Synthesis of Chain-End Functional Polydienes Using Diene Comonomer Bearing Boronic Acid Masked with Diaminonaphthalene
Abstract
1. Introduction
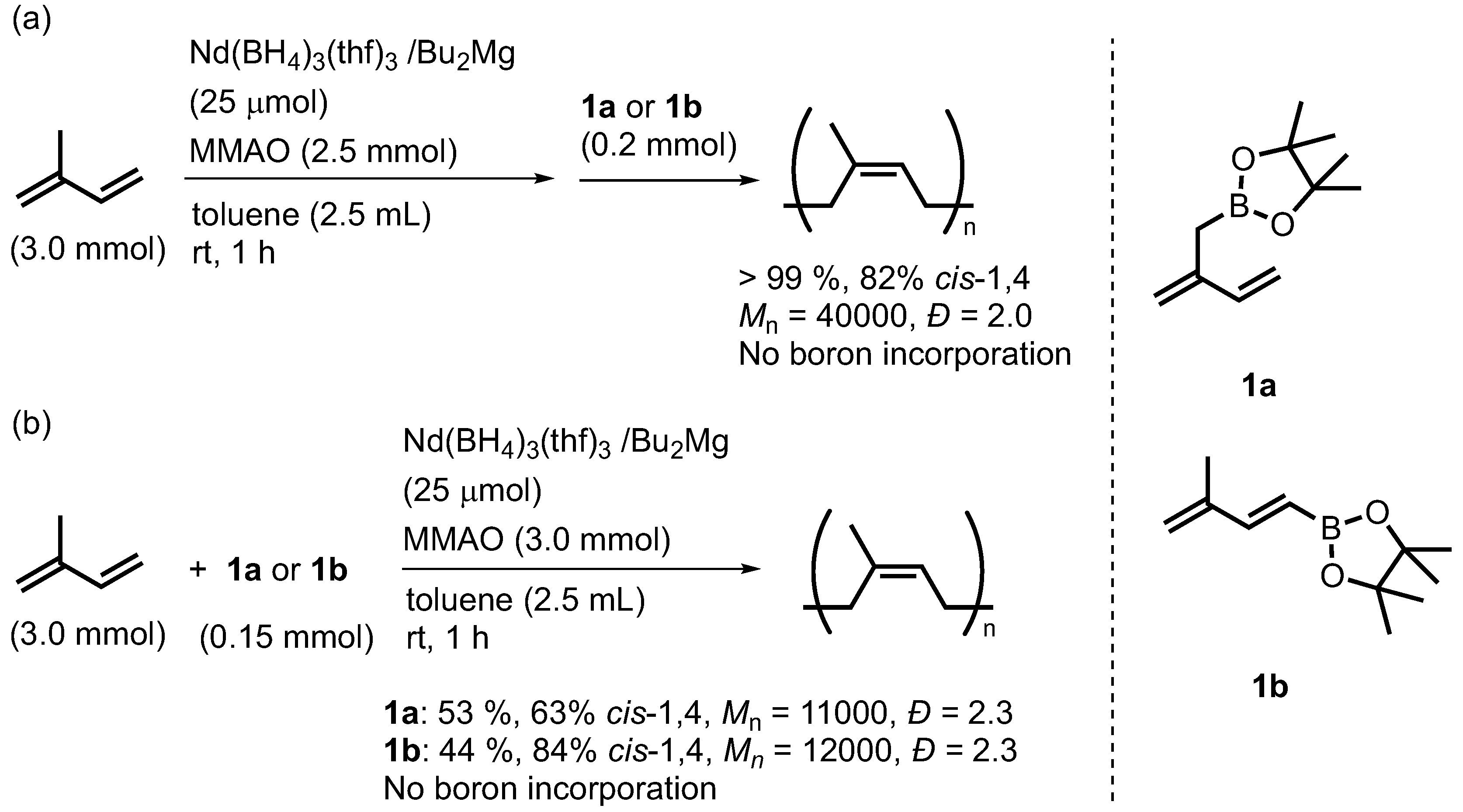
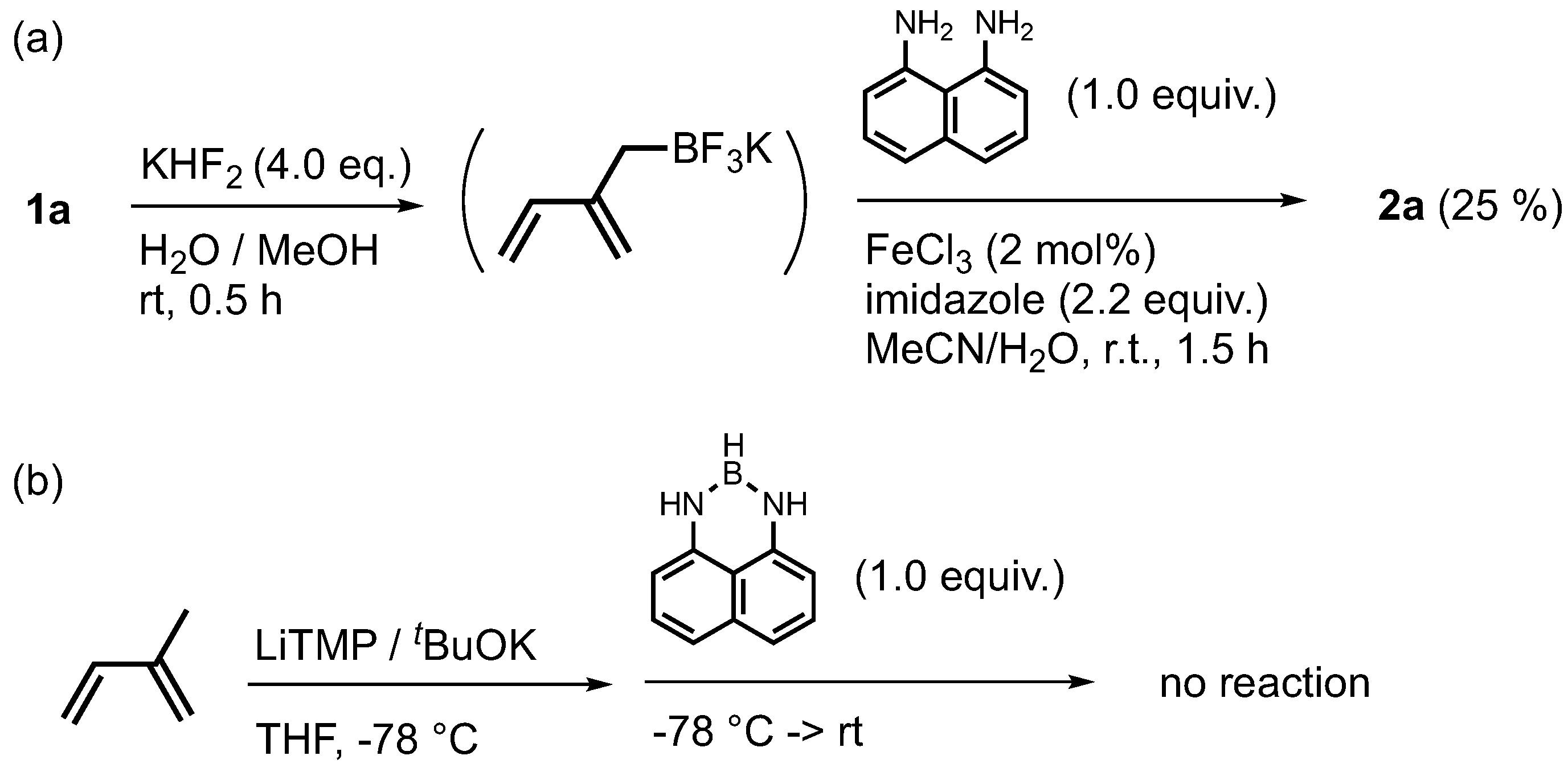
2. Results and Discussion
3. Experimental Section
3.1. General
3.1.1. Synthesis of Naphthalene Diamide Monomer 2a
3.1.2. Synthesis of Naphthalene Diamide Monomer 2b
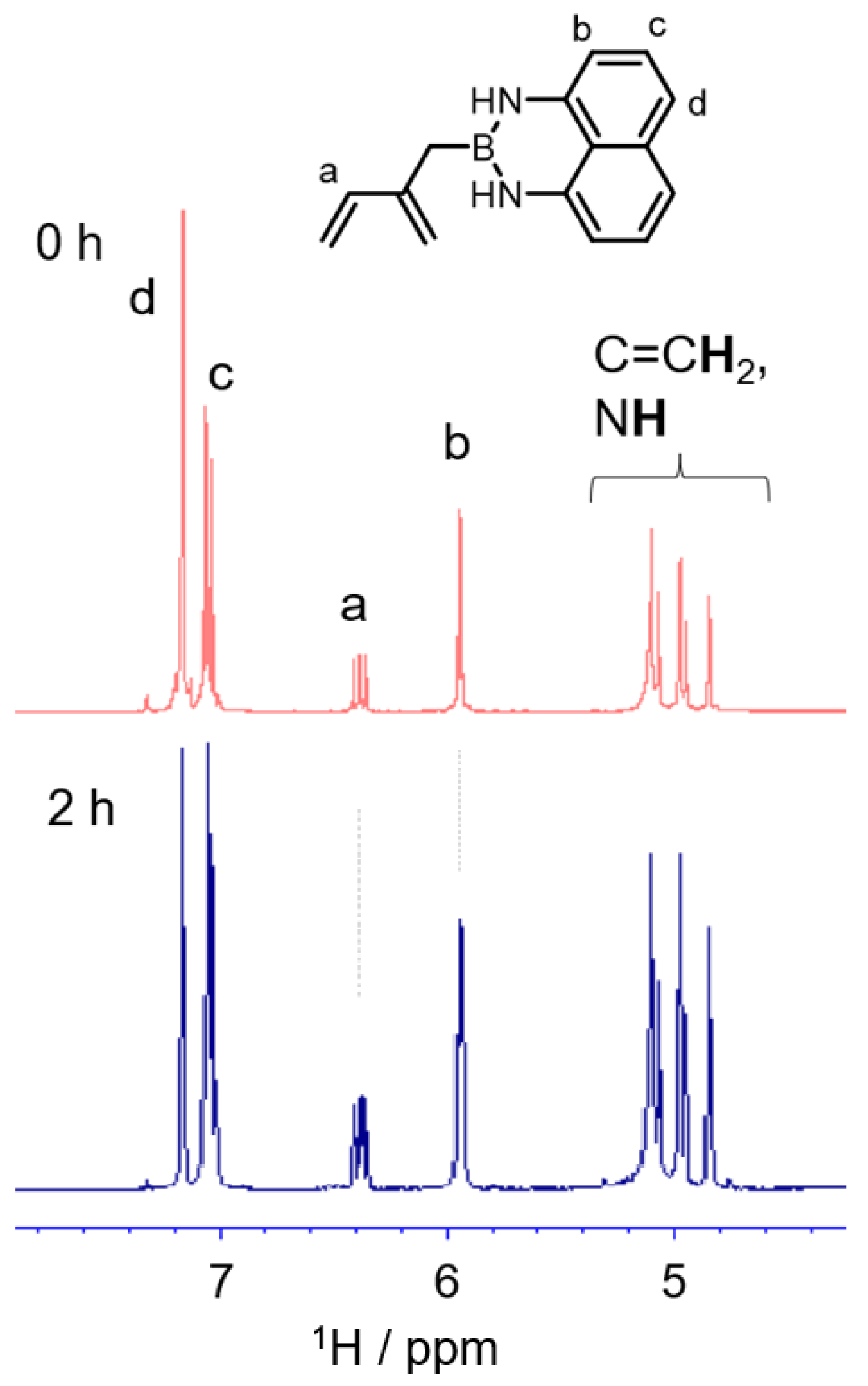
3.1.3. NMR Study on the Comonomer Degradation
3.1.4. Preparation of Neodymium Catalyst Stock Solution
3.1.5. Copolymerization of Butadiene and Boronic Acid-Containing Comonomer
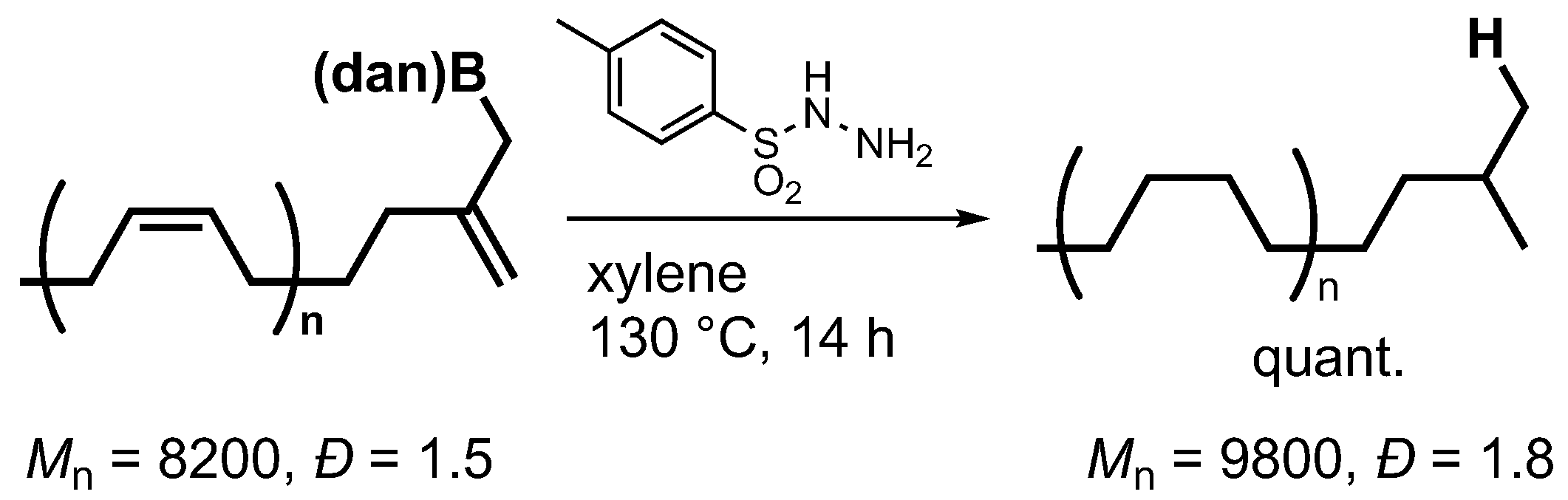
3.1.6. Hydrogenation of Boron-Functionalized Polybutadiene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Threadingham, D.; Obrecht, W.; Wieder, W.; Wachholz, G.; Engehausen, R. Rubber, 3. Synthetic Rubbers, Introduction and Overview. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Hoboken, NJ, USA, 2011. [Google Scholar]
- Dhanorkar, R.J.; Mohanty, S.; Gupta, V.K. Synthesis of Functionalized Styrene Butadiene Rubber and Its Applications in SBR–Silica Composites for High Performance Tire Applications. Ind. Eng. Chem. Res. 2021, 60, 4517–4535. [Google Scholar] [CrossRef]
- Ying, W.; Pan, W.; Gan, Q.; Jia, X.; Grassi, A.; Gong, D. Preparation and property investigation of chain end functionalized cis-1,4 polybutadienes via de-polymerization and cross metathesis of cis-1,4 polybutadienes. Polym. Chem. 2019, 10, 3525–3534. [Google Scholar] [CrossRef]
- Lu, B.; Chung, T.C. Maleic Anhydride Modified Polypropylene with Controllable Molecular Structure: New Synthetic Route via Borane-Terminated Polypropylene. Macromolecules 1998, 31, 5943–5946. [Google Scholar] [CrossRef]
- Ottou, W.N.; Norsic, S.; Belaid, I.; Boisson, C.; D’Agosto, F. Amino End-Functionalized Polyethylenes and Corresponding Telechelics by Coordinative Chain Transfer Polymerization. Macromolecules 2017, 50, 8372–8377. [Google Scholar] [CrossRef]
- Leicht, H.; Bauer, J.; Göttker-Schnetmann, I.; Mecking, S. Heterotelechelic and In-Chain Polar Functionalized Stereoregular Poly(dienes). Macromolecules 2018, 51, 763–770. [Google Scholar] [CrossRef]
- Göttker-Schnetmann, I.; Kenyon, P.; Mecking, S. Coordinative Chain Transfer Polymerization of Butadiene with Functionalized Aluminum Reagents. Angew. Chem. Int. Ed. 2019, 58, 17777–17781. [Google Scholar] [CrossRef]
- Ozawa, Y.; Takata, T.J. Synthesis and property of end-functionalized poly(cis-1,4-butadiene) and its application to rubber compound. Appl. Polym. Sci. 2019, 136, 47985. [Google Scholar] [CrossRef]
- Georges, S.; Hashmi, O.H.; Bria, M.; Zinck, P.; Champouret, Y.; Visseaux, M. Efficient One-Pot Synthesis of End-Functionalized trans-Stereoregular Polydiene Macromonomers. Macromolecules 2019, 52, 1210–1219. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhu, H.; Huang, X.; Wu, Y. Amphiphilic Silicon Hydroxyl-Functionalized cis-Polybutadiene: Synthesis, Characterization, and Properties. Macromolecules 2021, 54, 2427–2438. [Google Scholar] [CrossRef]
- Brooks, W.L.A.; Sumerlin, B.S. Synthesis and Applications of Boronic Acid-Containing Polymers: From Materials to Medicine. Chem. Rev. 2016, 116, 1375–1397. [Google Scholar] [CrossRef]
- Leicht, H.; Göttker-Schnetmann, I.; Mecking, S. Stereoselective Copolymerization of Butadiene and Functionalized 1,3-Dienes. ACS Macro Lett. 2016, 5, 777–780. [Google Scholar] [CrossRef]
- Jacobs, B.P.; Brantley, J.N. Vinyl-Addition Polymerizations of Borylated Allenes. Macromolecules 2021, 54, 8822–8828. [Google Scholar] [CrossRef]
- Ricci, G.; Pampaloni, G.; Sommazzi, A.; Masi, F. Dienes Polymerization: Where We Are and What Lies Ahead. Macromolecules 2021, 54, 5879–5914. [Google Scholar] [CrossRef]
- Yao, C.; Liu, N.; Long, S.; Cui, C.W.D. Highly cis-1,4-selective coordination polymerization of polar 2-(4-methoxyphenyl)-1,3-butadiene and copolymerization with isoprene using a β-diketiminato yttrium bis(alkyl) complex. Polym. Chem. 2016, 7, 1264–1270. [Google Scholar] [CrossRef]
- Long, S.; Lin, F.; Yao, C.; Cui, D. Highly cis-1,4 Selective Living Polymerization of Unmasked Polar 2-(2-Methylidenebut-3-enyl)Furan and Diels–Alder Addition. Macromol. Rapid Commun. 2017, 38, 1700227. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Long, S.; Wu, C.; Li, S.; Yao, C.; Hua, X.; Na, H.; Liu, D.; Tang, T.; Cui, D. Highly selective cis-1,4 copolymerization of dienes with polar 2-(3-methylidenepent-4-en-1-yl) pyridine: An approach for recyclable elastomers. Polym. Chem. 2020, 11, 1646–1652. [Google Scholar] [CrossRef]
- Leicht, H.; Göttker-Schnetmann, I.; Mecking, S. Stereoselective Copolymerization of Butadiene and Functionalized 1,3-Dienes with Neodymium-Based Catalysts. Macromolecules 2017, 50, 8464–8468. [Google Scholar] [CrossRef]
- Tanaka, R.; Yuuya, K.; Sato, H.; Eberhardt, P.; Nakayama, Y.; Shiono, T. Synthesis of stereodiblock polyisoprene consisting of cis-1,4 and trans-1,4 sequences by neodymium catalyst: Change of the stereospecificity triggered by aluminum compound. Polym. Chem. 2016, 7, 1239–1243. [Google Scholar] [CrossRef]
- Tanaka, R.; Shinto, Y.; Nakayama, Y.; Shiono, T. Synthesis of Stereodiblock Polybutadiene Using Cp*Nd(BH4)2(thf)2 as a Catalyst. Catalysts 2017, 7, 284. [Google Scholar] [CrossRef]
- Tanaka, R.; Tonoko, N.; Kihara, S.; Nakayama, Y.; Shiono, T. Reversible star assembly of polyolefins using interconversion between boroxine and boronic acid. Polym. Chem. 2018, 9, 3774–3779. [Google Scholar] [CrossRef]
- Tanaka, R.; Fujii, H.; Kida, T.; Nakayama, Y.; Shiono, T. Incorporation of Boronic Acid Functionality into Isotactic Polypropylene and Its Application as a Cross-Linking Point. Macromolecules 2021, 54, 1267–1272. [Google Scholar] [CrossRef]
- Mendis, S.N.; Zhou, T.; Klausen, R.S. Syndioselective Polymerization of a BN Aromatic Vinyl Monomer. Macromolecules 2018, 51, 6859–6864. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, Y.; Zhang, Z.; Li, S.; Cui, D. Stereoselective Polymerization of an Aromatic Vinyl Monomer to Access Highly Syndiotactic Poly(vinyl alcohol). Macromol. Rapid Commun. 2020, 41, 2000038. [Google Scholar] [CrossRef] [PubMed]
- Kamio, S.; Kageyuki, I.; Osaka, I.; Hatano, S.; Abe, M.; Yoshida, H. Anthranilamide (aam)-substituted diboron: Palladium-catalyzed selective B(aam) transfer. Chem. Commun. 2018, 54, 9290–9293. [Google Scholar] [CrossRef]
- Hahn, S.F. An improved method for the diimide hydrogenation of butadiene and isoprene containing polymers. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 397–408. [Google Scholar] [CrossRef]
- Cheng, H.N.; Smith, D.A. Carbon-13 NMR studies of low-molecular-weight ethylene-propylene copolymers and characterization of polymer chain ends. Macromolecules 1986, 17, 2065–2072. [Google Scholar] [CrossRef]
- Derlin, S.; Kaminsky, W. Copolymerization of ethylene and propylene with the sterically hindered monomer 3-methyl-1-butene by homogeneous catalysis. Macromolecules 2007, 40, 4130–4137. [Google Scholar] [CrossRef]
- Erver, F.; Hilt, G. Multi-Component Regio- and Diastereoselective Cobalt-catalyzed Hydrovinylation/Allylboration Reaction Sequence. Org. Lett. 2011, 13, 5700–5703. [Google Scholar] [CrossRef]

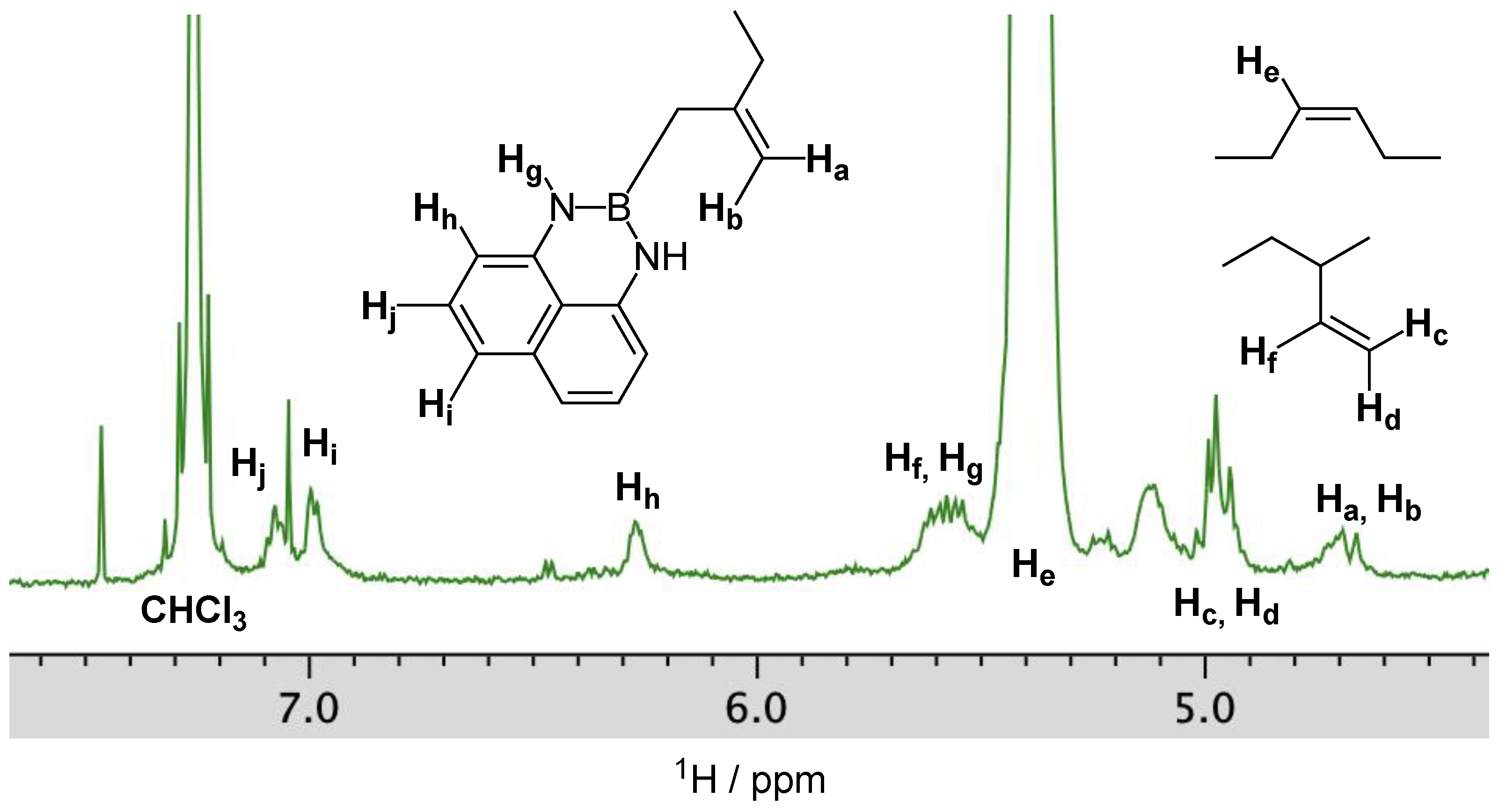
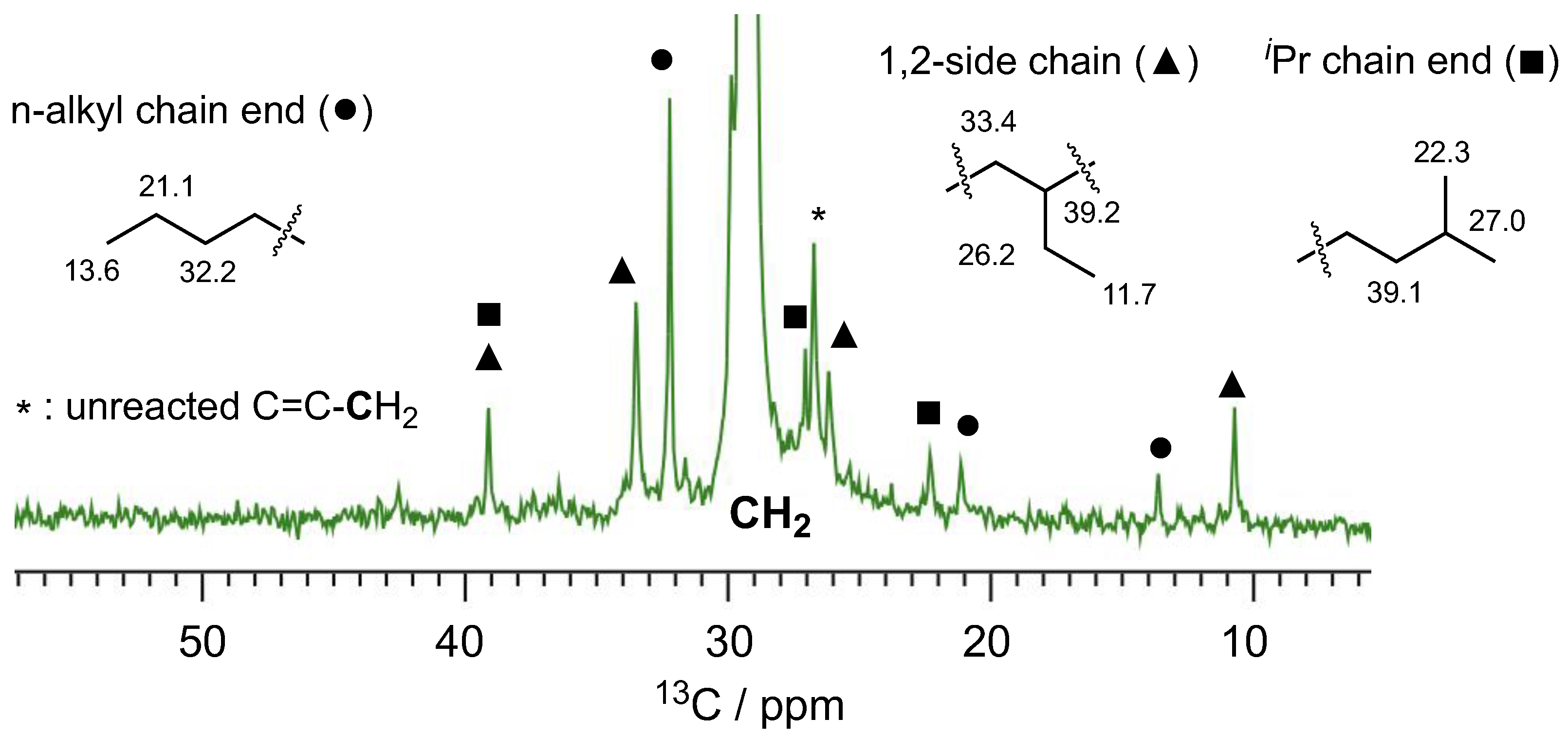
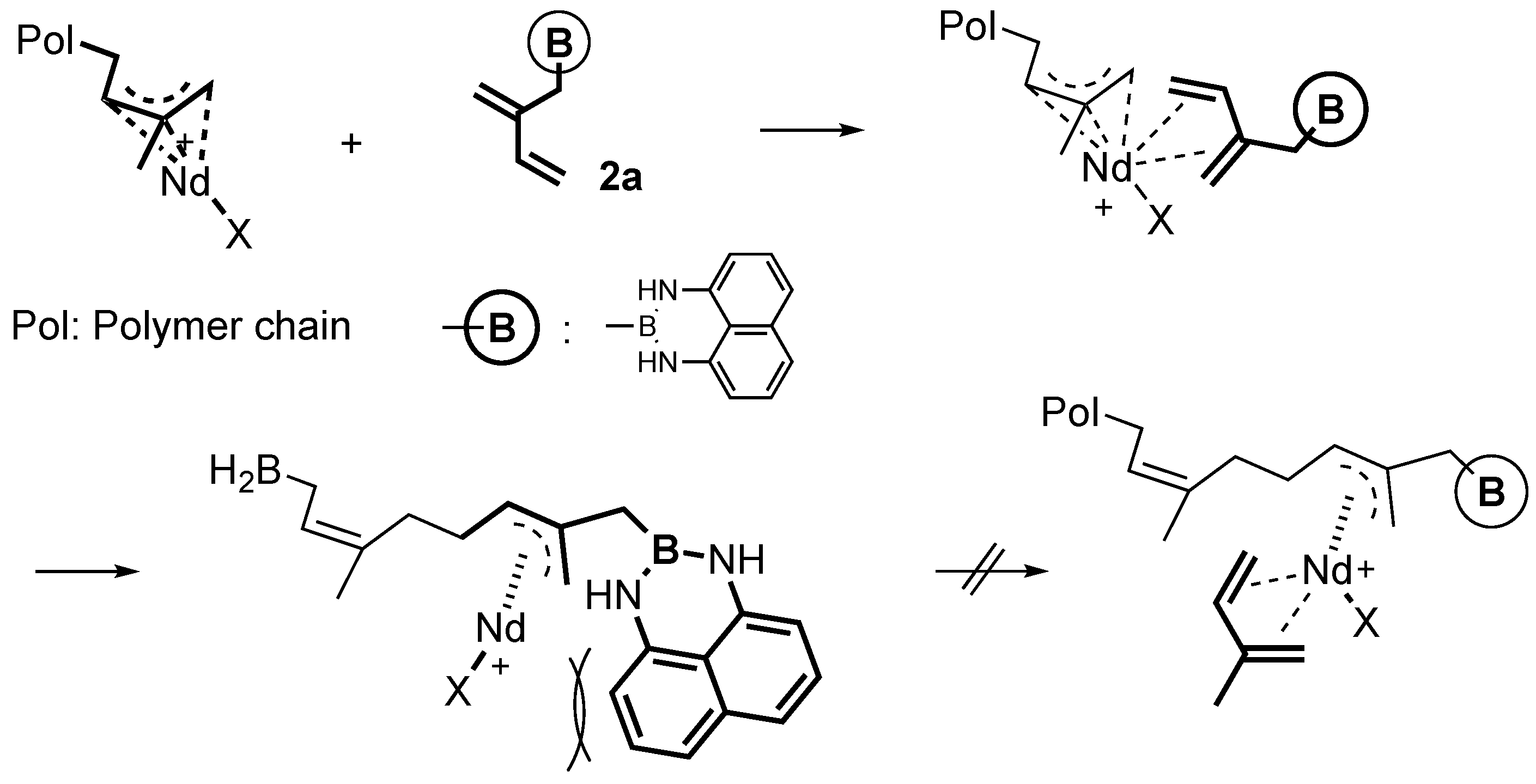
| Run | R | Mg/Nd | B(dan) | Time (h) | Yield (%) | Mna (103) | Đa | Number of B per Polymer Chain b | cis:trans:3,4 c |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Me | 1 | 2a | 1 | 37 | 3.1 | 2.6 | 1.2 | 64:19:17 |
| 2 d | Me | 1 | 2a | 1 | 0 | - e | - e | - e | - e |
| 3 | Me | 2 | 2b | 1 | 13 | 3.3 | 2.9 | 0 | 71:10:19 |
| 4 f | Me | 2 | 2a | 2.5 | 0 | - e | - e | - e | - e |
| 5 | H | 1 | 2a | 2 | 18 | 8.2 | 1.5 | 1.6 | 76:21:3 |
| 6 g | H | 1 | none | 1 | 30 | 6.7 | 1.3 | - e | 65:33:2 |
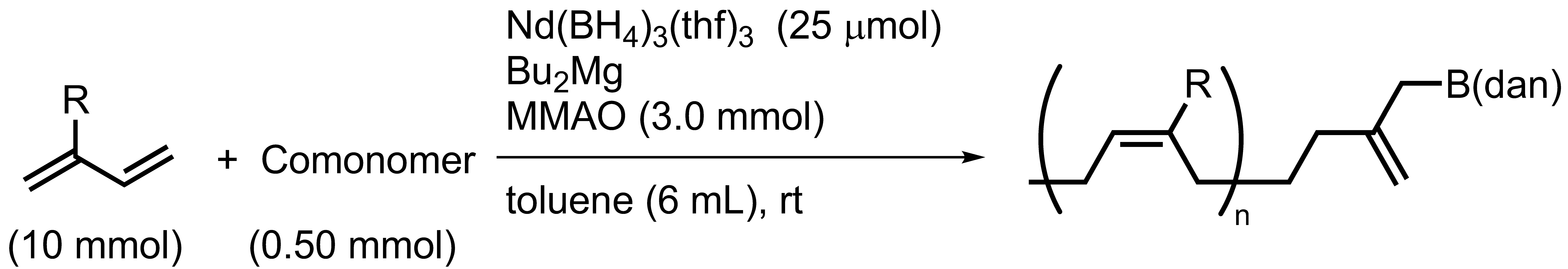 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, R.; Kuwabara, Y.; Nakayama, Y.; Shiono, T. Synthesis of Chain-End Functional Polydienes Using Diene Comonomer Bearing Boronic Acid Masked with Diaminonaphthalene. Molecules 2022, 27, 9007. https://doi.org/10.3390/molecules27249007
Tanaka R, Kuwabara Y, Nakayama Y, Shiono T. Synthesis of Chain-End Functional Polydienes Using Diene Comonomer Bearing Boronic Acid Masked with Diaminonaphthalene. Molecules. 2022; 27(24):9007. https://doi.org/10.3390/molecules27249007
Chicago/Turabian StyleTanaka, Ryo, Yuina Kuwabara, Yuushou Nakayama, and Takeshi Shiono. 2022. "Synthesis of Chain-End Functional Polydienes Using Diene Comonomer Bearing Boronic Acid Masked with Diaminonaphthalene" Molecules 27, no. 24: 9007. https://doi.org/10.3390/molecules27249007
APA StyleTanaka, R., Kuwabara, Y., Nakayama, Y., & Shiono, T. (2022). Synthesis of Chain-End Functional Polydienes Using Diene Comonomer Bearing Boronic Acid Masked with Diaminonaphthalene. Molecules, 27(24), 9007. https://doi.org/10.3390/molecules27249007







