Adhesion and Anti-Adhesion Abilities of Potentially Probiotic Lactic Acid Bacteria and Biofilm Eradication of Honeybee (Apis mellifera L.) Pathogens
Abstract
1. Introduction
2. Results and Discussion
2.1. Cell Surface Properties of LAB Strains: Auto-aggregation, Coaggregation and Hydrophobicity
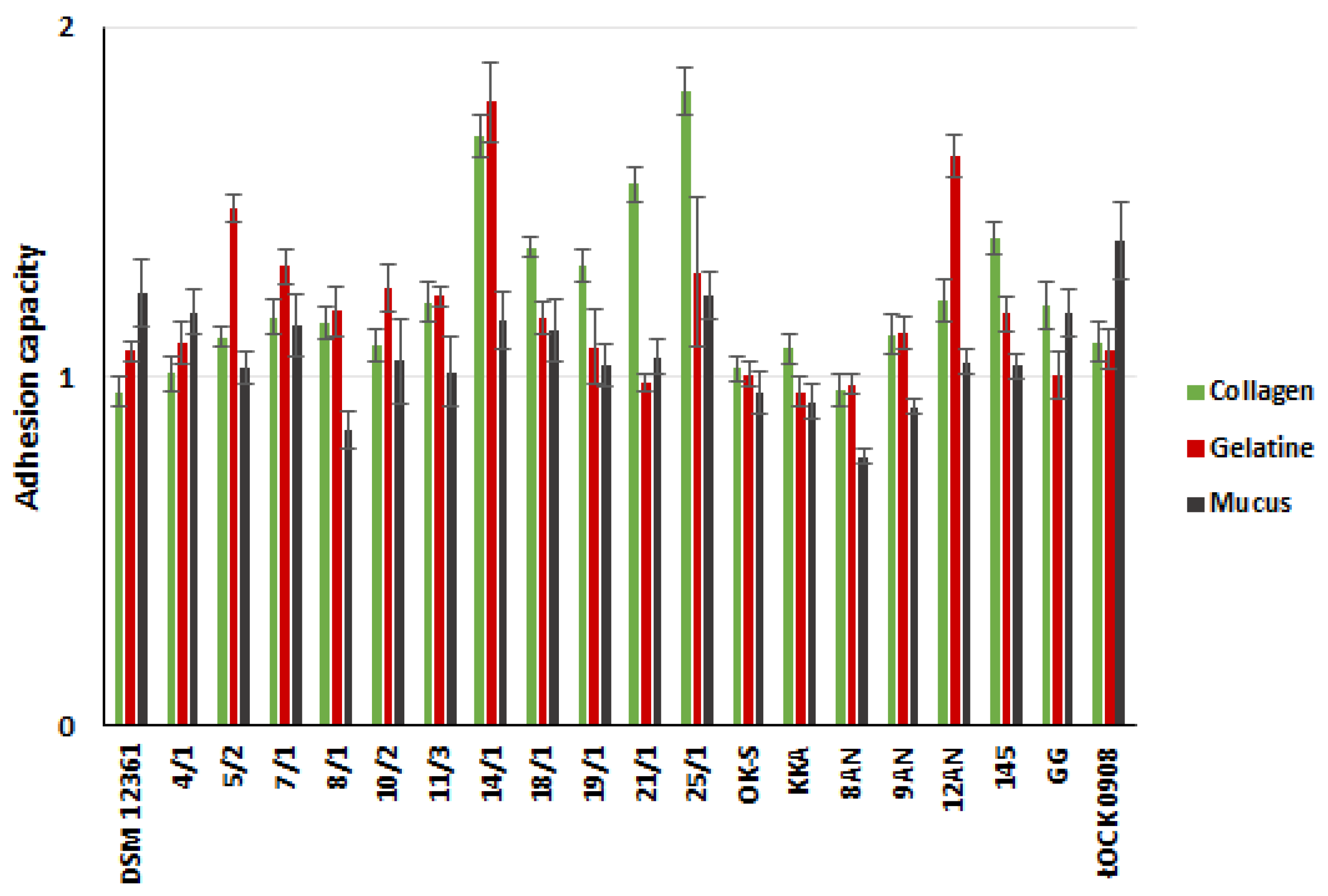
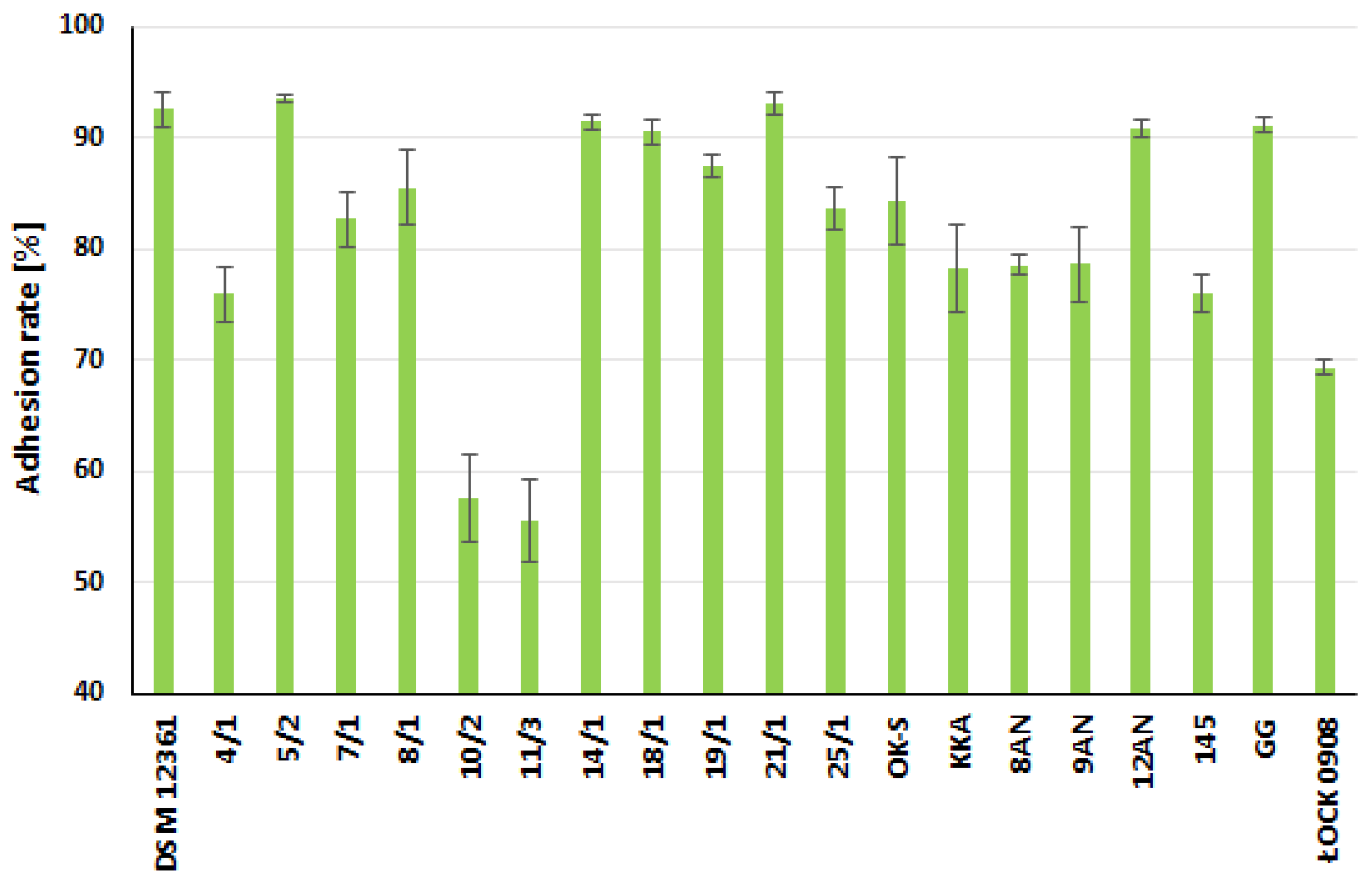
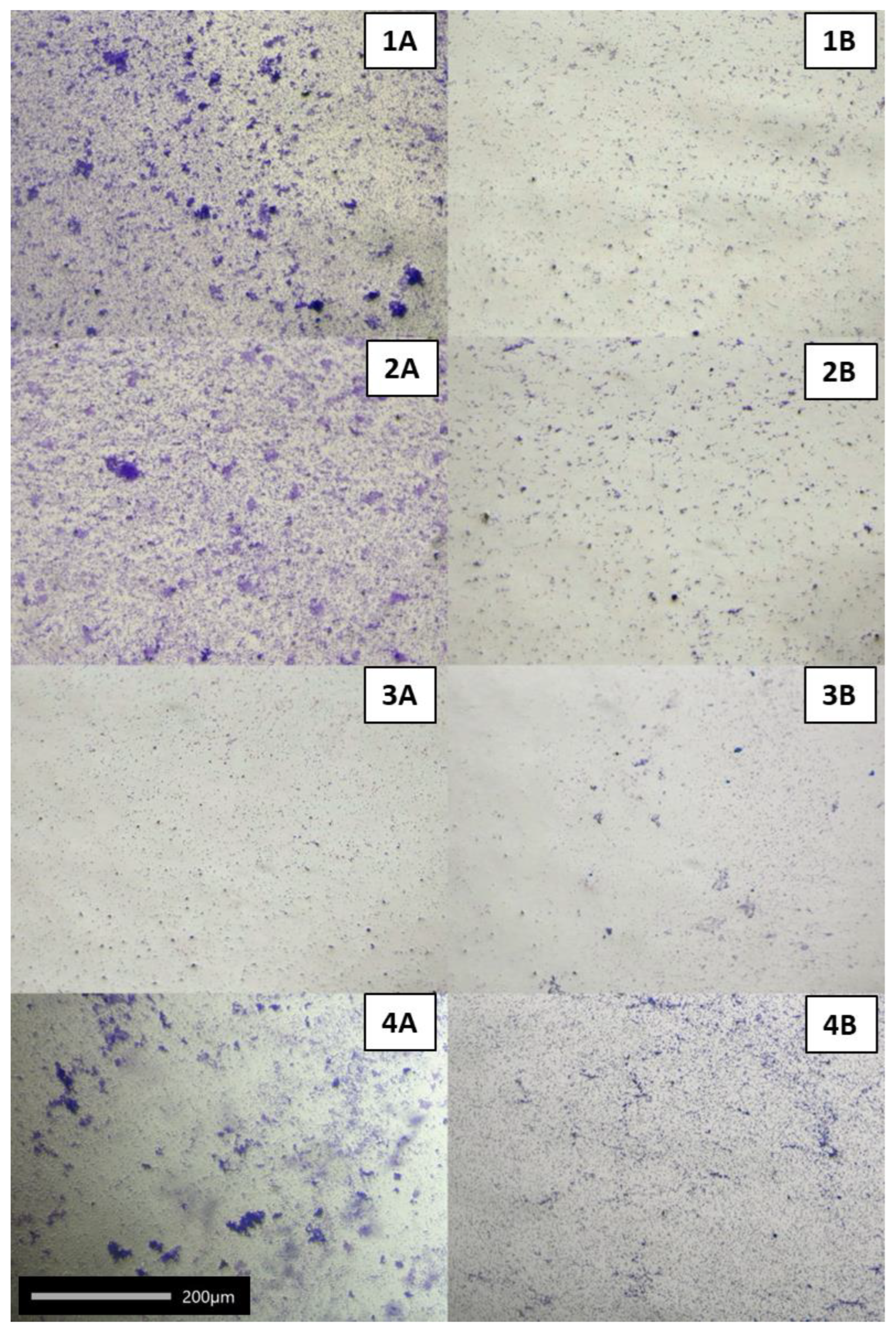
2.2. Adhesive Properties of LAB
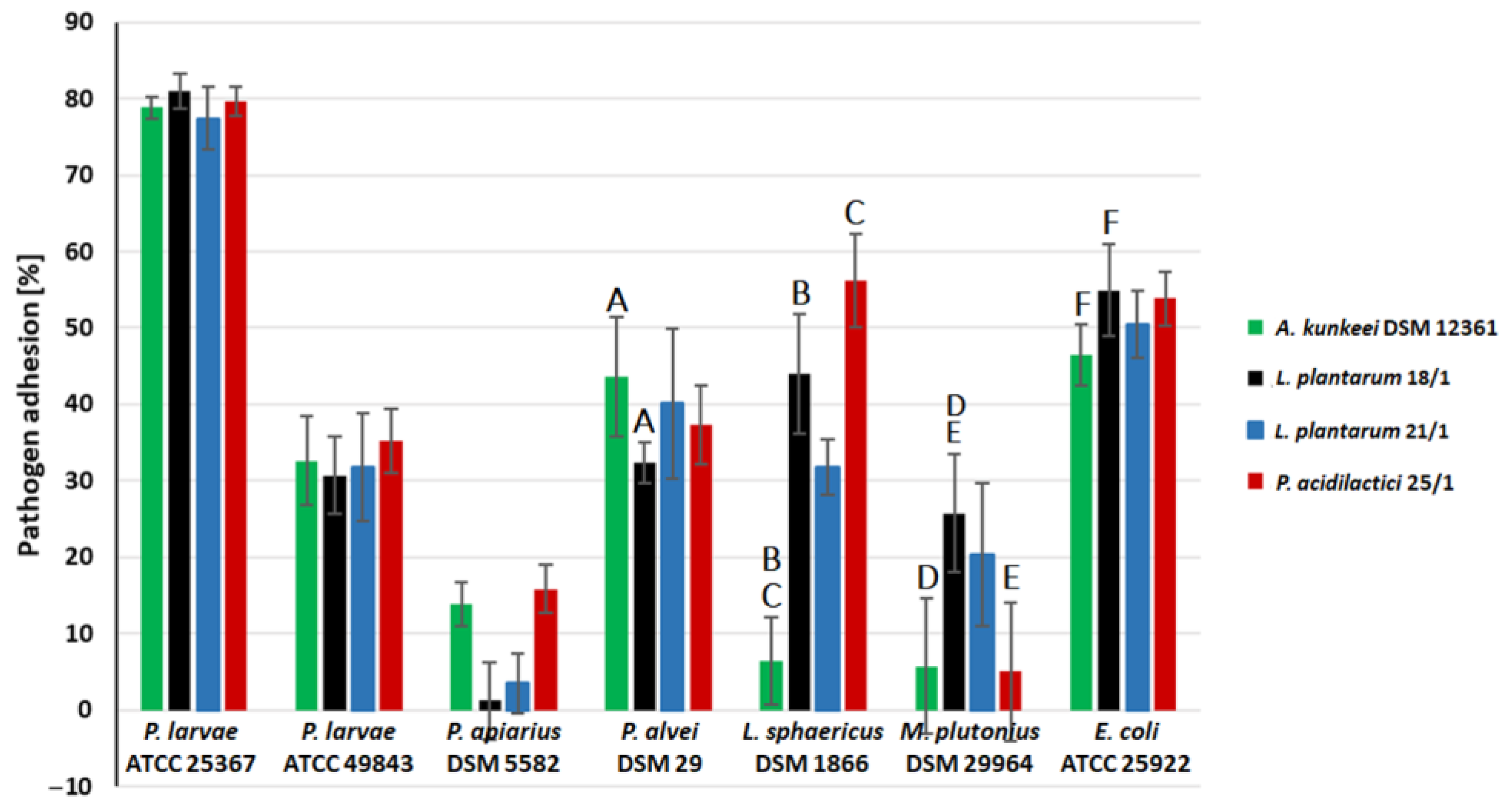
2.3. Coaggregation of LAB with Honeybee Pathogens
2.4. Effect of LAB Cell-Free Supernatants (CFSs)
2.4.1. On Adhesion of Honeybee Pathogens to Polystyrene
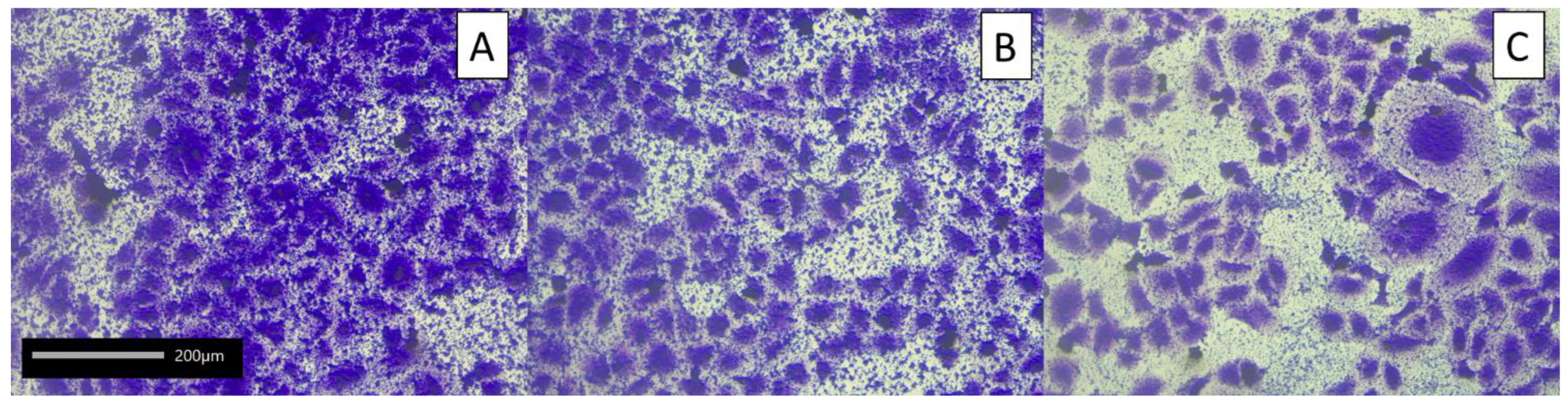
2.4.2. On Biofilm Eradication of Honeybee Pathogens
3. Materials and Methods
3.1. Chemicals, Vessels, and Other Materials
3.2. Bacterial Strains and Growth Conditions
3.3. Preparation of Bacterial Suspensions
3.4. Hydrophobicity and Auto-Aggregation of LAB
3.5. Adhesive Abilities
3.5.1. To Abiotic Surfaces (PS and Glass)
3.5.2. To Biotic Surfaces (Collagen, Mucus, and Gelatin)
3.5.3. To Human Colon Adenocarcinoma Cell Line Caco-2
3.6. Coaggregation of Selected LAB with Honeybee Pathogens
3.7. Anti-Adhesion Ability of LAB Cell-Free Supernatants (CFSs)
3.7.1. Preparation of CFSs
3.7.2. Anti-Adhesion Test
3.8. Eradication of Pathogens Biofilm by LAB Strains
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zielińska, D.; Kolożyn-Krajewska, D. Food-origin lactic acid bacteria may exhibit probiotic properties: Review. Biomed. Res. Int. 2018, 2018, 5063185. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.R.; de Sardi, J.; de Pitangui, N.; Roque, S.M.; Silva, A.C.; Rosalen, P.L. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 2020, 73, 104080. [Google Scholar] [CrossRef]
- Du, Y.; Li, H.; Shao, J.; Wu, T.; Xu, W.L.; Hu, X.; Chen, J. Adhesion and colonization of the probiotic Lactobacillus plantarum HC-2 in the intestine of Litopenaeus vannamei are associated with bacterial surface proteins. Front. Microbiol. 2022, 13, 878874. [Google Scholar] [CrossRef]
- Alp, D.; Kuleasan, H. Determination of competition and adhesion abilities of lactic acid bacteria against gut pathogens in a whole-tissue model. Biosci. Microbiota. Food Health 2020, 39, 250–258. [Google Scholar] [CrossRef]
- Vásquez, A.; Forsgren, E.; Fries, I.; Paxton, R.J.; Flaberg, E.; Szekely, L.; Olofsson, T.C. Symbionts as major modulators of insect health: Lactic acid bacteria and honeybees. PLoS ONE 2012, 7, e33188. [Google Scholar] [CrossRef]
- Silva-Dias, A.; Miranda, I.M.; Branco, J.; Monteiro-Soares, M.; Pina-Vaz, C.; Rodrigues, A.G. Adhesion, biofilm formation, cell surface hydrophobicity, and antifungal planktonic susceptibility: Relationship among Candida spp. Front. Microbiol. 2015, 6, 205. [Google Scholar] [CrossRef]
- Marshall, K.C.; Blainey, B.L. Role of bacterial adhesion in biofilm formation and biocorrosion. In Biofouling and Biocorrosion in Industrial Water Systems; Springer: Berlin/Heidelberg, Germany, 1991; pp. 29–46. [Google Scholar] [CrossRef]
- Merritt, K.; An, Y.H. Factors influencing bacterial adhesion. In Handbook of Bacterial Adhesion: Principles, Methods, and Applications; An, Y.H., Friedman, R.J., Eds.; Humana Press: Totowa, NJ, USA, 2000. [Google Scholar]
- Tatsaporn, T.; Kornkanok, K. Using potential lactic acid bacteria biofilms and their compounds to control biofilms of foodborne pathogens. Biotechnol. Rep. 2020, 26, e00477. [Google Scholar] [CrossRef]
- Lee, S.-J.; Jeon, H.-S.; Yoo, J.-Y.; Kim, J.-H. Some Important Metabolites Produced by Lactic Acid Bacteria Originated from Kimchi. Foods 2021, 10, 2148. [Google Scholar] [CrossRef]
- Werning, M.L.; Hernández-Alcántara, A.M.; Ruiz, M.J.; Soto, L.P.; Dueñas, M.T.; López, P.; Frizzo, L.S. Biological Functions of Exopolysaccharides from Lactic Acid Bacteria and Their Potential Benefits for Humans and Farmed Animals. Foods 2022, 11, 1284. [Google Scholar] [CrossRef]
- Nieminen, M.T.; Novak-Frazer, L.; Rautemaa, V.; Rajendran, R.; Sorsa, T.; Ramage, G.; Bowyer, P.; Rautemaa, R. A novel antifungal is active against Candida albicans biofilms and inhibits mutagenic acetaldehyde production in vitro. PLoS ONE 2014, 9, e97864. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, V.; Khan, M.S.; Jamal, Q.M.; Alzohairy, M.A.; Al Karaawi, M.A.; Siddiqui, M.U. Antimicrobial potential of bacteriocins: In therapy, agriculture and Food Preservation. Int. J. Antimicrob. Agents 2017, 49, 1–11. [Google Scholar] [CrossRef] [PubMed]
- van der Sluijs, J.P.; Vaage, N.S. Pollinators and global food security: The Need for Holistic Global Stewardship. Food Ethics 2016, 1, 75–91. [Google Scholar] [CrossRef]
- Zheng, H.; Powell, J.E.; Steele, M.I.; Dietrich, C.; Moran, N.A. Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc. Natl. Acad. Sci. USA 2017, 114, 4775–4780. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.E.; Ricigliano, V.A. Honey Bee Gut Dysbiosis: A novel context of disease ecology. Curr. Opin. Insect. Sci. 2017, 22, 125–132. [Google Scholar] [CrossRef]
- Engel, P.; Martinson, V.G.; Moran, N.A. Functional diversity within the simple gut microbiota of the honey bee. Proc. Natl. Acad. Sci. USA 2012, 109, 11002–11007. [Google Scholar] [CrossRef]
- Bielik, B.; Molnár, L.; Vrabec, V.; Andrášiová, R.; Maruščáková, I.C.; Nemcová, R.; Toporčák, J.; Mudroňová, D. Biofilm-forming lactic acid bacteria of honey bee origin intended for potential probiotic use. Acta Vet. Hung. 2021, 68, 345–353. [Google Scholar] [CrossRef]
- Berríos, P.; Fuentes, J.A.; Salas, D.; Carreño, A.; Aldea, P.; Fernández, F.; Trombert, A.N. Inhibitory effect of biofilm-forming Lactobacillus kunkeei strains against virulent Pseudomonas aeruginosa in vitro and in honeycomb moth (Galleria mellonella) infection model. Benef. Microbes 2018, 9, 257–268. [Google Scholar] [CrossRef]
- Brudzynski, K. Honey as an Ecological Reservoir of Antibacterial Compounds Produced by Antagonistic Microbial Interactions in Plant Nectars, Honey and Honey Bee. Antibiotics 2021, 10, 551. [Google Scholar] [CrossRef]
- Rowland, B.W.; Rushton, S.P.; Shirley, M.D.; Brown, M.A.; Budge, G.E. Identifying the climatic drivers of Honey Bee Disease in England and Wales. Sci. Rep. 2021, 11, 21953. [Google Scholar] [CrossRef]
- Daisley, B.A.; Pitek, A.P.; Chmiel, J.A.; Al, K.F.; Chernyshova, A.M.; Faragalla, K.M.; Burton, J.P.; Thompson, G.J.; Reid, G. Novel probiotic approach to counter Paenibacillus larvae infection in honey bees. ISME J. 2019, 14, 476–491. [Google Scholar] [CrossRef] [PubMed]
- Fünfhaus, A.; Göbel, J.; Ebeling, J.; Knispel, H.; Garcia-Gonzalez, E.; Genersch, E. Swarming motility and biofilm formation of paenibacillus larvae, the etiological agent of American Foulbrood of honey bees (Apis mellifera). Sci Rep 2018, 8, 8840. [Google Scholar] [CrossRef] [PubMed]
- Arai, R.; Tominaga, K.; Wu, M.; Okura, M.; Ito, K.; Okamura, N.; Onishi, H.; Osaki, M.; Sugimura, Y.; Yoshiyama, M.; et al. Diversity of Melissococcus plutonius from honeybee larvae in Japan and experimental reproduction of European Foulbrood with cultured atypical isolates. PLoS ONE 2012, 7, e33708. [Google Scholar] [CrossRef] [PubMed]
- de Lacerda, J.R.; da Silva, T.F.; Vollú, R.E.; Marques, J.M.; Seldin, L. Generally recognized as safe (GRAS) Lactococcus lactis strains associated with Lippia sidoides Cham. are able to solubilize/mineralize phosphate. SpringerPlus 2016, 5, 828. [Google Scholar] [CrossRef]
- Ferreira, C.L.; Grześkowiak, Ł.; Collado, M.C.; Salminen, S. In vitro evaluation of Lactobacillus gasseri strains of infant origin on adhesion and aggregation of specific pathogens. J. Food Prot. 2011, 74, 1482–1487. [Google Scholar] [CrossRef]
- Malik, S.; Petrova, M.I.; Claes, I.J.; Verhoeven, T.L.; Busschaert, P.; Vaneechoutte, M.; Lievens, B.; Lambrichts, I.; Siezen, R.J.; Balzarini, J.; et al. The highly autoaggregative and adhesive phenotype of the vaginal Lactobacillus plantarum strain CMPG5300 is sortase dependent. Appl. Environ. Microbiol. 2013, 79, 4576–4585. [Google Scholar] [CrossRef]
- Tuo, Y.; Yu, H.; Ai, L.; Wu, Z.; Guo, B.; Chen, W. Aggregation and adhesion properties of 22 lactobacillus strains. J. Dairy Sci. 2013, 96, 4252–4257. [Google Scholar] [CrossRef]
- Grigoryan, S.; Bazukyan, I.; Trchounian, A. Aggregation and adhesion activity of lactobacilli isolated from fermented products in vitro and in vivo: A potential probiotic strain. Probiotics Antimicrob Proteins 2017, 10, 269–276. [Google Scholar] [CrossRef]
- Zawistowska-Rojek, A.; Kośmider, A.; Stępień, K.; Tyski, S. Adhesion and aggregation properties of Lactobacillaceae strains as protection ways against enteropathogenic bacteria. Arch. Microbiol. 2022, 204, 285. [Google Scholar] [CrossRef]
- Saito, K.; Tomita, S.; Nakamura, T. Aggregation of Lactobacillus brevis associated with decrease in pH by glucose fermentation. Biosci. Biotechnol. Biochem. 2019, 83, 1523–1529. [Google Scholar] [CrossRef]
- Neupane, K.R.; Thapa, R.B. Alternative to off-season sugar supplement feeding of honeybees. J. Inst. Agric. Anim. Sci. 2005, 26, 77–81. [Google Scholar] [CrossRef]
- Vlková, E.; Rada, V.; Šmehilová, M.; Killer, J. Auto-aggregation and co-aggregation ability in Bifidobacteria and Clostridia. Folia Microbiol. 2008, 53, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Ayala, K.; Ascencio-Valle, F.J.; Gutiérrez-González, P.; Estrada-Girón, Y.; Torres-Vitela, M.R.; Macías-Rodríguez, M.E. Hydrophobic and adhesive patterns of lactic acid bacteria and their antagonism against foodborne pathogens on tomato surface (Solanum lycopersicum L.). J. Appl. Microbiol. 2020, 129, 876–891. [Google Scholar] [CrossRef] [PubMed]
- Krausova, G.; Hyrslova, I.; Hynstova, I. In Vitro Evaluation of Adhesion Capacity, Hydrophobicity, and Auto-Aggregation of Newly Isolated Potential Probiotic Strains. Fermentation 2019, 5, 100. [Google Scholar] [CrossRef]
- Marín, M.L.; Benito, Y.; Pin, C.; Fernández, M.F.; García, M.L.; Selgas, M.D.; Casas, C. Lactic acid bacteria: Hydrophobicity and strength of attachment to meat surfaces. Lett. Appl. Microbiol. 1997, 24, 14–18. [Google Scholar] [CrossRef]
- Somashekaraiah, R.; Shruthi, B.; Deepthi, B.V.; Sreenivasa, M.Y. Probiotic properties of lactic acid bacteria isolated from Neera: A naturally fermenting coconut palm nectar. Front. Microbiol. 2019, 10, 1382. [Google Scholar] [CrossRef]
- Guan, C.; Chen, X.; Jiang, X.; Zhao, R.; Yuan, Y.; Chen, D.; Zhang, C.C.; Lu, M.; Lu, Z.; Gu, R. In vitro studies of adhesion properties of six lactic acid bacteria isolated from the longevous population of China. RSC Adv. 2020, 10, 24234–24240. [Google Scholar] [CrossRef]
- Tlais, A.Z.; Polo, A.; Filannino, P.; Cantatore, V.; Gobbetti, M.; Di Cagno, R. Biofilm Formation as an extra gear for Apilactobacillus kunkeei to counter the threat of agrochemicals in honeybee crop. Microbiol. Biotechnol. 2022, 15, 2160–2175. [Google Scholar] [CrossRef]
- Djukic, M.; Poehlein, A.; Strauß, J.; Tann, F.J.; Leimbach, A.; Hoppert, M.; Daniel, R. High quality draft genome of Lactobacillus kunkeei efb6, isolated from a German European foulbrood outbreak of Honeybees. Stand. Genom. Sci. 2015, 10, 16. [Google Scholar] [CrossRef]
- Iorizzo, M.; Lombardi, S.J.; Ganassi, S.; Testa, B.; Ianiro, M.; Letizia, F.; Succi, M.; Tremonte, P.; Vergalito, F.; Cozzolino, A.; et al. Antagonistic Activity against Ascosphaera apis and Functional Properties of Lactobacillus kunkeei Strains. Antibiotics 2020, 9, 262. [Google Scholar] [CrossRef]
- Simões, L.C.; Simões, M.; Vieira, M.J. Adhesion and biofilm formation on polystyrene by drinking water-isolated bacteria. Antonie Leeuwenhoek 2010, 98, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Elfazazi, K.; Zahir, H.; Tankiouine, S.; Mayoussi, B.; Zanane, C.; Lekchiri, S.; Ellouali, M.; Mliji, E.M.; Latrache, H. Adhesion behavior of Escherichia coli strains on glass: Role of cell surface qualitative and quantitative hydrophobicity in their attachment ability. Int. J. Microbiol. 2021, 2021, 5580274. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Torres, A.; Chen, L.; Kong, Y.; Cirillo, J.D.; Liang, H. Fluid-shear method to evaluate bacterial adhesion to glass surfaces. J. Appl. Phys. 2012, 112, 014703. [Google Scholar] [CrossRef]
- An, Y.H.; Friedman, R.J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. Biomed. Mater. Res. 1998, 43, 338–348. [Google Scholar] [CrossRef]
- Wallis, J.K.; Krömker, V.; Paduch, J.-H. Biofilm formation and adhesion to bovine udder epithelium of potentially probiotic lactic acid bacteria. AIMS Microbiol. 2018, 4, 209–224. [Google Scholar] [CrossRef]
- Gómez, N.C.; Ramiro, J.M.; Quecan, B.X.; de Melo Franco, B.D. Use of potential probiotic lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes, Salmonella typhimurium, and Escherichia coli O157:H7 biofilms formation. Front. Microbiol. 2016, 7, 863. [Google Scholar] [CrossRef]
- Mustar, S.; Ibrahim, N. A Sweeter Pill to Swallow: A Review of Honey Bees and Honey as a Source of Probiotic and Prebiotic Products. Foods 2022, 11, 2102. [Google Scholar] [CrossRef]
- Kiňová Sepová, H.; Florová, B.; Bilková, A.; Drobná, E.; Březina, V. Evaluation of adhesion properties of lactobacilli probiotic candidates. Monatsh. Chem. 2018, 149, 893–899. [Google Scholar] [CrossRef]
- Balcázar, J.L.; Vendrell, D.; de Blas, I.; Ruiz-Zarzuela, I.; Gironés, O.; Múzquiz, J.L. In vitro competitive adhesion and production of antagonistic compounds by lactic acid bacteria against fish pathogens. Vet. Microbiol. 2007, 122, 373–380. [Google Scholar] [CrossRef]
- Haddaji, N.; Khouadja, S.; Fdhila, K.; Krifi, B.; Ben Ismail, M.; Lagha, R.; Bakir, K.; Bakhrouf, A. Acid stress suggests different determinants for polystyrene and Hela cell adhesion in Lactobacillus casei. J. Dairy Sci. 2015, 98, 4302–4309. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Dave, D.; Shahidi, F. Sea Cucumber Derived Type I Collagen: A Comprehensive Review. Mar. Drugs 2020, 18, 471. [Google Scholar] [CrossRef] [PubMed]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile Perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Pop, O.L.; Pop, C.R.; Dufrechou, M.; Vodnar, D.C.; Socaci, S.A.; Dulf, F.V.; Minervini, F.; Suharoschi, R. Edible films and coatings functionalization by Probiotic Incorporation: A Review. Polymers 2019, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Groulx, J.-F.; Gagné, D.; Benoit, Y.D.; Martel, D.; Basora, N.; Beaulieu, J.-F. Collagen VI is a basement membrane component that regulates epithelial cell–fibronectin interactions. Matrix Biol. 2011, 30, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Gomand, F.; Borges, F.; Guerin, J.; El-Kirat-Chatel, S.; Francius, G.; Dumas, D.; Burgain, J.; Gaiani, C. Adhesive interactions between lactic acid bacteria and β-lactoglobulin: Specificity and impact on bacterial location in whey protein isolate. Front. Microbiol. 2019, 10, 1512. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Tyagi, A.; Kaushik, J.K.; Saklani, A.C.; Grover, S.; Batish, V.K. Role of surface layer collagen binding protein from indigenous Lactobacillus plantarum 91 in adhesion and its anti-adhesion potential against gut pathogen. Microbiol. Res. 2013, 168, 639–645. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A Review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Rodrigues, N.P.; Garcia, E.F.; de Souza, E.L. Selection of lactic acid bacteria with promising probiotic aptitudes from fruit and ability to survive in different food matrices. Braz. J. Microbiol. 2021, 52, 2257–2269. [Google Scholar] [CrossRef]
- Koryszewska-Bagińska, A.; Gawor, J.; Nowak, A.; Grynberg, M.; Aleksandrzak-Piekarczyk, T. Comparative genomics and functional analysis of a highly adhesive dairy Lactobacillus paracasei subsp. paracasei IBB3423 strain. Appl. Microbiol. Biotechnol. 2019, 103, 7617–7634. [Google Scholar] [CrossRef]
- Nowak, A.; Motyl, I. In vitro anti-adherence effect of probiotic Lactobacillus strains on human enteropathogens. Food Sci. Biotechnol. 2007, 81, 103–112. [Google Scholar]
- Nowak, A.; Motyl, I.; Śliżewska, K.; Libudzisz, Z.; Klewicka, E. Adherence of probiotic bacteria to human colon epithelial cells and inhibitory effect against enteric pathogens—In vitro study. Int. J. Dairy Technol. 2016, 69, 532–539. [Google Scholar] [CrossRef]
- Nishiyama, K.; Sugiyama, M.; Mukai, T. Adhesion Properties of Lactic Acid Bacteria on Intestinal Mucin. Microorganisms 2016, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, Y.; Hagi, T.; Hoshino, T. Correlation between in vitro mucus adhesion and the in vivo colonization ability of lactic acid bacteria: Screening of new candidate Carp probiotics. Biosci. Biotechnol. Biochem. 2011, 75, 511–515. [Google Scholar] [CrossRef]
- Li, X.J.; Yue, L.Y.; Guan, X.F.; Qiao, S.Y. The adhesion of putative probiotic lactobacilli to cultured epithelial cells and porcine intestinal mucus. J. Appl. Microbiol. 2008, 104, 1082–1091. [Google Scholar] [CrossRef]
- Salas-Jara, M.J.; Ilabaca, A.; Vega, M.; García, A. Biofilm Forming Lactobacillus: New Challenges for the Development of Probiotics. Microorganisms 2016, 4, 35. [Google Scholar] [CrossRef]
- Dimitrov, Z.; Gotova, I.; Chorbadjiyska, E. In vitro characterization of the adhesive factors of selected probiotics to Caco-2 epithelium cell line. Biotechnol. Biotechnol. Equip. 2014, 28, 1079–1083. [Google Scholar] [CrossRef]
- Wang, J.; Pu, Y.; Zeng, Y.; Chen, Y.; Zhao, W.; Niu, L.; Chen, B.; Yang, Z.; Wu, L.; Pan, K.; et al. Multi-functional potential of five lactic acid bacteria strains derived from giant panda (Ailuropoda Melanoleuca). Probiotics Antimicrob. Proteins 2022, 11–14. [Google Scholar] [CrossRef]
- Hernández-Alcántara, A.M.; Pardo, S.; Mohedano, M.L.; Vignolo, G.M.; de Moreno de LeBlanc, A.; LeBlanc, J.G.; Aznar, R.; López, P. The ability of riboflavin-overproducing Lactiplantibacillus plantarum strains to survive under gastrointestinal conditions. Front. Microbiol. 2020, 11, 591945. [Google Scholar] [CrossRef]
- Morita, H.; He, F.; Fuse, T.; Ouwehand, A.C.; Hashimoto, H.; Hosoda, M.; Mizumachi, K.; Kurisaki, J. Adhesion of lactic acid bacteria to Caco-2 cells and their effect on cytokine secretion. Microbiol. Immunol. 2002, 46, 293–297. [Google Scholar] [CrossRef]
- Datta, A.; Stapleton, F.; Willcox, M.D. Bacterial coaggregation among the most commonly isolated bacteria from contact lens cases. Investig. Ophthalmol. Vis. Sci. 2017, 58, 50. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, M.; Dai, X. Biological characteristics and probiotic effect of Leuconostoc lactis strain isolated from the intestine of black porgy fish. Braz. J. Microbiol. 2013, 44, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Cui, H.; Li, Y.; Sun, Y.; Qiu, H.-J. Characterization of lactic acid bacteria isolated from the gastrointestinal tract of a wild boar as potential probiotics. Front. Vet. Sci. 2020, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Brandel, A.; Becker, M.C.; Balles, R.; Abdelmohsen, U.R.; Ankenbrand, M.J.; Sickel, W. Wild bees and their nests host Paenibacillus bacteria with functional potential of avail. Microbiome 2018, 6, 229. [Google Scholar] [CrossRef] [PubMed]
- Forsgren, E. European foulbrood in honey bees. J. Invertebr. Pathol. 2010, 103, S5–S9. [Google Scholar] [CrossRef] [PubMed]
- Fünfhaus, A.; Ebeling, J.; Genersch, E. Bacterial pathogens of bees. Curr. Opin. Insect. Sci. 2018, 26, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Biová, J.; Charrière, J.-D.; Dostálková, S.; Škrabišová, M.; Petřivalský, M.; Bzdil, J.; Danihlík, J. Melissococcus plutonius Can Be Effectively and Economically Detected Using Hive Debris and Conventional PCR. Insects 2021, 12, 150. [Google Scholar] [CrossRef]
- Azzami, K.; Ritter, W.; Tautz, J.; Beier, H. Infection of honey bees with acute bee paralysis virus does not trigger humoral or cellular immune responses. Arch. Virol. 2012, 157, 689–702. [Google Scholar] [CrossRef]
- Ekmekci, H.; Aslim, B.; Ozturk, S. Characterization of vaginal lactobacilli coaggregation ability with Escherichia coli. Microbiol. Immunol. 2009, 53, 59–65. [Google Scholar] [CrossRef]
- Good, A.P.; Gauthier, M.-P.L.; Vannette, R.L.; Fukami, T. Honey bees avoid nectar colonized by three bacterial species, but not by a yeast species, isolated from the bee gut. PLoS ONE 2014, 9, e86494. [Google Scholar] [CrossRef]
- Mafu, A.A.; Plumety, C.; Deschênes, L.; Goulet, J. Adhesion of pathogenic bacteria to food contact surfaces: Influence of ph of culture. Int. J. Microbiol. 2011, 2011, 972494. [Google Scholar] [CrossRef]
- Leska, A.; Nowak, A.; Szulc, J.; Motyl, I.; Czarnecka-Chrebelska, K.H. Antagonistic Activity of Potentially Probiotic Lactic Acid Bacteria against Honeybee (Apis mellifera L.) Pathogens. Pathogens 2022, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- El Hage, R.; El Hage, J.; Snini, S.P.; Ammoun, I.; Touma, J.; Rachid, R.; Mathieu, F.; Sabatier, J.-M.; Abi Khattar, Z.; El Rayess, Y. The Detection of Potential Native Probiotics Lactobacillus spp. against Salmonella Enteritidis, Salmonella Infantis and Salmonella Kentucky ST198 of Lebanese Chicken Origin. Antibiotics 2022, 11, 1147. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, S.; Karthikeyan, R.; Nithyalakshmi, S.; Ranjani, J.; Gunasekaran, P.; Rajendhran, J. Anti-adhesion property of the potential probiotic strain Lactobacillus fermentum 8711 against methicillin-resistant Staphylococcus aureus (MRSA). Front. Microbiol. 2018, 9, 411. [Google Scholar] [CrossRef] [PubMed]
- Bulgasem, B.; Hassan, Z.; Abdalsadiq, N.; Yusoff, W.; Tibin, E.; Lani, M. Anti-Adhesion Activity of Lactic Acid Bacteria Supernatant against Human Pathogenic Candida Species Biofilm. Health Sci. J. 2015, 9, 1–9. [Google Scholar]
- Gutiérrez, S.; Martínez-Blanco, H.; Rodríguez-Aparicio, L.B.; Ferrero, M.A. Effect of fermented broth from lactic acid bacteria on pathogenic bacteria proliferation. J. Dairy Sci. 2016, 99, 2654–2665. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Wu, H.; Song, Z.; Høiby, N.; Molin, S.; Givskov, M. Combating biofilms. FEMS Microbiol. Immunol. 2012, 65, 146–157. [Google Scholar] [CrossRef]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug. Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Roberts, P.A.; Huebinger, R.M.; Keen, E.; Krachler, A.-M.; Jabbari, S. Predictive modelling of a novel anti-adhesion therapy to combat bacterial colonisation of burn wounds. PLoS Comput. Biol. 2018, 14, e1006071. [Google Scholar] [CrossRef]
- Leska, A.; Nowak, A.; Miśkiewicz, K.; Rosicka-Kaczmarek, J. Binding and Detoxification of Insecticides by Potentially Probiotic Lactic Acid Bacteria Isolated from Honeybee (Apis mellifera L.) Environment—An In Vitro Study. Cells 2022, 11, 3743. [Google Scholar] [CrossRef]
- Leska, A.; Nowak, A.; Motyl, I. Isolation and Some Basic Characteristics of Lactic Acid Bacteria from Honeybee (Apis mellifera L.) Environment—A Preliminary Study. Agriculture 2022, 12, 1562. [Google Scholar] [CrossRef]
- Lebeer, S.; Claes, I.; Tytgat, H.L.; Verhoeven, T.L.; Marien, E.; von Ossowski, I.; Reunanen, J.; Palva, A.; Vos, W.M.; Keersmaecker, S.C.; et al. Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl. Environ. Microbiol. 2012, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]

| LAB Strains | Auto-Aggregation [%] ± S.E.M. | Hydrophobicity [%] ± S.E.M. |
|---|---|---|
| A. kunkeei DSM 12361 | 32.91 ± 9.06 | 18.87 ± 0.94 |
| P. acidilactici 4/1 | 27.74 ± 4.50 | 1.83 ± 0.46 a,b |
| P. acidilactici 5/2 | 52.58 ± 4.47 | 21.65 ± 3.66 |
| P. acidilactici 7/1 | 52.09 ± 7.90 | 26.49 ± 5.12 |
| P. acidilactici 8/1 | 33.74 ± 9.06 | 33.64 ± 6.09 |
| L. plantarum 10/2 | 61.61 ± 3.12 | 45.13 ± 4.76 |
| P. pentosaceus 11/3 | 52.03 ± 9.75 | 31.31 ± 1.99 |
| P. pentosaceus 14/1 | 68.25 ± 5.97 | 19.85 ± 2.86 |
| L. plantarum 18/1 | 71.91 ± 5.44 | 52.45 ± 2.80 |
| P. pentosaceus 19/1 | 68.04 ± 5.31 | 63.16 ± 5.27 a |
| L. plantarum 21/1 | 66.63 ± 3.00 | 47.05 ± 4.12 |
| P. acidilactici 25/1 | 65.04 ± 0.90 | 73.49 ± 2.72 b |
| P. parvulus OK-S | 65.56 ± 2.71 | 37.43 ± 2.78 |
| L. brevis KKA | 54.68 ± 7.18 | 22.69 ± 4.69 |
| L. plantarum 8AN | 54.63 ± 3.53 | 22.45 ± 8.40 |
| L. salivarius 9AN | 53.98 ± 3.08 | 27.50 ± 2.81 |
| L. casei 12AN | 59.47 ± 4.56 | 14.31 ± 0.89 |
| L. plantarum 145 | 30.96 ± 0.69 | 4.77 ± 2.01 |
| L. rhamnosus GG | 60.83 ± 0.75 | 16.43 ± 2.80 |
| L. rhamnosus ŁOCK 0908 | 53.87 ± 0.52 | 13.03 ± 4.90 |
| Honeybee Pathogens | Coaggregation [%] ± S.E.M. | ||||
|---|---|---|---|---|---|
| A. kunkeei DSM 12361 | L. plantarum 18/1 | L. plantarum 21/1 | P. acidilactici 25/1 | p-Value (KW Test) | |
| P. larvae ATCC 25367 | 89.37 ± 10.41 | 54.79 ± 4.36 | 55.77 ± 7.51 | 80.40 ± 5.95 | p = 0.075 |
| P. larvae ATCC 49843 | 87.16 ± 1.27 * | 42.96 ± 1.38 * | 47.04 ± 2.29 | 48.33 ± 2.56 | p = 0.043 |
| P. apiarius DSM 5582 | 89.61 ± 7.44 | 76.52 ± 1.17 | 86.12 ± 5.49 | 80.53 ± 6.57 | p = 0.264 |
| P. alvei DSM 29 | 84.70 ± 3.69 | 77.16 ± 4.24 | 85.74 ± 1.65 | 77.22 ± 5.31 | p = 0.459 |
| L. sphaericus DSM 1866 | 96.11 ± 3.52 | 97.13 ± 1.45 | 95.15 ± 7.76 | 73.86 ± 1.90 | p = 0.092 |
| M. plutonius DSM 29964 | 29.27 ± 8.74 | 44.82 ± 10.75 | 43.22 ± 13.14 | 39.20 ± 2.90 | p = 0.281 |
| E. coli ATCC 25922 | 93.09 ± 3.65 | 96.06 ± 1.35 | 61.97 ± 0.51 | 60.06 ± 2.65 | p = 0.072 |
| Honeybee Pathogens | Eradication (+) and Stimulation (−) of Pathogen Biofilms [%] ± S.E.M. | p-Value KW Test | |||
|---|---|---|---|---|---|
| A. kunkeei DSM 12361 | L. plantarum 18/1 | L. plantarum 21/1 | P. acidilactici 25/1 | ||
| P. larvae ATCC 25367 | −4.86 ± 4.31 | −8.91 ± 6.68 | −18.18 ± 2.53 | −15.50 ± 4.58 | |
| P. larvae ATCC 49843 | −5.43 ± 2.88 A | 10.36 ± 1.90 B | 17.53 ± 2.41 A,C | −10.13 ± 4.12 B,C | A 0.0092, B 0.0451, C 0.0007 |
| P. apiarius DSM 5582 | −29.96 ± 2.79 A | −5.04 ± 3.21 | 0.12 ± 3.94 A,B | −27.27 ± 3.32 B | A 0.008, B 0.028 |
| P. alvei DSM 29 | −8.10 ± 3.10 | −11.14 ± 5.50 | −8.37 ± 4.50 | −5.47 ± 6.88 | |
| L. sphaericus DSM 1866 | −0.35 ± 3.69 A | 0.99 ± 2.26 | 16.31 ± 2.51 A,B | −6.73 ± 4.54 B | A 0.028, B 0.003 |
| M. plutonius DSM 29964 | −16.89 ± 6.28 | 19.87 ± 1.00 A | 19.33 ± 2.28 B | −32.59 ± 4.31 A,B | A 0.003, B 0.004 |
| E. coli ATCC 25922 | −42.30 ± 6.76 A,B | 8.52 ± 2.74 A | 9.22 ± 2.96 B | −7.04 ± 3.00 | A 0.003, B 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leska, A.; Nowak, A.; Czarnecka-Chrebelska, K.H. Adhesion and Anti-Adhesion Abilities of Potentially Probiotic Lactic Acid Bacteria and Biofilm Eradication of Honeybee (Apis mellifera L.) Pathogens. Molecules 2022, 27, 8945. https://doi.org/10.3390/molecules27248945
Leska A, Nowak A, Czarnecka-Chrebelska KH. Adhesion and Anti-Adhesion Abilities of Potentially Probiotic Lactic Acid Bacteria and Biofilm Eradication of Honeybee (Apis mellifera L.) Pathogens. Molecules. 2022; 27(24):8945. https://doi.org/10.3390/molecules27248945
Chicago/Turabian StyleLeska, Aleksandra, Adriana Nowak, and Karolina Henryka Czarnecka-Chrebelska. 2022. "Adhesion and Anti-Adhesion Abilities of Potentially Probiotic Lactic Acid Bacteria and Biofilm Eradication of Honeybee (Apis mellifera L.) Pathogens" Molecules 27, no. 24: 8945. https://doi.org/10.3390/molecules27248945
APA StyleLeska, A., Nowak, A., & Czarnecka-Chrebelska, K. H. (2022). Adhesion and Anti-Adhesion Abilities of Potentially Probiotic Lactic Acid Bacteria and Biofilm Eradication of Honeybee (Apis mellifera L.) Pathogens. Molecules, 27(24), 8945. https://doi.org/10.3390/molecules27248945







