Hydroethanolic Extract of Urtica dioica L. (Stinging Nettle) Leaves as Disaccharidase Inhibitor and Glucose Transport in Caco-2 Hinderer
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of Phenolics Using HPLC-DAD-ESI/MS2
2.2. α-Glucosidase Activity
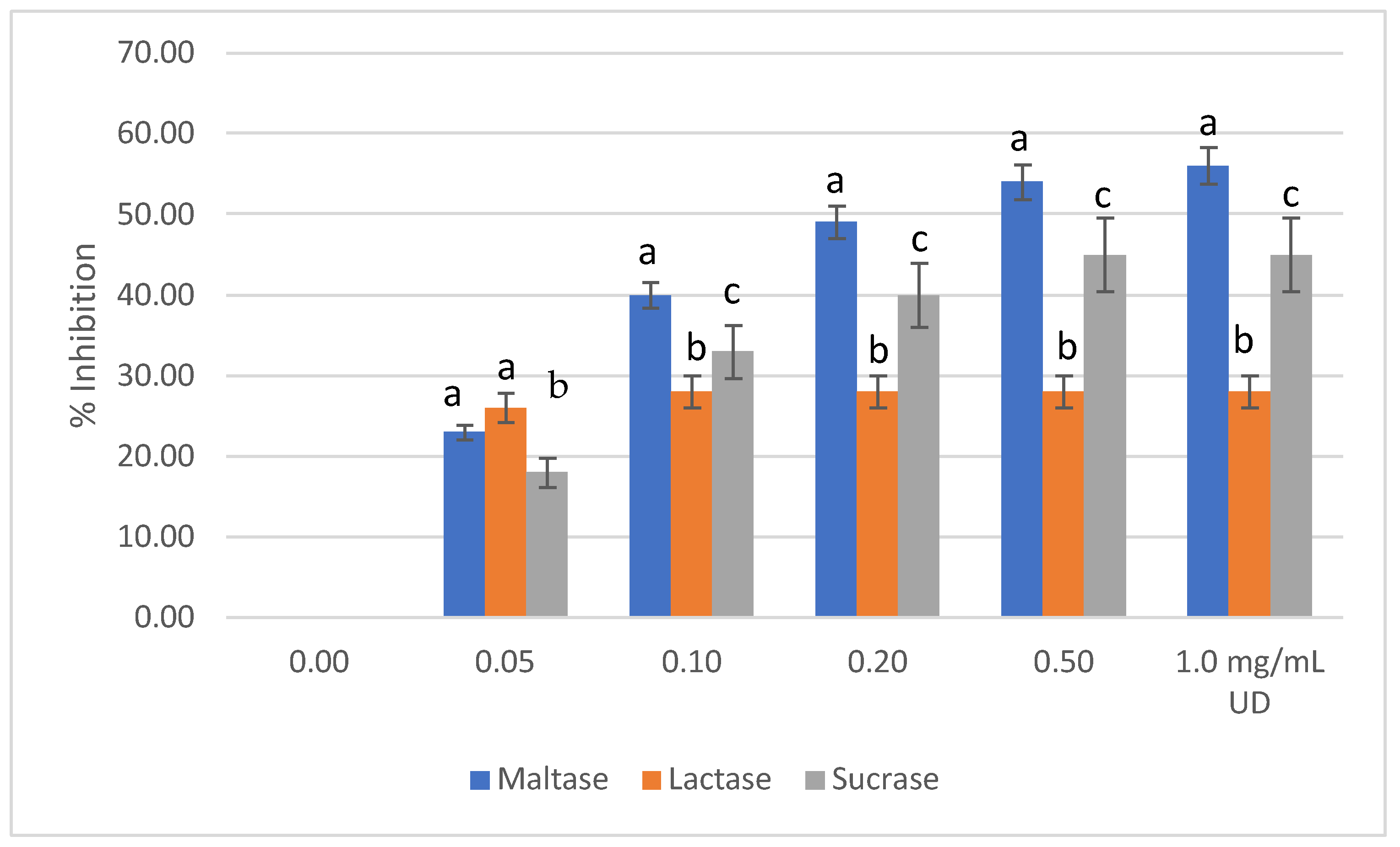
2.3. Disaccharidase Activity
2.4. Glucose Transport
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Preparation of the Extracts
3.4. Phenolics Analysis by HPLC-DAD-ESI/MS2
3.5. Cell Culture Maintenance
3.6. Glucose-Oxidase Assay
3.7. Measurement of α-Glucosidase Activity
3.8. Disaccharidase Activity
3.9. Glucose Transport
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dardano, A.; Penno, G.; Del Parto, S.; Miccoli, R. Optimal therapy of type 2 diabetes: A controversial challenge. Aging 2014, 6, 187–206. [Google Scholar] [CrossRef]
- Reinehr, T. Type 2 diabetes mellitus in children and adolescents. World J. Diabetes 2013, 4, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Domola, M.S.; Vu, V.; Robson-Doucette, C.A.; Sweeney, G.; Wheeler, M.B. Insulin mimetics in Urtica dioica: Structural and computational analyses of Urtica dioica extracts. Phytother. Res. 2009, 24, S175–S182. [Google Scholar] [CrossRef] [PubMed]
- Jan, K.N.; Zarafshan, K.; Singh, S. Stinging nettle (Urtica dioica L.): A reservoir of nutrition and bioactive components with great functional potential. J. Food Meas. Charact. 2016, 11, 423–433. [Google Scholar] [CrossRef]
- Shonte, T.; De Kock, H. Descriptive sensory evaluation of cooked stinging nettle (Urtica dioica L.) leaves and leaf infusions: Effect of using fresh or oven-dried leaves. S. Afr. J. Bot. 2017, 110, 167–176. [Google Scholar] [CrossRef]
- Rutto, L.K.; Xu, Y.; Ramirez, E.; Brandt, M. Mineral Properties and Dietary Value of Raw and Processed Stinging Nettle (Urtica dioica L.). Int. J. Food Sci. 2013, 2013, 857120. [Google Scholar] [CrossRef]
- Dhouibi, R.; Affes, H.; Ben Salem, M.; Hammami, S.; Sahnoun, Z.; Zeghal, K.M.; Ksouda, K. Screening of pharmacological uses of Urtica dioica and others benefits. Prog. Biophys. Mol. Biol. 2020, 150, 67–77. [Google Scholar] [CrossRef]
- Vogl, S.; Picker, P.; Mihaly-Bison, J.; Fakhrudin, N.; Atanasov, A.G.; Heiss, E.H.; Wawrosch, C.; Reznicek, G.; Dirsch, V.M.; Saukel, J.; et al. Ethnopharmacological in vitro studies on Austria’s folk medicine—An unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs. J. Ethnopharmacol. 2013, 149, 750–771. [Google Scholar] [CrossRef]
- Mittman, P. Randomized, Double-Blind Study of Freeze-Dried Urtica dioica in the Treatment of Allergic Rhinitis. Planta Med. 1990, 56, 44–47. [Google Scholar] [CrossRef]
- Behzadi, A.A.; Kalalian-Moghaddam, H.; Ahmadi, A.H. Effects of Urtica dioica supplementation on blood lipids, hepatic enzymes and nitric oxide levels in type 2 diabetic patients: A double blind, randomized clinical trial. Avicenna J. Phytomed. 2016, 6, 686–697. [Google Scholar]
- Kellett, G.L.; Brot-Laroche, E. Apical GLUT2: A Major Pathway of Intestinal Sugar Absorption. Diabetes 2005, 54, 3056–3062. [Google Scholar] [CrossRef] [PubMed]
- Drozdowski, L. Intestinal sugar transport. World J. Gastroenterol. 2006, 12, 1657–1670. [Google Scholar] [CrossRef]
- Rosak, C.; Mertes, G. Critical evaluation of the role of acarbose in the treatment of diabetes: Patient considerations. Diabetes Metab. Syndr. Obes. Targets Ther. 2012, 5, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, J.-L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. Lancet 2002, 359, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Narwal, S.; Kumar, V.; Parakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Kianbakht, S.; Khalighi-Sigaroodi, F.; Dabaghian, F.H. Improved Glycemic Control in Patients with Advanced Type 2 Diabetes Mellitus Taking Urtica dioica Leaf Extract: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Clin. Lab. 2013, 59, 1071–1076. [Google Scholar] [CrossRef]
- Ziaei, R.; Foshati, S.; Hadi, A.; Kermani, M.A.H.; Ghavami, A.; Clark, C.C.; Tarrahi, M.J. The effect of nettle (Urtica dioica) supplementation on the glycemic control of patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Phytother. Res. 2019, 34, 282–294. [Google Scholar] [CrossRef]
- El Haouari, M.; Rosado, J.A. Phytochemical, Anti-diabetic and Cardiovascular Properties of Urtica dioica L. (Urticaceae): A Review. Mini-Rev. Med. Chem. 2018, 19, 63–71. [Google Scholar] [CrossRef]
- Bnouham, M.; Merhfour, F.-Z.; Ziyyat, A.; Mekhfi, H.; Aziz, M.; Legssyer, A. Antihyperglycemic activity of the hydroethanolic extract of Urtica dioica. Fitoterapia 2003, 74, 677–681. [Google Scholar] [CrossRef]
- Orčić, D.; Francišković, M.; Bekvalac, K.; Svirčev, E.; Beara, I.; Lesjak, M.; Mimica-Dukić, N. Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection. Food Chem. 2014, 143, 48–53. [Google Scholar] [CrossRef]
- Carvalho, A.R.; Costa, G.; Figueirinha, A.; Liberal, J.; Prior, J.A.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Urtica spp.: Phenolic composition, safety, antioxidant and anti-inflammatory activities. Food Res. Int. 2017, 99, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary Plants with Extraordinary Properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef] [PubMed]
- Pimpley, V.; Patil, S.; Srinivasan, K.; Desai, N.; Murthy, P.S. The chemistry of chlorogenic acid from green coffee and its role in attenuation of obesity and diabetes. Prep. Biochem. Biotechnol. 2020, 50, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Castilho, P. Evaluation of Asteraceae herbal extracts in the management of diabetes and obesity. Contribution of caffeoylquinic acids on the inhibition of digestive enzymes activity and formation of advanced glycation end-products (in vitro). Phytochemistry 2017, 143, 29–35. [Google Scholar] [CrossRef]
- Önal, S.; Timur, S.; Okutucu, B.; Zihnioğlu, F. Inhibition of α-Glucosidase by Hydroethanolic Extracts of Some Potent Antidiabetic Medicinal Herbs. Prep. Biochem. Biotechnol. 2005, 35, 29–36. [Google Scholar] [CrossRef]
- Rahimzadeh, M.; Jahanshahi, S.; Moein, S.; Moein, M. Evaluation of alpha- amylase inhibition by Urtica dioica and Juglans regia extracts. Iran. J. Basic Med. Sci. 2014, 17, 465. [Google Scholar]
- Cheng, M.W.; Chegeni, M.; Kim, K.H.; Zhang, G.; Benmoussa, M.; Quezada-Calvillo, R.; Nichols, B.L.; Hamaker, B.R. Different sucrose-isomaltase response of Caco-2 cells to glucose and maltose suggests dietary maltose sensing. J. Clin. Biochem. Nutr. 2014, 54, 55–60. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, F.; Yang, D.; Ding, K.; Fan, J. The ethanol extract of Eucommia ulmoides Oliv. leaves inhibits disaccharidase and glucose transport in Caco-2 cells. J. Ethnopharmacol. 2015, 163, 99–105. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C.; Liu, F.; Hu, T.; Shen, W.; Li, E.; Liao, S.; Zou, Y. Mulberry leaf polyphenols attenuated postprandial glucose absorption via inhibition of disaccharidases activity and glucose transport in Caco-2 cells. Food Funct. 2020, 11, 1835–1844. [Google Scholar] [CrossRef]
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2012, 57, 48–57. [Google Scholar] [CrossRef]
- Kadan, S.; Saad, B.; Sasson, Y.; Zaid, H. In Vitro Evaluations of Cytotoxicity of Eight Antidiabetic Medicinal Plants and Their Effect on GLUT4 Translocation. Evid. Based Complement. Altern. Med. 2013, 2013, 549345. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yao, L.; He, X.; Wang, L.; Li, M.; Yang, Y.; Wan, C. Hypoglycemic activity and constituents analysis of blueberry (Vaccinium corymbosum) fruit extracts. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ren, C.; Yan, S.; Wang, K.; Hrynets, Y.; Xiang, L.; Xue, X.; Betti, M.; Wu, L. Extract of Unifloral Camellia sinensis L. Pollen Collected by Apis mellifera L. Honeybees Exerted Inhibitory Effects on Glucose Uptake and Transport by Interacting with Glucose Transporters in Human Intestinal Cells. J. Agric. Food Chem. 2021, 69, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Lea, T. Caco-2 Cell Line. In The Impact of Food Bioactives on Health; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Blake, D.A.; McLean, N.V. A colorimetric assay for the measurement of d-glucose consumption by cultured cells. Anal. Biochem. 1989, 177, 156–160. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, L.; Yang, H. Screening α-glucosidase inhibitors from Coruns officinalis (I). Food Sci. 2007, 28, 167–170. [Google Scholar]
- Zhu, Y.-P.; Yin, L.-J.; Cheng, Y.-Q.; Yamaki, K.; Mori, Y.; Su, Y.-C.; Li, L.-T. Effects of sources of carbon and nitrogen on production of α-glucosidase inhibitor by a newly isolated strain of Bacillus subtilis B2. Food Chem. 2008, 109, 737–742. [Google Scholar] [CrossRef]
- Ogawa, N.; Satsu, H.; Watanabe, H.; Fukaya, M.; Tsukamoto, Y.; Miyamoto, Y.; Shimizu, M. Acetic Acid Suppresses the Increase in Disaccharidase Activity That Occurs during Culture of Caco-2 Cells. J. Nutr. 2000, 130, 507–513. [Google Scholar] [CrossRef]
- Xu, Z.; Li, W.; Sun, J. Properties of gastrointestinal disaccharidase in pig. Acta Zool. Sin. 2020, 48, 202–207. [Google Scholar]
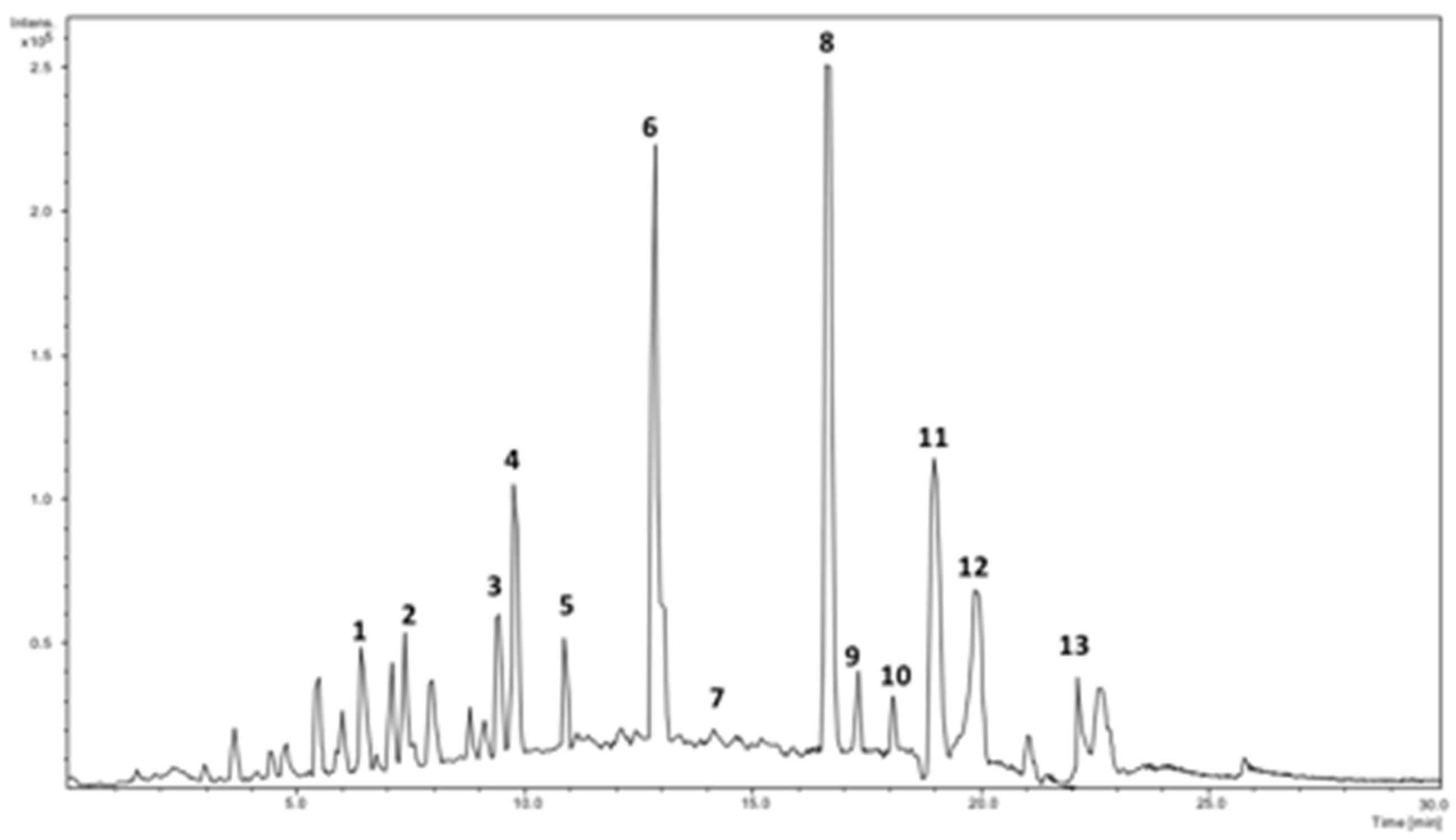

| Peak # | Rt (min) | [M − H]− | λmax (nm) | Fragment Ions (% Base Peak) | Assignment |
|---|---|---|---|---|---|
| 1 | 6.55 | 353 | 295sh, 325 | 191 (100), 179 (79), 135 (3) | Caffeoylquinic acid I |
| 2 | 7.65 | 311 | 296sh, 329 | 179 (70), 149 (100), 135 (8) | Caffeoyltartaric acid |
| 3 | 8.68 | 355 | 293sh, 325 | 209 (65), 191 (100) | Hydroxyferulic acid deoxyhexoside |
| 4 | 9.85 | 353 | 298sh, 323 | 173 (100) | Caffeoylquinic acid II |
| 5 | 10.89 | 591 | 291sh, 326 | 295 (100), 179 (30) | Caffeoylmalic acid |
| 6 | 12.91 | 353 | 294sh, 312 | 191 (100) | Caffeoylquinic acid III |
| 7 | 14.72 | 279 | 272sh, 313 | 163 (100), 119 (9) | Coumaroylmalic acid I |
| 8 | 15.83 | 609 | 273sh, 291sh, 351 | 301 (100) | Rutin |
| 9 | 17.31 | 725 | 266sh, 330 | 477 (100), 315 (25) | Isorhamnetin dihexoside malonate |
| 10 | 18.21 | 279 | 287sh,311 | 163 (100), 119 (3) | Coumaroylmalic acid II |
| 11 | 19.16 | 593 | 261, 332 | 285 (100) | Kaempferol-O-rutinoside |
| 12 | 19.97 | 447 | 266, 290sh, 348 | 285 (100) | Kaempferol-O-hexoside |
| 13 | 22.22 | 623 | 269sh, 296sh, 352 | 315 (100) | Isorhamnetin-O-rutinoside |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altamimi, M.A.; Abu-Reidah, I.M.; Altamimi, A.; Jaradat, N. Hydroethanolic Extract of Urtica dioica L. (Stinging Nettle) Leaves as Disaccharidase Inhibitor and Glucose Transport in Caco-2 Hinderer. Molecules 2022, 27, 8872. https://doi.org/10.3390/molecules27248872
Altamimi MA, Abu-Reidah IM, Altamimi A, Jaradat N. Hydroethanolic Extract of Urtica dioica L. (Stinging Nettle) Leaves as Disaccharidase Inhibitor and Glucose Transport in Caco-2 Hinderer. Molecules. 2022; 27(24):8872. https://doi.org/10.3390/molecules27248872
Chicago/Turabian StyleAltamimi, Mohammad A., Ibrahim M. Abu-Reidah, Almothana Altamimi, and Nidal Jaradat. 2022. "Hydroethanolic Extract of Urtica dioica L. (Stinging Nettle) Leaves as Disaccharidase Inhibitor and Glucose Transport in Caco-2 Hinderer" Molecules 27, no. 24: 8872. https://doi.org/10.3390/molecules27248872
APA StyleAltamimi, M. A., Abu-Reidah, I. M., Altamimi, A., & Jaradat, N. (2022). Hydroethanolic Extract of Urtica dioica L. (Stinging Nettle) Leaves as Disaccharidase Inhibitor and Glucose Transport in Caco-2 Hinderer. Molecules, 27(24), 8872. https://doi.org/10.3390/molecules27248872







