Chitosan-Based Ciprofloxacin Extended Release Systems: Combined Synthetic and Pharmacological (In Vitro and In Vivo) Studies
Abstract
1. Introduction
2. Results and Discussion
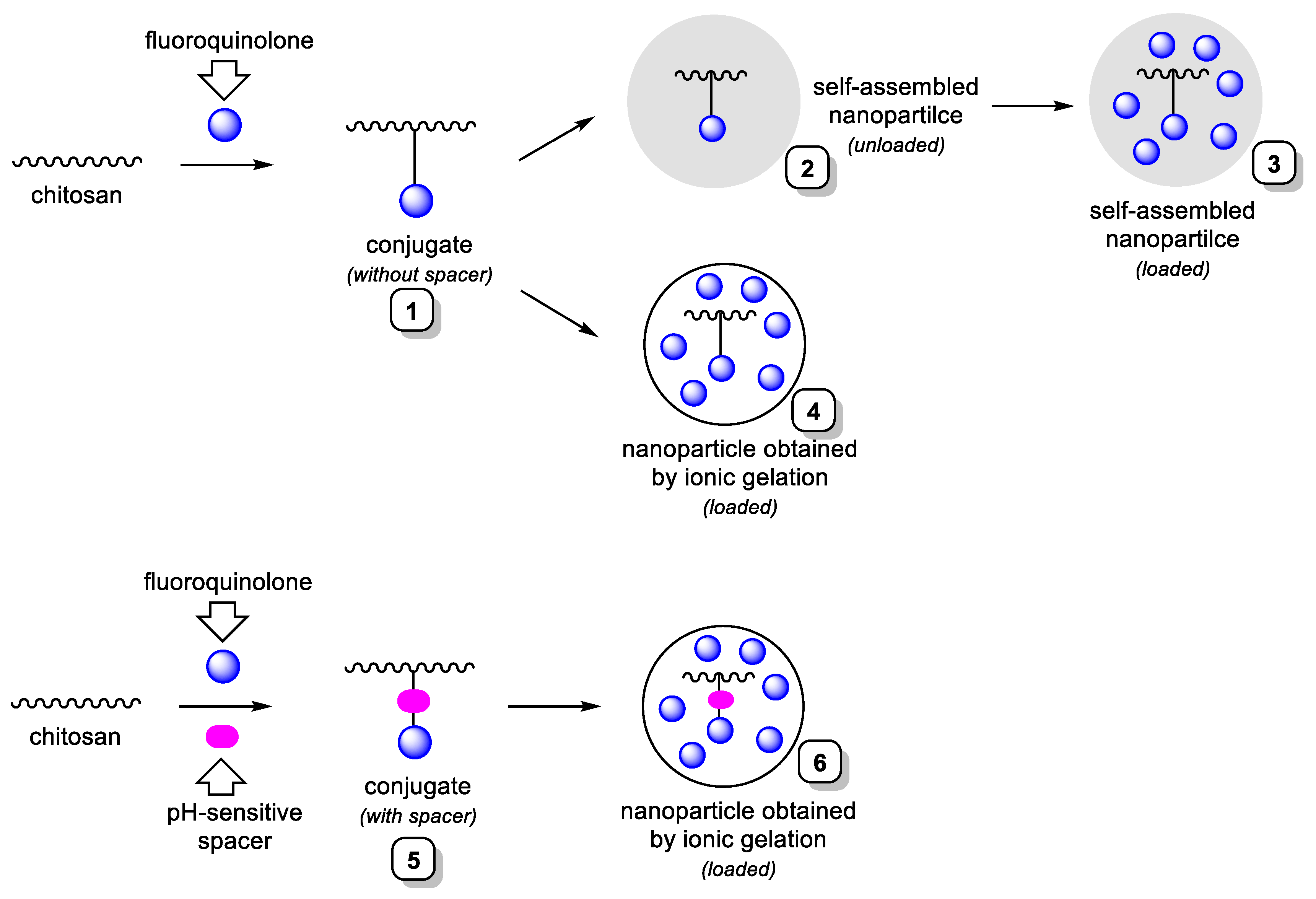
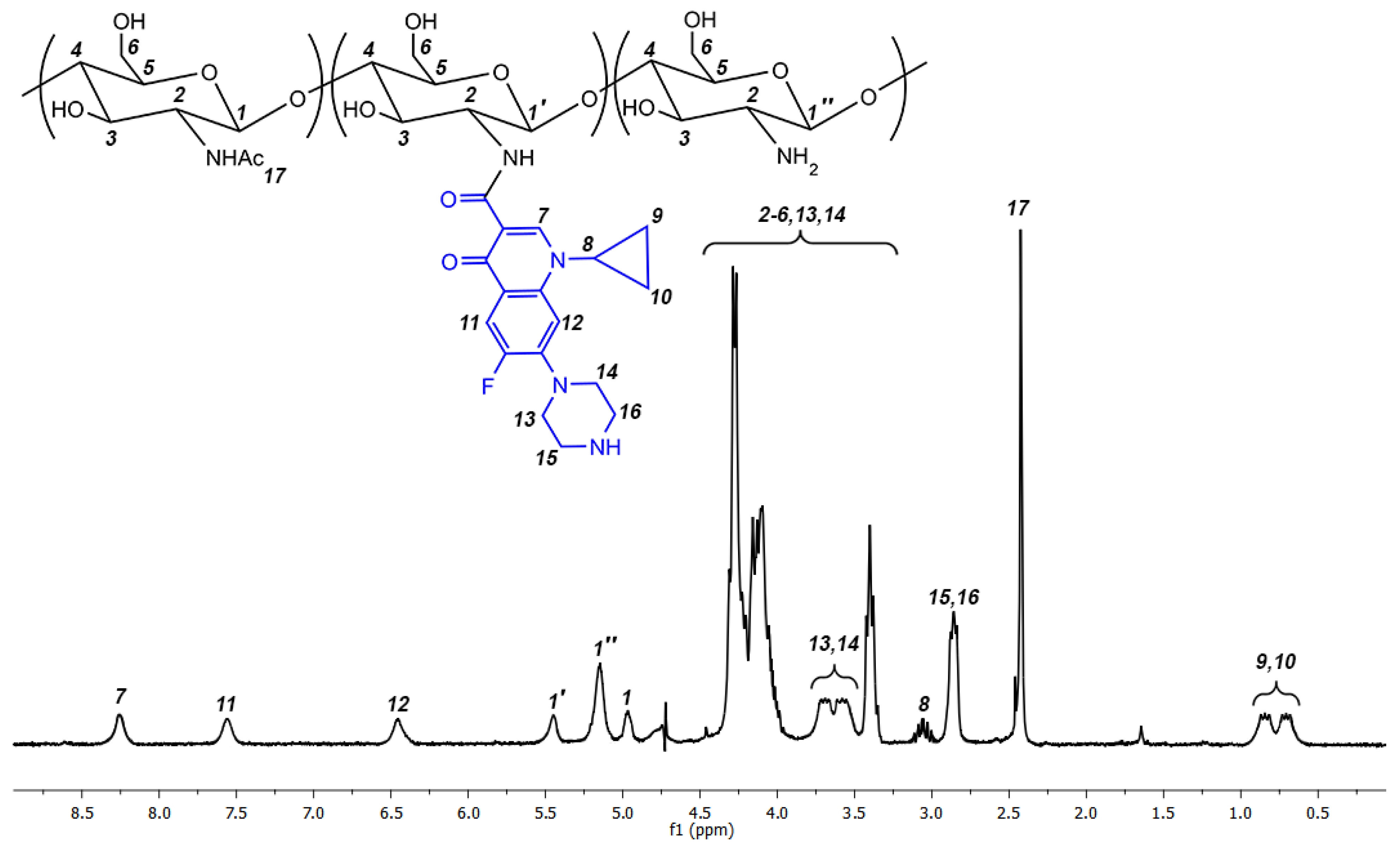
2.1. Synthesis of Ciprofloxacin-Chitosan Conjugates in Which Ciprofloxacin Is Directly Attached to the Polymer Chain (Scheme 1, System 1)
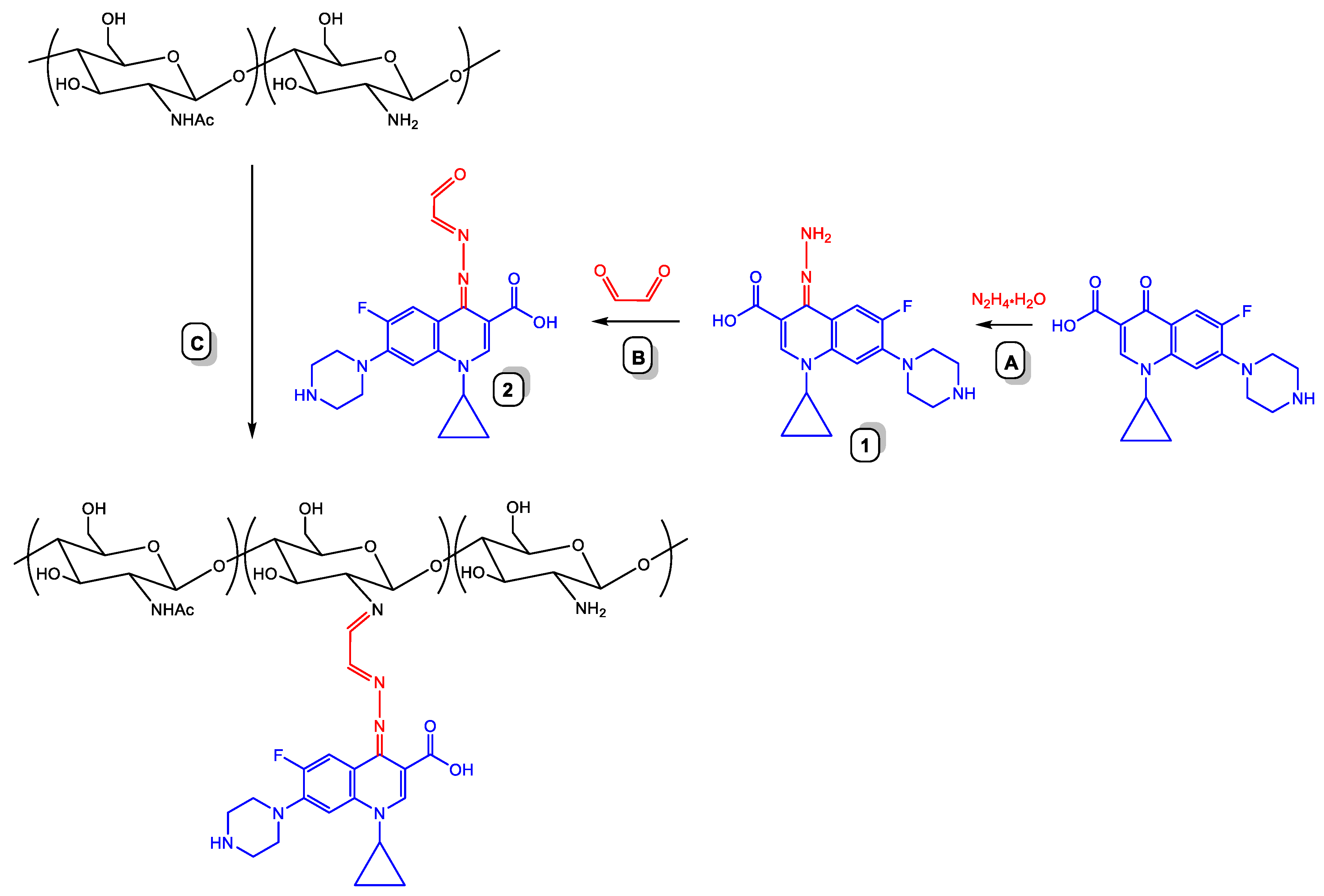
2.2. Synthesis of Ciprofloxacin-Chitosan Conjugates in Which Ciprofloxacin Is Linked to the Polymer Chain through a pH-Sensitive Linker (Scheme 1, System 5)
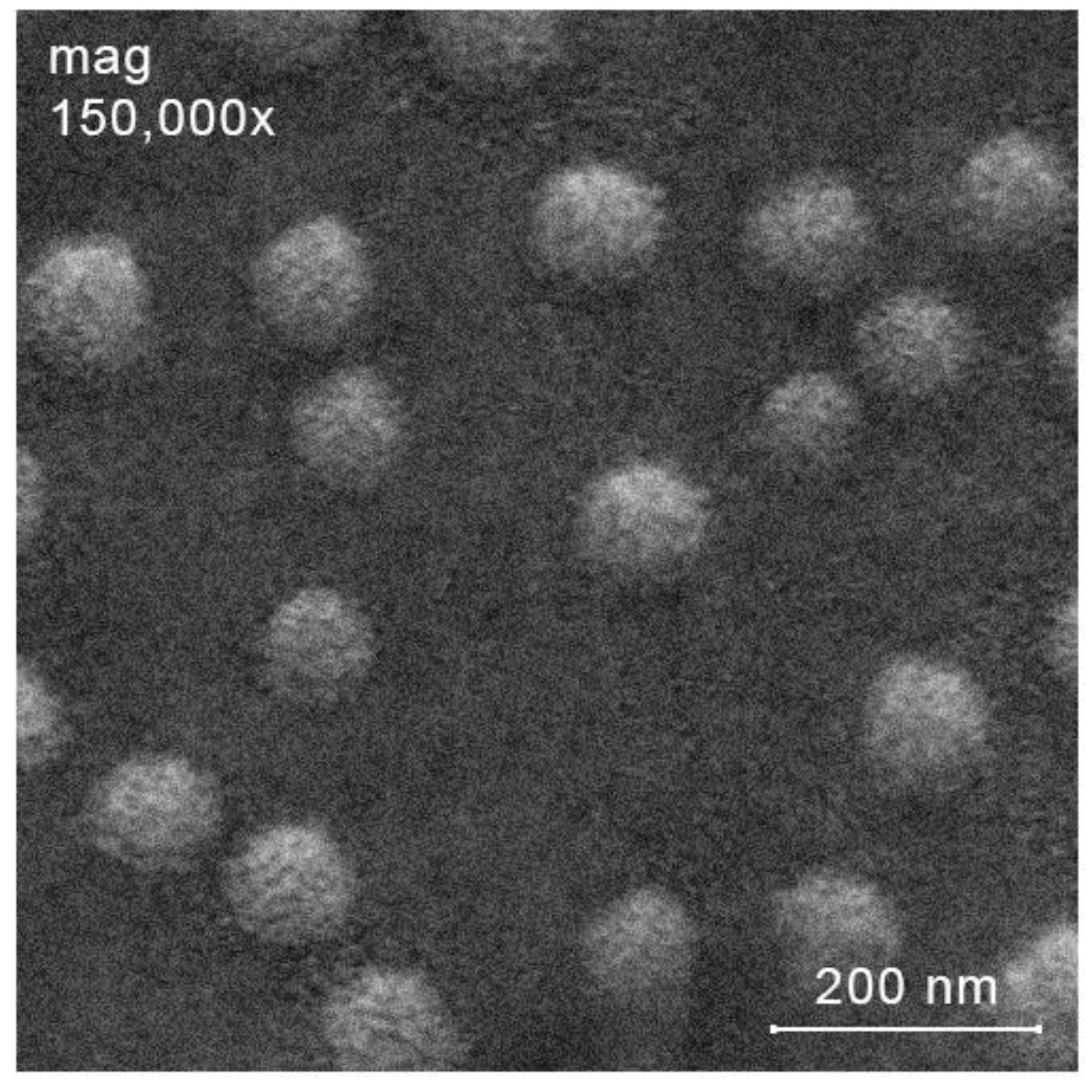
2.3. Preparation of Ciprofloxacin-Loaded Nanoparticles
2.3.1. Preparation of Ciprofloxacin-Loaded Nanoparticles from NPs-C-CS-I-M, NPs-C-CS-II-M and NPs-C-CS-III-M (Scheme 1, System 3)
2.3.2. Preparation of Ciprofloxacin-Loaded Nanoparticles from Other Polymers by Ionic Gelation Method (Scheme 1, System 4 and Scheme 1, System 6)
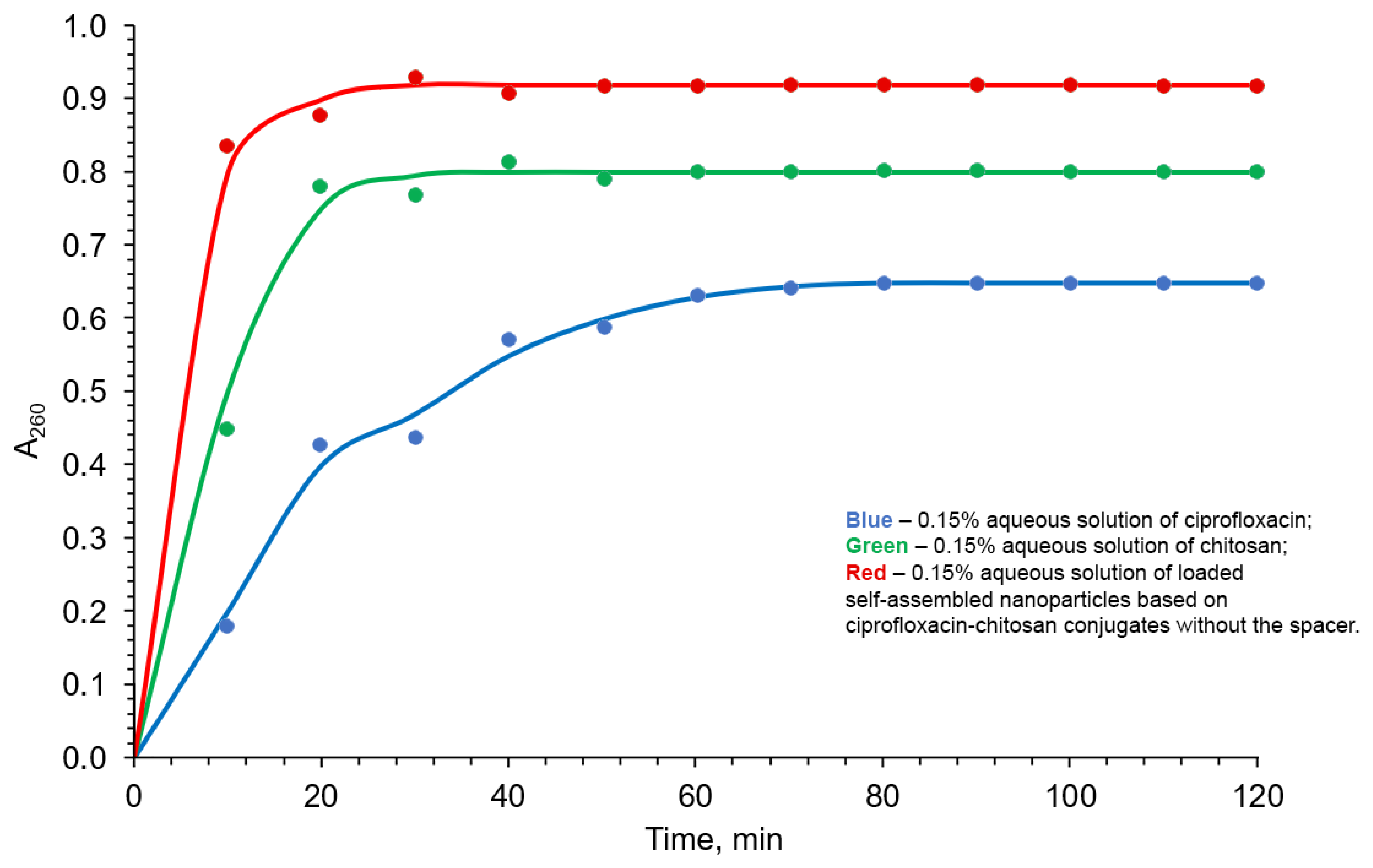
2.3.3. Drug Release Study
- (i)
- (ii)
- (iii)
- (iv)
- (v)
2.4. Antibacterial Activity of Nanoparticles and Conjugates In Vitro
2.5. Antibacterial Activity of the Loaded Self-Assembled Nanoparticles Based on Ciprofloxacin-Chitosan Conjugates without the Spacer In Vivo
2.6. Toxicity of the Loaded Self-Assembled Nanoparticles Based on Ciprofloxacin-Chitosan Conjugates without the Spacer
3. Materials and Methods
3.1. Raw Materials
3.2. General Methods
3.3. Synthetic Work
3.3.1. Model Reaction of Ciprofloxacin with n-Butylamine
3.3.2. Synthesis of Ciprofloxacin-Chitosan Conjugates in Which Ciprofloxacin Is Directly Attached to the Polymer Chain (without the Spacer, Scheme 1, System 1; Table 2)
3.3.3. Synthesis of Ciprofloxacin-Chitosan Conjugates in Which Ciprofloxacin Is Linked to the Polymer Chain through the pH-Sensitive Linker (Scheme 1, System 5; Table 5)
Synthesis of Hydrazone 1 (Scheme 4, A)
Synthesis 2 (Scheme 4, B)
Conjugation of 2 with Chitosan (Scheme 4, C)
3.3.4. Preparation of Unloaded Nanoparticles
3.3.5. Preparation of Loaded Nanoparticles
3.4. Drug Release Kinetics Study
3.5. Antibacterial Activity
3.6. Toxicity Study
3.7. Statistical Analysis
4. Conclusions
- We demonstrated that DCC-mediated coupling between COOH and NH2 groups can be efficiently promoted by ultrasound in water. Using this approach, we successfully prepared the first ciprofloxacin-chitosan conjugates, which do not contain any spacer.
- We synthesized the first ciprofloxacin-chitosan conjugates carrying an antibiotic, which is attached to the polymer chain through a pH-sensitive spacer.
- Thirdly, we elaborated three types of loaded by the ciprofloxacin nanoparticles based on both conjugates with or without a spacer (Scheme 1).Ciprofloxacin-loaded self-assembled nanoparticles, based on conjugates without any spacer, demonstrated to be capable to release the antibiotic featuring the best release profile. Moreover, their in vitro antibacterial effect is the best among all chitosan/ciprofloxacin-based systems regarded in the current work.
- We evaluated the in vivo antibacterial activity and the in vivo acute and sub-acute toxicity of the best-performing antibacterial nanoparticles (self-assembled ciprofloxacin-loaded nanoparticles based on conjugates without any spacer). The in vivo antibacterial effect of the nanoparticles exceeded even that of the starting ciprofloxacin. Moreover, the in vivo toxicity of the nanoparticles was almost identical to that of the chitosan, which is considered as the non-toxic biopolymer.
- The obtained results inspired us to regard the elaborated leading nanoparticles as the systems which are of interest for prolonged release of ciprofloxacin and its targeted delivery. The developed nanoparticles need to be turned into a suitable dosage form, as well as in further in vivo pharmacological experiments and this project is underway in our group.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Front Matter. A New History of Vaccines for Infectious Diseases; Rees, A.R., Ed.; Academic Press: Cambridge, MA, USA, 2022; p. Iii. [Google Scholar] [CrossRef]
- Grayson, M.L.; Cosgrove, S.; Crowe, S.M.; McCarthy, J.S.; Hope, W.; Mills, J.; Mouton, J.W.; Paterson, D.L. Principles of antimicrobial use. In Kucers the Use of Antibiotics; CRC Press: Boca Raton, FL, USA, 2017; pp. 3–8. [Google Scholar]
- Khaleel, A.K.; Shaari, R.B.; Nawi, M.A.A.; Al-Yassiri, A.M.H. Toxicological Aspects of Fluoroquinolones Administration: A Literature Review. Egypt. J. Chem. 2022, 65, 561–569. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Mercuro, N.J.; Davis, S.L.; Rybak, M.J. Delafloxacin: Place in Therapy and Review of Microbiologic, Clinical and Pharmacologic Properties. Infect. Dis. Ther. 2018, 7, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Estradé, O.; Vozmediano, V.; Carral, N.; Isla, A.; González, M.; Poole, R.; Suarez, E. Key Factors in Effective Patient-Tailored Dosing of Fluoroquinolones in Urological Infections: Interindividual Pharmacokinetic and Pharmacodynamic Variability. Antibiotics 2022, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J.; Kralova, K. Advances in Nanostructures for Antimicrobial Therapy. Materials 2022, 15, 2388. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Jin, Q. Polymeric Nanoplatforms for the Delivery of Antibacterial Agents. Macromol. Chem. Phys. 2022, 223, 2100440. [Google Scholar] [CrossRef]
- Ndayishimiye, J.; Kumeria, T.; Popat, A.; Falconer, J.R.; Blaskovich, M.A.T. Nanomaterials: The New Antimicrobial Magic Bullet. ACS Infect. Dis. 2022, 8, 693–712. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Zhaliazniak, N.V.; Egorov, A.R.; Lobanov, N.N.; Volkova, O.V.; Zabodalova, L.A.; Suchkova, E.P.; Kurliuk, A.V.; Shakola, T.V.; Rubanik, V.V.; et al. Chitosan derivatives and their based nanoparticles: Ultrasonic approach to the synthesis, antimicrobial and transfection properties. Carbohydr. Polym. 2020, 242, 116478. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Gopi, S.; Thomas, S.; Pius, A. (Eds.) Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. i–iii. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan: Drug Delivery and Biomedical Applications; Jana, S., Jana, S., Eds.; Springer: Singapore, 2019; pp. 457–489. [Google Scholar]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial–a review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, L.A.M.; Boeriu, C.G. (Eds.) Chitin and Chitosan; John Wiley and Sons: New York, NY, USA, 2019; p. 510. [Google Scholar]
- Ahmed, S.; Ikram, S. Chitosan: Derivatives, Composites and Applications; John Wiley & Sons: New York, NY, USA, 2017; p. 516. [Google Scholar]
- Cheng, Y.; Morovvati, M.R.; Huang, M.; Shahali, M.; Saber-Samandari, S.; Niazi Angili, S.; Ghadiri Nejad, M.; Shakibaie, M.; Toghraie, D. A multilayer biomimetic chitosan-gelatin-fluorohydroxyapatite cartilage scaffold using for regenerative medicine application. J. Mater. Res. Technol. 2021, 14, 1761–1777. [Google Scholar] [CrossRef]
- Ahmady, A.R.; Razmjooee, K.; Saber-Samandari, S.; Toghraie, D. Fabrication of chitosan-gelatin films incorporated with thymol-loaded alginate microparticles for controlled drug delivery, antibacterial activity and wound healing: In-vitro and in-vivo studies. Int. J. Biol. Macromol. 2022, 223, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Saber-Samandari, S.; Saber-Samandari, S. Biocompatible nanocomposite scaffolds based on copolymer-grafted chitosan for bone tissue engineering with drug delivery capability. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaidi, H.; Kalgudi, R.; Zariwala, M.G. Fabrication of inhaled hybrid silver/ciprofloxacin nanoparticles with synergetic effect against Pseudomonas aeruginosa. Eur. J. Pharm. Biopharm. 2018, 128, 27–35. [Google Scholar] [CrossRef]
- Levofloxacin-Loaded Nanoparticles Decrease Emergence of Fluoroquinolone Resistance in Escherichia coli. Microb. Drug Resist. 2018, 24, 1098–1107. [CrossRef]
- Anwer, M.K.; Iqbal, M.; Muharram, M.M.; Mohammad, M.; Ezzeldin, E.; Aldawsari, M.F.; Alalaiwe, A.; Imam, F. Development of Lipomer Nanoparticles for the Enhancement of Drug Release, Anti-Microbial Activity and Bioavailability of Delafloxacin. Pharmaceutics 2020, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Vodovotz, Y.; An, G. (Eds.) Complex Systems and Computational Biology Approaches to Acute Inflammation; Springer: New York, NY, USA, 2013; pp. 1–291. [Google Scholar]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. MedChemComm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Nozaki, S. Effects of amounts of additives on peptide coupling mediated by a water-soluble carbodiimide in alcohols. J. Pept. Res. 1999, 54, 162–167. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Dysin, A.P.; Volkova, O.V.; Zabodalova, L.A.; Suchkova, E.P.; Kurliuk, A.V.; Shakola, T.V. Ultrasound-assisted Cu(I)-catalyzed azide-alkyne click cycloaddition as polymer-analogous transformation in chitosan chemistry. High antibacterial and transfection activity of novel triazol betaine chitosan derivatives and their nanoparticles. Int. J. Biol. Macromol. 2019, 137, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Kritchenkov, A.S.; Egorov, A.R.; Artemjev, A.A.; Kritchenkov, I.S.; Volkova, O.V.; Kurliuk, A.V.; Shakola, T.V.; Rubanik, V.V.; Tskhovrebov, A.G.; Yagafarov, N.Z.; et al. Ultrasound-assisted catalyst-free thiol-yne click reaction in chitosan chemistry: Antibacterial and transfection activity of novel cationic chitosan derivatives and their based nanoparticles. Int. J. Biol. Macromol. 2020, 143, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Semenov, K.N.; Charykov, N.A.; Keskinov, V.A.; Kritchenkov, A.S.; Murin, I.V. Fullerenol-d Solubility in Fullerenol-d-Inorganic Salt-Water Ternary Systems at 25 degrees C. Ind. Eng. Chem. Res. 2013, 52, 16095–16100. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Artemjev, A.A.; Kritchenkov, I.S.; Volkova, O.V.; Kiprushkina, E.I.; Zabodalova, L.A.; Suchkova, E.P.; Yagafarov, N.Z.; Tskhovrebov, A.G.; et al. Novel heterocyclic chitosan derivatives and their derived nanoparticles: Catalytic and antibacterial properties. Int. J. Biol. Macromol. 2020, 149, 682–692. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Kletskov, A.V.; Egorov, A.R.; Tskhovrebov, A.G.; Kurliuk, A.V.; Zhaliazniak, N.V.; Shakola, T.V.; Khrustalev, V.N. New water-soluble chitin derivative with high antibacterial properties for potential application in active food coatings. Food Chem. 2020, 343, 128696. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Volkova, O.V.; Kritchenkov, I.S.; Kurliuk, A.V.; Shakola, T.V.; Khrustalev, V.N. Ultrasound-assisted catalyst-free phenol-yne reaction for the synthesis of new water-soluble chitosan derivatives and their nanoparticles with enhanced antibacterial properties. Int. J. Biol. Macromol. 2019, 139, 103–113. [Google Scholar] [CrossRef]
- Inamuddin; Boddula, R.; Asiri, A. (Eds.) Green Sustainable Process for Chemical and Environmental Engineering and Science: Sonochemical Organic Synthesis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–375. [Google Scholar]
- Goddard, J.-P.; Malacria, M.; Ollivier, C. (Eds.) Activation Methods: Sonochemistry and High Pressure; Wiley-ISTE: London, UK, 2019; pp. 1–157. [Google Scholar]
- Zhanel, G.G.; Walkty, A.; Vercaigne, L.; Karlowsky, J.A.; Embil, J.; Gin, A.S.; Hoban, D.J. The New Fluoroquinolones: A Critical Review. Can. J. Infect. Dis. 1999, 10, 378394. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kim, K.D.; Chun, S.C. Antibacterial Activity of Chitosan Nanoparticles: A Review. Processes 2020, 8, 1173. [Google Scholar] [CrossRef]
- Confederat, L.G.; Tuchilus, C.G.; Dragan, M.; Sha’at, M.; Dragostin, O.M. Preparation and Antimicrobial Activity of Chitosan and Its Derivatives: A Concise Review. Molecules 2021, 26, 3694. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, S.S.; Bora, R.S.; Al-Garni, S.M. Antimicrobial activity of chitosan nanoparticles. Biotechnol. Biotechnol. Equip. 2021, 35, 1874–1880. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Kurasova, M.N.; Volkova, O.V.; Meledina, T.V.; Lipkan, N.A.; Tskhovrebov, A.G.; Kurliuk, A.V.; Shakola, T.V.; Dysin, A.P.; et al. Novel non-toxic high efficient antibacterial azido chitosan derivatives with potential application in food coatings. Food Chem. 2019, 301, 125247. [Google Scholar] [CrossRef]
- Rahman, A.-U.; Choudhary, M.I.; Thomson, W.J. Bioassay Techniques for Drug Development; CRC Press: Boca Raton, FL, USA, 2005; Volume 2. [Google Scholar]
- Kritchenkov, A.S.; Egorov, A.R.; Skorik, Y.A. Azide pre-click modification of chitosan: N-(2-azidoethyl)chitosan. Russ. Chem. Bull. 2018, 67, 1915–1919. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Krytchankou, I.S.; Dubashynskaya, N.V.; Volkova, O.V.; Shakola, T.V.; Kurliuk, A.V.; Skorik, Y.A. Synthesis of novel 1H-tetrazole derivatives of chitosan via metal-catalyzed 1,3-dipolar cycloaddition. Catalytic and antibacterial properties of [3-(1H-tetrazole-5-yl)ethyl]chitosan and its nanoparticles. Int. J. Biol. Macromol. 2019, 132, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Sahm, D.H. Antibacterial susceptibility tests: Dilution methods. In Manual of Clinical Microbiology; Murray, P.R., Ed.; ASM Press: Washington, DC, USA, 1991; pp. 1105–1116. [Google Scholar]


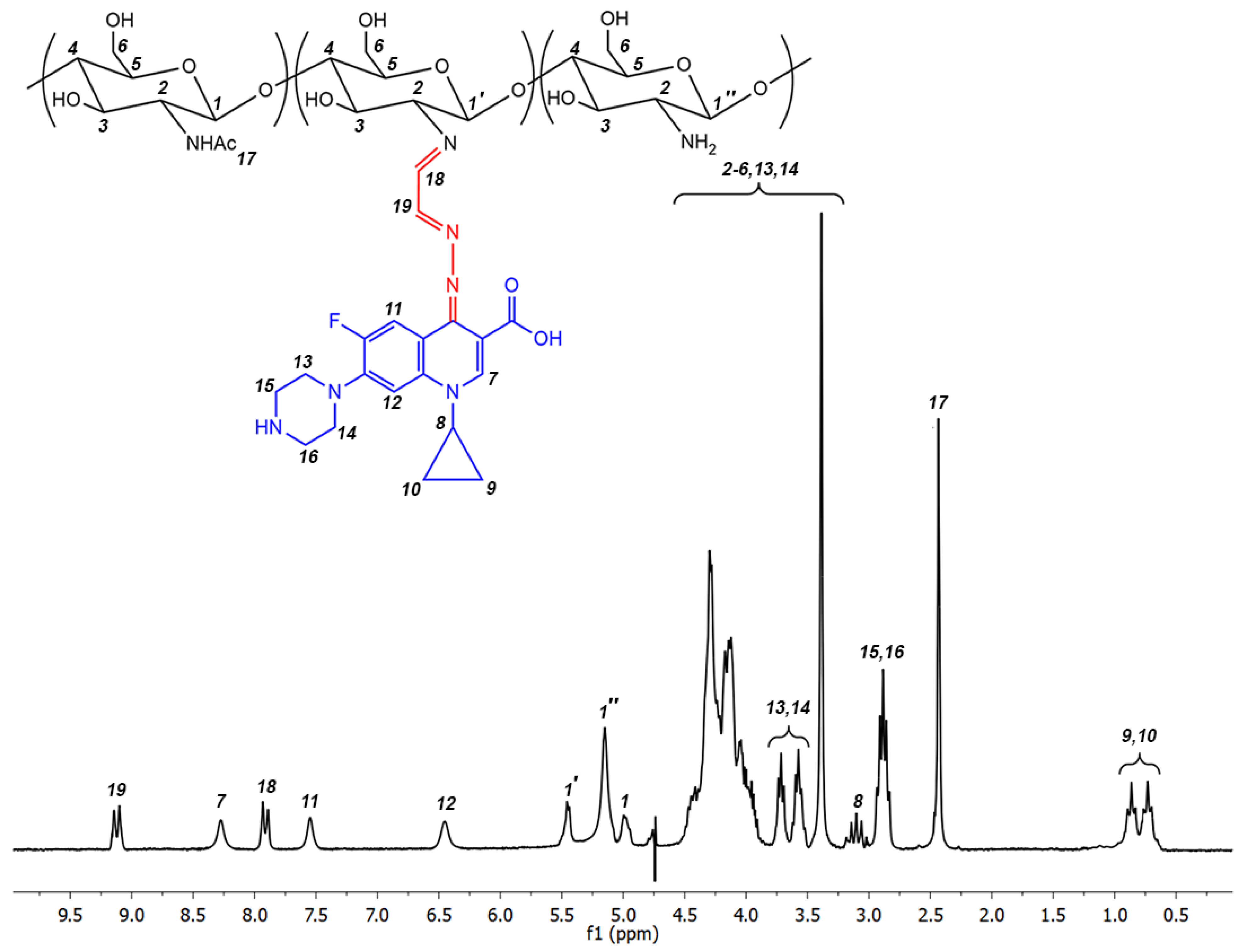
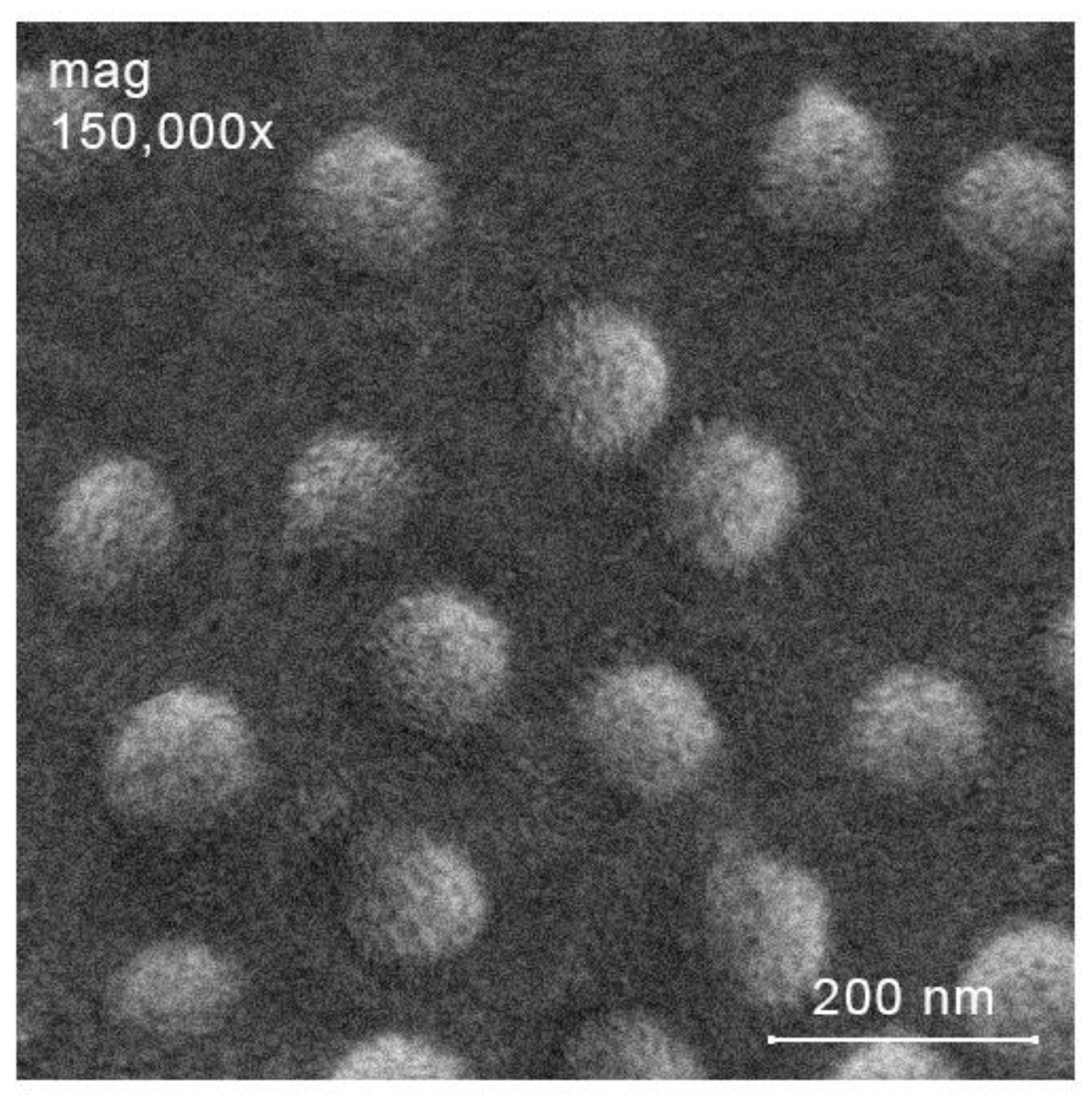
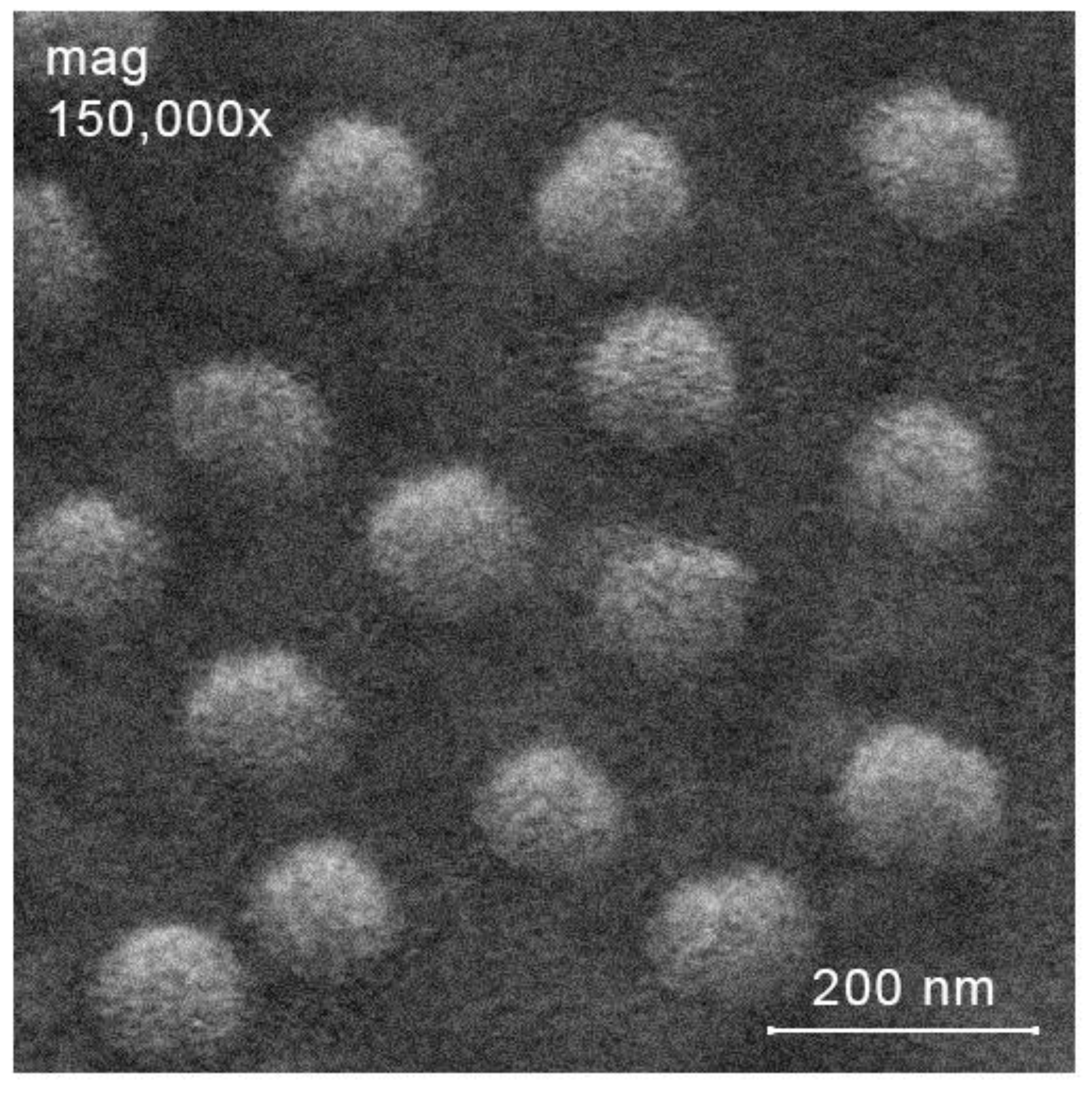


| Parameters, Identical for Both Ultrasound-Free and Ultrasonic Conditions | Ultrasound-Free Conditions | Ultrasonic Conditions | ||||
|---|---|---|---|---|---|---|
| DS | T, °C | pH | Molar Ratio | Reaction Time | Molar Ratio | Reaction Time |
| 0.05 | 50 °C | 3 | 0.5 | 5 h | 0.2 | 20 min |
| 0.10 | 50 °C | 3 | 1.5 | 5 h | 0.5 | 20 min |
| 0.20 | 50 °C | 3 | 4 | 5 h | 1.6 | 20 min |
| Ciprofloxacin/Chitosan Conjugate | The Molecular Weight of the Starting Chitosan | The Degree of Substitution of the Derivative |
|---|---|---|
| C-CS-I-L | 3.5 × 104 | 0.05 |
| C-CS-I-M | 7.1 × 104 | 0.05 |
| C-CS-I-H | 17.2 × 104 | 0.05 |
| C-CS-II-L | 3.5 × 104 | 0.10 |
| C-CS-II-M | 7.1 × 104 | 0.10 |
| C-CS-II-H | 17.2 × 104 | 0.09 |
| C-CS-III-L | 3.5 × 104 | 0.20 |
| C-CS-III-M | 7.1 × 104 | 0.20 |
| C-CS-III-H | 17.2 × 104 | 0.20 |
| Sample | 2Rh, nm * | PDI * | ζ-Potential, mV * |
|---|---|---|---|
| NPs-C-CS-I-M | 35 ± 5 | 0.18 ± 0.03 | 32.4 ± 0.3 |
| NPs-C-CS-II-M | 59 ± 3 | 0.16 ± 0.02 | 35.2 ± 0.1 |
| NPs-C-CS-III-M | 97 ± 3 | 0.18 ± 0.02 | 39.7 ± 0.3 |
| Parameters, Identical for Both Ultrasound-Free and Ultrasonic Conditions | Ultrasound-Free Conditions | Ultrasonic Conditions | ||||
|---|---|---|---|---|---|---|
| DS | T, °C | pH | Molar Ratio | Reaction Time | Molar Ratio | Reaction Time |
| 0.05 | 25 °C | 3 | 0.9 | 3 h | 0.5 | 20 min |
| 0.10 | 25 °C | 3 | 1.7 | 3 h | 0.9 | 20 min |
| 0.20 | 25 °C | 3 | 3.5 | 3 h | 1.6 | 20 min |
| Ciprofloxacin/Chitosan Conjugate | The Molecular Weight of the Starting Chitosan | The Degree of Substitution of the Derivative |
|---|---|---|
| C-SP-CS-I-L | 3.5 × 104 | 0.06 |
| C-SP-CS-I-M | 7.1 × 104 | 0.05 |
| C-SP-CS-I-H | 17.2 × 104 | 0.04 |
| C-SP-CS-II-L | 3.5 × 104 | 0.11 |
| C-SP-CS-II-M | 7.1 × 104 | 0.10 |
| C-SP-CS-II-H | 17.2 × 104 | 0.09 |
| C-SP-CS-III-L | 3.5 × 104 | 0.22 |
| C-SP-CS-III-M | 7.1 × 104 | 0.20 |
| C-SP-CS-III-H | 17.2 × 104 | 0.20 |
| Sample | 2Rh, nm * | PDI * | ζ-Potential, mV * |
|---|---|---|---|
| NPs-C-SP-CS-I-M | 39 ± 5 | 0.11 ± 0.02 | 31.0 ± 0.4 |
| NPs-C-SP-CS-II-M | 67 ± 3 | 0.14 ± 0.03 | 37.9 ± 0.3 |
| NPs-C-SP-CS-III-M | 110 ± 4 | 0.15 ± 0.03 | 40.3 ± 0.2 |
| Sample | 2Rh, nm * | PDI * | ζ-Potential, mV * | EE, % * | LE, μg/mg * (%) |
|---|---|---|---|---|---|
| C-L-NPs-C-CS-I-M | 48 ± 3 | 0.13 ± 0.04 | 33.0 ± 0.5 | 73 ± 5 | 264 ± 3 (ca. 26%) |
| C-L-NPs-C-CS-II-M | 83 ± 4 | 0.15 ± 0.03 | 38.4 ± 0.2 | 81 ± 2 | 283 ± 5 (ca. 28%) |
| C-L-NPs-C-CS-III-M | 137 ± 2 | 0.12 ± 0.02 | 40.0 ± 0.4 | 93 ± 3 | 317 ± 2 (ca. 32%) |
| Sample | Volume of TPP (mL) | 2Rh, nm * | PDI * | ζ-Potential, mV * | EE, % * | LE, μg/mg * (%) |
|---|---|---|---|---|---|---|
| Part I(nanoparticles based on the conjugates without the spacer, (Scheme 1, system 4)) | ||||||
| C-L-NPs-C-CS-I-L | 3.0 | 43 ± 2 | 0.09 ± 0.02 | 34.3 ± 0.1 | 31 ± 3 | 80 ± 2 |
| (ca. 8%) | ||||||
| C-L-NPs-C-CS-I-H | 3.0 | 49 ± 3 | 0.12 ± 0.03 | 33.2 ± 0.5 | 32 ± 4 | 80 ± 2 |
| (ca. 8%) | ||||||
| C-L-NPs-C-CS-II-L | 2.0 | 63 ± 3 | 0.13 ± 0.04 | 38.2 ± 0.3 | 28 ± 2 | 82 ± 3 |
| (ca. 8%) | ||||||
| C-L-NPs-C-CS-II-H | 2.0 | 69 ± 1 | 0.10 ± 0.03 | 39.1 ± 0.1 | 30 ± 3 | 91 ± 2 |
| (ca. 9%) | ||||||
| C-L-NPs-C-CS-III-L | 1.5 | 128 ± 4 | 0.15 ± 0.02 | 43.3 ± 0.2 | 27 ± 6 | 88 ± 4 |
| (ca. 9%) | ||||||
| C-L-NPs-C-CS-III-H | 1.5 | 126 ± 3 | 0.12 ± 0.03 | 45.8 ± 0.3 | 30 ± 3 | 98 ± 2 |
| (ca. 10%) | ||||||
| Part II(nanoparticles based on the conjugates with the spacer, Scheme 1, system 6) | ||||||
| C-L-NPs-C-SP-CS-I-L | 3.0 | 48 ± 2 | 0.12 ± 0.04 | 32.9 ± 0.3 | 31 ± 5 | 78 ± 4 |
| (ca. 8%) | ||||||
| C-L-NPs-C-SP-CS-I-M | 3.0 | 51 ± 3 | 0.14 ± 0.03 | 33.1 ± 0.4 | 33 ± 5 | 81 ± 2 |
| (ca. 8%) | ||||||
| C-L-NPs-C-SP-CS-I-H | 3.0 | 59 ± 2 | 0.12 ± 0.03 | 32.5 ± 0.2 | 30 ± 3 | 78 ± 8 |
| (ca. 8%) | ||||||
| C-L-NPs-C-SP-CS-II-L | 2.0 | 64 ± 4 | 0.16 ± 0.02 | 35.3 ± 0.4 | 27 ± 4 | 80 ± 4 |
| (ca. 8%) | ||||||
| C-L-NPs-C-SP-CS-II-M | 2.0 | 66 ± 2 | 0.12 ± 0.02 | 36.4 ± 0.1 | 31 ± 1 | 87 ± 1 |
| (ca. 9%) | ||||||
| C-L-NPs-C-SP-CS-II-H | 2.0 | 71 ± 3 | 0.14 ± 0.05 | 36.9 ± 0.2 | 30 ± 2 | 84 ± 2 |
| (ca. 8%) | ||||||
| C-L-NPs-C-SP-CS-III-L | 1.5 | 118 ± 4 | 0.16 ± 0.02 | 45.8 ± 0.2 | 30 ± 3 | 97 ± 3 |
| (ca. 10%) | ||||||
| C-L-NPs-C-SP-CS-III-M | 1.5 | 125 ± 3 | 0.13 ± 0.04 | 45.3 ± 0.4 | 28 ± 2 | 91 ± 3 |
| (ca. 9%) | ||||||
| C-L-NPs-C-SP-CS-III-H | 1.5 | 129 ± 3 | 0.12 ± 0.02 | 46.1 ± 0.3 | 26 ± 4 | 84 ± 3 |
| (ca. 8%) | ||||||
| Tested Sample | Inhibition Zone, mm * | |
|---|---|---|
| E. coli | S. aureus | |
| Ciprofloxacin-chitosan conjugates without the spacer (Scheme 1, system 1; Table 2) | ||
| C-CS-I-M | 14.2 ± 0.3 | 11.3 ± 0.1 |
| C-CS-II-M | 16.2 ± 0.4 | 13.1 ± 0.2 |
| C-CS-III-M | 20.0 ± 0.2 | 14.9 ± 0.1 |
| Ciprofloxacin-chitosan conjugates with the spacer (Scheme 1, system 5; Table 4) | ||
| C-SP-CS-I-M | 23.7 ± 0.4 | 18.4 ± 0.5 |
| C-SP-CS-II-M | 27.4 ± 0.1 | 21.6 ± 0.3 |
| C-SP-CS-III-M | 31.1 ± 0.1 | 23.9 ± 0.4 |
| Loaded self-assembled nanoparticles (based on ciprofloxacin-chitosan conjugates without the spacer, Scheme 1, system 3; Table 6) | ||
| C-L-NPs-C-CS-I-M | 47.2 ± 0.1 | 42.5 ± 0.5 |
| C-L-NPs-C-CS-II-M | 47.8 ± 0.3 | 42.3 ± 0.4 |
| C-L-NPs-C-CS-III-M | 47.9 ± 0.2 | 42.6 ± 0.3 |
| Loaded nanoparticles based on ciprofloxacin-chitosan conjugates without the spacer prepared by ionic gelation method (Scheme 1, system 4; Table 7, part I) | ||
| C-L-NPs-C-CS-I-M | 36.0 ± 0.5 | 30.9 ± 0.7 |
| C-L-NPs-C-CS-II-M | 36.5 ± 0.1 | 30.2 ± 0.3 |
| C-L-NPs-C-CS-III-M | 36.2 ± 0.4 | 31.1 ± 0.2 |
| Loaded nanoparticles based on ciprofloxacin-chitosan conjugates with the spacer prepared by ionic gelation method (Scheme 1, system 6; Table 7, part II) | ||
| C-L-NPs-C-SP-CS-I-M | 36.3 ± 0.1 | 29.9 ± 0.1 |
| C-L-NPs-C-SP-CS-II-M | 36.9 ± 0.4 | 31.2 ± 0.3 |
| C-L-NPs-C-SP-CS-III-M | 36.4 ± 0.3 | 30.5 ± 0.1 |
| Reference antibacterials | ||
| Ciprofloxacin | 43.6 ± 0.3 | 38.4 ± 0.5 |
| Chitosan | 8.7 ± 0.2 | 7.9 ± 0.4 |
| Error Functions | Equations | Drug Release Study | Antibacterial Activity of Nanoparticles and Conjugates In Vitro |
|---|---|---|---|
| Root mean square error | 3.683 | 0.0130 | |
| Mean absolute percentage error | 6.499 | 2.284 | |
| Mean absolute error | 2.835 | 0.0112 | |
| Mean Square Error | 13.561 | 0.000169 | |
| Determination coefficient | 0.976 | 0.994 |
| Sample | CFU/mL of Exudate (7 h after Injection or 31 h after Infection) |
|---|---|
| Control without treatment (24 h after infection) | 2825 |
| Chitosan | 1930 |
| C-L-NPs-C-CS-I-M | 0 (no growth) |
| C-L-NPs-C-CS-II-M | 0 (no growth) |
| C-L-NPs-C-CS-III-M | 0 (no growth) |
| Ciprofloxacin | 175 |
| Hematological Parameters | Acute Toxicity | Subacute Toxicity | ||
|---|---|---|---|---|
| Control | C-L-NPs-C-CS-III-M | Control | C-L-NPs-C-CS-III-M | |
| Male rats | ||||
| MCV (fL) | 67.31 ± 3.45 | 66.99 ± 3.43 | 64.98 ± 1.41 | 64.87 ± 1.40 |
| MCH (pg) | 18.87 ± 0.71 | 18.78 ± 0.70 | 19.01 ± 0.57 | 18.79 ± 0.54 |
| MCHC (g/dL) | 28.23 ± 0.78 | 28.12 ± 0.74 | 29.22 ± 0.27 | 29.15 ± 0.26 |
| RBC (×106/μL) | 9.09 ± 0.34 | 9.07 ± 0.31 | 8.35 ± 0.67 | 8.32 ± 0.65 |
| WBC (×103/μL) | 8.51 ± 1.67 | 8.42 ± 1.59 | 8.03 ± 2.01 | 8.01 ± 1.97 |
| Platelets (×103/μL) | 843.00 ± 135.94 | 837.00 ± 132.62 | 1207.00 ± 193.54 | 1189.00 ± 187.73 |
| Hct (%) | 63.01 ± 3.96 | 62.96 ± 3.87 | 53.74 ± 3.53 | 53.04 ± 3.48 |
| Hgb (g/dL) | 18.78 ± 1.45 | 18.64 ± 1.44 | 15.68 ± 1.07 | 15.24 ± 1.04 |
| Female rats | ||||
| MCV (fL) | 64.36 ± 3.26 | 64.07 ± 3.25 | 61.04 ± 1.26 | 60.94 ± 1.24 |
| MCH (pg) | 18.13 ± 0.69 | 17.95 ± 0.67 | 18.59 ± 0.49 | 18.45 ± 0.47 |
| MCHC (g/dL) | 27.94 ± 0.73 | 27.78 ± 0.71 | 29.45 ± 0.34 | 29.37 ± 0.33 |
| RBC (×106/μL) | 9.01 ± 0.33 | 8.97 ± 0.30 | 9.11 ± 0.56 | 9.07 ± 0.55 |
| WBC (×103/μL) | 5.79 ± 0.98 | 5.78 ± 0.96 | 4.62 ± 0.27 | 4.59 ± 0.25 |
| Platelets (×103/μL) | 987.00 ± 141.76 | 981.00 ± 140.25 | 1099.00 ± 206.23 | 1086.00 ± 204.65 |
| Hct (%) | 58.08 ± 3.71 | 57.87 ± 3.67 | 55.97 ± 3.73 | 55.81 ± 3.71 |
| Hgb (g/dL) | 16.32 ± 1.32 | 16.27 ± 1.31 | 16.72 ± 1.31 | 16.68 ± 1.30 |
| Blood Biochemical Parameters | Acute Toxicity | Subacute Toxicity | ||
|---|---|---|---|---|
| Control | C-L-NPs-C-CS-III-M | Control | C-L-NPs-C-CS-III-M | |
| Male rats | ||||
| Albumin (g/dL) | 4.20 ± 0.16 | 4.17 ± 0.15 | 3.59 ± 0.23 | 3.63 ± 0.24 |
| AST (U/L) | 84.00 ± 6.74 | 82.75 ± 6.71 | 97.12 ± 11.84 | 93.34 ± 11.56 |
| ALT (U/L) | 11.70 ± 5.78 | 11.50 ± 5.76 | 27.09 ± 6.27 | 26.86 ± 6.19 |
| BUN (mg/dL) | 15.41 ± 1.38 | 13.88 ± 1.37 | 13.45 ± 1.48 | 13.49 ± 1.50 |
| Creatinine (mg/dL) | 0.35 ± 0.05 | 0.29 ± 0.05 | 0.34 ± 0.05 | 0.33 ± 0.05 |
| Glucose (mg/dL) | 209.75 ± 31.46 | 191.50 ± 30.96 | 79.12 ± 10.87 | 93.48 ± 12.48 |
| Total bilirubin (mg/dL) | 0.30 ± 0.05 | 0.29 ± 0.05 | 0.35 ± 0.05 | 0.38 ± 0.05 |
| Total protein (g/dL) | 6.32 ± 0.31 | 6.32 ± 0.31 | 6.16 ± 0.27 | 6.21 ± 0.28 |
| Female rats | ||||
| Albumin (g/dL) | 4.60 ± 0.19 | 4.67 ± 0.20 | 4.41 ± 0.31 | 4.34 ± 0.30 |
| AST (U/L) | 66.75 ± 6.24 | 67.46 ± 6.27 | 79.25 ± 6.87 | 79.00 ± 6.82 |
| ALT (U/L) | 12.47 ± 2.48 | 12.54 ± 2.51 | 21.97 ± 6.19 | 21.71 ± 6.11 |
| BUN (mg/dL) | 14.01 ± 1.51 | 14.13 ± 1.52 | 16.72 ± 1.15 | 15.98 ± 1.03 |
| Creatinine (mg/dL) | 0.29 ± 0.05 | 0.29 ± 0.05 | 0.34 ± 0.05 | 0.33 ±= 0.05 |
| Glucose (mg/dL) | 159.00 ± 35.27 | 167.00 ± 36.74 | 44.50 ± 8.96 | 49.00 ± 9.17 |
| Total bilirubin (mg/dL) | 0.44 ± 0.05 | 0.45 ± 0.05 | 0.53 ± 0.06 | 0.49 ± 0.06 |
| Total protein (g/dL) | 6.71 ± 0.36 | 6.76 ± 0.37 | 6.21 ± 0.29 | 6.14 ± 0.26 |
| Relative Organ Weight (g/100 g of Body Weight) | Acute Toxicity | Subacute Toxicity | ||
|---|---|---|---|---|
| Control | C-L-NPs-C-CS-III-M | Control | C-L-NPs-C-CS-III-M | |
| Male rats | ||||
| Heart | 0.75 ± 0.07 | 0.79 ± 0.08 | 0.23 ± 0.02 | 0.24 ± 0.02 |
| Kidney | 1.38 ± 0.18 | 1.40 ± 0.21 | 0.41 ± 0.04 | 0.38 ± 0.03 |
| Liver | 10.67 ± 1.01 | 10.81 ± 1.12 | 3.18 ± 0.17 | 3.23 ± 0.19 |
| Spleen | 2.41 ± 0.19 | 2.43 ± 0.20 | 0.83 ± 0.02 | 0.87 ± 0.03 |
| Female rats | ||||
| Heart | 0.55 ± 0.06 | 0.59 ± 0.07 | 0.31 ± 0.03 | 0.30 ± 0.03 |
| Kidney | 0.91 ± 0.13 | 0.92 ± 0.14 | 0.45 ± 0.04 | 0.44 ± 0.05 |
| Liver | 6.34 ± 0.51 | 6.37 ± 0.53 | 3.09 ± 0.27 | 3.14 ± 0.24 |
| Spleen | 1.54 ± 0.16 | 1.53 ± 0.15 | 0.68 ± 0.05 | 0.69 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorov, A.R.; Kurliuk, A.V.; Rubanik, V.V.; Kirichuk, A.A.; Khubiev, O.; Golubev, R.; Lobanov, N.N.; Tskhovrebov, A.G.; Kritchenkov, A.S. Chitosan-Based Ciprofloxacin Extended Release Systems: Combined Synthetic and Pharmacological (In Vitro and In Vivo) Studies. Molecules 2022, 27, 8865. https://doi.org/10.3390/molecules27248865
Egorov AR, Kurliuk AV, Rubanik VV, Kirichuk AA, Khubiev O, Golubev R, Lobanov NN, Tskhovrebov AG, Kritchenkov AS. Chitosan-Based Ciprofloxacin Extended Release Systems: Combined Synthetic and Pharmacological (In Vitro and In Vivo) Studies. Molecules. 2022; 27(24):8865. https://doi.org/10.3390/molecules27248865
Chicago/Turabian StyleEgorov, Anton R., Aleh V. Kurliuk, Vasili V. Rubanik, Anatoly A. Kirichuk, Omar Khubiev, Roman Golubev, Nikolai N. Lobanov, Alexander G. Tskhovrebov, and Andreii S. Kritchenkov. 2022. "Chitosan-Based Ciprofloxacin Extended Release Systems: Combined Synthetic and Pharmacological (In Vitro and In Vivo) Studies" Molecules 27, no. 24: 8865. https://doi.org/10.3390/molecules27248865
APA StyleEgorov, A. R., Kurliuk, A. V., Rubanik, V. V., Kirichuk, A. A., Khubiev, O., Golubev, R., Lobanov, N. N., Tskhovrebov, A. G., & Kritchenkov, A. S. (2022). Chitosan-Based Ciprofloxacin Extended Release Systems: Combined Synthetic and Pharmacological (In Vitro and In Vivo) Studies. Molecules, 27(24), 8865. https://doi.org/10.3390/molecules27248865





