiang, R.; Wang, Y.; Ding, D.; Xue, J.; Ma, J.; Zhao, H.; Han, X. Constructing uniform core-shell PPy@PANI composites with tunable shell thickness toward enhancement in microwave absorption. ACS Appl. Mater. Inter. 2015, 7, 20090–20099. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Chen, X. Recent progress of nanomaterials for microwave absorption. J. Mater. 2019, 5, 503–541. [Google Scholar] [CrossRef]
- Qin, M.; Lan, D.; Wu, G.; Qiao, X.; Wu, H. Sodium citrate assisted hydrothermal synthesis of nickel cobaltate absorbers with tunable morphology and complex dielectric parameters toward efficient electromagnetic wave absorption. Appl. Surf. Sci. 2020, 504, 144480. [Google Scholar] [CrossRef]
- Lan, D.; Qin, M.; Yang, R.; Wu, H.; Jia, Z.; Kou, K.; Wu, G.; Fan, Y.; Fu, Q.; Zhang, F. Synthesis, characterization and microwave transparent properties of Mn3O4 microspheres. J. Mater. Sci. Mater. Electron. 2019, 30, 8771–8776. [Google Scholar] [CrossRef]
- Lan, D.; Qin, M.; Yang, R.; Chen, S.; Wu, H.; Fan, Y.; Fu, Q.; Zhang, F. Facile synthesis of hierarchical chrysanthemum-like copper cobaltate-copper oxide composites for enhanced microwave absorption performance. J. Colloid Interface Sci. 2019, 533, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, M.; Xie, A.; Wang, Y.; Xu, X. Recent advances in design and fabrication of nanocomposites for electromagnetic wave shielding and absorbing. Materials 2021, 14, 4148. [Google Scholar] [CrossRef] [PubMed]
- Ragni, R.; Cicco, S.R.; Vona, D.; Farinola, G.M. Multiple routes to smart nanostructured materials from diatom microalgae: A chemical perspective. Adv. Mater. 2018, 30, e1704289. [Google Scholar] [CrossRef]
- Li, K.; Liu, X.; Zheng, T.; Jiang, D.; Zhou, Z.; Liu, C.; Zhang, X.; Zhang, Y.; Losic, D. Tuning MnO2 to FeOOH replicas with bio-template 3D morphology as electrodes for high performance asymmetric supercapacitors. Chem. Eng. J. 2019, 370, 136–147. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Dong, X.; Jiang, H.; Hu, T.; Meng, C.; Huang, C. Alkali etching metal silicates derived from bamboo leaves with enhanced electrochemical properties for solid-state hybrid supercapacitors. Chem. Eng. J. 2021, 417, 127964. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Cheng, Y.; Zhao, W.; Chen, W.; Meng, C.; Huang, C. Synthesis of Co2SiO4/Ni(OH)2 core–shell structure as the supercapacitor electrode material with enhanced electrochemical properties. Mater. Lett. 2021, 282, 128774. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Y.; Jiang, H.; Dong, X.; Zheng, J.; Meng, C. Synthesis of amorphous cobalt silicate nanobelts@manganese silicate core-shell structures as enhanced electrode for high-performance hybrid supercapacitors. J. Colloid Interface Sci. 2020, 561, 762–771. [Google Scholar] [CrossRef]
- Sista, K.S.; Dwarapudi, S.; Kumar, D.; Sinha, G.R.; Moon, A.P. Carbonyl iron powders as absorption material for microwave interference shielding: A review. J. Alloys Compd. 2021, 853, 157251. [Google Scholar] [CrossRef]
- Lan, D.; Gao, Z.; Zhao, Z.; Wu, G.; Kou, K.; Wu, H. Double-shell hollow glass microspheres@Co2SiO4 for lightweight and efficient electromagnetic wave absorption. Chem. Eng. J. 2021, 408, 127313. [Google Scholar] [CrossRef]
- Huang, L.; Duan, Y.; Dai, X.; Zeng, Y.; Ma, G.; Liu, Y.; Gao, S.; Zhang, W. Bioinspired metamaterials: Multibands electromagnetic wave adaptability and hydrophobic characteristics. Small 2019, 15, 1902730. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Pei, K.; Xing, L.; Yu, X.; You, W.; Che, R. Enhanced microwave absorption performance from magnetic coupling of magnetic nanoparticles suspended within hierarchically tubular composite. Adv. Funct. Mater. 2019, 29, 1901448. [Google Scholar] [CrossRef]
- Zhong, G.; Xu, S.; Chen, C.; Kline, D.J.; Giroux, M.; Pei, Y.; Jiao, M.; Liu, D.; Mi, R.; Xie, H.; et al. Synthesis of metal oxide nanoparticles by rapid, high-temperature 3D microwave heating. Adv. Funct. Mater. 2019, 29, 1904282. [Google Scholar] [CrossRef]
- Gao, S.; Wang, G.S.; Guo, L.; Yu, S.H. Tunable and ultraefficient microwave absorption properties of trace N-doped two-dimensional carbon-based nanocomposites loaded with multi-rare earth oxides. Small 2020, 16, e1906668. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).