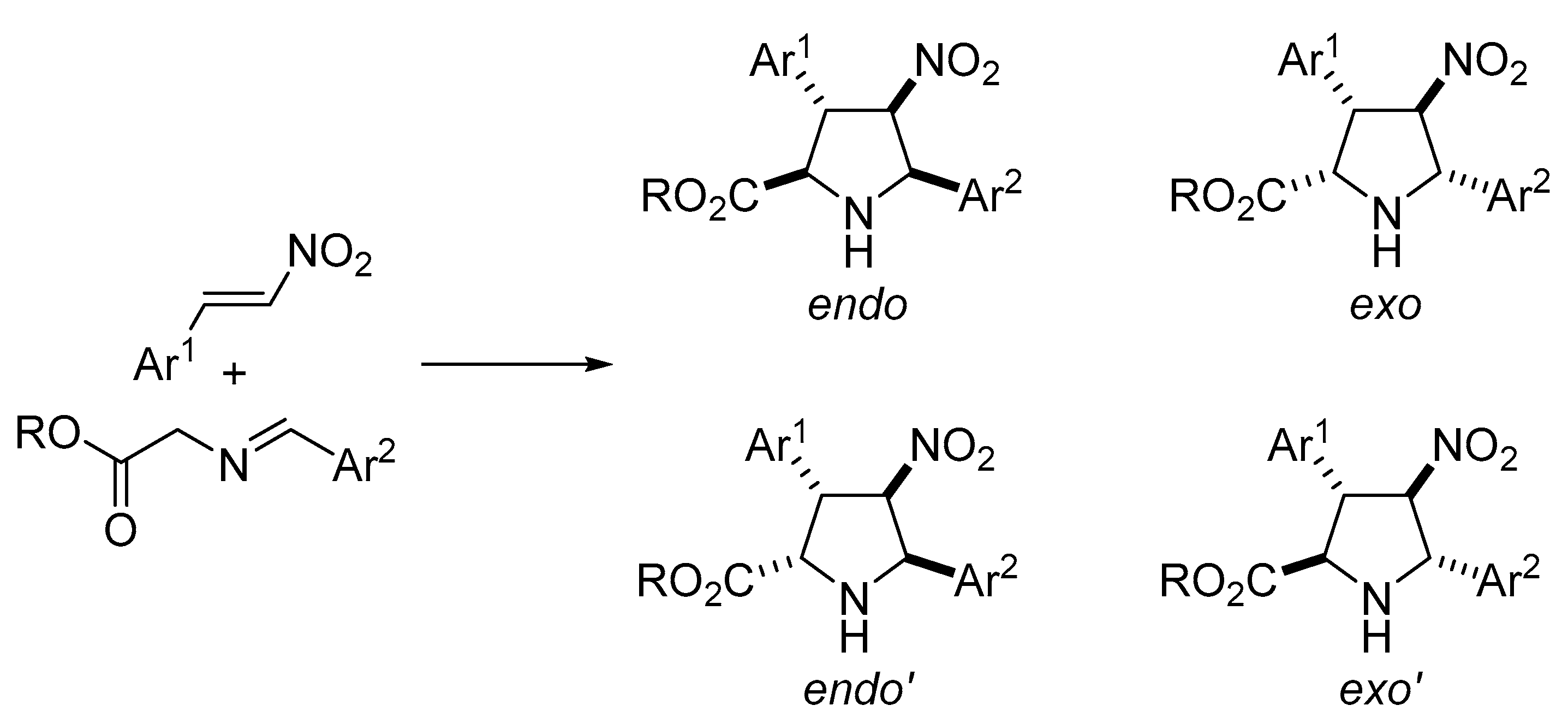
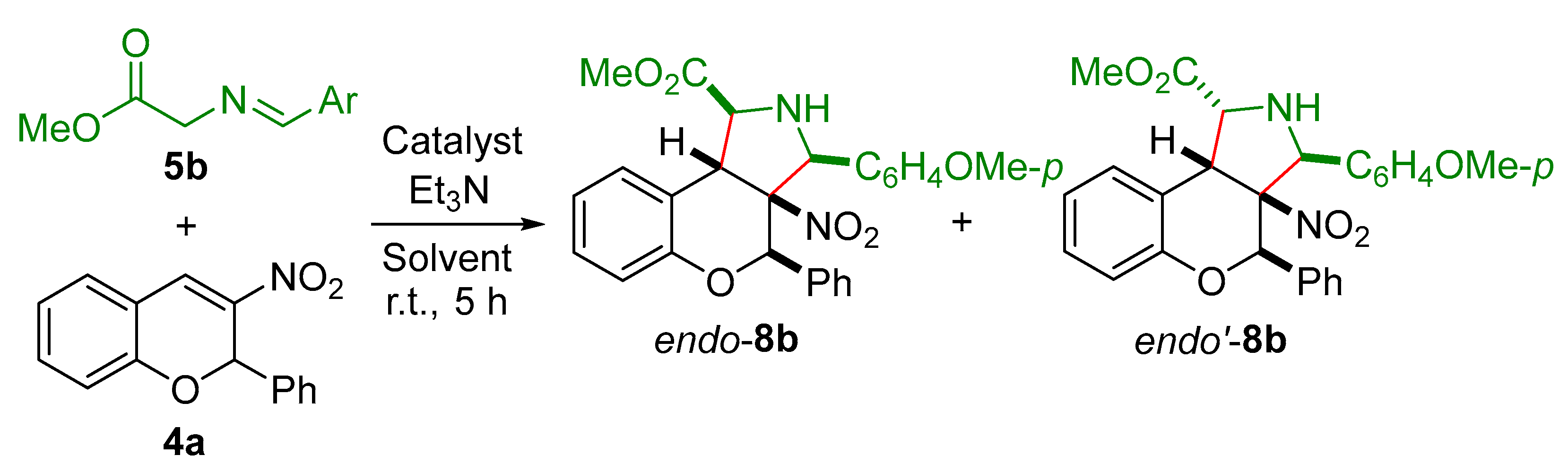
3. Materials and Methods
3.1. General
IR spectra were recorded on a Shimadzu IRSpirit-T spectrometer (Shimadzu Corp., Kyoto, Japan) using an attenuated total reflectance (ATR) unit (FTIR mode, diamond prism), and the absorbance maxima (
ν) are reported in cm
–1. NMR spectra were recorded on Bruker Avance III-500 (work frequencies:
1H—500 MHz,
19F—471 MHz,
13C—126 MHz) and Bruker DRX-400 (Bruker BioSpin GmbH, Ettlingen, Germany, work frequencies:
1H—400 MHz;
19F—376 MHz) spectrometers in CDCl
3. The chemical shifts (
δ) are reported in ppm relative to the internal standard TMS (
1H NMR), C
6F
6 (
19F NMR), and residual signal of the solvent (
13C NMR). 2D NMR spectra were acquired on Bruker AVANCE NEO (600 MHz) and Bruker AVANCE 400 spectrometers. The HRMS spectra were obtained using the UHR-QqTOF maXis Impact HD mass spectrometer. Melting points were determined on an SMP40 apparatus. Column chromatography was performed on silica gel (Merck 60, 70–230 mesh, Darmstadt, Germany). All solvents used were dried and distilled by standard procedures. The starting chromenes
4a−c and
4d−n were prepared according to described procedures [
40,
41]. Schiff bases
5 were obtained according to the described procedure [
42].
3.2. Synthesis of Compounds 8a–g
General procedure. A mixture of the appropriate 3-nitro-2-phenyl-2H-chromene 4 (0.5 mmol), azomethine 5 (0.55 mmol), Et3N (7 μL, 5 mg, 0.05 mmol) and AgOAc (5.8 mg, 0.05 mmol) was stirred in dichloromethane (2 mL) for 5 h at room temperature (TLC control, EtOAc−hexane (1:2)). Upon completion of the reaction, the residue was evaporated under reduced pressure to complete dryness. The residue was purified by silica gel column chromatography (eluent−EtOAc−hexane (1:2)) to give products endo-8 and exo’-8.
Methyl(1S*,3S*,3aS*,4R*,9bR*)-3a-nitro-3,4-diphenyl-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo-8a). Yield 120 mg (56%), white powder, mp 183–185 °C. IR (ATR) ν 3381 (NH), 1748 (C=O), 1547, 1340 (NO2). 1H NMR (500 MHz) δ 3.14 (dd, 1H, J = 10.8, 7.8 Hz, NH), 4.02 (s, 3H, MeO2C), 4.15 (dd, J = 7.8, 3.8 Hz, 1H, H-1), 4.79 (d, J = 3.8 Hz, 1H, H-9b), 4.97 (d, J = 10.8 Hz, 1H, H-3), 5.58 (s, 1H, H-4), 6.82 (d, J = 8.2 Hz, 1H, H-6), 7.07 (t, J = 7.6 Hz, 1H, H-8), 7.11–7.21 (m, 6H, H-7, H Ph), 7.35–7.45 (m, 2H, H Ph), 7.55 (d, J = 7.6 Hz, 1H, H-9); 13C NMR (126 MHz) δ 46.0, 53.0, 68.6, 70.5, 75.5, 96.7, 118.3, 123.3, 125.1, 126.9 (2C), 128.3 (2C), 128.5 (2C), 128.8, 128.88, 128.90, 129.0 (2C), 129.6, 133.9, 135.1, 149.9, 172.4. HRMS (ESI) m/z: [M + H]+ calcd for C25H23N2O5 431.1601, found 431.1595.
Methyl(1R*,3S*,3aS*,4R*,9bR*)-3a-nitro-3,4-diphenyl-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo’-8a). Yield 50 mg (23%), beige powder, mp 90–92 °C. IR (ATR) ν 3350 (NH), 1735 (C=O), 1542, 1355 (NO2). 1H NMR (500 MHz) δ 2.78 (br. s, 1H, NH), 3.36 (s, 3H, MeO2C), 5.00 (d, J = 9.8 Hz, 1H, H-1), 5.06 (d, J = 9.8 Hz, 1H, H-9b), 5.38 (s, 1H, H-3), 5.68 (s, 1H, H-4), 6.81 (d, J = 8.2, 1.2 Hz, 1H, H-6), 6.96 (td, J = 7.6, 1.2 Hz, 1H, H-8), 7.11 (ddd, J = 8.2, 7.8, 1.4 Hz, 1H, H-7), 7.22–7.34 (m, 11H, H-9, H Ph); 13C NMR (126 MHz) δ 45.2, 51.7, 64.3, 68.6, 77.5, 97.8, 118.1, 120.6, 121.9, 127.3 (2C), 128.2 (2C), 128.4 (2C), 128.7 (2C), 128.87, 128.92, 129.0, 129.7, 134.8, 136.7, 152.6, 173.3. HRMS (ESI) m/z: [M + H]+ calcd for C25H23N2O5 431.1601, found 431.1604.
Methyl(1S*,3S*,3aS*,4R*,9bR*)-3-(4-methoxyphenyl)-3a-nitro-4-phenyl-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo-8b). Yield 145 mg (63%), white powder, mp 159–161 °C. IR (ATR) ν 3323 (NH), 1745 (C=O), 1536, 1361 (NO2). 1H NMR (600 MHz) δ 3.09 (br. s, 1H, NH), 3.83 (s, 3H, MeO), 4.02 (s, 3H, MeO2C), 4.13 (br. s, 1H, H-1), 4.77 (d, J = 3.7 Hz, 1H, H-9b), 4.93 (d, J = 4.9 Hz, 1H, H-3), 5.53 (s, 1H, H-4), 6.81 (d, J = 8.1 Hz, 1H, H-6), 6.95 (d, J = 8.6 Hz, 2H, H-3,5 4-MeOC6H4), 7.06 (td, J = 7.5, 0.9 Hz, 1H, H-8), 7.12–7.20 (m, 6H, H-7, H Ph), 7.30 (d, J = 8.6 Hz, 2H, H-2,6 4-MeOC6H4), 7.55 (d, J = 7.6 Hz, 1H, H-9); 13C NMR (151 MHz) δ 45.9 (C-9b), 53.2 (MeO2C), 55.3 (MeO), 68.4 (C-1), 70.1 (C-3), 75.5 (C-4), 96.4 (C-3a), 114.4 (C-3,5 4-MeOC6H4), 118.4 (C-6), 123.3 (C-8), 124.9 (C-9a), 125.3 (C-7), 128.1 (C-2,6 4-MeOC6H4), 128.3 (C-2,6 Ph), 128.5 (C-3,5 Ph), 128.7 (C-9), 128.9 (C-4 Ph, C-1 4-MeOC6H4), 135.0 (C-1 Ph), 149.8 (C-5a), 160.6 (C-4 4-MeOC6H4), 172.3 (C=O). HRMS (ESI) m/z: [M + H]+ calcd for C26H25N2O6 461.1707, found 461.1710.
Methyl(1R*,3S*,3aS*,4R*,9bR*)-3-(4-methoxyphenyl)-3a-nitro-4-phenyl-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo’-8b). Yield 48 mg (21%), beige powder, mp 132–133 °C. IR (ATR) ν 3356 (NH), 1732 (C=O), 1543, 1351 (NO2). 1H NMR (600 MHz) δ 2.74 (br. s, 1H, NH), 3.35 (s, 3H, MeO2C), 3.78 (s, 3H, MeO), 4.98 (d, J = 9.8 Hz, 1H, H-1), 5.04 (d, J = 9.8 Hz, 1H, H-9b), 5.35 (s, 1H, H-3), 5.64 (s, 1H, H-4), 6.80 (dd, J = 8.2, 1.0 Hz, 1H, H-6), 6.85 (d, J = 8.7 Hz, 2H, H-3,5 4-MeOC6H4), 6.95 (td, J = 7.6, 1.0 Hz, 1H, H-8), 7.10 (ddd, J = 8.2, 7.6, 1.0 Hz, 1H, H-7), 7.21 (d, J = 8.7 Hz, 2H, H-2,6 4-MeOC6H4), 7.23 (d, J = 8.0 Hz, 1H, H-9), 7.25–7.28 (m, 5H, H Ph); 13C NMR (151 MHz) δ 45.2, 51.9, 55.3, 64.4, 68.6, 77.5, 97.9, 114.2 (2C), 118.3, 120.9, 122.1, 128.3 (2C), 128.36, 128.40, 128.5 (2C), 128.6 (2C), 129.0, 129.8, 134.9, 152.6, 160.3, 173.4. HRMS (ESI) m/z: [M + H]+ calcd for C26H25N2O6 461.1707, found 461.1706.
Methyl(1S*,3S*,3aS*,4R*,9bR*)-3-(3,4-dimethoxyphenyl)-3a-nitro-4-phenyl-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo-8c). Yield 132 mg (54%), white powder, mp 191–193 °C. IR (ATR) ν 3327 (NH), 1732 (C=O), 1535, 1341 (NO2). 1H NMR (500 MHz) δ 3.12 (br. s, 1H, NH), 3.90 (s, 3H, MeO), 3.91 (s, 3H, MeO), 4.02 (s, 3H, MeO2C), 4.14 (d, J = 3.8 Hz, 1H, H-1), 4.77 (d, J = 3.8 Hz, 1H, H-9b), 4.92 (c, 1H, H-3), 5.55 (s, 1H, H-4), 6.81 (dd, J = 7.5, 1.0 Hz, 1H, H-6), 6.85 (d, J = 1.8 Hz, 1H, H-2 (MeO)2C6H3), 6.90 (d, J = 8.3 Hz, 1H, H-5 (MeO)2C6H3), 6.95 (dd, J = 8.3, 1.8 Hz, 1H, H-6 (MeO)2C6H3), 7.06 (td, J = 7.5, 1.0 Hz, 1H, H-8), 7.12–7.21 (m, 6H, H-7, H Ph), 7.54 (d, J = 7.5 Hz, 1H, H-9); 13C NMR (126 MHz) δ 45.9, 53.0, 55.9, 56.1, 68.4, 70.5, 75.7, 96.4, 109.8, 111.3, 118.3, 119.6, 123.2, 125.1, 126.3, 128.3 (2C), 128.5 (2C), 128.8, 128.9 (2C), 135.1, 149.3, 149.9, 150.1, 172.5. HRMS (ESI) m/z: [M + H]+ calcd for C27H27N2O6 491.1813, found 491.1814.
Methyl (1R*,3S*,3aS*,4R*,9bR*)-3-(3,4-dimethoxyphenyl)-3a-nitro-4-phenyl-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo’-8c). Yield 66 mg (27%), beige powder, mp 113–115 °C. IR (ATR) ν 3338 (NH), 1732 (C=O), 1536, 1340 (NO2). 1H NMR (500 MHz) δ 2.73 (br. s, 1H, NH), 3.36 (s, 3H, MeO2C), 3.80 (s, 3H, MeO), 3.85 (s, 3H, MeO), 4.96 (d, J = 9.5 Hz, 1H, H-1), 5.07 (d, J = 9.5 Hz, 1H, H-9b), 5.40 (s, 1H, H-3), 5.59 (s, 1H, H-4), 6.71 (d, J = 1.8 Hz, 1H, H-2 (MeO)2C6H3), 6.78–6.83 (m, 2H, H-6, H-5 (MeO)2C6H3), 6.90 (dd, J = 8.0, 1.8 Hz, 1H, H-6 (MeO)2C6H3), 6.96 (t, J = 7.6 Hz, 1H, H-8), 7.10 (t, J = 7.8 Hz, 1H, H-7), 7.23–7.31 (m, 6H, H-9, H Ph); 13C NMR (126 MHz) δ 45.4, 51.7, 55.8, 56.0, 64.0, 68.4, 77.8, 97.9, 110.5, 111.0, 118.2, 119.7, 122.0, 127.9, 128.1 (2C), 128.3, 128.6 (2C), 128.9 (2C), 129.7, 134.9, 149.0, 149.6, 152.6, 173.5. HRMS (ESI) m/z: [M + H]+ calcd for C27H27N2O6 491.1813, found 491.1806.
Methyl (1S*,3S*,3aS*,4R*,9bR*)-3a-nitro-4-phenyl-3-(3,4,5-trimethoxyphenyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo-8d). Yield 143 mg (55%), white powder, mp 200–202 °C. IR (ATR) ν 3356 (NH), 1735 (C=O), 1537, 1332 (NO2). 1H NMR (500 MHz) δ 3.02 (br. s, 1H, NH), 3.87 (s, 3H, MeO), 3.89 (s, 6H, MeO), 4.02 (s, 3H, MeO2C), 4.13 (d, J = 3.8 Hz, 1H, H-1), 4.80 (d, J = 3.8 Hz, 1H, H-9b), 4.89 (c, 1H, H-3), 5.59 (s, 1H, H-4), 6.58 (s, 2H, H-2,6 3,4,5-(MeO)3C6H2), 6.82 (d, J = 8.1 Hz, 1H, H-6), 7.07 (t, J = 7.6 Hz, 1H, H-8), 7.13–7.24 (m, 6H, H-7, H Ph), 7.53 (d, J = 7.6 Hz, 1H, H-9); 13C NMR (126 MHz) δ 45.8, 52.9 (3C), 56.3, 68.1, 70.2, 75.7, 96.3, 104.1 (2C), 118.1, 123.1, 124.7, 128.1 (2C), 128.3 (2C), 128.6, 128.77, 128.80, 129.8, 134.9, 138.9, 149.9, 153.4 (2C), 172.4. HRMS (ESI) m/z: [M + H]+ calcd for C28H29N2O8 521.1918, found 521.1921.
Methyl (1R*,3S*,3aS*,4R*,9bR*)-3a-nitro-4-phenyl-3-(3,4,5-trimethoxyphenyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo’-8d). This product was not isolated in pure form. 1H NMR (400 MHz) δ 3.02 (br. s, 1H, NH), 3.39 (s, 3H, MeO2C), 3.77 (s, 6H, MeO), 3.81 (s, 3H, MeO), 4.94 (d, J = 9.3 Hz, 1H, H-1), 5.10 (d, J = 9.3 Hz, 1H, H-9b), 5.42 (c, 1H, H-3), 5.54 (s, 1H, H-4), 6.46 (s, 2H, H-2,6 3,4,5-(MeO)3C6H2), 6.79 (d, J = 8.2 Hz, 1H, H-6), 6.98 (td, J = 7.6, 1.1 Hz, 1H, H-8), 7.10 (t, J = 8.1 Hz, 1H, H-7), 7.21–7.37 (m, 6H, H-9, H Ph).
Methyl (1S*,3S*,3aS*,4R*,9bR*)-3-(benzo[d][1,3]dioxol-5-yl)-3a-nitro-4-phenyl-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo-8e). Yield 133 mg (56%), white powder, mp 177–179 °C. IR (ATR) ν 3328 (NH), 1744 (C=O), 1536, 1346 (NO2). 1H NMR (500 MHz) δ 3.00 (dd, J = 10.2, 7.7 Hz, 1H, NH), 4.01 (s, 3H, MeO2C), 4.11 (dd, J = 7.7, 3.8 Hz, 1H, H-1), 4.77 (d, J = 3.8 Hz, 1H, H-9b), 4.89 (d, J = 10.2 Hz, 1H, H-3), 5.57 (s, 1H, H-4), 5.99 (d, J = 1.4 Hz, 1H, OCH2O), 6.00 (d, J = 1.4 Hz, 1H, OCH2O), 6.80 (dd, J = 8.1, 1.1 Hz, 1H, H-6), 6.85 (d, J = 8.0 Hz, 1H, H-7 benzo[d][1,3]dioxol-5-yl), 6.86 (d, J = 1.6 Hz, 1H, H-4 benzo[d][1,3]dioxol-5-yl), 6.88 (dd, J = 8.1, 1.6 Hz, 1H, H-6 benzo[d][1,3]dioxol-5-yl), 7.06 (td, J = 7.6, 1.1 Hz, 1H, H-8), 7.11–7.22 (m, 6H, H-7, H Ph), 7.53 (d, J = 7.6 Hz, 1H, H-9); 13C NMR (126 MHz) δ 45.8, 53.0, 68.3, 70.2, 75.5, 96.3, 101.4, 107.0, 108.6, 118.3, 120.8, 123.2, 125.1, 127.6, 128.3 (2C), 128.5 (2C), 128.8, 128.9 (2C), 135.1, 148.3, 148.7, 149.9, 172.4. HRMS (ESI) m/z: [M + H]+ calcd for C26H23N2O7 475.1500, found 475.1486.
Methyl (1R*,3S*,3aS*,4R*,9bR*)-3-(benzo[d][1,3]dioxol-5-yl)-3a-nitro-4-phenyl-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo’-8e). Yield 47 mg (20%), beige powder, mp 111–113 °C. IR (ATR) ν 3362 (NH), 1731 (C=O), 1543, 1340 (NO2). 1H NMR (500 MHz) δ 2.69 (br. s, 1H, NH), 3.36 (s, 3H, MeO2C), 4.95 (d, J = 9.7 Hz, 1H, H-1), 5.04 (d, J = 9.7 Hz, 1H, H-9b), 5.32 (br. s, 1H, H-3), 5.64 (s, 1H, H-4), 5.94 (d, J = 1.3 Hz, 1H, OCH2O), 5.95 (d, J = 1.3 Hz, 1H, OCH2O), 6.73–6.82 (m, 4H, H-6, H-4,6,7 benzo[d][1,3]dioxol-5-yl), 6.96 (td, J = 7.5, 1.0 Hz, 1H, H-8), 7.10 (td, J = 7.6, 1.2 Hz, 1H, H-8), 7.22 (dd, J = 7.6, 1.2 Hz, 1H, H-9), 7.25–7.29 (m, 5H, H Ph); 13C NMR (126 MHz) δ 45.0, 51.7, 64.1, 68.4, 77.5, 97.6, 101.2, 107.5, 108.3, 118.2, 120.7, 121.2, 121.9, 128.2 (2C), 128.4 (2C), 128.87, 128.92, 129.7, 130.4, 134.7, 147.9, 148.2, 152.5, 173.4. HRMS (ESI) m/z: [M + H]+ calcd for C26H23N2O7 475.1500, found 475.1503.
Methyl(1S*,3S*,3aS*,4R*,9bR*)-8-bromo-3-(4-methoxyphenyl)-3a-nitro-4-phenyl-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo-8f). Yield 164 mg (61%), white powder, mp 208–210 °C. IR (ATR) ν 3359 (NH), 1755 (C=O), 1547, 1362 (NO2). 1H NMR (500 MHz) δ 3.07 (dd, J = 10.6, 7.7 Hz, 1H, NH), 3.82 (s, 3H, MeO), 4.03 (s, 3H, MeO2C), 4.10 (dd, J = 7.7, 3.8 Hz, 1H, H-1), 4.74 (d, J = 3.8 Hz, 1H, H-9b), 4.88 (d, J = 10.6 Hz, 1H, H-3), 5.53 (s, 1H, H-4), 6.69 (d, J = 8.7 Hz, 1H, H-6), 6.94 (d, J = 8.7 Hz, 2H, H-3,5 4-MeOC6H4), 7.11–7.15 (m, 2H, H Ph), 7.16–7.22 (m, 3H, H Ph), 7.24 (dd, J = 8.7, 2.5 Hz, 1H, H-7), 7.28 (d, J = 8.7 Hz, 2H, H-2,6 4-MeOC6H4), 7.67 (d, J = 2.5 Hz, 1H, H-9); 13C NMR (126 MHz) δ 45.8, 53.2, 55.3, 68.3, 70.3, 75.7, 96.0, 114.4 (2C), 115.4, 120.2, 125.4, 127.3, 128.0 (2C), 128.2 (2C), 128.6 (2C), 129.0, 131.4, 131.9, 134.7, 149.1, 160.6, 172.1. HRMS (ESI) m/z: [M + H]+ calcd for C26H24BrN2O6 539.0812, found 539.0809.
Methyl(1R*,3S*,3aS*,4R*,9bR*)-8-bromo-3-(4-methoxyphenyl)-3a-nitro-4-phenyl-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo’-8f). Yield 57 mg (21%), beige powder, mp 188–190 °C. IR (ATR) ν 3382 (NH), 1715 (C=O), 1546, 1362 (NO2). 1H NMR (500 MHz) δ 2.72 (br. s, 1H, NH), 3.47 (s, 3H, MeO2C), 3.78 (s, 3H, MeO), 4.96 (d, J = 9.8 Hz, 1H, H-1), 4.99 (d, J = 9.8 Hz, 1H, H-9b), 5.29 (s, 1H, H-3), 5.63 (s, 1H, H-4), 6.70 (d, J = 8.7 Hz, 1H, H-6), 6.85 (d, J = 8.7 Hz, 2H, H-3,5 4-MeOC6H4), 7.21 (d, J = 8.7 Hz, 2H, H-2,6 4-MeOC6H4), 7.23–7.29 (m, 7H, H-7,9, Ph); 13C NMR (126 MHz) δ 44.7, 51.9, 55.2, 63.9, 68.3, 77.5, 97.2, 114.1 (2C), 120.0, 122.9, 124.3, 128.26 (2C), 128.32 (2C), 128.5 (2C), 129.0, 131.6, 131.8, 132.3, 134.4, 151.2, 160.0, 170.4. HRMS (ESI) m/z: [M + H]+ calcd for C26H24BrN2O6 539.0812, found 539.0810.
Methyl (1S*,3S*,3aS*,4R*,9bR*)-8-methoxy-3-(4-methoxyphenyl)-3a-nitro-4-phenyl-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo-8g). Yield 140 mg (57%), white powder, mp 173–175 °C. IR (ATR) ν 3364 (NH), 1752 (C=O), 1544, 1365 (NO2). 1H NMR (400 MHz) δ 3.09 (t, J = 8.7 Hz, 1H, NH), 3.79 (s, 3H, MeO), 3.82 (s, 3H, MeO), 4.02 (s, 3H, MeO2C), 4.12 (dd, J = 6.7, 3.9 Hz, 1H, H-1), 4.72 (d, J = 3.9 Hz, 1H, H-9b), 4.93 (d, J = 10.4 Hz, 1H, H-3), 5.50 (s, 1H, H-4), 6.69 (dd, J = 8.8, 2.5 Hz, 1H, H-7), 6.72 (d, J = 8.8 Hz, 1H, H-6), 6.94 (d, J = 8.7 Hz, 2H, H-3,5 4-MeOC6H4), 7.07 (d, J = 2.5 Hz, 1H, H-9), 7.11–7.21 (m, 5H, H Ph), 7.29 (d, J = 8.7 Hz, 2H, H-2,6 4-MeOC6H4); 13C NMR (126 MHz) δ 46.4, 53.0, 55.2, 55.6, 68.4, 70.3, 75.7, 96.7, 113.1, 114.4 (2C), 114.7, 119.1, 125.6, 126.0, 128.0 (2C), 128.3 (2C), 128.4 (2C), 128.8, 135.1, 143.6, 155.3, 160.6, 172.3. HRMS (ESI) m/z: [M + H]+ calcd for C27H27N2O7 491.1813, found 491.1812.
Methyl (1R*,3S*,3aS*,4R*,9bR*)-8-methoxy-3-(4-methoxyphenyl)-3a-nitro-4-phenyl-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo’-8g). Yield 54 mg (22%), beige powder, mp 95–97 °C. IR (ATR) ν 3344 (NH), 1733 (C=O), 1542, 1358 (NO2). 1H NMR (500 MHz) δ 2.73 (br. s, 1H, NH), 3.40 (s, 3H, MeO2C), 3.76 (s, 3H, MeO), 3.79 (s, 3H, MeO), 4.97 (d, J = 9.7 Hz, 1H, H-1), 5.01 (d, J = 9.7 Hz, 1H, H-9b), 5.36 (s, 1H, H-3), 5.59 (s, 1H, H-4), 6.66 (dd, J = 8.8, 2.8 Hz, 1H, H-7), 6.71 (d, J = 8.8 Hz, 1H, H-6), 6.75 (d, J = 2.8 Hz, 1H, H-9), 6.86 (d, J = 8.7 Hz, 2H, H-3,5 4-MeOC6H4), 7.20–7.26 (m, 7H, H-2,6 4-MeOC6H4, H Ph); 13C NMR (126 MHz) δ 45.5, 51.7, 55.2, 55.7, 64.3, 68.6, 77.4, 98.0, 114.0, 114.1 (2C), 115.0, 118.9, 121.6, 128.2 (2C), 128.36 (2C), 128.44 (2C), 128.8, 130.1, 134.9, 146.2, 154.4, 160.2, 173.2. HRMS (ESI) m/z: [M + H]+ calcd for C27H27N2O7 491.1813, found 491.1811.
3.3. Synthesis of Compounds 9a–j
General procedure. A mixture of the appropriate 3-nitro-2-(trifluoromethyl)-2H-chromene 4 (0.5 mmol), azomethine 5 (0.55 mmol), Et3N (7 μL, 5 mg, 0.05 mmol) and AgOAc (5.8 mg, 0.05 mmol) was stirred in dichloromethane (2 mL) for 5 h at room temperature (TLC control, EtOAc−hexane (1:3)). Upon completion of the reaction, the residue was evaporated under reduced pressure to complete dryness. The residue was purified by silica gel column chromatography (eluent−EtOAc−hexane (1:3)) to give products endo-9.
Methyl (1S*,3S*,3aS*,4S*,9bR*)-3a-nitro-3-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo-9a). Yield 190 mg (90%), beige powder, mp 125–127 °C. IR (ATR) ν 3320 (NH), 1743 (C=O), 1546, 1361 (NO2). 1H NMR (500 MHz) δ 3.13 (dd, J = 11.3, 7.5 Hz, 1H, NH), 4.02 (s, 3H, MeO2C), 4.09 (dd, J = 7.5, 3.1 Hz, 1H, H-1), 4.57 (d, J = 3.1 Hz, 1H, H-9b), 4.83 (d, J = 11.3 Hz, 1H, H-3), 5.11 (q, J = 7.0 Hz, 1H, H-4), 7.07 (dd, J = 8.2, 1.0 Hz, 1H, H-6), 7.18 (ddd, J = 8.2, 7.6, 1.0 Hz, 1H, H-8), 7.23–7.32 (m, 3H, H-7, H Ph), 7.43–7.47 (m, 3H, H Ph), 7.52 (d, J = 7.7 Hz, 1H, H-9); 19F NMR (471 MHz) δ 96.6 (d, J = 7.0 Hz, CF3); 13C NMR (126 MHz) δ 45.6, 53.1, 68.4, 70.2, 72.3 (q, 2JCF = 31.8 Hz, C-4), 93.6, 117.6, 123.3 (q, 1JCF = 288.9 Hz, CF3), 124.0, 124.4, 126.5 (2C), 129.09, 129.14, 129.2 (2C), 130.0, 132.6, 149.0, 171.9. HRMS (ESI) m/z: [M + H]+ calcd for C20H18F3N2O5 423.1162, found 423.1160.
Methyl (1S*,3S*,3aS*,4S*,9bR*)-3-(4-methoxyphenyl)-3a-nitro-3-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo-9b). Yield 208 mg (92%), white powder, mp 163–165 °C. IR (ATR): ν 3321 (NH), 1742 (C=O), 1547, 1362 (NO2). 1H NMR (500 MHz) δ 3.07 (dd, J = 11.2, 7.6 Hz, 1H, NH), 3.83 (s, 3H, MeO), 4.02 (s, 3H, MeO2C), 4.07 (dd, J = 7.6, 3.0 Hz, 1H, H-1), 4.54 (d, J = 3.0 Hz, 1H, H-9b), 4.79 (d, J = 11.2 Hz, 1H, H-3), 5.07 (q, J = 7.0 Hz, 1H, H-4), 6.95 (d, J = 8.7 Hz, 2H, H-3,5 4-MeOC6H4), 7.06 (dd, J = 8.1, 1.0 Hz, 1H, H-6), 7.15 (td, J = 7.6, 1.0 Hz, 1H, H-8), 7.22 (d, J = 8.7 Hz, 2H, H-2,6 4-MeOC6H4), 7.28 (td, J = 8.1, 1.0 Hz, 1H, H-7), 7.51 (dd, J = 7.6, 1.0 Hz, 1H, H-9); 19F NMR (471 MHz) δ 96.7 (d, J = 7.0 Hz, CF3); 13C NMR (126 MHz) δ 45.5, 53.1, 55.3, 68.4, 70.0, 72.4 (q, 2JCF = 31.4 Hz, C-4), 93.5, 114.6 (2C), 117.5, 123.4 (q, 1JCF = 288.7 Hz, CF3), 124.0, 124.4 (2C), 127.7 (2C), 129.1 (2C), 149.0, 160.9, 172.0. HRMS (ESI) m/z: [M + H]+ calcd for C21H20F3N2O6 453.1268, found 453.1272.
Methyl (1R*,3S*,3aS*,4S*,9bR*)-3-(4-methoxyphenyl)-3a-nitro-3-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo’-9b). This product was obtained according to the general procedure at −20 °C for 5 h and was not isolated in pure form. 1H NMR (400 MHz) δ 2.67 (br. s, 1H), 3.36 (s, 3H, MeO2C), 3.83 (s, 3H, MeO), 4.83 (d, J = 9.0 Hz, 1H, H-1), 4.90 (d, J = 9.0 Hz, 1H, H-9b), 5.13 (q, J = 7.0 Hz, 1H, H-4), 5.30 (s, 1H, H-3), 6.94 (d, J = 8.7 Hz, 2H, H-3,5 4-MeOC6H4), 6.98 (dd, J = 8.0, 1.0 Hz, 1H, H-6), 7.02–7.08 (m, 2H, H-7,8), 7.15 (dd, J = 7.6, 1.4 Hz, 1H, H-9), 7.27 (d, J = 8.7 Hz, 2H, H-2,6 4-MeOC6H4); 19F NMR (376 MHz) δ 98.0 (d, J = 7.0 Hz, CF3).
Methyl (1R*,3R*,3aS*,4S*,9bR*)-3-(4-methoxyphenyl)-3a-nitro-3-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (exo-9b). This product was obtained according to the general procedure without AgOAc and was not isolated in pure form. 1H NMR (400 MHz) δ 3.02 (dd, J = 10.8, 10.0 Hz, 1H, NH), 3.74 (s, 3H, CO2Me), 3.87 (s, 3H, MeO), 4.07 (dd, J = 10.0, 5.8 Hz, 1H, H-1), 4.38 (d, J = 5.8 Hz, 1H, H-9b), 4.51 (d, J = 10.8 Hz, 1H, H-3), 4.67 (q, J = 7.2 Hz, 1H, H-4) (other signals overlapped with signals of major isomers). 19F NMR (471 MHz) δ 95.8 (d, J = 7.2 Hz, CF3).
Methyl (1S*,3S*,3aS*,4S*,9bR*)-3-(3,4-dimethoxyphenyl)-3a-nitro-3-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo-9c). Yield 222 mg (92%), white powder, mp 150–152 °C. IR (ATR) ν 3386 (NH), 1745 (C=O), 1547, 1363 (NO2). 1H NMR (400 MHz) δ 3.07 (dd, J = 10.8, 7.5 Hz, 1H, NH), 3.83 (s, 6H, 2MeO), 4.03 (s, 3H, MeO2C), 4.07 (dd, J = 7.5, 3.0 Hz, 1H, H-1), 4.55 (d, J = 3.0 Hz, 1H, H-9b), 4.78 (d, J = 10.8 Hz, 1H, H-3), 5.10 (q, J = 7.0 Hz, 1H, H-4), 6.76 (d, J = 1.8 Hz, 1H, H-2 3,4-(MeO)2C6H3), 6.87 (dd, J = 8.3, 1.8 Hz, 1H, H-6 3,4-(MeO)2C6H3), 6.91 (d, J = 8.3 Hz, H-5 3,4-(MeO)2C6H3), 7.06 (dd, J = 8.2, 1.0 Hz, 1H, H-6), 7.17 (td, J = 7.6, 1.0 Hz, 1H, H-8), 7.28 (td, J = 8.2, 1.0 Hz, 1H, H-7), 7.51 (dd, J = 7.6, 1.0 Hz, 1H, H-9); 19F NMR (376 MHz) δ 96.9 (d, J = 7.0 Hz, CF3); 13C NMR (126 MHz) δ 45.4, 53.1, 55.9, 56.1, 68.3, 70.3, 72.4 (q, 2JCF = 31.5 Hz, C-4), 93.4, 109.3, 111.5, 117.6, 119.3, 123.4 (q, 1JCF = 288.9 Hz, CF3), 124.0, 124.4, 124.9, 129.08, 129.11, 149.0, 149.6, 150.4, 172.0. HRMS (ESI) m/z: [M + H]+ calcd for C22H22F3N2O7 483.1374, found 483.1374.
Methyl (1S*,3S*,3aS*,4S*,9bR*)-3a-nitro-4-(trifluoromethyl)-3-(3,4,5-trimethoxyphenyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo-9d). Yield 218 mg (85%), white powder, mp 115–117 °C. IR (ATR) ν 3326 (NH), 1747 (C=O), 1549, 1334 (NO2). 1H NMR (400 MHz) δ 3.01 (dd, J = 10.4, 7.2 Hz, 1H, NH), 3.88 (s, 3H, MeO), 3.89 (s, 6H, 2MeO), 4.03 (s, 3H, MeO2C), 4.07 (dd, J = 7.2, 3.1 Hz, 1H, H-1), 4.56 (d, J = 3.1 Hz, 1H, H-9b), 4.75 (d, J = 10.4 Hz, 1H, H-3), 5.13 (q, J = 7.0 Hz, 1H, H-4), 6.50 (s, 2H, H-2,6 3,4,5-(MeO)2C6H2), 7.07 (dd, J = 8.2, 1.2 Hz, 1H, H-6), 7.18 (td, J = 7.7, 1.2 Hz, 1H, H-8), 7.29 (td, J = 8.2, 1.0 Hz, 1H, H-7), 7.51 (dd, J = 7.7, 1.0 Hz, 1H, H-9); 19F NMR (376 MHz) δ 96.9 (d, J = 7.0 Hz, CF3); 13C NMR (126 MHz) δ 45.3, 53.1, 56.4 (2C), 60.9, 68.3, 70.5, 72.5 (q, 2JCF = 31.6 Hz, C-4), 93.3, 103.8 (2C), 117.6, 123.3 (q, 1JCF = 288.8 Hz, CF3), 124.0, 124.5, 128.2, 129.1, 129.2, 149.0, 153.8 (3C), 172.0. HRMS (ESI) m/z: [M + H]+ calcd for C23H24F3N2O8 513.1479, found 513.1472.
Methyl (1S*,3S*,3aS*,4S*,9bR*)-3-(benzo[d][1,3]dioxol-5-yl)-3a-nitro-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo-9e). Yield 203 mg (87%), white powder, mp 168–170 °C. IR (ATR) ν 3336 (NH), 1736 (C=O), 1544, 1362 (NO2). 1H NMR (400 MHz) δ 2.97 (dd, J = 10.7, 7.6 Hz, 1H, NH), 4.01 (s, 3H, MeO2C), 4.05 (dd, J = 7.6, 3.0 Hz, 1H, H-1), 4.55 (d, J = 3.0 Hz, 1H, H-9b), 4.75 (d, J = 10.7 Hz, 1H, H-3), 5.11 (q, J = 7.0 Hz, 1H, H-4), 6.01 (d, J = 1.4 Hz, 1H, OCH2O), 6.02 (d, J = 1.4 Hz, 1H, OCH2O), 6.76–6.82 (m, 2H, H-4,7 benzo[d][1,3]dioxol-5-yl), 6.85 (d, J = 7.9 Hz, 1H, H-6 benzo[d][1,3]dioxol-5-yl), 7.05 (dd, J = 8.2, 1.1 Hz, 1H, H-6), 7.17 (td, J = 7.7, 1.1 Hz, 1H, H-8), 7.27 (td, J = 8.2, 1.1 Hz, 1H, H-7), 7.50 (d, J = 7.7, 1.1 Hz, 1H, H-9); 19F NMR (376 MHz) δ 96.7 (d, J = 7.0 Hz, CF3); 13C NMR (126 MHz) δ 45.3, 53.1, 68.2, 70.0, 72.3 (q, 2JCF = 31.4 Hz, C-4), 93.3, 101.6, 106.6, 108.8, 117.6, 120.5, 123.4 (q, 1JCF = 289.0 Hz, CF3), 124.0, 124.4, 126.3, 129.09, 129.13, 148.5, 148.99, 149.02, 171.9. HRMS (ESI) m/z: [M + H]+ calcd for C21H18F3N2O7 467.1061, found 467.1064.
Methyl (1S*,3S*,3aS*,4S*,9bR*)-8-chloro-3-(4-methoxyphenyl)-3a-nitro-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo-9f). Yield 219 mg (90%), white powder, mp 191–193 °C. IR (ATR) ν 3316 (NH), 1745 (C=O), 1547, 1360 (NO2). 1H NMR (500 MHz) δ 3.06 (dd, J = 11.0, 7.7 Hz, 1H, NH), 3.83 (s, 3H, MeO), 4.01–4.05 (m, 4H, MeO2C, H-1), 4.50 (d, J = 3.3 Hz, 1H, H-9b), 4.74 (d, J = 11.0 Hz, 1H, H-3), 5.07 (q, J = 6.9 Hz, 1H, H-4), 6.95 (d, J = 8.6 Hz, 2H, H-3,5 4-MeOC6H4), 7.01 (d, J = 8.7 Hz, 1H, H-6), 7.20 (d, J = 8.6 Hz, 2H, H-2,6 4-MeOC6H4), 7.24 (dd, J = 8.7, 2.3 Hz, 1H, H-7), 7.49 (d, J = 2.3 Hz, 1H, H-9); 19F NMR (471 MHz) δ 96.8 (d, J = 6.9 Hz, CF3); 13C NMR (126 MHz, CDCl3) δ 45.3, 53.3, 55.3, 68.1, 70.1, 72.5 (q, 2JCF = 31.7 Hz, C-4), 93.1, 114.7 (2C), 119.1, 123.2 (q, 1JCF = 288.9 Hz, CF3), 124.1, 125.8, 127.7 (2C), 128.7, 129.4, 129.5, 147.7, 161.0, 171.6. HRMS (ESI) m/z: [M + H]+ calcd for C21H19ClF3N2O6 487.0878, found 487.0879.
Methyl (1S*,3S*,3aS*,4S*,9bR*)-8-bromo-3-(4-methoxyphenyl)-3a-nitro-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo-9g). Yield 250 mg (94%), white powder, mp 191–192 °C. IR (ATR) ν 3315 (NH), 1746 (C=O), 1546, 1361 (NO2). 1H NMR (500 MHz) δ 3.06 (br. s, 1H, NH), 3.83 (s, 3H, MeO), 4.01–4.05 (m, 4H, MeO2C, H-1), 4.50 (d, J = 3.3 Hz, 1H, H-9b), 4.73 (s, 1H, H-3), 5.07 (q, J = 6.9 Hz, 1H, H-4), 6.94 (d, J = 8.7 Hz, 2H, H-3,5 4-MeOC6H4), 6.97 (d, J = 8.8 Hz, 1H, H-6), 7.20 (d, J = 8.7 Hz, 2H, H-2,6 4-MeOC6H4), 7.38 (dd, J = 8.8, 2.2 Hz, 1H, H-7), 7.63 (d, J = 2.2 Hz, 1H, H-9); 19F NMR (471 MHz) δ 96.8 (d, J = 6.9 Hz, CF3); 13C NMR (126 MHz) δ 45.2, 53.3, 55.3, 68.1, 70.1, 72.4 (q, 2JCF = 31.7 Hz, C-4), 93.0, 114.6 (2C), 116.8, 119.4, 123.2 (q, 1JCF = 288.9 Hz, CF3), 124.0, 126.2, 127.7 (2C), 131.7, 132.3, 148.2, 160.9, 171.6. HRMS (ESI) m/z: [M + H]+ calcd for C21H19BrF3N2O6 531.0373, found 531.0374.
Methyl (1S*,3S*,3aS*,4S*,9bR*)-6,8-dibromo-3-(4-methoxyphenyl)-3a-nitro-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo-9h). Yield 284 mg (93%), white powder, mp 189–191 °C. IR (ATR) ν 3321 (NH), 1750 (C=O), 1547, 1362 (NO2). 1H NMR (400 MHz) δ 3.07 (dd, J = 10.8, 7.7 Hz, 1H, NH), 3.80 (s, 3H, MeO), 4.00 (dd, J = 7.7, 3.2 Hz, 1H, H-1), 4.03 (s, 3H, MeO2C), 4.53 (d, J = 3.2 Hz, 1H, H-9b), 4.72 (d, J = 10.8 Hz, 1H, H-3), 5.18 (q, J = 6.8 Hz, 1H, H-4), 6.96 (d, J = 8.6 Hz, 2H, H-3,5 4-MeOC6H4), 7.22 (d, J = 8.6 Hz, 2H, H-2,6 4-MeOC6H4), 7.61 (d, J = 1.9 Hz, 1H, H-9), 7.67 (d, J = 1.9 Hz, 1H, H-7); 19F NMR (376 MH) δ 97.0 (d, J = 6.8 Hz, CF3); 13C NMR (126 MHz) δ 45.5, 53.5, 55.3, 68.1, 70.3, 73.1 (q, 2JCF = 32.2 Hz, C-4), 93.2, 112.9 114.8 (2C), 116.9, 122.9 (q, 1JCF = 288.5 Hz, CF3), 123.8, 127.5, 127.7 (2C), 130.9, 135.3, 145.5, 161.1, 171.3. HRMS (ESI) m/z: [M + H]+ calcd for C21H18Br2F3N2O6 608.9478, found 608.9475.
Methyl (1S*,3S*,3aS*,4S*,9bR*)-8-methoxy-3-(4-methoxyphenyl)-3a-nitro-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo-9i). Yield 215 mg (89%), white powder, mp 125–127 °C. IR (ATR) ν 3368 (NH), 1744 (C=O), 1547, 1348 (NO2). 1H NMR (400 MHz) δ 3.07 (dd, J = 11.3, 7.8 Hz, 1H, NH), 3.82 (s, 3H, MeO), 3.83 (s, 3H, MeO), 4.02 (s, 3H, MeO2C), 4.07 (dd, J = 7.8, 3.2 Hz, 1H, H-1), 4.49 (d, J = 3.2 Hz, 1H, H-9b), 4.78 (d, J = 11.3 Hz, 1H, H-3), 5.03 (q, J = 7.0 Hz, 1H, H-4), 6.83 (dd, J = 9.0, 2.8 Hz, 1H, H-7), 6.95 (d, J = 8.7 Hz, 2H, H-3,5 4-MeOC6H4), 6.98 (d, J = 9.0 Hz, 1H, H-6), 7.01 (d, J = 2.2 Hz, 1H, H-9), 7.21 (d, J = 8.7 Hz, 2H, H-2,6 4-MeOC6H4); 19F NMR (376 MHz) δ 97.0 (d, J = 7.0 Hz, CF3); 13C NMR (126 MHz) δ 45.9, 53.1, 55.3, 55.7, 68.3, 70.1, 72.6 (q, 2JCF = 31.4 Hz, C-4), 93.8, 113.1, 114.6 (2C), 115.2, 118.4, 123.4 (q, 1JCF = 289.2 Hz, CF3), 124.4, 124.8, 127.7 (2C), 142.8, 156.2, 160.9, 171.9. HRMS (ESI) m/z: [M + H]+ calcd for C22H22F3N2O7 483.1374, found 483.1374.
Methyl (1S*,3S*,3aS*,4S*,9bR*)-6-ethoxy-3-(4-methoxyphenyl)-3a-nitro-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4-c]pyrrole-1-carboxylate (endo-9j). Yield 216 mg (87%), white powder, mp 123–125 °C. IR (ATR) ν 3348 (NH), 1752 (C=O), 1557, 1361 (NO2). 1H NMR (400 MHz) δ 1.48 (t, J = 7.0 Hz, Me), 3.06 (dd, J = 10.9, 7.6 Hz, 1H, NH), 3.83 (s, 3H, MeO), 4.01 (s, 3H, MeO2C), 4.05 (dd, J = 7.6, 3.2 Hz, 1H, H-1), 4.17 (d, J = 7.0 Hz, OCH2), 4.52 (d, J = 3.2 Hz, 1H, H-9b), 4.81 (d, J = 10.9 Hz, 1H, H-3), 5.19 (q, J = 7.0 Hz, 1H, H-4), 6.82–6.89 (m, 1H, H-8), 6.94 (d, J = 8.7 Hz, 2H, H-3,5 4-MeOC6H4), 7.06–7.10 (m, 2H, H-7,9), 7.25 (d, J = 8.7 Hz, 2H, H-2,6 4-MeOC6H4); 19F NMR (376 MHz) δ 97.1 (d, J = 7.0 Hz, CF3); 13C NMR (126 MHz) δ 14.7, 45.7, 53.1, 55.3, 64.7, 68.4, 70.2, 72.7 (q, 2JCF = 31.5 Hz, C-4), 94.0, 112.3, 114.6 (2C), 120.1, 123.3 (q, 1JCF = 288.8 Hz, CF3), 124.3, 124.4, 125.3, 127.8 (2C), 139.1, 148.1, 160.9, 172.0. HRMS (ESI) m/z: [M + H]+ calcd for C23H23F3N2NaO7 519.1350, found 519.1347.
3.4. Synthesis of Compounds 10a–g
General procedure. A mixture of the appropriate 3-nitro-2-(trichloromethyl)-2H-chromene 4 (0.5 mmol), azomethine 5 (0.55 mmol), Et3N (7 μL, 5 mg, 0.05 mmol) and AgOAc (5.8 mg, 0.05 mmol) was stirred in dichloromethane (2 mL) for 5 h at room temperature (TLC control, EtOAc−hexane (1:3)). Upon completion of the reaction, the residue was evaporated under reduced pressure to complete dryness. The residue was purified by by silica gel column chromatography using (eluent−EtOAc−hexane (1:3)) to give products 10 as white powders.
Methyl (S)-2-[((E)-benzylidene)amino]-2-((2S*,3R*,4R*)-3-nitro-2-(trichloromethyl)chroman-4-yl)acetate (10a). Yield 101 mg (43%), mp 225–227 °C. IR (ATR) ν 1737 (C=O), 1552, 1311 (NO2). 1H NMR (400 MHz) δ 3.86 (s, 3H, MeO2C), 4.14 (br. d, J = 2.3 Hz, 1H, H-4’), 4.53 (d, J = 2.3 Hz, 1H, H-2), 5.22 (d, J = 1.4 Hz, 1H, H-2’), 6.29 (br. d, J = 1.4 Hz, 1H, H-3’), 7.04 (d, J = 8.0 Hz, 1H, H-8’), 7.09 (t, J = 7.5 Hz, 1H, H-6’), 7.20–7.29 (m, 2H, H-5’,7’), 7.39 (t, J = 7.3 Hz, 2H, H Ph), 7.46 (tt, J = 7.3, 1.3 Hz, 1H, H Ph), 7.63 (dd, J = 7.3, 1.3 Hz, 2H, H Ph), 8.02 (s, 1H, =CH); 13C NMR (126 MHz) δ 42.7, 53.2, 76.1, 78.8, 82.7, 95.5, 117.6, 118.4, 123.1, 127.8, 128.7 (2C), 128.8, 128.9 (2C), 132.1, 134.5, 153.8, 166.6, 169.9. HRMS (ESI) m/z: [M + H]+ calcd for C20H18Cl3N2O5 471.0276, found 471.0276.
Methyl (S)-2-[((E)-4-methoxybenzylidene)amino]-2-((2S*,3R*,4R*)-3-nitro-2-(trichloromethyl)chroman-4-yl)acetate (10b). Yield 170 mg (66%), mp 155–157 °C. IR (ATR) ν 1737 (C=O), 1553, 1310 (NO2). 1H NMR (500 MHz) δ 3.82 (s, 3H, MeO), 3.85 (s, 3H, MeO2C), 4.11 (br. s, 1H, H-4’), 4.48 (d, J = 2.3 Hz, 1H, H-2), 5.25 (d, J = 1.6 Hz, 1H, H-2’), 6.29 (br. d, J = 1.6 Hz, 1H, H-3’), 6.89 (d, J = 8.7 Hz, 2H, H-3,5 4-MeOC6H4), 7.03 (d, J = 8.0 Hz, 1H, H-8’), 7.09 (t, J = 7.5 Hz, 1H, H-6’), 7.20–7.27 (m, 2H, H-5’,7’), 7.54 (d, J = 8.7 Hz, 2H, H-2,6 4-MeOC6H4), 7.92 (s, 1H, =CH); 13C NMR (126 MHz) δ 42.7 (C-4’), 53.1 (MeO), 55.4 (MeO), 76.0 (C-2), 78.7 (C-3’), 82.6 (C-2’), 95.6 (CCl3), 114.2 (C-3,5 4-MeOC6H4), 117.5 (C-5’), 118.6 (C-4a’), 123.0 (C-6’), 127.5 (C-1 4-MeOC6H4), 127.8 (C-8’), 128.7 (C-7’), 130.4 (C-2,6 4-MeOC6H4), 153.8 (C-8a’), 162.8 (C-4 4-MeOC6H4), 165.7 (C=N), 170.2 (C=O). HRMS (ESI) m/z: [M + H]+ calcd for C21H20Cl3N2O6 501.0381, found 501.0372.
Methyl (S)-2-[((E)-benzo[d][1,3]dioxol-5-ylmethylene)amino]-2-((2S*,3R*,4R*)-3-nitro-2-(trichloromethyl)chroman-4-yl)acetate (10c). Yield 146 mg (55%), mp 189–191 °C. IR (ATR) ν 1732 (C=O), 1552, 1339 (NO2). 1H NMR (500 MHz) δ 3.85 (s, 3H, MeO2C), 4.11 (br. d, J = 2.4 Hz, 1H, H-4’), 4.48 (d, J = 2.4 Hz, 1H, H-2), 5.21 (d, J = 1.8 Hz, 1H, H-2’), 6.00 (s, 2H, OCH2O), 6.26 (dd, J = 1.8, 1.0 Hz, 1H, H-3’), 6.78 (d, J = 8.0 Hz, 1H, H-7 benzo[d][1,3]dioxol-5-yl), 7.01 (dd, J = 8.0, 1.5 Hz, 1H, H-6 benzo[d][1,3]dioxol-5-yl), 7.05 (dd, J = 8.3, 1.1 Hz, 1H, H-8’), 7.09 (dd, J = 7.6, 1.1 Hz, 1H, H-6’), 7.20 (d, J = 1.5 Hz, 1H, H-4 benzo[d][1,3]dioxol-5-yl), 7.21–7.27 (m, 2H, H-5’,7’), 7.87 (s, 1H, =CH); 13C NMR (126 MHz) δ 42.6, 53.1, 75.9, 78.7, 82.6, 95.5, 101.7, 106.5, 108.2, 117.6, 118.5, 123.1, 125.9, 127.8, 128.8, 129.3, 148.5, 151.1, 153.8, 165.5, 170.1. HRMS (ESI) m/z: [M + H]+ calcd for C21H18Cl3N2O7 515.0174, found 515.0181.
Methyl (S)-2-((2S*,3R*,4R*)-6-chloro-3-nitro-2-(trichloromethyl)chroman-4-yl)-2-[((E)-4-methoxybenzylidene)amino]acetate (10d). Yield 161 mg (60%), mp 127–129 °C. IR (ATR) ν 1743 (C=O), 1556, 1337 (NO2). 1H NMR (500 MHz) δ 3.83 (s, 3H, MeO), 3.85 (s, 3H, MeO2C), 4.08 (br. s, 1H, H-4’), 4.44 (d, J = 1.8 Hz, 1H, H-2), 5.29 (d, J = 1.3 Hz, 1H, H-2’), 6.26 (br. s, 1H, H-3’), 6.90 (d, J = 8.6 Hz, 2H, H-3,5 4-MeOC6H4), 6.98 (d, J = 8.8 Hz, 1H, H-8’), 7.19 (dd, J = 8.8, 2.1 Hz, 1H, H-7’), 7.59 (d, J = 2.1 Hz, 1H, H-5’), 7.59 (d, J = 8.6 Hz, 2H, H-2,6 4-MeOC6H4), 7.99 (s, 1H, =CH); 13C NMR (126 MHz) δ 42.5, 53.2, 55.5, 75.7, 78.3, 82.8, 95.3, 114.3 (2C), 119.0, 120.4, 127.4, 127.5, 127.9, 129.0, 130.5 (2C), 152.4, 162.9, 166.1, 169.8. HRMS (ESI) m/z: [M + H]+ calcd for C21H19Cl4N2O6 534.9992, found 534.9993.
Methyl (S)-2-((2S*,3R*,4R*)-6-bromo-3-nitro-2-(trichloromethyl)chroman-4-yl)-2-[((E)-4-methoxybenzylidene)amino]acetate (10e). Yield 194 mg (67%), mp 158–160 °C. IR (ATR) ν 1743 (C=O), 1557, 1337 (NO2). 1H NMR (500 MHz) δ 3.83 (s, 3H, MeO), 3.85 (s, 3H, MeO2C), 4.08 (s, 1H, H-4’), 4.44 (s, 1H, H-2), 5.30 (s, 1H, H-2’), 6.26 (s, 1H, H-3’), 6.90 (d, J = 8.3 Hz, 2H, H-3,5 4-MeOC6H4), 6.93 (d, J = 8.8 Hz, 1H, H-8’), 7.32 (d, J = 8.8 Hz, 1H, H-7’), 7.39 (s, 1H, H-5’), 7.59 (d, J = 8.3 Hz, 2H, H-2,6 4-MeOC6H4), 7.99 (s, 1H, =CH); 13C NMR (126 MHz) δ 42.4, 53.2, 55.5, 75.7, 78.3, 82.7, 95.3, 114.3 (2C), 115.2, 119.4, 120.9, 127.4, 130.50 (2C), 130.53, 131.8, 152.9, 162.9, 166.1, 169.8. HRMS (ESI) m/z: [M + H]+ calcd for C21H19BrCl3N2O6 578.9487, found 578.9486.
Methyl (S)-2-((2S*,3R*,4R*)-6,8-dibromo-3-nitro-2-(trichloromethyl)chroman-4-yl)-2-[((E)-4-methoxybenzylidene)amino]acetate (10f). Yield 165 mg (50%), mp 112–115 °C. IR (ATR) ν 1735 (C=O), 1561, 1341 (NO2). 1H NMR (500 MHz) δ 3.81 (s, 3H, MeO), 3.84 (s, 3H, MeO2C), 4.11 (br. s, 1H, H-4’), 4.43 (d, J = 1.8 Hz, 1H, H-2), 5.35 (d, J = 1.6 Hz, 1H, H-2’), 6.25 (br. s, J = 1.5 Hz, 1H, H-3’), 6.92 (d, J = 8.6 Hz, 2H, H-3,5 4-MeOC6H4), 7.35 (d, J = 1.6 Hz, 1H, H-5’), 7.60 (d, J = 8.6 Hz, 2H, H-2,6 4-MeOC6H4), 7.62 (d, J = 1.6 Hz, 1H, H-7’), 8.03 (s, 1H, =CH); 13C NMR (126 MHz) δ 42.6, 53.3, 55.5, 75.5, 78.3, 83.2, 94.8, 114.4 (2C), 115.0, 122.9, 127.3, 128.1, 129.7, 130.6 (2C), 134.9, 149.8, 163.0, 166.5, 169.6. HRMS (ESI) m/z: [M + H]+ calcd for C21H18Br2Cl3N2O6 656.8592, found 656.8590.
Methyl (S)-2-((2S*,3R*,4R*)-6-methoxy-3-nitro-2-(trichloromethyl)chroman-4-yl)-2-[((E)-4-methoxybenzylidene)amino]acetate (10g). Yield 106 mg (40%), mp 168–170 °C. IR (ATR) ν 1746 (C=O), 1562, 1326 (NO2). 1H NMR (500 MHz) δ 3.80 (s, 3H, MeO), 3.83 (s, 3H, MeO), 3.85 (s, 3H, MeO2C), 4.08 (dd, J = 2.3, 1.7 Hz, 1H, H-4’), 4.46 (d, J = 2.3 Hz, 1H, H-2), 5.18 (d, J = 1.7 Hz, 1H, H-2’), 6.25 (dd, J = 1.7, 1.0 Hz, 1H, H-3’), 6.89 (d, J = 8.8 Hz, 2H, H-3,5 4-MeOC6H4), 6.75 (d, J = 2.9 Hz, 1H, H-5’), 6.79 (dd, J = 8.9, 2.9 Hz, 1H, H-7’), 6.90 (d, J = 8.9 Hz, 1H, H-8’), 7.59 (d, J = 8.8 Hz, 2H, H-2,6 4-MeOC6H4), 7.95 (s, 1H, =CH); 13C NMR (126 MHz) δ 42.9, 53.1, 55.4, 75.8, 75.9, 78.6, 82.9, 95.6, 112.3, 114.2 (2C), 114.6, 118.3, 119.2, 127.5, 130.4 (2C), 147.9, 155.2, 162.8, 165.6, 170.2. HRMS (ESI) m/z: [M + H]+ calcd for C22H22Cl3N2O7 531.0487, found 531.9486.
3.5. Biology
3.5.1. Cell Cultures
The human cervical carcinoma (HeLa) cell line was purchased from the Bank of Cell Cultures of the Institute of Cytology of the Russian Academy of Sciences, St. Petersburg, Russia. The normal human dermal fibroblasts (HDF) cell line was obtained from the Institute of Medical Cell Technologies, Ekaterinburg, Russia.
3.5.2. Assessment of In Vitro Cytotoxic Activity
The cells were seeded in 96-well microplates at a seeding density of 2 × 10
5 cells per mL and cultured for 24 h in DMEM medium with glutamine (1%) in the presence of 10% fetal bovine serum and gentamicin (50 mg/L) at 37 °C in a humidified atmosphere containing 5% CO
2. Then the tested compounds were added to the wells in various concentrations (10
−7 M, 10
−6 M, 10
−5 M, 10
−4 M). Cells with compounds were incubated for 72 h, after which cell viability was assessed using the standard MTT test [
43] based on the reduction of the yellow tetrazole salt by living cell mitochondrial dehydrogenases to formazan crystals, soluble in DMSO. Experiments were performed in triplicates with negative control (culture medium), positive control (camptothecin, 3 mM) and solvent control (DMSO). The results of the MTT test were evaluated by comparing the optical density of the formazan solution measured on a flatbed scanner Tecan Infinite M200 PRO (Tecan Austria GmbH, Austria) at a wavelength of 570 nm in the experimental and control wells and control wells and calculating the cytotoxicity index (IC). The cytotoxicity index was determined for each concentration of the studied substances by AAT Bioquest-calculator:
https://www.aatbio.com/tools/ic50-calculator (accessed on 15 November 2022). The parameters of the arithmetic mean value and the standard error were calculated. The differences in the average values according to the Mann-Whitney U test with
p < 0.05 were considered reliable. For the statistical analysis, Microsoft Excel 2019 (Microsoft corp., Redmond, DC, USA) and Statistika 13.3 (Tibco, Palo Alto, CA, USA) were used.