Screening and Structure–Activity Relationship for Selective and Potent Anti-Melanogenesis Agents Derived from Species of Mulberry (Genus Morus)
Abstract
1. Introduction
2. Polyphenols
2.1. Flavones
2.2. Flavanones and Flavonones
2.3. Chalcones
2.4. Stilbenes
2.5. Arylobenzofurans
3. Application in Cosmetic and Pharmaceutical Industry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms regulating skin pigmentation: The rise and fall of complexion coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef] [PubMed]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tymińska, A. Skin melanocytes: Biology and development. Postepy Dermatol. Alergol. 2013, 30, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Choudhary, R.K.; Naaz, I.; Ali, A.S. Understanding the challenges of melanogenesis: Key role of bioactive compounds in the treatment of hyperpigmentory disorders. J. Pigment. Disord. 2015, 2, 1000223. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.S.; Oh, Y.N.; Hyun, S.K.; Kwon, H.J.; Kim, B.W. Betulinic acid isolated from Vitis amurensis root inhibits 3-isobutyl-1-methylxanthine induced melanogenesis via the regulation of MEK/ERK and PI3K/Akt pathways in B16F10 cells. Food Chem. Toxicol. 2014, 68, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Nokinsee, D.; Shank, L.; Lee, V.S.; Nimmanpipug, P. Estimation of inhibitory effect against tyrosinase activity through homology modeling and molecular docking. Enzyme Res. 2015, 2015, 262364. [Google Scholar] [CrossRef]
- Bagherzadeh, K.; Shirgahi Talari, K.F.; Sharifi, A.; Ganjali, M.R.; Saboury, A.A.; Amanlou, M. A new insight into mushroom tyrosinase inhibitors: Docking, pharmacophore-based virtual screening, and molecular modeling studies. J. Biomol. Struct. Dyn. 2015, 33, 487–501. [Google Scholar] [CrossRef]
- Zakęs, N.; Studzińska-Sroka, E.; Bylka, W. Tyrosinase inhibitors of fungi and lichens as regulators of melanogenesis. Post. Fitoter. 2015, 4, 244–249. Available online: http://www.postepyfitoterapii.pl 2016/02 (accessed on 12 May 2021).
- Zaidi, K.U.; Ali, A.S.; Ali, S.A.; Naaz, I. Microbial Tyrosinases: Promising enzymes for pharmaceutical, food bioprocessing, and environmental industry. Biochem. Res. Int. 2014, 2014, 854687. [Google Scholar] [CrossRef]
- Chang, T.S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, T.; Nakashima, A.; Watanabe, H.; Ito, S.; Wakamatsu, K. Neuromelanin in Parkinson’s disease: Tyrosine hydroxylase and tyrosinase. Int. J. Mol. Sci. 2022, 10, 4176. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Li, X.; Jankovic, J. The association between Parkinson’s disease and melanoma. Int. J. Cancer 2011, 128, 2251–2260. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Tseng, T.S.; Hsiao, N.W.; Lin, Y.L.; Wen, Z.H.; Tsai, C.C.; Lee, Y.C.; Lin, H.H.; Tsai, K.C. Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis a ility by zebrafish in vivo assay and computational molecular modeling. Sci. Rep. 2015, 5, 7995. [Google Scholar] [CrossRef] [PubMed]
- Phasha, V.; Senabe, J.; Ndzotoyi, P.; Okole, B.; Fouche, G.; Chuturgoon, A. Review on the use of kojic acid—A skin-lightening ingredient. Cosmetics 2022, 9, 64. [Google Scholar] [CrossRef]
- Juliano, C.C.A. Spreading of dangerous skin-lightening products as a result of colourism: A review. Appl. Sci. 2022, 12, 3177. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Final report of the safety assessment of Kojic acid as used in cosmetics. Int. J. Toxicol. 2010, 29, 244S–273S. [Google Scholar] [CrossRef]
- Zhu, W.; Gao, J. The use of botanical extracts as topical skin-lightening agents for the improvement of skin pigmentation disorders. J. Investig. Dermatol. Symp. Proc. 2008, 13, 20–24. [Google Scholar] [CrossRef]
- Hsiao, N.W.; Tseng, T.S.; Lee, Y.C.; Chen, W.C.; Lin, H.H.; Chen, Y.R.; Wang, Y.T.; Hsu, H.J.; Tsai, K.C. Serendipitous discovery of short peptides from natural products as tyrosinase inhibitors. J. Chem. Inf. Model. 2014, 54, 3099–3111. [Google Scholar] [CrossRef]
- Curto, E.V.; Kwong, C.; Hermersdörfer, H.; Glatt, H.; Santis, C.; Virador, V.; Hearing, V.J., Jr.; Dooley, T.P. Inhibitors of mammalian melanocyte tyrosinase: In vitro comparisons of alkyl esters of gentisic acid with other putative inhibitors. Biochem. Pharmacol. 1999, 57, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Pintus, F.; Spanò, D.; Corona, A.; Medda, R. Antityrosinase activity of Euphorbia characias extracts. PeerJ. 2015, 3, e1305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fawole, O.A.; Makunga, N.P.; Opara, U.L. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement. Altern. Med. 2012, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Sindhuja, P.R.; Prabhakaran, G.; Gokulshankar, S. Synergistic effect of anti-oxidant, anti-tyrosinase and anti-bacterial activities of Tridax procumbens, Lantana camara, Euphorbia hirta and Thevetia peruviana plant extracts for cosmetic and personal care applications. Int. J. Pharm. Pharm. Sci. 2014, 6, 91–94. [Google Scholar]
- Gryn-Rynko, A.; Bazylak, G.; Olszewska-Slonina, D. New potential phytotherapeutics obtained from white mulberry (Morus alba L.) leaves. Biomed. Pharmacother. 2016, 84, 628–636. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Wang, Y.; Zhang, Y. Bioassay-guided screening and isolation of α-glucosidase and tyrosinase inhibitors from leaves of Morus alba. Food Chem. 2012, 131, 617–625. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Tan, H.Y.; Wang, M. Tyrosinase inhibition constituents from the roots of Morus australis. Fitoterapia 2012, 83, 1008–1013. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Cheng, K.W.; Zhu, Q.; Wang, X.C.; Lin, Z.X.; Wang, M. Tyrosinase inhibitory constituents from the roots of Morus nigra: A structure-activity relationship study. J. Agric. Food Chem. 2010, 58, 5368–5373. [Google Scholar] [CrossRef]
- Kang, K.B.; Kim, S.D.; Kim, T.B.; Jeong, E.J.; Kim, Y.C.; Sung, J.H.; Sung, S.H. Tyrosinase inhibitory constituents of Morus bombycis cortex. Nat. Prod. Sci. 2011, 17, 198–201. [Google Scholar]
- Arfan, M.; Khan, R.; Rybarczyk, A.; Amarowicz, R. Antioxidant activity of mulberry fruit extracts. Int. J. Mol. Sci. 2012, 13, 2472–2480. [Google Scholar] [CrossRef]
- Jan, B.; Parveen, R.; Zahiruddin, S.; Khan, M.U.; Mohapatra, S.; Ahmad, S. Nutritional constituents of mulberry and their potential applications in food and pharmaceuticals: A review. Saudi J. Biol. Sci. 2021, 28, 3909–3921. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Rao, J.; Wang, K.; Wang, M.; Yao, T.; Qiu, F. Highly potent inhibition of tyrosinase by mulberrosides and the inhibitory mechanism in vitro. Chem. Biodivers. 2022, 19, 202100740. [Google Scholar] [CrossRef]
- Kumar, V.R.; Chauhan, S. Mulberry: Life enhancer. J. Med. Plants Res. 2008, 2, 271–278. [Google Scholar] [CrossRef]
- Rizza, L.; Bonina, C.; Frasca, G.; Puglia, C. Skin-whitening effects of Mediterranean herbal extracts by in vitro and in vivo models. J. Cosmet. Sci. 2012, 63, 311–320. Available online: https://library.scconline.org/v063n05/21 (accessed on 12 May 2021).
- Nerya, O.; Musa, R.; Khatib, S.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The effect of hydroxyl positions and numbers. Phytochemistry 2004, 65, 1389–1395. [Google Scholar] [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Mocanu, M.M.; Nagy, P.; Szöllősi, J. Chemoprevention of breast cancer by dietary polyphenols. Molecules 2015, 20, 22578–22620. [Google Scholar] [CrossRef]
- Ackar, D.; Valek Lendić, K.; Valek, M.; Šubarić, D.; MiliIević, B.; Babić, J.; Nedić, I. Cocoa polyphenols: Can we consider cocoa and chocolate as potential functional food? J. Chem. 2013, 2013, 289392. [Google Scholar] [CrossRef]
- Bellik, Y.; Boukraâ, L.; Alzahrani, H.A.; Bakhotmah, B.A.; Abdellah, F.; Hammoudi, S.M.; Iguer-Ouada, M. Molecular mechanism underlying anti-inflammatory and anti-allergic activities ofphytochemicals: An update. Molecules 2012, 18, 322–353. [Google Scholar] [CrossRef]
- Jeong, S.H.; Ryu, Y.B.; Curtis-Long, M.J.; Ryu, H.W.; Baek, Y.S.; Kang, J.E.; Lee, W.S.; Park, K.H. Tyrosinase inhibitory polyphenols from roots of Morus lhou. J. Agric. Food Chem. 2009, 57, 1195–1203. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Ha, T.J.; Curtis-Long, M.J.; Ryu, H.W.; Gal, S.W.; Park, K.H. Inhibitory effects on mushroom tyrosinase by flavones from the stem barks of Morus lhou (S.) Koidz. J. Enzyme Inhib. Med. Chem. 2008, 23, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Dej-Adisai, S.; Parndaeng, K.; Wattanapiromsakul, C.; Hwang, J.S. Three New Isoprenylated Flavones from Artocarpus chama Stem and Their Bioactivities. Molecules 2021, 27, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tao, G.; Chen, J.; Zheng, Z.-P. Characterization of a New Flavone and Tyrosinase Inhibition Constituents from the Twigs of Morus alba L. Molecules 2016, 21, 1130. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Park, J.; Kim, J.; Han, C.; Yoon, J.; Kim, N.; Seo, J.; Lee, C. Flavonoids as mushroom tyrosinase inhibitors: A fluorescence quenching study. J. Agric. Food Chem. 2006, 54, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Ding, H.; Zhang, G.; Hu, X.; Gong, D. Relationships of dietary flavonoid structure with its tyrosinase inhibitory activity and affinity. LWT 2019, 107, 25–34. [Google Scholar] [CrossRef]
- Gębalski, J.; Graczyk, F.; Załuski, D. Paving the way towards effective plant-based inhibitors of hyaluronidase and tyrosinase: A critical review on a structure–activity relationship. J. Enzyme. Inhib. Med. Chem. 2022, 37, 1120–1195. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Liu, Q.; Kim, S.B.; Jo, Y.H.; Mo, E.J.; Yang, H.H.; Song, D.H.; Hwang, B.Y.; Lee, M.K. Characterization of melanogenesis inhibitory constituents of Morus alba leaves and optimization of extraction conditions using response surface methodology. Molecules 2015, 20, 8730–8741. [Google Scholar] [CrossRef] [PubMed]
- Macaev, F.; Boldescu, V.; Pogrebnoi, S.; Duca, G. Chalcone scaffold based antimycobacterial agents. Med. Chem. 2014, 4, 487–493. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, X.; Hou, A.; Wang, H. Inhibitory effect of 2,4,2′,4′-tetrahydroxy-3-(3-methyl-2-butenyl)-chalcone on tyrosinase activity and melanin biosynthesis. Biol. Pharm. Bull. 2009, 32, 86–90. [Google Scholar] [CrossRef]
- Takahashi, M.; Takara, K.; Toyozato, T.; Wada, K. A novel bioactive chalcone of Morus australis inhibits tyrosinase activity and melanin biosynthesis in B16 melanoma cells. J. Oleo Sci. 2012, 61, 585–592. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural and bioinspired phenolic compounds as tyrosinase inhibitors for the treatment of skin hyperpigmentation: Recent advances. Cosmetics 2019, 6, 57. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.M.; Zhang, J.; Zhang, Y.Q. An efficient preparation of mulberroside a from the branch bark of mulberry and its effect on the inhibition of tyrosinase activity. PLoS ONE 2014, 9, e109396. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, M.; Cho, S.G.; Kim, M.K.; Kim, S.W.; Lim, Y.H. Biotransformation of mulberroside A from Morus alba results in enhancement of tyrosinase inhibition. J. Ind. Microbiol. Biotechnol. 2010, 37, 631–637. [Google Scholar] [CrossRef]
- Komaikul, J.; Kitisripanya, T.; Tanaka, H.; Sritularak, B.; Putalun, W. Enhanced mulberroside a production from cell suspension and root cultures of Morus alba using elicitation. Nat. Prod. Commun. 2015, 10, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Park, K.T.; Kim, J.K.; Hwang, D.; Yoo, Y.; Lim, Y.H. Inhibitory effect of mulberroside A and its derivatives on melanogenesis induced by ultraviolet B irradiation. Food Chem. Toxicol. 2011, 49, 3038–3045. [Google Scholar] [CrossRef]
- Inyai, C.; Komaikul, J.; Kitisripanya, T.; Tanaka, H.; Sritularak, B.; Putalun, W. Development of a Rapid Immunochromatographic Strip Test for the Detection of Mulberroside A. Phytochem. Anal. 2015, 26, 423–427. [Google Scholar] [CrossRef]
- Zhaxi, M.; Chen, L.; Li, X.; Komatsu, K.; Yao, X.; Qiu, F. Three major metabolites of mulberroside A in rat intestinal contents and feces. Planta Med. 2010, 76, 362–364. [Google Scholar] [CrossRef]
- Zhou, J.; Li, S.X.; Wang, W.; Guo, X.Y.; Lu, X.Y.; Yan, X.P.; Huang, D.; Wei, B.Y.; Cao, L. Variations in the leaves of mulberroside A, oxyresveratrol, and resveratrol in mulberries in different seasons and during growth. Sci. World J. 2013, 2013, 380692. [Google Scholar] [CrossRef]
- Zhao, L.J.; Zhang, Y.Q. Rapid analysis of oxyresveratrol-4-3’-β-O-D-diglycosie in branch bark of mulberry by HPCE-DAD. Adv. Biomed. Eng. Res. 2013, 1, 69–74. [Google Scholar]
- Sarkar, R.; Arora, P.; Garg, K.V. Cosmeceuticals for hyperpigmentation: What is available? J. Cutan. Aesthet. Surg. 2013, 6, 4–11. [Google Scholar] [CrossRef]
- Qiu, F.; Komatsu, K.I.; Saito, K.I.; Kawasaki, K.; Yao, X.; Kano, Y. Pharmacological properties of traditional medicines (XXI) analysis of plasma, urine and bile of rats after oral administration of water extract of Mori Cortex. Nat. Med. 1996, 50, 103–108. [Google Scholar]
- Mei, M.; Ruan, J.Q.; Wu, W.J.; Zhou, R.N.; Lei, J.P.; Zhao, H.Y.; Yan, R.; Wang, Y.T. In vitro pharmacokinetic characterization of mulberroside A, the main polyhydroxylated stilbene in mulberry (Morus alba L.), and its bacterial metabolite oxyresveratrol in traditional oral use. J. Agric. Food Chem. 2012, 60, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, K.T.; Lee, H.S.; Kim, M.; Lim, Y.H. Evaluation of the inhibition of mushroom tyrosinase and cellular tyrosinase activities of oxyresveratrol: Comparison with mulberroside A. J. Enzyme Inhib. Med. Chem. 2012, 27, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Ayinampudi, S.; Wang, Z.H.; Avula, B.; Smillie, T.; Khan, I. Quantitative analysis of oxyresveratrol in different plant parts of Morus species and related genera by HPTLC and HPLC. J. Planar. Chromatogr. Mod. TLC 2011, 24, 125–129. [Google Scholar] [CrossRef]
- Huang, H.; Chen, G.; Lu, Z.; Zhang, J.; Guo, D.A. Identification of seven metabolites of oxyresveratrol in rat urine and bile using liquid chromatography/tandem mass spectrometry. Biomed. Chromatogr. 2010, 24, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, J.H.; Suh, H.J.; Lee, I.C.; Koh, J.; Boo, Y.C. Effects of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis. Arch. Dermatol. Res. 2014, 306, 475–487. [Google Scholar] [CrossRef]
- Lee, J.J.; Yun, S.R.; Jun, J.G. Facile synthesis of natural moracin compounds using Pd(OAc)2/P(tBu)3-HBF4 as a sonogashira coupling reagent. Bull. Korean Chem. Soc. 2014, 35, 3453–3458. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, S.Y.; Kim, H.; Hwang, J.S.; Lee, B.G.; Gao, J.J.; Kim, S.Y. Mulberroside F Isolated from the leaves of Morus alba inhibits melanin biosynthesis. Biol. Pharm. Bull. 2002, 25, 1045–1048. [Google Scholar] [CrossRef]
- Naik, R.; Harmalkar, D.S.; Xu, X.; Jang, K.; Lee, K. Bioactive benzofuran derivatives: Moracins A-Z in medicinal chemistry. Eur. J. Med. Chem. 2015, 90, 379–393. [Google Scholar] [CrossRef]
- Dej-adisai, S.; Parndaeng, K.; Wattanapiromsakul, C. Determination of phytochemical compounds, and tyrosinase inhibitory and antimicrobial activities of bioactive compounds from Streblus ilicifolius (S Vidal) Corner. Trop. J. Pharm. Res. 2016, 15, 497–506. [Google Scholar] [CrossRef][Green Version]
- Li, H.X.; Park, J.U.; Su, X.D.; Kim, K.T.; Kang, J.S.; Kim, Y.R.; Kim, Y.H.; Yang, S.Y. Identification of anti-melanogenesis constituents from Morus alba L. leaves. Molecules 2018, 23, 2559. [Google Scholar] [CrossRef] [PubMed]
- Budiman, A.; Zelika, M.R.; Nadiatul, K.Y.; Aulifa, D.L. Peel-off mask formulation from black mulberries (Morus nigra L.) leaves extract as a tyrosinase inhibitor. Int. J. Drug Deliv. Technol. 2019, 9, 517–524. [Google Scholar] [CrossRef]
- Budiman, A.; Praditasari, A.; Rahayu, D.; Aulifa, D.L. Formulation of antioxidant gel from black mulberry fruit extract (Morus nigra L.). J. Pharm. Bioallied. Sci. 2019, 11, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Hisham, J.; Khan, H.M.S.; Khan, B.A.; Mahmood, T.; Saeed, T. Whitening and antierythemic effect of a cream containing Morus alba extract. Hygeia. J. D Med. 2012, 4, 97–103. [Google Scholar]
- Memete, A.R.; Timar, A.V.; Vuscan, A.; Miere Groza, F.; Venter, A.C.; Vicas, S.I. Phytochemical composition of different botanical parts of Morus species, health benefits and application in food industry. Plants 2022, 11, 152. [Google Scholar] [CrossRef]
- Zakeri, E.S.; Hadjiakhoondi, A.; Nasrollahi, A.S.; Vazirian, M.; Naeimifar, A. Formulation and physicochemical evaluation of a 3% cream using crude 70% ethanolic extract of Morus alba L. leaves for skin hyperpigmentation. Jundishapur J. Nat. Pharm. Prod. 2022, 17, e115266. [Google Scholar] [CrossRef]
- Abhijith, T.M.; Azeez, A.A.; Rajagopalan, D.; Boopathi, C.; Shyam, A. Formulation and evaluation of Morus alba anti-aging cream. Nat. J. Pharm. 2021, 1, 85–87. Available online: https://www.pharmajournal.net/archives/2021.v1.i2.B.21 (accessed on 20 May 2022).
- Alvin, G.; Catambay, N.; Vergara, A.; Jamora, M.J. A comparative study of the safety and efficacy of 75% mulberry (Morus alba) extract oil versus placebo as a topical treatment for melasma: A randomized, single-blind, placebo-controlled trial. J. Drugs Dermatol. 2011, 10, 1025–1031. [Google Scholar]
- Oyama, T.; Yoshimori, A.; Ogawa, H.; Shirai, Y.; Abe, H.; Kamiya, T.; Tanuma, S. The structural differences between mushroom and human tyrosinase cleared by investigating the inhibitory activities of stilbenes. J. Mol. Struct. 2023, 1272, 134180. [Google Scholar] [CrossRef]
- Hałdys, K.; Latajka, R. Thiosemicarbazones with tyrosinase inhibitory activity. Medchemcomm 2019, 10, 378–389. [Google Scholar] [CrossRef]
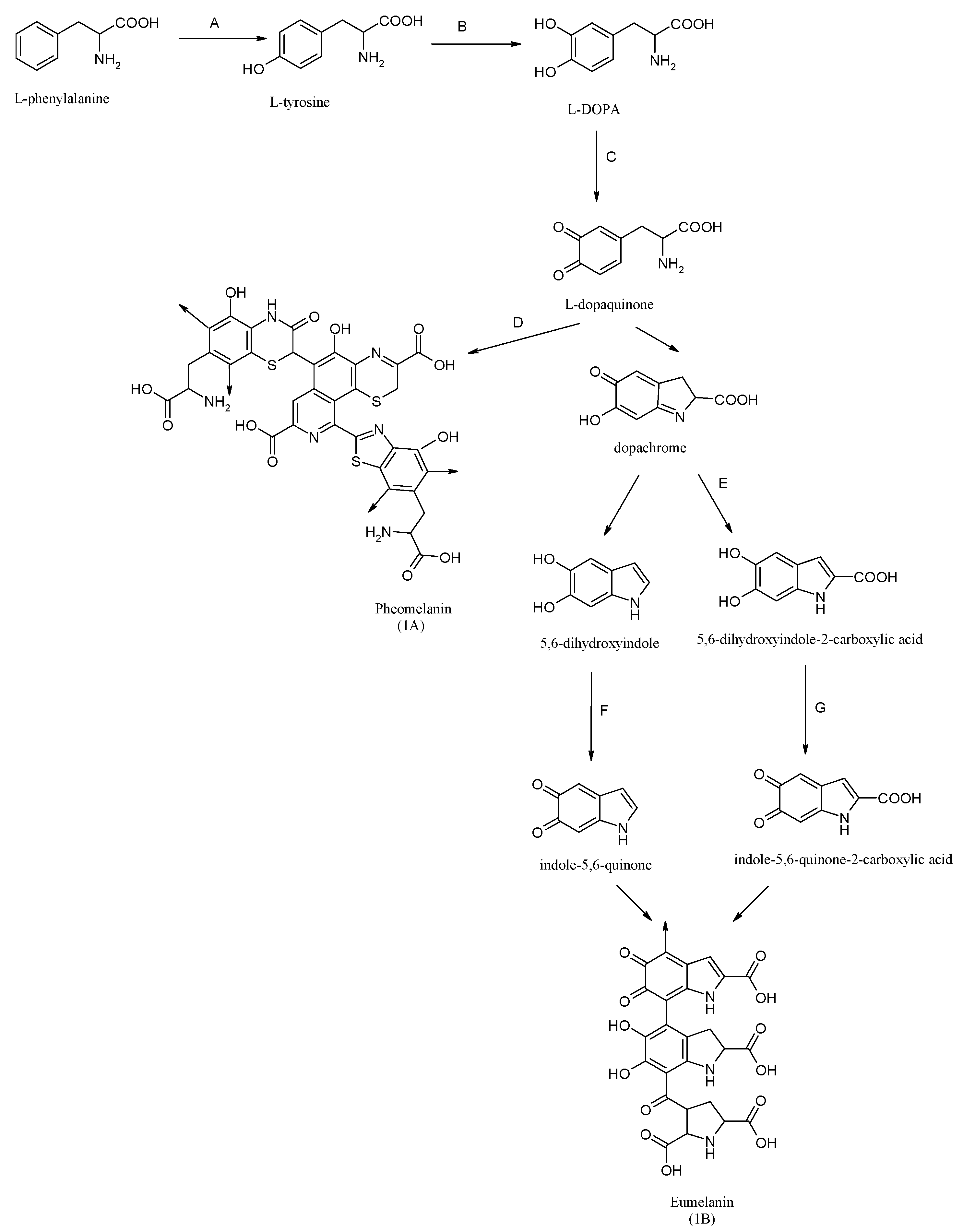
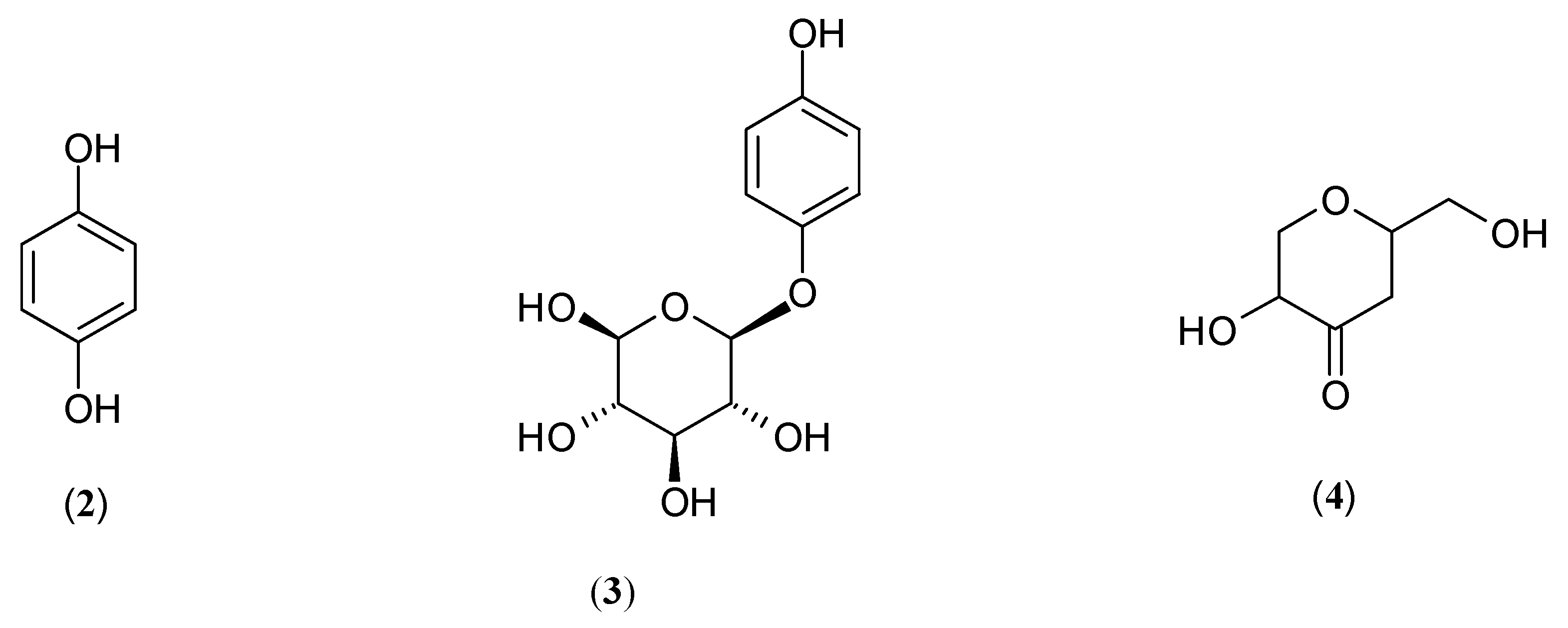
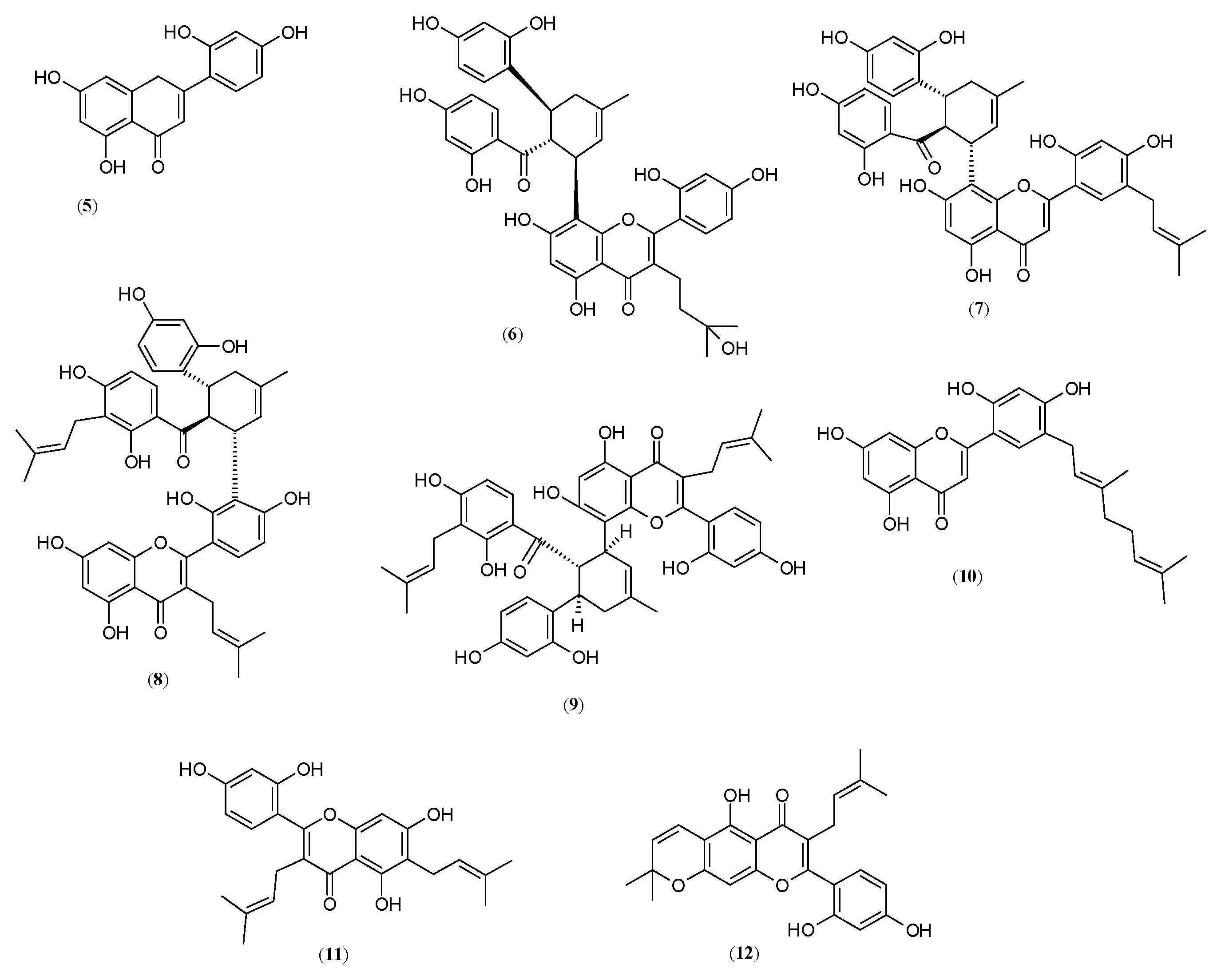
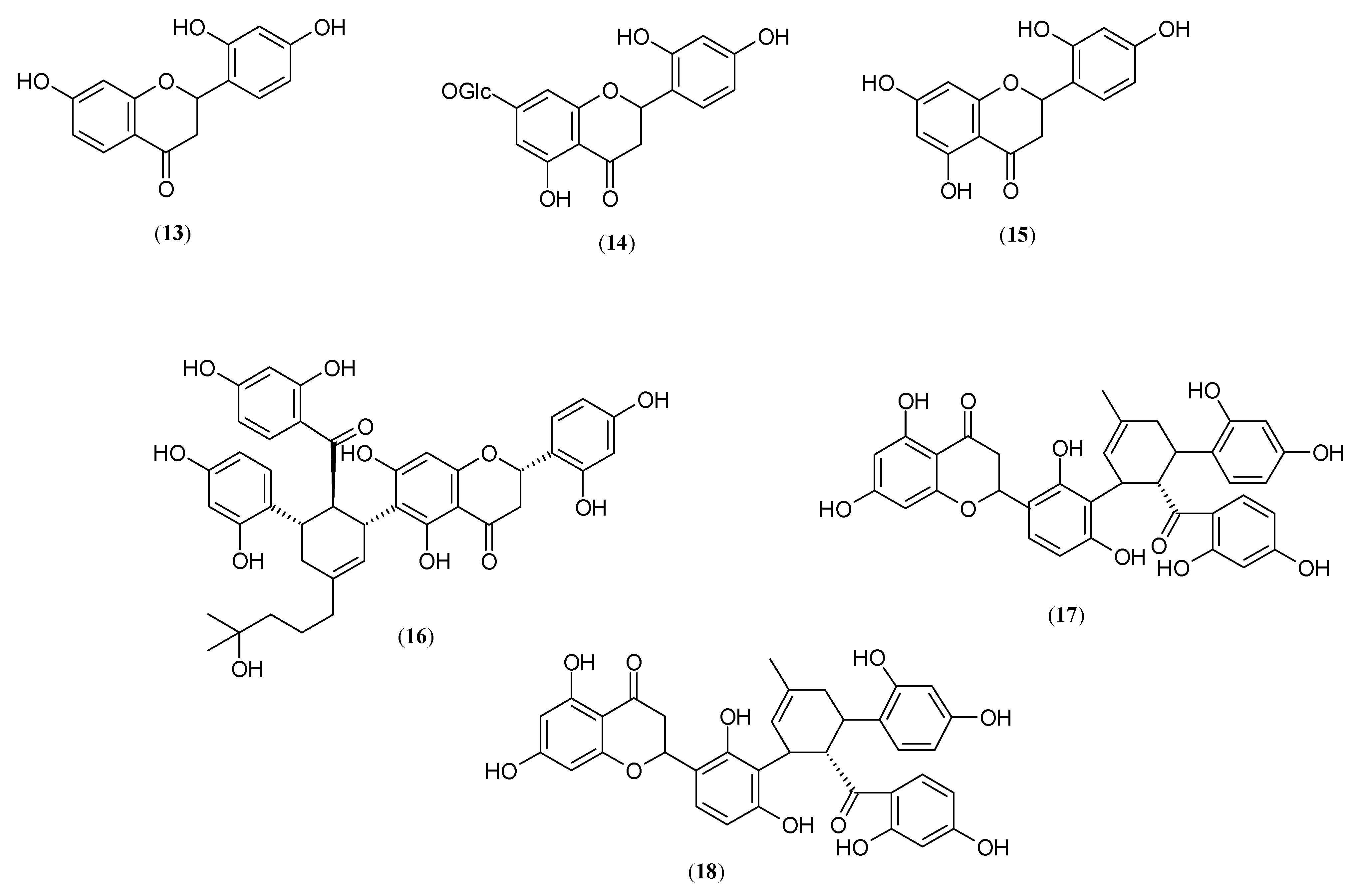
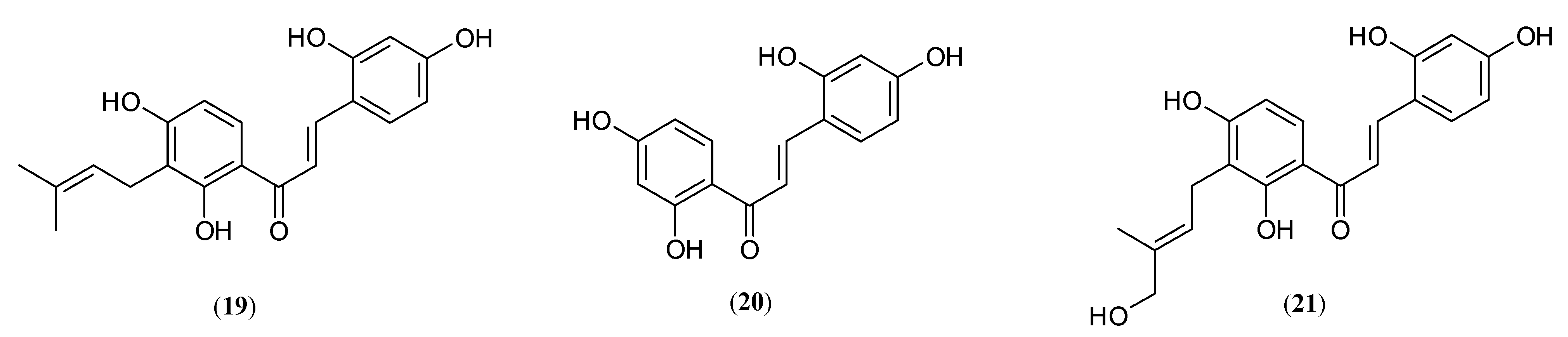
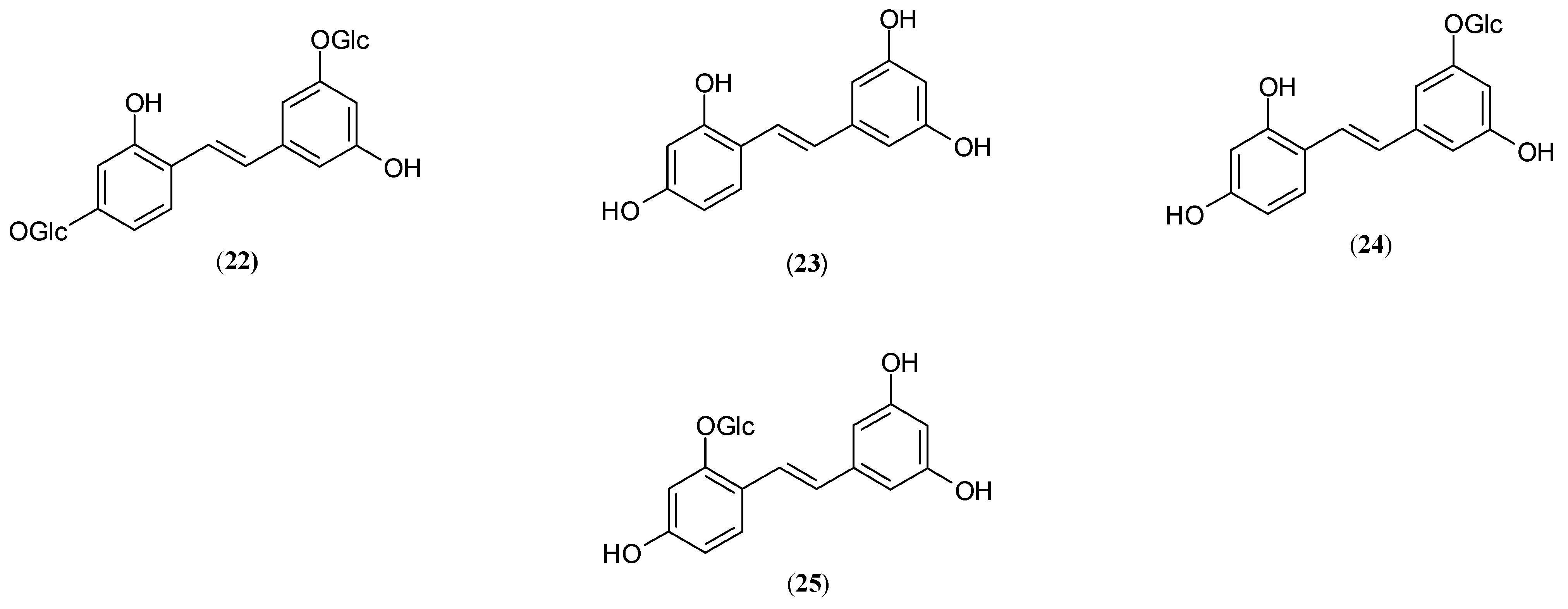
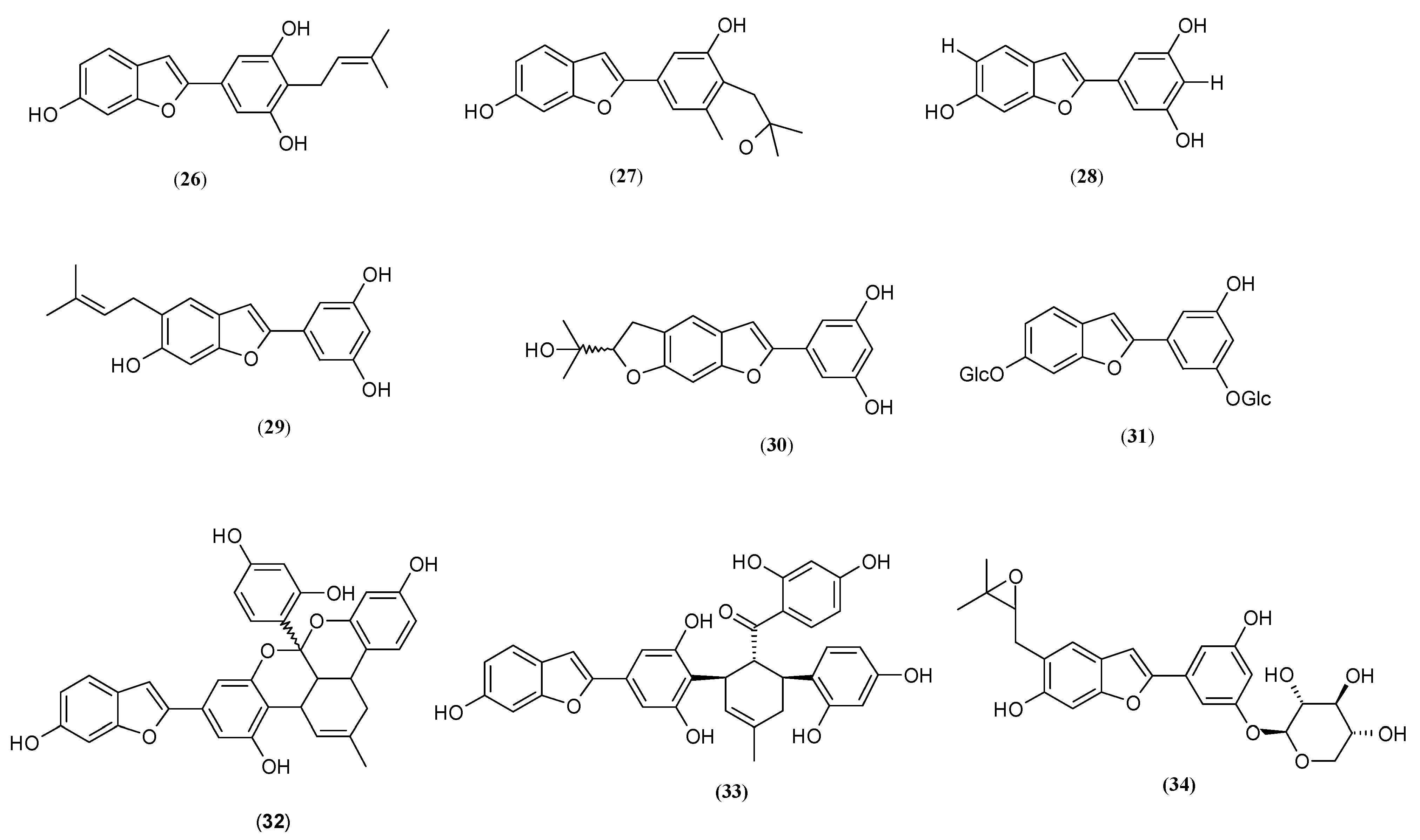
| Name | Part of Plant | Isolated Compound | IC50 (µM) | Relative Inhibitory Strength c | Ref. |
|---|---|---|---|---|---|
| M. alba | leaf | Norartocarpetin (5) | 0.0824 ± 0.0089 | 192.96 | [26,58,68] |
| Moracin C (26) | 2.27 ± 0.44 | 7.0 | |||
| Moracin D (27) | 12.0 ± 2.5 | 1.325 | |||
| Moracin N (29) | 0.924 ± 0.048 | 17.20 | |||
| Mulberroside F (31) | 0.29 | 4.48 | |||
| twig | Steppogenin (15) | 0.98 ± 0.01 | 59.49 | [43] | |
| Morachalcone A (19) | 0.08 ± 0.02 | 728.75 | |||
| 2,4,2′,4′-Tetrahydroxychalcone (20) | 0.07 ± 0.02 | 832.86 | |||
| Oxyresveratrol (23) | 0.10 ± 0.01 | 583 | |||
| Moracin M (28) | 8.00 ± 0.22 | 7.28 | |||
| root | Oxyresveratrol (23) | 0.49 a, 11.9 b | 1503.06 d 32.60 | [53,58] | |
| Mulberroside A (22) | 53.6 ± 2.3 a | 13.74 d | |||
| M. australis | root | Moracenin D (6) | 4.61 ± 0.13 | 10.94 | [27] |
| Sanggenon T (16) | 1.20 ± 0.04 | 42.00 | |||
| Kuwanon O (17) | 1.81 ± 0.08 | 27.86 | |||
| Oxyresveratrol (23) | 2.85 ± 0.26 | 17.69 | |||
| Mulberrofuran G (32) | 14.65 ± 0.32 | 3.44 | |||
| stem | Morachalcone A (19) | 0.82 | 200.0 d | [50] | |
| 2,4,2′,4′-Tetrahydroxychalcone (20) | 0.21 | 780.95 d | |||
| HMBCH (21) | 0.17 | 964.70 d | |||
| M. bombycis | cortex | 7,2′,4′-Trihydroxyflavanone (13) | 5.228 | 21.56 | [29] |
| 2,4,2′,4′-Tetrahydroxychalcone (20) | 0.9724 | 115.90 | |||
| Oxyresveratrol (23) | 3.66 | 30.80 | |||
| Moracinoside M (34) | 61.3 | 1.84 | |||
| M. lhou | root | Norartocarpetin (5) | 1.2 ± 0.40 a | 13.58 | [40] |
| Steppogenin (15) | 1.3 ± 0.30 a | 12.53 | |||
| Moracin M (28) | 7.4 ± 1.0 a | 2.20 | |||
| stem bark | Norartocarpetin (5) | 1.2 a | 10.42 | [41] | |
| M. multicaulis | branchbark | Mulberroside A (22) | 1.29 a, 7.45 b | nd | [52] |
| Oxyresveratrol (23) | 0.12 a, 0.39 b | nd | |||
| M. nigra | root | Kuwanon H (9) | 10.34 ± 0.19 | 4.54 | [28] |
| 5′-Geranyl-5,7,2′,4′-tetrahydroxyflavone (10) | 37.09 ± 1.74 | 1.26 | |||
| Steppogenin-7-O-β-D-glucoside (14) | 5.99 ± 0.03 | 7.84 | |||
| Morachalcone A (19) | 0.14 ± 0.01 | 335.36 | |||
| 2,4,2′,4′-Tetrahydroxychalcone (20) | 0.062 ± 0.002 | 757.26 | |||
| Moracin N (30) | 30.52 ± 1.46 | 1.54 | |||
| Oxyresveratrol-3′-O-β-D-glucopyranoside (24) | 1.64 ± 0.10 | 28.63 | |||
| Oxyresveratrol-2-O-β-D-glucopyranoside (25) | 29.75 ± 2.07 | 1.58 | |||
| Mulberrofuran G (32) | 17.53 ± 0.26 | 2.68 | |||
| stem | Morachalcone A (19) | 0.95 ± 0.040 b | 26.19 | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryn-Rynko, A.; Sperkowska, B.; Majewski, M.S. Screening and Structure–Activity Relationship for Selective and Potent Anti-Melanogenesis Agents Derived from Species of Mulberry (Genus Morus). Molecules 2022, 27, 9011. https://doi.org/10.3390/molecules27249011
Gryn-Rynko A, Sperkowska B, Majewski MS. Screening and Structure–Activity Relationship for Selective and Potent Anti-Melanogenesis Agents Derived from Species of Mulberry (Genus Morus). Molecules. 2022; 27(24):9011. https://doi.org/10.3390/molecules27249011
Chicago/Turabian StyleGryn-Rynko, Anna, Beata Sperkowska, and Michał S. Majewski. 2022. "Screening and Structure–Activity Relationship for Selective and Potent Anti-Melanogenesis Agents Derived from Species of Mulberry (Genus Morus)" Molecules 27, no. 24: 9011. https://doi.org/10.3390/molecules27249011
APA StyleGryn-Rynko, A., Sperkowska, B., & Majewski, M. S. (2022). Screening and Structure–Activity Relationship for Selective and Potent Anti-Melanogenesis Agents Derived from Species of Mulberry (Genus Morus). Molecules, 27(24), 9011. https://doi.org/10.3390/molecules27249011






