Abstract
Sophora japonica L. (SJL) and Robinia pseudoacacia L. (RPL) are widely cultivated in China. However, the utilization of their main by-products are limited due to a lack of comprehensive nutritional attributes. Herein, the proximate composition, mineral elements, fatty acids, amino acids, monosaccharides, and phenolics were analyzed to investigate the nutritional attributes of SJL and RPL. Dietary fiber was the main ingredient in SJL and RPL, followed by protein and lipids. The content of Fe in SJL and RPL was highest, especially in flowers of SJL, reaching about 1179.51 mg/kg. The total unsaturated fatty acids accounted for 89.67% of the bud of SJL. Meanwhile, the essential amino acids contents of the flower and bud of SJL and RPL accounted for 35.95–40.59% of total amino acids. The flower of SJL (373.75 mg/g) exhibited the most abundant monosaccharides. Meanwhile, the total phenolics and flavonoid contents in the buds of SJL and RPL were significantly higher than that of the flower, implying the buds possessed better biological activity. Moreover, the bud of SJL possessed the most abundant phenolics. The results provided a reference for the development of functional food derived from SJL and RPL.
1. Introduction
The buds of Sophora japonica L. (SJL), also named “Guohuai” in China, are known as Flos Sophorae Immaturus, a functional food widely used in China, Korea, and Japan. The functional properties of Flos Sophorae Immaturus, such as antioxidant properties, free radical scavenging, anti-inflammatory properties, hemostasis, blood sugar lowering, uric acid lowering, etc., were confirmed through extensive studies [1]. Xie et al. [2] determined 16 flavonoids in Flos Sophorae Immaturus through HPLC-DAD-ESI-MS/MS. Li et al. [3] found that polysaccharides from the buds of SJL protected HaCaT keratinocytes from ultraviolet-irradiation-induced skin injuries. Moreover, Flos Sophorae Immaturus contains various mineral elements, including K, Ca, Mg, Fe, Zn, Cu, etc. [1]. Therefore, Flos Sophorae Immaturus is mainly consumed as a functional tea. Unfortunately, the flowers of SJL are often ignored and considered lost application value, and no studies have focused on the difference between the main components of the flower and bud of SJL.
Robinia pseudoacacia L. (RPL) is also named “False acacia” or “Yanghuai” in China. Although RPL is considered an invasive species, it has been widely cultivated in China since 1970 due to its good soil and water conservation and ecological restoration effects [4]. The flower is the most abundant by-product of RPL and is usually used as a nectar source, as well as a vegetable in some parts of China, with a low utilization rate. Tian et al. [5] isolated five flavonoids from the ethanolic extract of the whole plant of RPL, including acacetin, secundiflorol I, mucronulatol, isomucronulatol, and isovestitol. Veitch et al. [6] determined 11 flavonoid glycosides in the leaves of RPL. Bratu et al. [7] indicated that the flower of RPL exhibited high antioxidant activity and that it induced significant necrosis and apoptosis of HeLa cells, providing an antitumor effect in vitro. Stankov et al. [8] found that the flower of RPL contained abundant concrete compounds (1.06%), including n-nonacosane, n-heptacosane, α-linolenic acid, n-pentacosane, palmitic acid, diisooctyl phthalate, etc. Therefore, the flower of RPL may be a promising food material.
Importantly, nutrient elements, including phenolics, fatty acids, mineral elements, and amino acids, were crucial for the development of functional foods. Liang et al. [9] determined the nutrition-related compounds of byproducts of sunflower seeds and revealed that the florets of sunflowers were rich sources of dietary fiber, Fe, and phenols. Hu et al. [10] investigated the phenolic composition and nutritional attributes of diaphragma juglandis fructus and walnut shells and revealed that these byproducts of walnut could be utilized for the development of health-beneficial functional foods, e.g., as phenolic antioxidants and weight loss. Meanwhile, high cellulose content in SJL and RPL exhibited promising application prospects in the preparation of hemicelluloses [11,12]. Additionally, polysaccharides also played a considerable role in the bioactivities of SJL and RPL [13]. Therefore, the analysis of basic components in SJL and RPL was important for the application of these materials. As the same plant species from the family Fabaceae, both the SJL and RPL gained tremendous attention of researchers. However, there is still an absence of nutritional composition and two groups of polyphenols in these two species. Thus, the objectives of our study were (1) to comparatively explor the nutritional properties of flowers and buds of SJL and RPL and (2) to identify monosaccharide and phenolic composition in the flowers and buds of SJL and RPL. It is foreseen that a clearer understanding of the primary functional components in SJL and RPL was obtained, which further contributed to the development of functional food derived from SJL and RPL.
2. Results and Discussion
2.1. Proximate Chemical Composition
The primary chemical content of SJL and RPL were shown in Table 1. Dietary fiber was the primary ingredient in both SJL and RPL, followed by protein and lipid. The soluble dietary fiber in the flowers of SJL (17.25 g/100 g) and RPL (19.29 g/100 g) were significantly higher than in the buds (p ≤ 0.05). Notably, the soluble dietary fiber contents of SJL and RPL were higher than barley (4.73–5.70 g/100 g) [14] and oat bran (8.9–14.2 g/100 g) [15]. A previous study showed that the consumption of soluble dietary fiber played a considerable role in alleviating cardiovascular disease and diabetes, reducing inflammation and cholesterol, and regulating gut microbiota [16]. Therefore, the abundant amount of soluble dietary fiber suggests SJL and RPL are beneficial to human health, which can be developed as functional food materials. Furthermore, the total phenolics and flavonoids contents in SJL were also obvious and significantly higher than that of RPL (p ≤ 0.05). Polyphenols are closely related to a variety of biological activities, including antioxidant, hypoglycemic, hypolipidemic, anti-bacterial, anti-hyperuricemia, anti-inflammatory, etc. [1]. Thus, SJL has a better utilization value compared to RPL. Meanwhile, the total phenolics and flavonoid contents in the buds of SJL and RPL were significantly higher than in the flowers (p ≤ 0.05), implying the buds possessed better biological activity. Interestingly, the polyphenols in SJL mainly consist of free phenolics, the amounts of which are significantly higher than those of bound phenolics (p ≤ 0.05), indicating that the phenolics compounds of SJL are more easily utilized. Moreover, SJL and RPL showed abundant contents of reducing sugar, ranging from 19.21 to 27.11 g/100 g, except for the buds of SJL.

Table 1.
Proximate chemical composition (g/100 g dw), mineral and metal (mg/kg dw) contents of SJL and RPL.
2.2. Mineral Elements and Metals
The elemental compositions of SJL and RPL were shown in Table 1. The content of Fe in SJL and RPL was highest, especially in the flowers of SJL, reaching about 1179.51 mg/kg, which is similar to different types of tea [17]. Accumulating evidence has shown that Fe deficiency is closely related to fatigue, anemia, prematurity, and perinatal mortality [18]. Meanwhile, the recommended intake of Fe ions is 1, 10, 12, and 15 mg Fe kg/d for infants, children, and male and female adolescents, respectively, while the tolerable upper intake level is 45 mg/d [17]. Interestingly, the Fe contents in the flowers of SJL and RPL were higher than that of the buds, indicating the flowers might be a better Fe supplement for the human body. Furthermore, the Na contents in SJL and RPL were also in the leading position among all elements, which was beneficial to preserving the electrolyte environment in the human body. The contents of mineral compounds (Cu, Zn, Mn) in SJL and RPL were similar to herbal teas [19]. Cu, Zn, and Mn are essential components of plant enzymes and closely related to plant photosynthesis, respiration, and growth [20]. Notably, WHO has no allowable limits for mineral elements, as many of them are considered micronutrients. The contents of K, Ca, and Mg in SJL and RPL were lower than other elements, while heavy metals, such as Hg and Pb, were not detected. Moreover, a small amount of Se (0.029 mg/kg) was detected in the buds of RPL but not in other samples.
2.3. Fatty Acids
As shown in Table 2, twenty-seven fatty acids were measured in SJL and RPL, including twelve saturated fatty acids and fifteen unsaturated fatty acids. The total saturated fatty acid contents in the flower of SJL, flower, and bud of RPL were higher than unsaturated fatty acids, except in the bud of SJL. Palmitic acid was a primary saturated fatty acid in SJL and RPL, which accounted for 11.48%, 68.94%, 75.01%, and 63.12% in the flower of SJL, the bud of SJL, the flower of RPL, and the bud of RPL, respectively. Previous studies confirmed that palmitic acid exhibited analgesic, anti-inflammatory, antiviral, regulating lipid metabolism, and alleviating atherosclerosis properties [21]. Stearic acid, another major saturated fatty acid in SJL and RPL, has been shown to reduce low-density lipoprotein (LDL)-cholesterol [22]. The total unsaturated fatty acids accounted for 19.24%, 89.67%, 40.35%, and 40.81% of fatty acids in the flower of SJL, the bud of SJL, the flower of RPL, and the bud of RPL, respectively. The oleic content in the bud of SJL reached 1282.79 mg/kg, which is significantly higher than other samples (p ≤ 0.05). In contrast, the linoleic contents in the flower of SJL, flower and bud of RPL were dominant compared to oleic contents. Linoleic, a ω-6 series essential fatty acid, is beneficial to long-term glycemic control, cardiovascular risk, and insulin resistance [23]. Furthermore, linoleic can be metabolized into arachidonic acid, which is the precursor of eicosanoids, exhibiting proinflammatory or prothrombotic-vasoconstrictor properties [24]. The content of γ-linolenic (ω-6 family) was also abundant in the flower and bud of RPL, which is closely related to the decrease in blood concentrations of triacylglycerols, total cholesterol, and LDL [25]. SJL and RPL also contained a few polyunsaturated fatty acids (such as palmitoleic acid, eicosapentaenoic, and docosahexaenoic), which were beneficial to human health.

Table 2.
Fatty acid content of SJL and RPL (mg/kg dw).
2.4. Amino Acids
As shown in Table 3, eleven nonessential amino acids (NEAA) and six essential amino acids (EAA) were identified in SJL and RPL. The EAA and NEAA contents in the buds of SJL and RPL were significantly higher than those in the flowers of SJL and RPL (p ≤ 0.05). The leucine content in the flower of SJL, the bud of SJL, the flower of RPL, and the bud of RPL reached 6.94 mg/g, 9.43 mg/g, 7.65 mg/g, and 7.67 mg/g, respectively, which is the most abundant of all EAA. Leucine, a branched-chain amino acid, showed a close relation to type 2 diabetes, insulin resistance, obesity, and nonmetabolic diseases. Notably, other branched-chain amino acids (valine and isoleucine) contents were also abundant in both SJL and RPL, which exhibited similar beneficial effects on humans [26]. Previous studies confirmed that branched-chain amino acids were the primary nitrogen sources for glutamine and alanine synthesis in muscle [9].

Table 3.
Amino acid content of SJL and RPL (mg/g dw).
The most abundant NEAA in SJL and RPL was proline, with the contents ranging from 10.08 mg/g to 19.08 mg/g, respectively, while the bud of SJL possessed the highest content compared with other samples. Proline was also known as a primary precursor of extracellular collagens, against various potential harms (UV radiation, drought/salinity, heavy metals, reactive oxygen species), promotes beneficial tissue regeneration, and regulates cell signaling pathways [27]. The EAA contents of the flower of SJL, the bud of SJL, the flower of RPL, and the bud of RPL accounted for 40.59%, 35.95%, 35.95%, and 37.45% of total amino acids (TAA), respectively. The EAA/NEAA values of the flower of SJL, the bud of SJL, the flower of RPL, and the bud of RPL were 68.29, 56.13%, 56.14%, and 59.90%, respectively. Thus, the amino acid composition in the flower of SJL was more balanced than that in the bud of SJL according to the ideal protein standard for the human body suggested by FAO/WHO, which suggested 40% EAA/TAA and 60% EAA/NEAA as an appropriate amino acid composition. Interestingly, the opposite phenomenon occurred in the flower and bud of RPL.
To further evaluate the EAA composition of SJL and RPL, the amino acid scores of EAA (in 1 g of the sample protein) to the reference protein (in 1 g of the standard protein) of FAO/WHO were calculated. Lysine and threonine exhibited the lowest content in the flower and bud of SJL and RPL, implying these two amino acids were the first limiting amino acids. According to the standard amino acid composition recommended by FAO/WHO, an amino acid score close to 1 implies a more reasonable amino acid composition. As shown in Table 4, most amino acid scores of SJL and RPL were lower than 1, except methionine, cystine, and isoleucine, indicating a more reasonable composition of these three amino acids. Furthermore, the scores of the flower of SJL were higher than the bud of SJL, while the scores of the bud of RPL were higher than the flower of RPL, implying that the amino acid compositions of the flower of SJL and the bud of RPL were more balanced.

Table 4.
Essential amino acid composition of compared to the FAO/WHO pattern. (mg/100 g protein dw).
2.5. Monosaccharide Composition
The monosaccharide compositions of SJL and RPL were analyzed and shown in Table 5. The flower of SJL (373.75 mg/g) exhibited the most abundant monosaccharides, followed by the bud of SJL (198.92 mg/g), the flower of RPL (65.20 mg/g), and the bud of RPL (9.31 mg/g). The flowers of SJL and RPL showed the highest rhamnose contents, significantly higher than those of the buds. Rhamnose is a trace sugar that widely exists in plants and is often used as sweetener, flavor, and fragrance in the food industry [28]. The bud of SJL possessed the highest glucuronic acid content, significantly higher than that in the flower. However, glucuronic acid was not found in RPL. Previous studies confirmed that glucuronic acid could combine with a variety of harmful substances in the liver and provide a detoxification effect [29]. The arabinose content was abundant in both the flowers and buds of SJL and RPL. Arabinose can regulate intestinal peristalsis, control the accumulation of blood sugar and fat, and relieve a series of diseases such as diabetes and obesity [30]. Moreover, the xylose content was also abundant in these materials, which was widely used as xylitol material, low-calorie sweeteners, or food colorants in the food industry [31]. Furthermore, mannose, ribose, and galacturonic acid were also identified in SJL and RPL, indicating that hemicellulose was abundant in SJL and RPL. The results showed that SJL and RPL were important alternative renewable energy resources and provided a theoretical basis for future research and development.

Table 5.
Identification of monosaccharide compounds of SJL and RPL (mg/g dw).
2.6. Identification of Polyphenols
The chromatograms of phenolics compounds in SJL and RPL were shown in Figure 1a–h. Ten peaks were assigned to FP compounds (Figure 1a,c) in the flower and bud of SJL, while the FPs in the flower and bud of RPL had only five peaks. Moreover, the bud of SJL possessed the most abundant bound phenolics (10 peaks), followed by the bud of RPL (9 peaks), the flower of RPL (8 peaks), and the flower of SJL (4 peaks). Thus, SJL possessed more abundant free phenolics and RPL possessed more abundant bound phenolics. Compared to bound phenolics, free phenolics are easier to utilize. Therefore, SJL has greater advantages as a food material than RPL. Similar results showed that eight primary polyphenols were identified in Tartary buckwheat, consisting of six types of free polyphenols and six types of bound polyphenols [32]. Hu et al. [10] also found eleven types of free polyphenols and ten types of bound polyphenols in the diaphragma juglandis fructus. Therefore, SJL and RPL can be used as important sources of polyphenols.
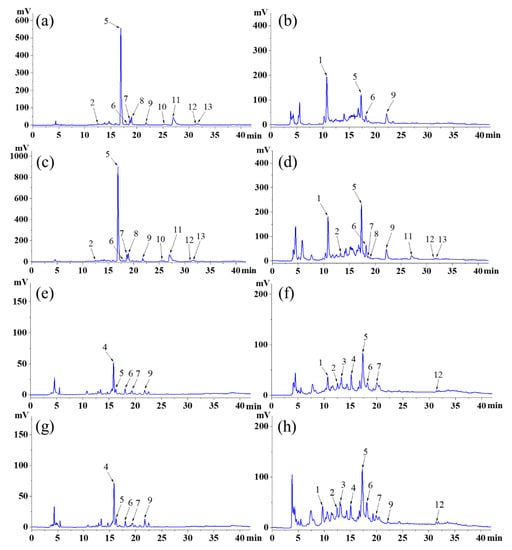
Figure 1.
High-performance liquid chromatograms of (a) free phenolic compounds of the flower of Sophora japonica L.; (b) bound phenolic compounds of the flower of Sophora japonica L.; (c) free phenolic compounds of the bud of Sophora japonica L.; (d) bound phenolic compounds of the bud of Sophora japonica L.; (e) free phenolic compounds of the flower of Robinia pseudoacacia L.; (f) bound phenolic compounds of the flower of Robinia pseudoacacia L.; (g) free phenolic compounds of the bud of Robinia pseudoacacia L.; and (h) bound phenolic compounds of the bud of Robinia pseudoacacia L. The numbers of the peaks in this figure coincide with the compound numbers in Table 6. The chromatograms were recorded at 280 nm.
The mass spectrometric data of identified phenolics are shown in Table 6. Compound 1 showed an [M-H]− ion at m/z 153 and only existed in bound phenolics of SJL and RPL, so it was considered protocatechuic acid according to the previous studies [7,33]. Compound 2 showed an [M-H]− ion at m/z 353, which was considered chlorogenic acid and also confirmed by other findings [7,33]. Compounds 3 and 4 only existed in RPL and revealed [M-H]− ions at m/z 289 and 739, respectively. They were tentatively assigned as catechin and robinin according to the previous study [7]. Compounds 5, 6, and 7 existed in both SJL and RPL; they showed [M-H]− ions at m/z 609, 463, and 593, so these compounds were tentatively assigned as rutin, hyperoside, and kaempferol-3-O-rutinoside, respectively [2,6]. Compounds 8, 10, 11, and 13 only existed in SJL and revealed [M-H]− ions at m/z 623, 577, 301, and 315, respectively, so these compounds were assigned as narcissoside, sophorabioside, quercetin, and isorhamnetin, respectively based on the previous studies [33,34]. Compounds 9 and 12 showed [M-H]− ions at m/z 447 and 285, which were assigned as quercitrin and kaempferol, respectively [6,35].

Table 6.
Mass spectrometric data, and identification of polyphenols extracted from SJL and RPL.
In general, the phenolics in SJL and RPL were mainly derivatives of quercetin, including rutin, kaempferol, isorhamnetin, hyperoside, quercitrin, etc. Interestingly, rutin was converted to quercetin and other derivatives by the rutin-degrading enzyme in SJL and RPL [36]. Previous studies also found that abundant derivatives of quercetin existed in SJL and RPL [33]. Therefore, the types of flavonoids in SJL and RPL are directly related to the planting environment and preservation methods. Moreover, accumulating evidence has shown that flavonoids exhibit better antihyperuricemia, antihyperglycemia, and antioxidant effects than phenolic acids [37,38]. Meanwhile, chemical reactions such as methylation, deglycosylation, and decarboxylation of flavonoids occurred during processing, which improved the conversion of these flavonoids with similar structures and further affected their bioactivities [39]. Therefore, studying appropriate processing methods in order to enhance the bioactivities of these flavonoids will be an interesting topic. Furthermore, SJL exhibited richer phenolic compositions and higher phenolic contents compared to RPL, implying that SJL possessed better application value in the food industry.
3. Materials and Methods
3.1. Materials and Chemicals
Fresh flowers and buds of SJL and RPL were obtained from indigenous trees (Zhaohe town, Nanyang, Henan Province, China), then dried in an oven (Binder, Neckarsulm, Germany) at 50 °C for 6 h. The dried samples were crushed to powder with a pulverizer (Wuyi Haina Electric Co., Ltd., Jinhua, China) and sieved through a 100-mesh screen. Rutin (≥98%), gallic acid (≥98%), α-amylase (10,000 u/mL), neutral protease (100 u/mg), and amyloglucosidase (100,000 u/mL) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
3.2. Proximate Composition Analysis
Moisture, crude lipid, crude protein, ash, and dietary fiber contents were measured according to the AOAC (2005) methods. Total and reducing sugars were measured using the 3,5-dinitrosalicylic acid method [36]. The total phenolics and flavonoid contents were analyzed via the Folin–Ciocalteu method and aluminum chloride colorimetric method, respectively [37].
3.3. Mineral Elements and Metals Analysis
An inductively coupled plasma mass spectrometer (ICP-MS) instrument (Plasma Quant MS, Analytik Jena AG, Jena, Germany) was employed to determine the elements according to the method of Potorti et al. [40] with minor revisions. ICP-MS operated in no-gas mode for the isotopes Zn, Mn, Na, K, Ca, and Pb and in helium mode for Cu, Fe, Mg, Se, and Hg. The parameters were as follows: sample depth, 9 mm; generators power, 1500 W; nebulization chamber temperature, 2 ℃; nebulizer pump, 0.1 rps; extract lens, 1.5 V; collision gas flow rate, 4 mL/min.
3.4. Fatty Acid Analysis
The fatty acid composition was analyzed using a QP2010 Ultra GC-MS instrument (Shimadzu, Tokyo, Japan) equipped with a TG-5MS (30 m × 0.25 mm × 0.25 μm) chromatographic column [10]. Briefly, 100 mg of samples, 2 mL of 2% NaOH-methanol solution and 100 μL of methyl nonadecylate (interior label) were mixed. Then, the mixture was incubated at 40 °C for 20 min and extracted with 1 mL of n-hexane. The obtained supernatant was filled to 1 mL with n-hexane. The GC-MS parameters were as follows: injection volume, 1 μL; inlet temperature, 250 °C; the initial temperature was 80 ℃, then was increased to 230 °C at 10 ℃/min and held for 15 min; electron bombardment (EI) energy, 70 eV; ion source temperature, 230 °C; transfer line temperature 270 °C; solvent delay, 2 min; scanning range, 50–450 amu; scanning mode, total ion scanning.
3.5. Amino Acid Analysis
The amino acid composition was measured with a 1260 HPLC instrument (Agilent, Palo Alto, CA, USA) equipped with a DAD detector and a C18 column (4.6 × 250 mm × 5 μm) (Shiseido, Tokyo, Japan) [41]. First, samples were hydrolyzed with 6 mol/L HCl at 110 °C for 24 h. Then, the hydrolyzed samples were redissolved with 0.02 mol/L HCl solution after being drained in vacuum. Finally, the sample solution was mixed with 0.1 mol/L phenyl isothiocyanate acetonitrile solution and 1.0 mol/L triethylamine-acetonitrile solution. The determined samples were extracted by n-hexane. The liquid chromatography conditions were as follows: Solvent A, water mixed with 0.8% sodium acetate and 7% acetonitrile; Solvent B, acetonitrile mixed with 20% methanol and 20% water; the elution conditions were performed from 100% to 98% A in 5 min, from 98% to 95% A in 1 min, from 95% to 91% A in 8 min, from 91% to 79% A in 4 min, from 79% to 55% A in 14 min, from 55% to 45% A in 2 min, from 45% to 0% A in 4 min, from 0% to 0% A in 4 min, from 0% to 100% A in 3 min, from 100% to 100% A in 5 min; the detection wavelength, 254 nm; the flow rate, 1.0 mL/min; the column temperature, 30 °C; the injection volume, 10 μL.
3.6. Monosaccharide Compounds Analysis
The monosaccharide compounds were measured with the method of Wen et al. [42] with minor revisions. Briefly, the sample was hydrolyzed with 2 mol/L trifluoroacetic acid at 110 °C for 4 h, then adjusted the pH to 7 with 2 mol/L NaOH and centrifuged at 10,000 r/min for 10 min. Secondly, 0.4 mL of polysaccharide hydrolysate was mixed with 0.8 mL of 0.3 mol/L NaOH, and 0.5 mol/L 1-phenyl-3-methyl-5-pyra-zolone (PMP)-methanol solution, then derivatized at 70 °C for 60 min. Finally, 0.8 mL of 0.3 mol/L HCl was added to the mixture and extracted with chloroform five times. The supernatant was subjected to an HPLC instrument (Shimadzu, Kyoto, Japan) equipped with a UV/vis detector and a C18 column (5 μm, 4.6 mm × 250 mm i.d.). The liquid chromatography conditions were as follows: Solvent A, 15% acetonitrile with 0.05 mol/L phosphate buffer solution (KH2-PO4-NaOH, pH 7.1); Solvent B, 40% acetonitrile with 0.05 mol/L phosphate buffer solution (KH2PO4-NaOH, pH 7.1). The elution conditions were performed from 0% to 10% B in 10 min, from 10% to 30% B in 30 min, from 30% to 0% B in 5 min; the wavelength, 250 nm; the flow rate, 0.7 mL/min; the injection volume, 20 μL.
3.7. Polyphenols Preparation
Extraction of free phenolic (FP) compounds: 1.0 g of SJL or RPL powder was mixed with 30 mL of 70% (v/v) ethanol. The mixture was extracted on a magnetic stirrer at 25 °C for 40 min, and the speed was 300 r/min, then centrifuged at 5000 r/min for 20 min. The supernatants were evaporated with a rotary evaporator (RV8, IKA, Staufen, Germany) at 60 °C. Finally, the residues were redissolved in methanol and filled to 10 mL [43].
Extraction of bound phenolic (BP) compounds: The residues obtained from FP extraction were mixed with 40 mL of 3 mol/L NaOH solution. The mixture was incubated at 40 °C for 4 h then centrifuged at 6000 r/min for 20 min after adjusted pH to 2.0. The supernatant was extracted with ethyl acetate and evaporated at 50 °C using a rotary evaporator. Finally, the BP residues were redissolved with 2 mL of methanol [32].
3.8. UPLC-QTOF-MS Analysis of Polyphenols
FP and BP extracts were determined using a UPLC-QTOF-MS (Waters, Milford, MA, USA) equipped with a C18 column (2.1 × 150 mm, 1.7 μm) according to our previous study [31]. The mobile phase was comprised of acetonitrile (solvent A) and 0.1% acetic acid aqueous solution (solvent B) with a flow rate of 0.3 mL/min. The elution conditions were performed as follows: 0–5 min, 7–20% A; 5–24 min, 20–40% A; 24–28 min, 40–50% A; 28–40 min, 50–40% A; 40–42 min, 40–7% A. The injection volume was 10 μL and the chromatograms were recorded at 280 nm. The QTOF-MS parameters were as follows: negative mode; desolvation temperature, 400 °C; drying gas (N2) flow rate, 700 L/h; spray voltage, 3000 V; ion source temperature, 100 °C; mass spectra, 50 to 1500 Da.
3.9. Statistical Analysis
All experiments were performed in triplicate. Statistical analyses were performed with SPSS (version 22) and Origin (version 2021) software. The differences in data (p ≤ 0.05) were measured using one-way analysis of variance (one-way ANOVA).
4. Conclusions
In general, the proximate composition, mineral elements, fatty acids, amino acids, monosaccharides, and phenolic composition of SJL and RPL were clarified. The flowers and buds of SJL and RPL contained abundant phenolic compositions and mainly existed in a free state. Meanwhile, the soluble dietary fiber in the flowers of SJL (17.25 g/100 g) and RPL (19.29 g/100 g) was abundant. Moreover, Fe was the primary mineral element in SJL and RPL, especially in the flowers of SJL (1179.51 mg/kg). The total unsaturated fatty acids accounted for 19.24–40.81% in the flowers and buds of SJL and RPL. In particular, SJL and RPL also contained a few polyunsaturated fatty acids, such as palmitoleic acid, eicosapentaenoic acid, and docosahexaenoic acid. Furthermore, the amino acid composition of the flower of SJL and bud of RPL was more balanced according to the amino acid scores. Thus, richness in dietary fiber and mineral elements indicated that the flowers and buds of SJL and RPL could be applied to health-beneficial functional food development, e.g., regulating the gut ecosystem, functional food additives, weight regulation, and iron supplements. Likewise, abundant monosaccharide composition indicated that SJL and RPL were important alternate renewable energy resources. Thereafter, developing a beverage incorporating with micronized flowers and buds of SJL and RPL may be a promising strategy. Thus, the chemical composition variation of the flower and bud of SJL and RPL during the micronization process should be addressed in the future.
Author Contributions
J.L. conceptualized the study, secured funding for the research, critically reviewed, and edited the paper; J.T. designed multifactorial experiments, performed experimental research, and prepared an initial draft of the paper; Y.G. performed statistical analysis and prepared figures and tables. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Sci-tech Plan Project of Guizhou Province ([2021] 174) and Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (2021L566).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Gong, Y.; Fan, L.; Wang, L.; Li, J. Flos Sophorae Immaturus: Phytochemistry, bioactivities, and its potential applications. Food Rev. Int. 2021, 1–19. [Google Scholar] [CrossRef]
- Xie, Z.; Lam, S.; Wu, J.; Yang, D.; Xu, X. Chemical fingerprint and simultaneous determination of flavonoids in Flos Sophorae Immaturus by HPLC-DAD and HPLC-DAD-ESI-MS/MS combined with chemometrics analysis. Anal. Methods 2014, 6, 4328–4335. [Google Scholar] [CrossRef]
- Li, L.; Huang, T.; Lan, C.; Ding, H.; Yan, C.; Dou, Y. Protective effect of polysaccharide from Sophora japonica L. flower buds against UVB radiation in a human keratinocyte cell line (HaCaT cells). J. Photochem. Photobiol. B 2019, 191, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Vitkova, M.; Mullerova, J.; Sadlo, J.; Pergl, J.; Pysek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. For. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; McLaughlin, J.L. Bioactive Flavonoids from the Black Locust Tree, Robinia Pseudoacacia. Pharm. Biol. 2000, 38, 229–234. [Google Scholar] [CrossRef]
- Veitch, N.C.; Elliott, P.C.; Kite, G.C.; Lewis, G.P. Flavonoid glycosides of the black locust tree, Robinia pseudoacacia (Leguminosae). Phytochemistry 2010, 71, 479–486. [Google Scholar] [CrossRef]
- Bratu, M.M.; Birghila, S.; Stancu, L.M.; Cenariu, M.C.; Emoke, P.; Popescu, A.; Radu, M.D.; Zglimbea, L. Evaluation of the antioxidant, cytotoxic and antitumoral activities of a polyphenolic extract of Robinia Pseudoacacia L. Flowers. J. Sci. Arts 2021, 21, 547–556. [Google Scholar] [CrossRef]
- Stankov, S.; Fidan, H.; Ivanova, T.; Stoyanova, A.; Damyanova, S.; Desyk, M. Chemical composition and application of flowers of false acacia (Robinia pseudoacacia L.). Ukr. Food J. 2018, 7, 577–588. [Google Scholar] [CrossRef]
- Liang, Q.; Cui, J.; Li, H.; Liu, J.; Zhao, G. Florets of sunflower (Helianthus annuus L.): Potential new sources of dietary fiber and phenolic acids. J. Agric. Food Chem. 2013, 61, 3435–3442. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, J.; Li, J.; Liu, H.; Dong, N.; Geng, Y.Y.; Lu, Y.; Wang, Y. Phenolic composition and nutritional attributes of diaphragma juglandis fructus and shell of walnut (Juglans regia L.). Food Sci. Biotechnol. 2020, 29, 187–196. [Google Scholar] [CrossRef]
- Borovkova, V.S.; Malyar, Y.N.; Sudakova, I.G.; Chudina, A.I.; Zimonin, D.V.; Skripnikov, A.M.; Miroshnikova, A.V.; Ionin, V.A.; Kazachenko, A.S.; Sychev, V.V.; et al. Composition and structure of aspen (Populus tremula) hemicelluloses obtained by oxidative delignification. Polymers 2022, 14, 4521. [Google Scholar] [CrossRef] [PubMed]
- Borovkova, V.S.; Malyar, Y.N.; Sudakova, I.G.; Chudina, A.I.; Skripnikov, A.M.; Fetisova, O.Y.; Kazachenko, A.S.; Miroshnikova, A.V.; Zimonin, D.V.; Ionin, V.A.; et al. Molecular characteristics and antioxidant activity of spruce (Picea abies) hemicelluloses isolated by catalytic oxidative delignification. Molecules 2022, 27, 266. [Google Scholar] [CrossRef] [PubMed]
- Kazachenko, A.S.; Vasilieva, N.Y.; Borovkova, V.S.; Fetisova, O.Y.; Issaoui, N.; Malyar, Y.N.; Elsuf’ev, E.V.; Karacharov, A.A.; Skripnikov, A.M.; Miroshnikova, A.V.; et al. Food xanthan polysaccharide sulfation process with sulfamic acid. Foods 2021, 10, 2571. [Google Scholar] [CrossRef] [PubMed]
- Bader Ul Ain, H.; Saeed, F.; Khan, M.A.; Niaz, B.; Rohi, M.; Nasir, M.A.; Tufail, T.; Anbreen, F.; Anjum, F.M. Modification of barley dietary fiber through thermal treatments. Food Sci. Nutr. 2019, 7, 1816–1820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bai, X.; Zhang, Z. Extrusion process improves the functionality of soluble dietary fiber in oat bran. J. Cereal Sci. 2011, 54, 98–103. [Google Scholar] [CrossRef]
- Chawla, R.; Patil, G.R. Soluble dietary fiber. Compr. Rev. Food Sci. Food Saf. 2010, 9, 178–196. [Google Scholar] [CrossRef]
- Karak, T.; Kutu, F.R.; Nath, J.R.; Sonar, I.; Paul, R.K.; Boruah, R.K.; Sanyal, S.; Sabhapondit, S.; Dutta, A.K. Micronutrients (B, Co, Cu, Fe, Mn, Mo, and Zn) content in made tea (Camellia sinensis L.) and tea infusion with health prospect: A critical review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2996–3034. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Ahmad, A.; Khalid, N.; David, A.; Sandhu, M.A.; Randhawa, M.A.; Suleria, H.A. A question mark on iron deficiency in 185 million people of Pakistan: Its outcomes and prevention. Crit. Rev. Food Sci. Nutr. 2014, 54, 1617–1635. [Google Scholar] [CrossRef]
- Martín-Domingo, M.C.; Pla, A.; Hernández, A.F.; Olmedo, P.; Navas-Acien, A.; Lozano-Paniagua, D.; Gil, F. Determination of metalloid, metallic and mineral elements in herbal teas. Risk assessment for the consumers. J. Food Compos. Anal. 2017, 60, 81–89. [Google Scholar] [CrossRef]
- Achari, G.A.; Kowshik, M. Recent Developments on nanotechnology in agriculture: Plant mineral nutrition, health, and interactions with soil microflora. J. Agric. Food Chem. 2018, 66, 8647–8661. [Google Scholar] [CrossRef]
- Zhu, S.; Jiao, W.; Xu, Y.; Hou, L.; Li, H.; Shao, J.; Zhang, X.; Wang, R.; Kong, D. Palmitic acid inhibits prostate cancer cell proliferation and metastasis by suppressing the PI3K/Akt pathway. Life Sci. 2021, 286, 120046. [Google Scholar] [CrossRef] [PubMed]
- van Rooijen, M.A.; Mensink, R.P. Palmitic acid versus stearic acid: Effects of interesterification and intakes on cardiometabolic risk markers—A systematic review. Nutrients 2020, 12, 615. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Khan, H.; Xiao, J.; Cheang, W.S. Effects of arachidonic acid metabolites on cardiovascular health and disease. Int. J. Mol. Sci. 2021, 22, 12029. [Google Scholar] [CrossRef] [PubMed]
- Shramko, V.S.; Polonskaya, Y.V.; Kashtanova, E.V.; Stakhneva, E.M.; Ragino, Y.I. The short overview on the relevance of fatty acids for human cardiovascular disorders. Biomolecules 2020, 10, 1127. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, P.; Capparelli, R.; Iannelli, A.; Iannelli, D. Role of branched-chain amino acid metabolism in type 2 diabetes, obesity, cardiovascular disease and non-alcoholic fatty liver disease. Int. J. Mol. Sci. 2022, 23, 4325. [Google Scholar] [CrossRef] [PubMed]
- Patriarca, E.J.; Cermola, F.; D’Aniello, C.; Fico, A.; Guardiola, O.; De Cesare, D.; Minchiotti, G. The multifaceted roles of proline in cell behavior. Front. Cell Dev. Biol. 2021, 9, 728576. [Google Scholar] [CrossRef]
- Jiang, N.; Dillon, F.M.; Silva, A.; Gomez-Cano, L.; Grotewold, E. Rhamnose in plants—From biosynthesis to diverse functions. Plant Sci. 2021, 302, 110687. [Google Scholar] [CrossRef]
- Monrad, R.N.; Eklof, J.; Krogh, K.; Biely, P. Glucuronoyl esterases: Diversity, properties and biotechnological potential. A review. Crit. Rev. Biotechnol. 2018, 38, 1121–1136. [Google Scholar] [CrossRef]
- Li, Y.; Pan, H.; Liu, J.X.; Li, T.; Liu, S.; Shi, W.; Sun, C.; Fan, M.; Xue, L.; Wang, Y.; et al. l-Arabinose inhibits colitis by modulating gut microbiota in mice. J. Agric. Food Chem. 2019, 67, 13299–13306. [Google Scholar] [CrossRef]
- Li, P.; Sun, H.; Chen, Z.; Li, Y.; Zhu, T. Construction of efficient xylose utilizing Pichia pastoris for industrial enzyme production. Microb. Cell Fact. 2015, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gong, Y.; Li, J.; Fan, L. In vitro inhibitory effects of polyphenols from Tartary buckwheat on xanthine oxidase: Identification, inhibitory activity, and action mechanism. Food Chem. 2022, 379, 132100. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Bai, Y.; Zhao, Z.; Wang, X.; Fang, J.; Huang, L.; Zeng, M.; Zhang, Q.; Zhang, Y.; Zheng, X. Local and traditional uses, phytochemistry, and pharmacology of Sophora japonica L.: A review. J. Ethnopharmacol. 2016, 187, 160–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cui, Q.; Yin, L.-J.; Li, Y.; Gao, M.-Z.; Meng, Y.; Li, J.; Zhang, S.-D.; Wang, W. Negative pressure cavitation based ultrasound-assisted extraction of main flavonoids from Flos Sophorae Immaturus and evaluation of its extraction kinetics. Sep. Purif. Technol. 2020, 244, 115805. [Google Scholar] [CrossRef]
- Wang, G.; Cui, Q.; Yin, L.J.; Zheng, X.; Gao, M.Z.; Meng, Y.; Wang, W. Efficient extraction of flavonoids from Flos Sophorae Immaturus by tailored and sustainable deep eutectic solvent as green extraction media. J. Pharm. Biomed. Anal. 2019, 170, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Khatri, D.; Chhetri, S.B.B. Reducing sugar, total phenolic content, and antioxidant potential of nepalese plants. Biomed. Res. Int. 2020, 2020, 7296859. [Google Scholar] [CrossRef]
- Li, J.; Gong, Y.; Li, J.; Fan, L. In vitro xanthine oxidase inhibitory properties of Flos Sophorae Immaturus and potential mechanisms. Food Biosci. 2022, 47, 101711. [Google Scholar] [CrossRef]
- Chen, L.; Pu, Y.; Xu, Y.; He, X.; Cao, J.; Ma, Y.; Jiang, W. Anti-diabetic and anti-obesity: Efficacy evaluation and exploitation of polyphenols in fruits and vegetables. Food Res. Int. 2022, 157, 111202. [Google Scholar] [CrossRef]
- Wan, F.; Feng, C.; Luo, K.; Cui, W.; Xia, Z.; Cheng, A. Effect of steam explosion on phenolics and antioxidant activity in plants: A review. Trends Food Sci. Tech. 2022, 124, 13–24. [Google Scholar] [CrossRef]
- Potortì, A.G.; Di Bella, G.; Mottese, A.F.; Bua, G.D.; Fede, M.R.; Sabatino, G.; Salvo, A.; Somma, R.; Dugo, G.; Lo Turco, V. Traceability of Protected Geographical Indication (PGI) Interdonato lemon pulps by chemometric analysis of the mineral composition. J. Food Compos. Anal. 2018, 69, 122–128. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, C.H.; Azmi, N.A.; Liew, R.K.; Hamdan, N.; Wong, S.L.; Ong, P.Y. Amino acid determination by HPLC combined with multivariate approach for geographical classification of Malaysian Edible Bird’s Nest. J. Food Compos. Anal. 2022, 107, 104399. [Google Scholar] [CrossRef]
- Wen, M.; Cui, Y.; Dong, C.X.; Zhang, L. Quantitative changes in monosaccharides of Keemun black tea and qualitative analysis of theaflavins-glucose adducts during processing. Food Res. Int. 2021, 148, 110588. [Google Scholar] [CrossRef] [PubMed]
- Martin-Garcia, B.; Pasini, F.; Verardo, V.; Gomez-Caravaca, A.M.; Marconi, E.; Caboni, M.F. Distribution of free and bound phenolic compounds in buckwheat milling fractions. Foods 2019, 8, 670. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).