2.1. Characterization of PCC Particles
To decrease the hydrophilic character of the chitosan-based coating, modified calcium carbonate particles with stearic acid were prepared before incorporation into the chitosan film-forming solution.
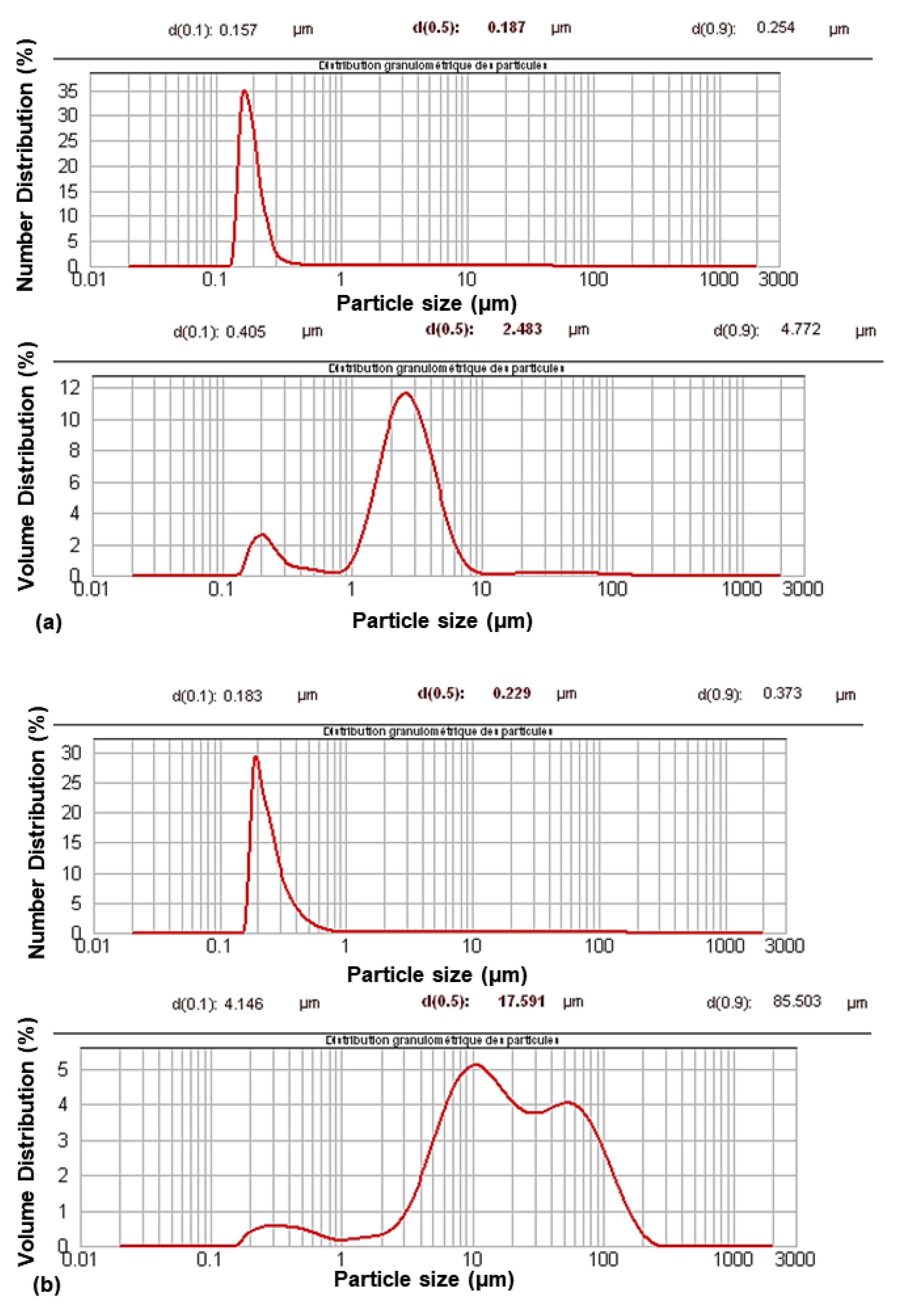
Their characterization using granulometry analysis (
Figure 1) showed a change in their size distribution after modification. The granulometric volume curves showed a multimodal distribution with an increase in particle size. The aggregation phenomenon occurred during the modification process. However, aggregation was limited, as shown by the monomodal distribution in the granulometric number curves.
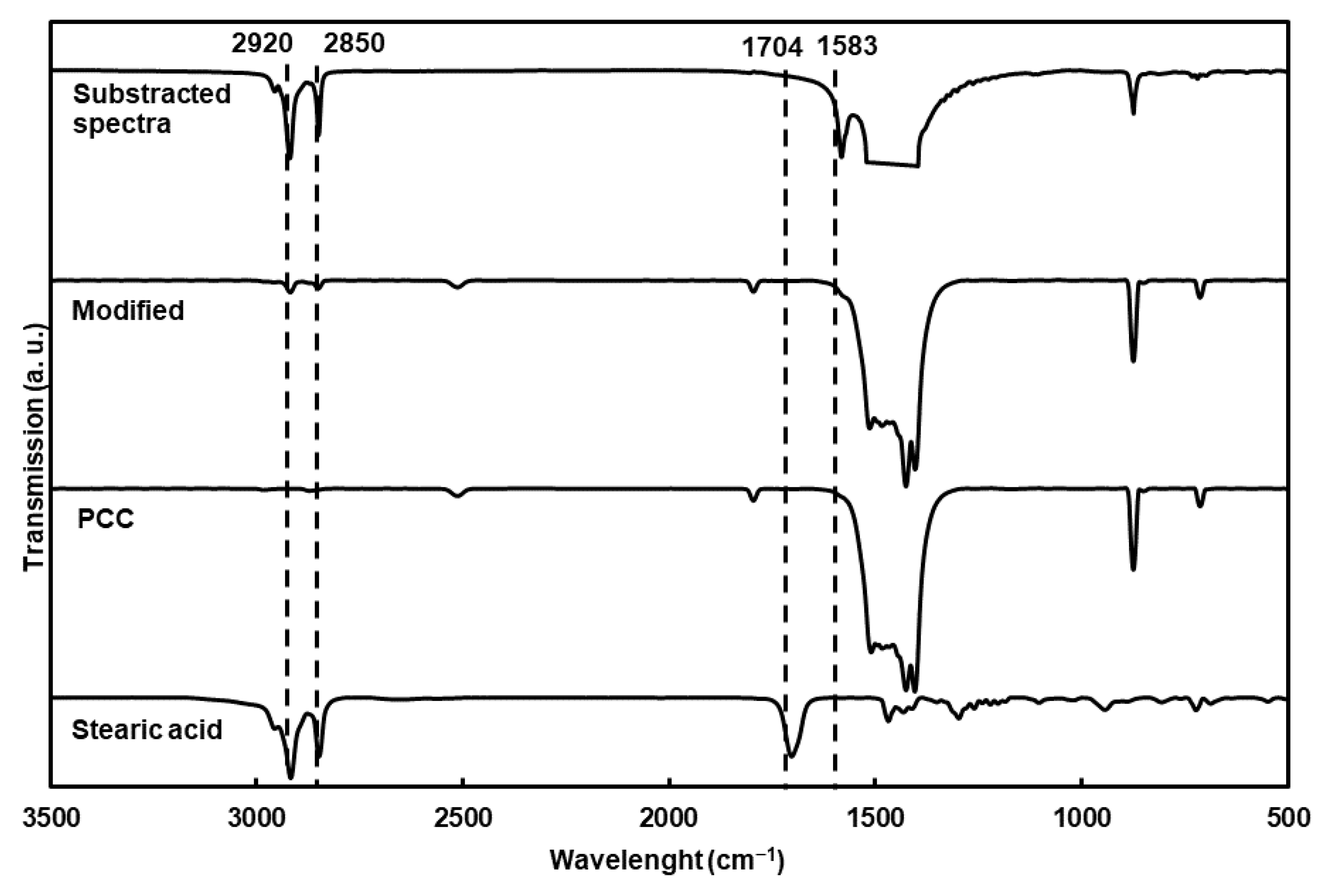
The chemical characterization of the particles using FTIR (
Figure 2) showed new bands compared to the nonmodified particles, corresponding to the asymmetric and symmetric elongation of the C-H bond (2920 and 2850 cm
−1). The substracted spectra clearly showed C-O stretching vibrations from the carboxylate group (1583 cm
−1) of the fatty acid. Moreover, stearic acid was completely adsorbed at the surface of the PCC on the carboxylate structure, as proven by the absence of the carbonyl band at 1704 cm
−1 [
12,
25,
26,
27].
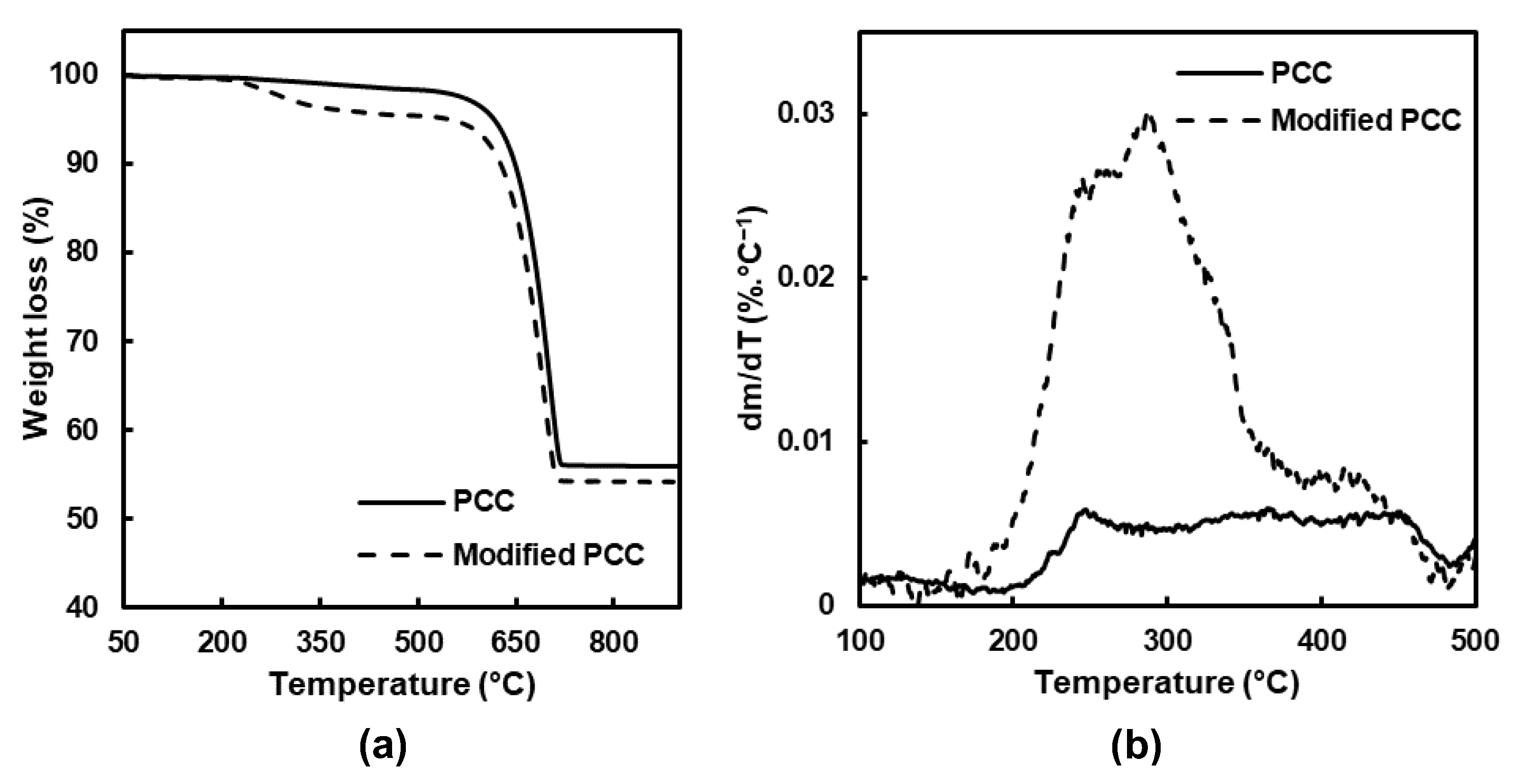
The thermal analysis (TGA) of the PCC particles was then carried out to complete the FTIR analysis and determine how stearic acid was deposited on the mineral surface.
The results before and after modification are showed
Figure 3, and three main weight losses were obtained. The first one between 25 and 150 °C corresponded to water loss. For the temperature range between 180 and 480 °C, the degradation of the adsorbed stearic acid and hydroxyl groups at the surface of the PCC was observed. At a temperature higher than 480 °C, the weight loss was attributed to calcium carbonate decarboxylation, leading to calcium oxide [
28].
The main difference when the PCC particles were modified was observed between 180 and 480 °C, and the mass loss was divided into two temperature ranges (
Table 1). Concerning the PCC particles before modification, a small degradation was observed related to bounded water and the hydroxyl groups’ removal [
29]. For the modified PCC, the main weight loss was observed between 180 and 380 °C, and it was attributed to the degradation of stearic acid which was physically linked [
30,
31]. For the second temperature range, the maximum of the degradation occurred around 420 °C and was assigned to monolayer decomposition, composed of stearic acid chemically bonded on the form of the stearate ion [
29,
30,
31].
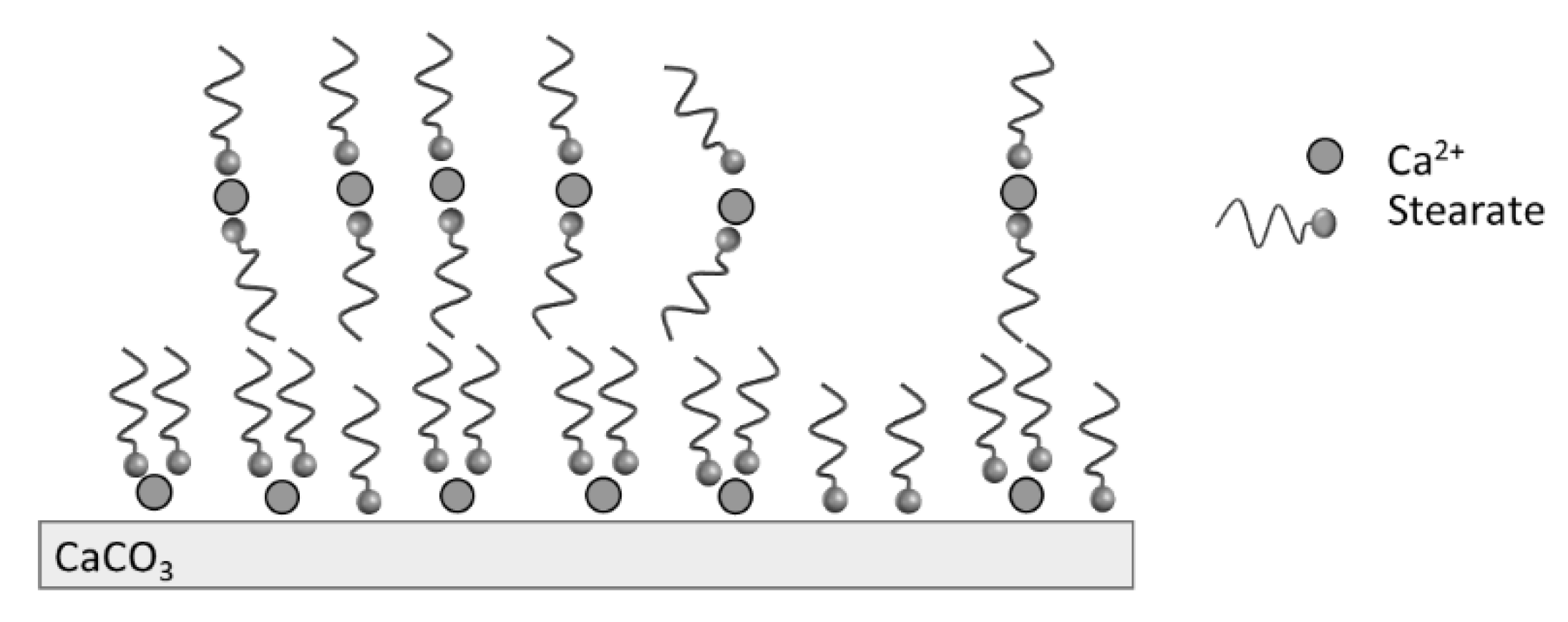
The TGA analysis showed that the modification of PCC was led mainly by the stearic acid physically bonded at the calcite surface, as illustrated in
Figure 4. The organization of molecules at the calcite surface is dependent on the stearic acid concentration when water is used as the solvent. For high stearic acid concentration up to the critical micelle concentration of stearic acid (7 × 10
−4 mol.L
−1 at 60 °C), there is a formation of micelles when the pH is basic. The presence of calcium ions from calcite in the medium leads to the decomposition of micelles and the precipitation of calcium stearate on the particles’ surface. Moreover, hydrophobic interactions between the organic tails of stearate may lead to the presence of multilayers [
31].
The investigation of the hydrophobicity of the particles before and after modification was also studied using water contact angle (WCA) measurements. Without any modification, the direct absorption of water drops was observed. In contrast, a WCA of 121 ± 2° was measured after modification, indicating an increase in hydrophobicity.
2.2. Characterization of Chitosan-Based Films Loaded with PCC Particles
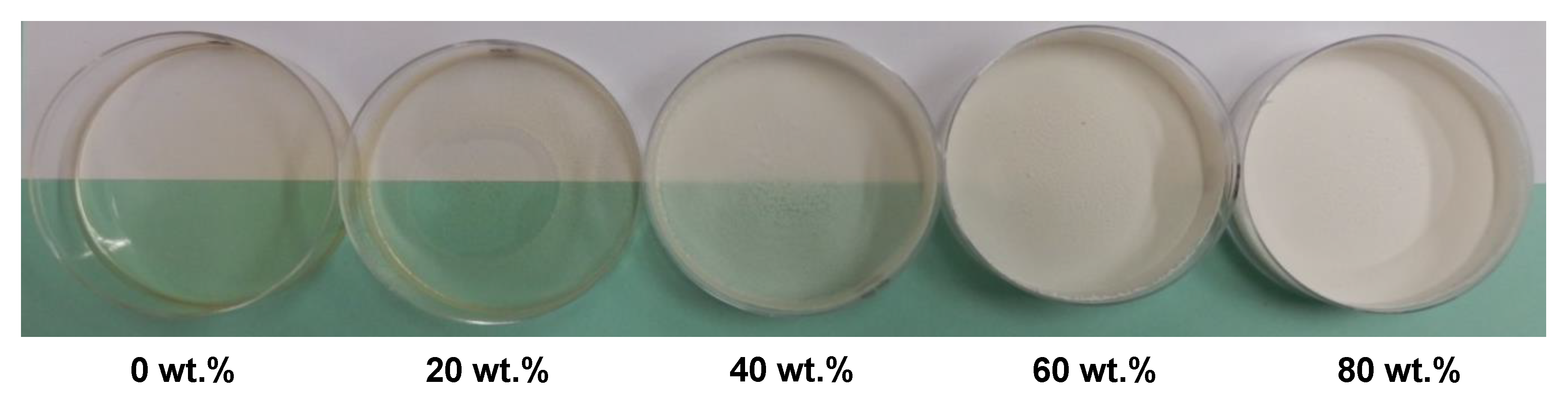
To study the chitosan-based coating, before deposition on paper material, modified PCCs were introduced in different amounts into a chitosan film-forming solution to prepare the composite films after film casting (
Figure 5). The visual aspect shows that the higher modified PCC content resulted in the higher opacity, whiteness and surface rugosity of the composite films. In addition, up to 40 wt.%, the films lost their plasticity and became brittle.
The water contact angle (WCA) measurements on the composite films showed a higher surface hydrophobicity, except for 80 wt.% modified PCC, in comparison with the pure chitosan film (
Table 2). The higher the WCA, the higher the surface hydrophobicity. However, with 80 wt.% of modified PCC, the water drop was directly absorbed. In this case, the roughness at the microscale was not sufficient to keep enough air between the film and the water drop. A change in the wetting regime from the Cassie–Baxter to Wenzel may happen [
32]. In the Wenzel regime, the surface of the solid is completely wet, leading to higher hydrophilicity for the hydrophilic surface [
33]. For the lower amount of modified PCC, after a few seconds, the film in contact with water swelled. As a consequence, it is assumed that the modified PCC decreased the hydrophilic behavior of the chitosan composite films, but the films could not be turned hydrophobic.
2.3. Characterization of Coated Paper
Taking into account the previous observations, paper was coated with selected formulations containing 20 and 50 wt.% of modified PCC.
Table 3 summarizes the thickness and grammage of the coatings and paper. As already observed in the literature, the coating showed no impact on the paper thickness due to the lower coated grammage and coating penetration in the paper [
17].
The coating penetration was investigated using FTIR-ATR characterization. As expected, the infrared spectra of the coated paper on both sides showed cellulosic absorption bands (
Figure 6) at 1630 cm
−1 (O-H bending vibration from absorbed water), 1420 cm
−1 (C-H deformation), 1370 cm
−1 (C-H bending), 1330 cm
−1 (CH
2 wagging), 1155 cm
−1 (asymmetric deformation of C-O-C), 1110 cm
−1 (C
2-O wagging) and 895 cm
−1 (C-H deformation of anomeric carbon) [
34,
35,
36].
On the coated side, the characteristic bands of chitosan were found at 1640 cm
−1 (C=O stretching of amide I) and 1583 cm
−1 (
N-H bond), in addition to the cellulose ones. Moreover, the intensity of the cellulose band at 1110 cm
−1 decreased after the coating, suggesting a partial coverage of the cellulose fibers by chitosan. The band at 873 cm
−1 corresponding to CaCO
3 [
37] has a higher intensity of formulation containing more calcium carbonate particles.
Considering the FTIR spectra of the coatings containing 50 wt.% of mineral particles, the intensity of the characteristic bands of chitosan and CaCO3 was more pronounced, suggesting a better retention of the coatings on the paper surface due to the incorporation of the particles.
For all materials, the noncoated side showed the same FTIR spectra without new absorption bands or band-intensity modification, which indicated that the coatings did not cross through the paper.
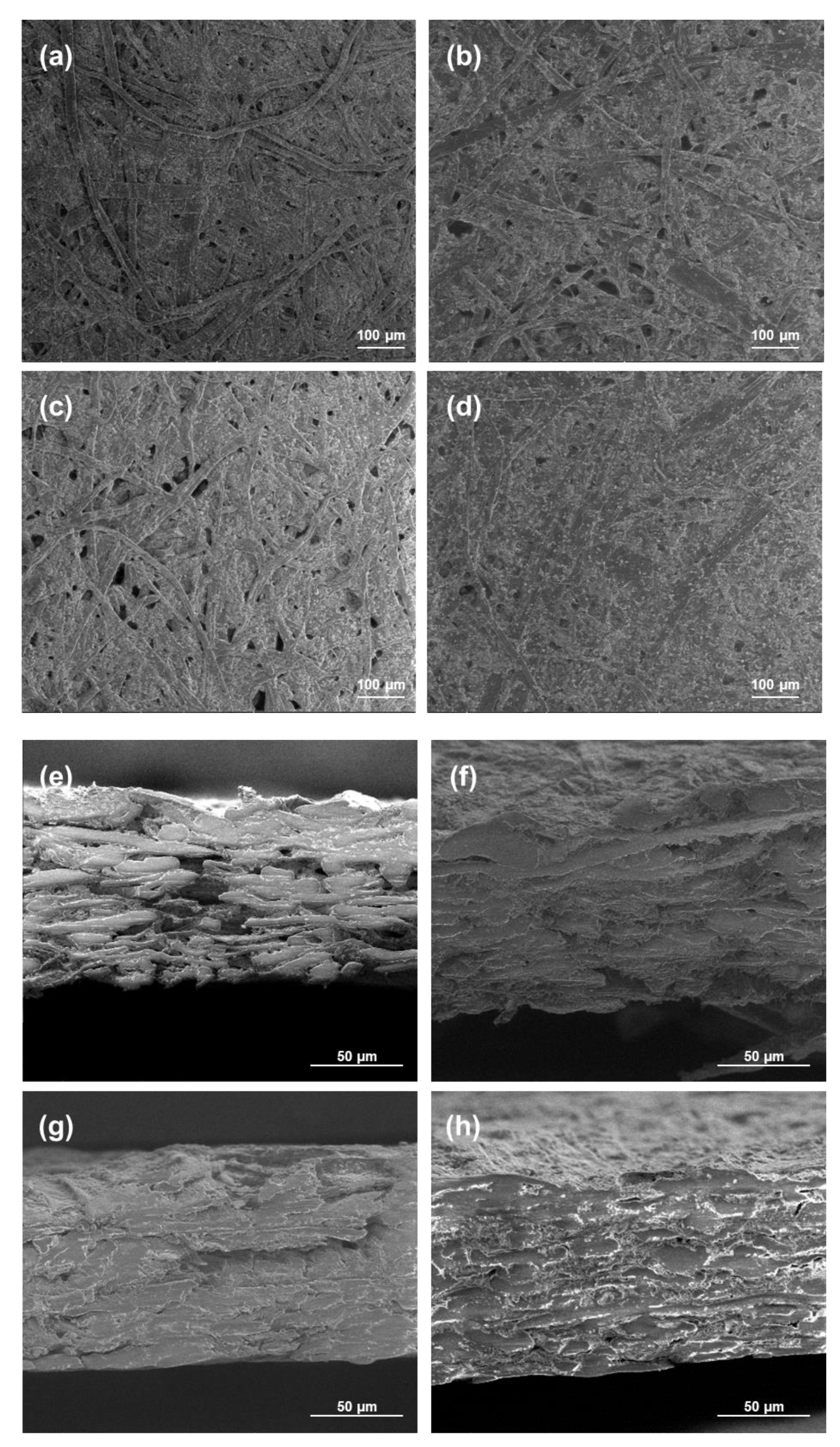
The morphology of the coated papers was studied using SEM analyses, and the views are presented in
Figure 7. The paper with only the chitosan coating or 20 wt.% of modified PCC showed a lot of fibers and pores that were not completely covered by the coating in opposition to the papers coated with more modified PCC. These results were in accordance with FTIR results showing better retention of coatings at the surface of the paper when the amount of modified PCC was at a sufficient concentration in the film-forming solution. The cross-section views show the penetration of chitosan in the material, leading to a more compact bulk structure.
2.4. Liquid Water Resistance
The investigation of liquid water resistance was first carried out using the measurements of WCA, surface wettability, wetting time and Cobb
60 (
Table 3). The WCA provided information about the surface hydrophobicity. The high WCA (110°) exhibited by the uncoated paper is due to the sizing used for its manufacture. After a coating with pure chitosan, the contact angle decreased to 82°, leading to the lowest WCA. As expected, chitosan provided the hydrophilicity of the paper. The introduction of modified PCC in the formulation reduced the hydrophilicity due to the chitosan developing a higher surface roughness. The highest WCA was obtained with the formulation containing 50 wt.% of modified PCC.
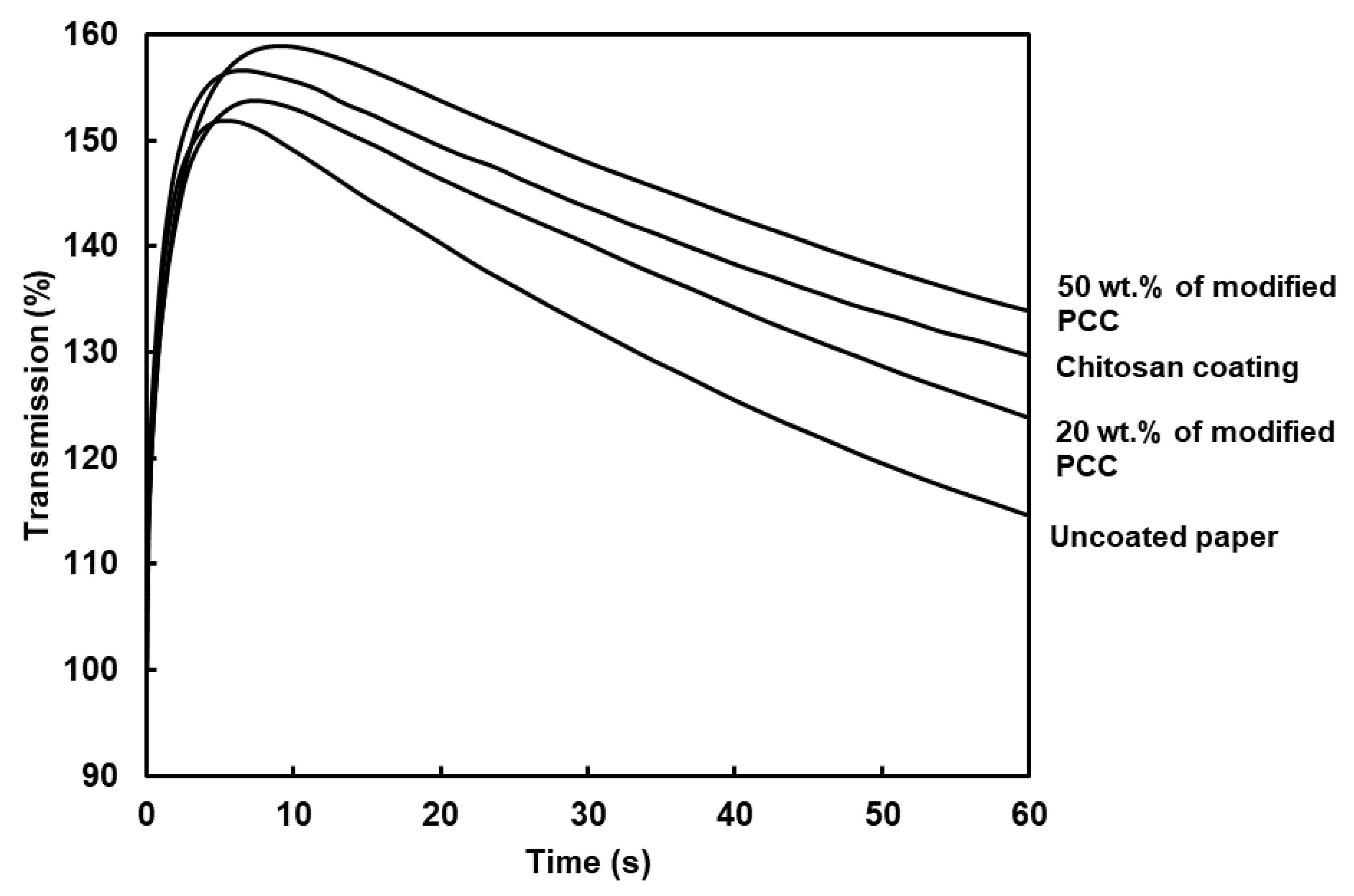
The PDA curves inform us about how the liquid water wets the paper. The surface wettability (W), wetting time and water penetration profile are shown in
Table 3 and
Figure 8, respectively. The curves showed two regions. The first one corresponded to the paper surface coming into contact with liquid water. The paper surface became wet when air was evacuated (the maximum of the curve). The second region was related to the rate of water penetration in the bulk material (the curve slope). The PDA curves showed similar behavior between coatings and allowed us to calculate the surface wettability and wetting time. The surface wettability (W) was assessed using the measure of the area between the ordinate axis and PDA curve at the wetting time. The higher the value, the lower the surface wettability [
38]. The highest value was found for the coating with 50 wt.% of modified PCC. The results show that surface wettability was not only dependent on the surface hydrophobicity provided using the WCA measurements. The dynamic of water penetration was related to the evacuation of the air layer at the surface of the paper. The combination of higher rugosity and higher WCA led to a lower surface wettability.
The wetting time was correlated to the surface roughness of coatings, i.e., the higher roughness, the higher the wetting time. Coating with 50 wt.% of modified PCC increased the wetting time of the paper by 172%. Considering previous characterizations, adding modified mineral particles with chitosan improved the coatings’ retention at the surface of paper and allowed the counter-balance with the chitosan hydrophilicity.
The Cobb60 value corresponds to the amount of liquid water absorbed by paper after 60 s of contact. The smaller the value, the higher the liquid water barrier properties. The coatings reduced the water absorptiveness (15%). However, the reported values were similar for all coatings, including the chitosan coating. The improvement in the surface hydrophobicity by the coating was not sufficient to drastically decrease the liquid water absorption. The best liquid water barrier was obtained with the 50 wt.% coating formulation. Clearly, the coatings with minerals had more impact on the surface properties than the bulk properties.
Water resistance is also illustrated by the water vapor permeability of materials. The results of the WVTR for the coated papers are shown in
Table 4. The chitosan coating led to a reduction in the water vapor transmission rate (WVTR) by 18% in our conditions, which was higher than the coated paper with particles. As previously observed using the SEM micrographs, the modified PCC maintained coatings at the surface of the paper. In that case, the lowest chitosan penetration led to a decrease in the efficiency of the coating to fill preferential water ways. As a result, the water vapor permeability appeared to be more highly affected by the bulk properties than the surface properties.
According to the literature, the influence of chitosan on the water barrier properties of coated paper is still under debate. Some authors observed that chitosan did not modify the WVTR [
17,
39], which is in contradiction with Vartiainen’s results and other publications [
1,
40,
41], which showed a significant reduction in WVTR [
22]. This could be due to the experimental conditions, such as the relative humidity adjusted for the tests, but also to the chitosan properties (molar mass and deacetylation degree).
Finally, as previously shown on the chitosan films or coatings, NMR relaxometry is a useful method to investigate the water interaction with a material [
1,
42]. The spin–spin relaxation time (
T2) is related to the fast interactions between water molecules and their macromolecular environment showing two types of populations called free or bounded water in the cellulose fiber network (
Table 5). The investigation of water–cellulose interactions provides information about paper hydrophobicity.
According to the literature, the lowest relaxation time,
T2a, for uncoated and coated papers was assigned to the first layers of bounded water on the polymer. Relaxation time,
T2b, is related to water-bounded molecules trapped in cell lumen [
43]. The
T2b values were similar between all paper samples, which meant that the surface of the lumen fiber is not modified by chitosan treatment.
The relaxation times associated with the chitosan film showed a main population at
T2a, representing 95% of the signal intensity and was extremely tight to the polymer backbone, as discussed in [
42]. In addition, the chitosan film was very hydrophilic and contained 22.5% of water. Despite low coated grammage, the paper coated with chitosan increased its initial water content from 4.3 to around 5.0% for coatings with modified PCC compared with 7.2% for the only chitosan coating. The increase in the water content was related to the coating penetration in the paper and the surface fiber coverage by chitosan. For all materials,
T2a was lower after coating, showing a reduction in the water molecule mobility, meaning an increase in fiber hydrophilicity [
1]. This behavior could be caused by strong interactions between the cellulose fibers and chitosan.
The relaxometry data led to a better comprehension of the previous water resistance results. Generally, to control the paper water absorption, two ways were chosen: cellulose hydrophobic modification and the reduction of water’s physical access to the cellulose fibers. In the case of chitosan-based coatings, the limitation of water access to cellulose was the main factor. Water absorption was mainly controlled by chitosan hydrogel formation in contact with water, despite the increasing hydrophilicity of the fibers after the chitosan coating.
2.6. Antifungal Activity
The antifungal activity of the chitosan film-forming solutions containing modified PCC was evaluated against
C. globosum by measuring the radial growth every day for 14 days. As displayed in
Table 7, three series were identified as the function of the lag phase for the chitosan-coating formulation. The first one corresponds to the control and the film-forming solution containing 50 wt.% of modified PCC, and no antifungal properties were observed. In the second series, an increase in the lag phase was observed for the chitosan film-forming solution. The incorporation of modified PCC into the film-forming solution led to an increase in pH from 4.2 to close to 6.2. At this pH, there was around 67% of the ammonium groups compared with 100% at the initial pH (pKa of chitosan is close to 6.5 [
45]). As a consequence, an investigation into the impact of pH on the fungal radial growth was carried out on the chitosan film-forming solution without modified PCC, but no significant difference was observed, as shown in
Table 7. The nonbioactivity of the formulation with 50 wt.% of modified PCC can be explained by a large amount of chitosan aggregation on the surface of the PCC particles resulting in a reduction in chitosan availability. Finally, the last series, with the longest lag phase, corresponds to the formulation with 20 wt.% of modified PCC.
The bioactivity of the modified PCC was also investigated using HPMC in place of chitosan to evaluate the impact of the oxygen barrier on fungal growth. Indeed, HMPC, a cellulose derivative with film-forming properties, showed similar oxygen barrier properties to chitosan but without any bioactive properties [
46]. The activation effect was observed with HMPC and attributed to the cellulose derivative metabolization by microbial strains, used as a carbon source (
Table 7). However, the growth rate was not affected.
Considering these results and the literature [
47], the association of chitosan with bicarbonate ions, from partial particle solubilization in acidic medium, may have a synergistic effect on the fungal lag phase. Chitosan is known to affect mycelial growth and the sporulation of fungi by notably acting on membrane permeability [
48]. Carbonate salts caused a reduction in the cellular turgor pressure, resulting in hyphae collapse and sporulation inhibition [
47]. Concerning HPMC solutions, the bicarbonate ions could present difficulties when going through the cellular membrane, reducing their bioactivity. Hence, their presence in the chitosan solution improved bioactivity due to the membrane permeability reduction caused by chitosan.
The antifungal properties of the coated paper were then determined (
Table 8). A comparison with the control paper showed no delay on the lag phase and sporulation. The growth rate was mainly affected by the coatings, probably caused by a lack of the fibers’ access to the spores and nutrient medium. The chitosan coating and 20 wt.% chitosan-modified PCC coating showed no efficient antifungal effect which is attributed to the low dry matter coated on the paper. The 50 wt.% chitosan-modified PCC formulation exhibited a reduction in the radial growth rate, which could be due to the highest water surface resistance, limiting the access of the spores to the fibers and nutrient medium.
To develop higher bioactivity with the 20 wt.% chitosan-modified PCC formulation, a second coated layer was applied on the paper. No delay on the lag phase was observed, but an inhibition by 44% was obtained at day 3, and the growth rate was lower. These results showed a cumulative effect between the reduction in the surface hydrophilicity and the antifungal behavior developed by the coatings.