Synthesis and Reactions of 3-Halogenated 2-CF3-Indoles
Abstract
1. Introduction
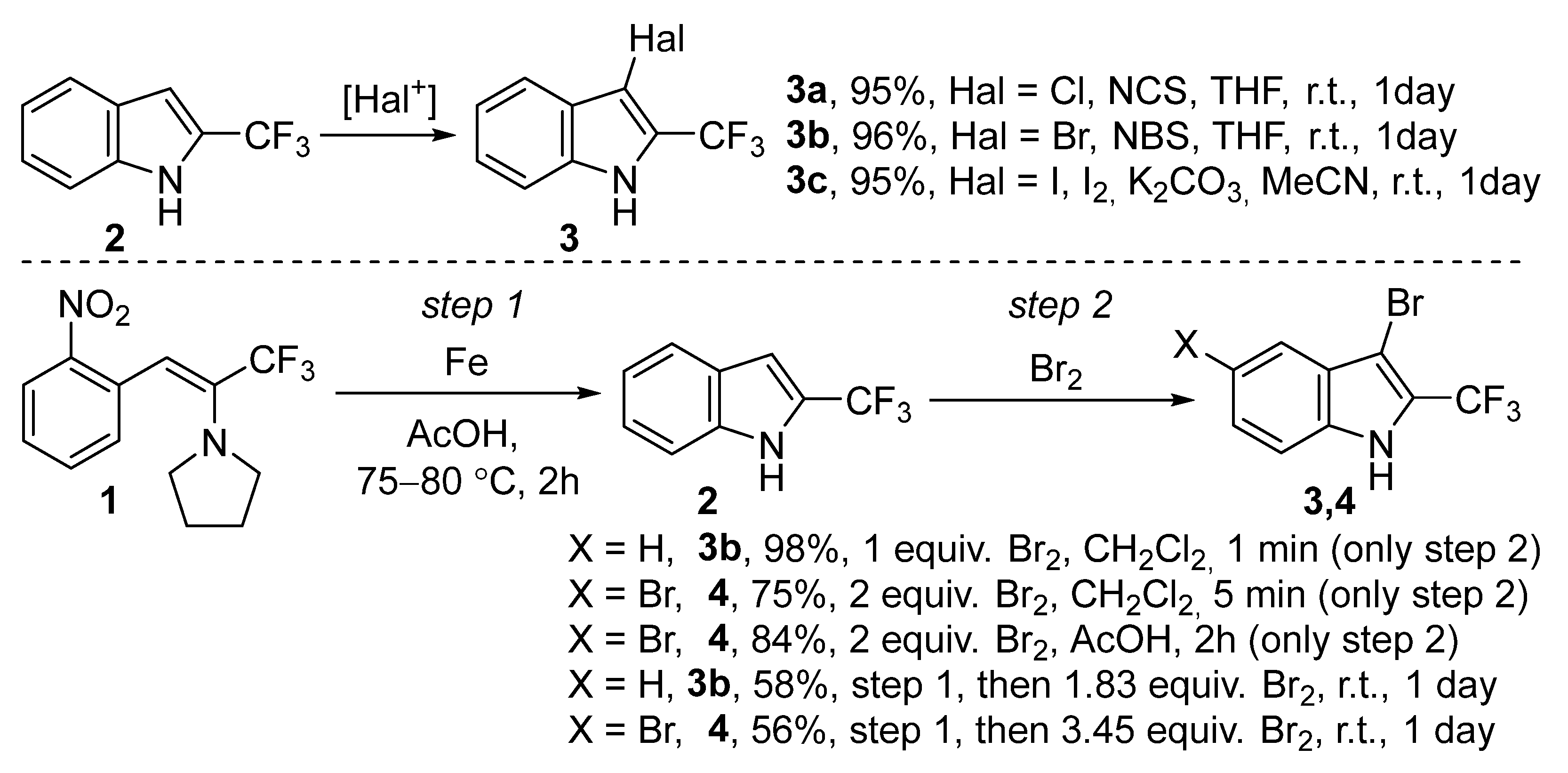
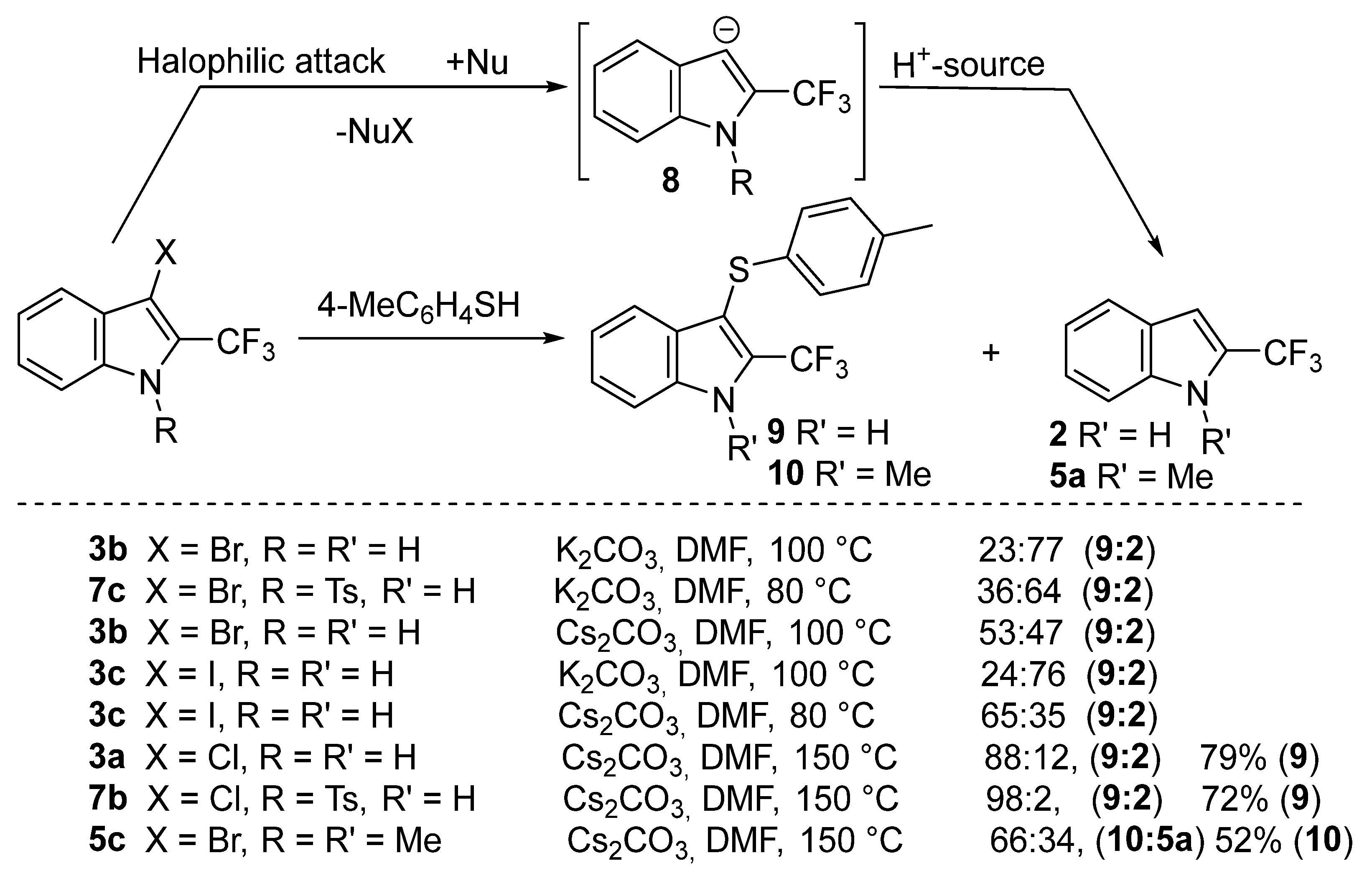
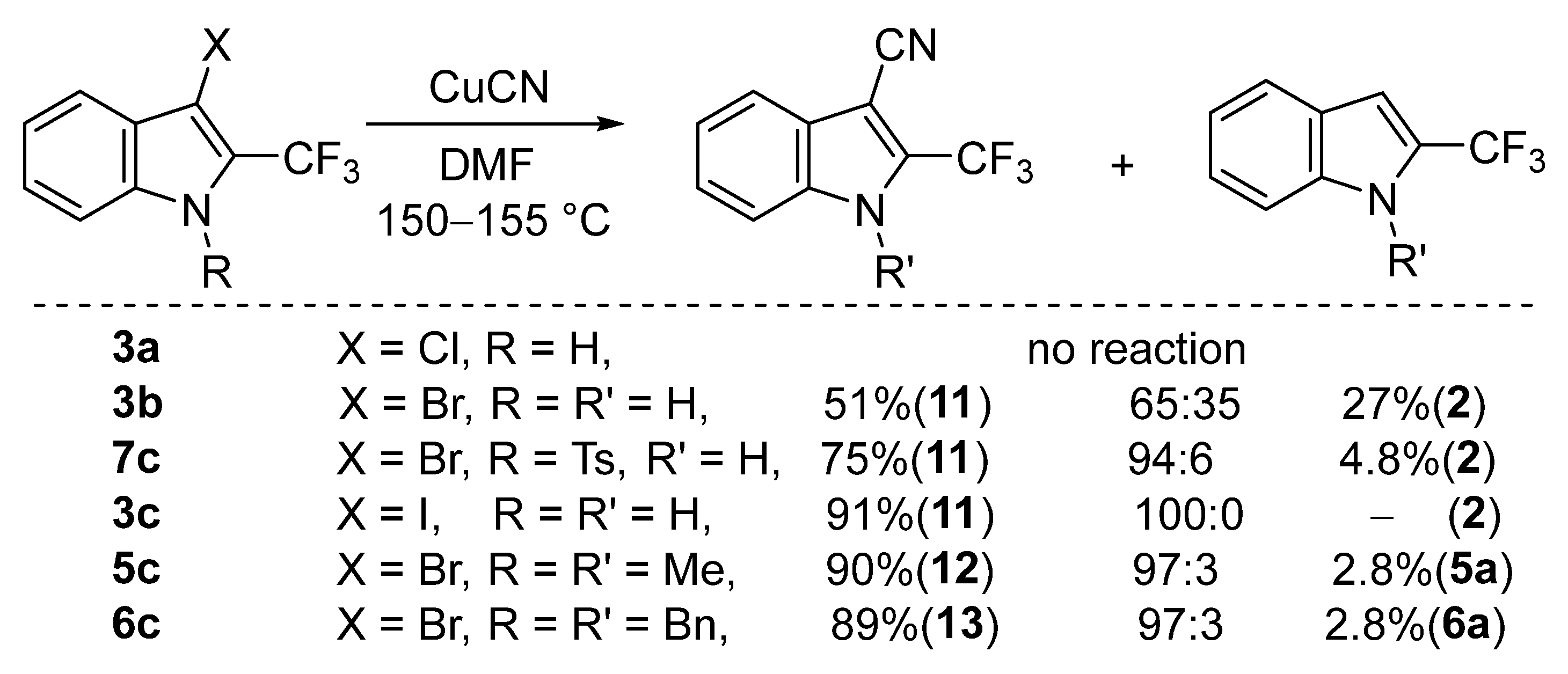
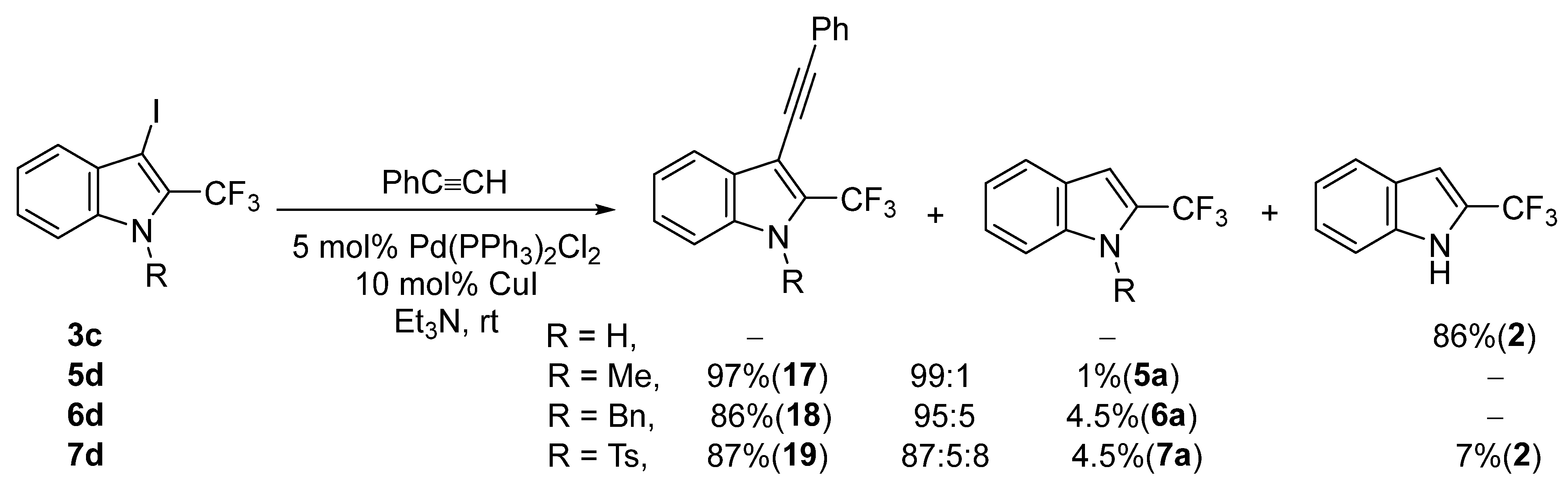
2. Results
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Baeyer, A. On the reduction of aromatic compounds by means of zinc dust. Ann. Chem. Pharm. 1866, 140, 295–296. (In German) [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Metal-free synthesis of fluorinated indoles enabled by oxidative dearomatization. Angew. Chem. 2016, 128, 2283–2287. [Google Scholar] [CrossRef]
- Pindur, U.; Adam, R. Synthetically attractive indolization processes and newer methods for the preparation of selectively substituted indole. J. Heterocycl. Chem. 1988, 25, 1–8. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G. Synthesis and functionalization of indoles through palladium-catalyzed reactions. Chem. Rev. 2005, 105, 2873–2920, Update 1: Chem. Rev. 2011, 111, PR215–PR283. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, G.R.; Kuethe, J.T. Practical methodologies for the synthesis of indoles. Chem. Rev. 2006, 106, 2875–2911. [Google Scholar] [CrossRef] [PubMed]
- Taber, D.F.; Tirunahari, P.K. Indole synthesis: A review and proposed classification. Tetrahedron 2011, 67, 7195–7210. [Google Scholar] [CrossRef]
- Platon, M.; Amardeil, R.; Djakovitch, L.; Hierso, J.C. Progress in palladium-based catalytic systems for the sustainable synthesis of annulated heterocycles: A focus on indole backbones. Chem. Soc. Rev. 2012, 41, 3929–3968. [Google Scholar] [CrossRef]
- Sundberg, R. Indoles; Academic Press: UK, 1996. [Google Scholar]
- Gribble, G.W. (Ed.) Indole Ring Synthesis; John Wiley & Sons: Chichester, UK, 2016. [Google Scholar]
- De Sa Alves, F.R.; Barreiro, E.J.; Fraga, C.A.M. From nature to drug discovery: The indole scaffold as a “privileged structure”. Mini-Rev. Med. Chem. 2009, 9, 782–793. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Thanikachalam, P.V.; Maurya, R.K.; Garg, V.; Monga, V. An insight into the medicinal perspective of synthetic analogs of indole: A review. Eur. J. Med. Chem. 2019, 180, 562–612. [Google Scholar] [CrossRef]
- McGrath, N.A.; Brichacek, M.; Njardarson, J.T. A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 2010, 87, 1348–1349. [Google Scholar] [CrossRef]
- Liang, T.; Neumann, C.N.; Ritter, T. Introduction of fluorine and fluorine-containing functional groups. Angew. Chem. Int. Ed. 2013, 52, 8214–8264. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, T.; Phipps, R.J.; Toste, F.D. Advances in catalytic enantioselective fluorination, mono-, di-, and trifluoromethylation, and trifluoromethylthiolation reactions. Chem. Rev. 2015, 115, 826–870. [Google Scholar] [CrossRef]
- Ahrens, T.; Kohlmann, J.; Ahrens, M.; Braun, T. Functionalization of fluorinated molecules by transition metal mediated C−F bond activation to access fluorinated building blocks. Chem. Rev. 2015, 115, 931–972. [Google Scholar] [CrossRef]
- Nenajdenko, V.G.; Muzalevskiy, V.M.; Shastin, A.V. Polyfluorinated ethanes as versatile fluorinated C2-building blocks for organic synthesis. Chem. Rev. 2015, 115, 973–1050. [Google Scholar] [CrossRef] [PubMed]
- Yerien, D.E.; Barata-Vallejo, S.; Postigo, A. Difluoromethylation reactions of organic compounds. Chem. Eur. J. 2017, 23, 14676–14701. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P. The unique role of fluorine in the design of active ingredients for modern crop protection. ChemBioChem 2004, 5, 570–589. [Google Scholar] [CrossRef]
- Jeschke, P. The unique role of halogen substituents in the design of modern agrochemicals. Pest Manag. Sci. 2010, 66, 10–27. [Google Scholar] [CrossRef]
- Fujiwara, T.; O’Hagan, D. Successful fluorine-containing herbicide agrochemicals. J. Fluor. Chem. 2014, 167, 16–29. [Google Scholar] [CrossRef]
- Jeschke, P. Latest generation of halogen-containing pesticides. Pest Manag. Sci. 2017, 73, 1053–1056. [Google Scholar] [CrossRef]
- Bégué, J.P.; Bonnet-Delpon, D. Bioorganic and Medicinal Chemistry of Fluorine; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Tressaud, A.; Haufe, G. (Eds.) Fluorine and Health. Molecular Imaging, Biomedical Materials and Pharmaceuticals; Elsevier: Amsterdam, The Netherlands, 2008; pp. 553–778. [Google Scholar]
- Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Hagmann, W.K. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sánchez-Rosellό, M.; Aceña, J.L.; del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in pharmaceutical industry: Fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef] [PubMed]
- Ilardi, E.A.; Vitaku, E.; Njardarson, J.T. Data-mining for sulfur and fluorine: An evaluation of pharmaceuticals to reveal opportunities for drug design and discovery. J. Med. Chem. 2014, 57, 2832–2842. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, J.; Wang, S.; Gu, Z.; Aceña, J.L.; Izawa, K.; Liu, H.; Soloshonok, V.A. Recent advances in the trifluoromethylation methodology and new CF3-containing drugs. J. Fluor. Chem. 2014, 167, 37–54. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next generation of fluorine containing pharmaceuticals, compounds currently in phase II−III clinical trials of major pharmaceutical companies: New structural trends and therapeutic areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef]
- Meanwell, N.A. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J. Med. Chem. 2018, 61, 5822–5880. [Google Scholar] [CrossRef]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of organofluorine compounds to pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef]
- De la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2021. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2022, 27, 1075. [Google Scholar] [CrossRef]
- Nenajdenko, V.G. (Ed.) Fluorine in Heterocyclic Chemistry; Springer: Heidelberg, Germany, 2014; Volume 1, p. 681. [Google Scholar]
- Nenajdenko, V.G. (Ed.) Fluorine in Heterocyclic Chemistry; Springer: Heidelberg, Germany, 2014; Volume 2, p. 760. [Google Scholar]
- Petrov, V.A. (Ed.) Fluorinated Heterocyclic Compounds: Synthesis, Chemistry, and Applications; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Gakh, A.; Kirk, K.L. (Eds.) Fluorinated Heterocycles; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Muzalevskiy, V.M.; Nenajdenko, V.G.; Shastin, A.V.; Balenkova, E.S.; Haufe, G. Synthesis of trifluoromethyl pyrroles and their benzo analogues. Synthesis 2009, 20, 3905–3929. [Google Scholar]
- Serdyuk, O.V.; Abaev, V.T.; Butin, A.V.; Nenajdenko, V.G. Synthesis of fluorinated thiophenes and their analogues. Synthesis 2011, 2011, 2505–2529. [Google Scholar] [CrossRef]
- Serdyuk, O.V.; Muzalevskiy, V.M.; Nenajdenko, V.G. Synthesis and properties of fluoropyrroles and their analogues. Synthesis 2012, 2012, 2115–2137. [Google Scholar]
- Politanskaya, L.V.; Selivanova, G.A.; Panteleeva, E.V.; Tretyakov, E.V.; Platonov, V.E.; Nikul’shin, P.V.; Vinogradov, A.S.; Zonov, Y.A.V.; Karpov, V.M.; Mezhenkova, T.V.; et al. Organofluorine chemistry: Promising growth areas and challenges. Russ. Chem. Rev. 2019, 88, 425–569. [Google Scholar] [CrossRef]
- Muzalevskiy, V.M.; Nenajdenko, V.G.; Rulev, A.Y.U.; Ushakov, I.A.; Romanenko, G.V.; Shastin, A.V.; Balenkova, E.S.; Haufe, G. Selective synthesis of α-trifluoromethyl-β-arylenamines or vinylogous guanidinium salts by treatment of β-halo-β-trifluoromethylstyrenes with secondary amines under different conditions. Tetrahedron 2009, 65, 6991–7000. [Google Scholar] [CrossRef]
- Nenajdenko, V.G.; Muzalevskiy, V.M.; Shastin, A.V.; Balenkova, E.S.; Kondrashov, E.V.; Ushakov, I.A.; Rulev, A.Y. Fragmentation of Trifluoromethylated Alkenes and Acetylenes by N,N-Binucleophiles. Synthesis of Imidazolines or Imidazolidines (Oxazolidines) Controlled by Substituent. J. Org. Chem. 2010, 75, 5679–5688. [Google Scholar] [CrossRef]
- Rulev, A.Y.; Muzalevskiy, V.M.; Kondrashov, E.V.; Ushakov, I.A.; Shastin, A.V.; Balenkova, E.S.; Haufe, G.; Nenajdenko, V.G. A cascade approach to captodative trifluoromethylated enamines or vinylogous guanidinium salts: Aromatic substituents as switches of reaction direction. Eur. J. Org. Chem. 2010, 2010, 300–310. [Google Scholar]
- Muzalevskiy, V.M.; Shastin, A.V.; Balenkova, E.S.; Haufe, G.; Nenajdenko, V.G. Synthesis of alpha-trifluoromethyl-phenethylamines from alpha-trifluoromethyl beta-aryl enamines and beta-chloro-beta-(trifluoromethyl)styrenes. J. Fluor. Chem. 2011, 132, 1247–1253. [Google Scholar] [CrossRef]
- Muzalevskiy, V.M.; Sizova, Z.A.; Panyushkin, V.V.; Chertkov, V.A.; Khrustalev, V.N.; Nenajdenko, V.G. α,β-Disubstituted CF3-enones as a trifluoromethyl building block: Regioselective preparation of totally substituted 3-CF3-pyrazoles. J. Org. Chem. 2021, 86, 2385–2405. [Google Scholar] [CrossRef]
- Muzalevskiy, V.M.; Sizova, Z.A.; Abaev, V.T.; Nenajdenko, V.G. Synthesis of 2-trifluoromethylated quinolines from CF3-alkenes. Org. Biomol. Chem. 2021, 19, 4303–4319. [Google Scholar] [CrossRef]
- Muzalevskiy, V.M.; Sizova, Z.A.; Nenajdenko, V.G. Modular construction of functionalized 2-CF3-indoles. Org. Lett. 2021, 23, 5973–5977. [Google Scholar] [CrossRef] [PubMed]
- Muzalevskiy, V.M.; Nenajdenko, V.G.; Shastin, A.V.; Balenkova, E.S.; Haufe, G. α-Trifluoromethyl-β-aryl enamines in the synthesis of trifluoromethylated heterocycles by the Fischer and the Pictet–Spengler reactions. Tetrahedron 2009, 65, 7553–7561. [Google Scholar] [CrossRef]
- Muzalevskiy, V.M.; Sizova, Z.A.; Nenajdenko, V.G. An Efficient Synthesis of 2-CF3-3-Benzylindoles. Molecules 2021, 26, 5084. [Google Scholar] [CrossRef]
- Muzalevskiy, V.M.; Shastin, A.V.; Balenkova, E.S.; Haufe, G.; Nenajdenko, V.G. New approaches to the synthesis of 2-(trifluoromethyl)indole and 2-amino-3-(trifluoromethyl)quinoline. Russ. Chem. Bull. 2008, 57, 2217–2219. [Google Scholar] [CrossRef]
- Muzalevskiy, V.M.; Sizova, Z.A.; Abaev, V.T.; Nenajdenko, V.G. An Efficient Approach to 2-CF3-Indoles Based on ortho-Nitrobenzaldehydes. Molecules 2021, 26, 7365. [Google Scholar] [CrossRef] [PubMed]
- Blobaum, A.L.; Uddin, J.; Felts, A.S.; Crews, B.C.; Rouzer, C.A.; Marnett, L.J. The 2′-trifluoromethyl analogue of indomethacin is a potent and selective COX-2 inhibitor. ACS Med. Chem. Lett. 2013, 4, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, T.; Jiang, J.; Yang, M.-S.; Dingledine, R. Optimization studies of cinnamic amide EP2 antagonists. J. Med. Chem. 2014, 57, 4173–4184. [Google Scholar] [CrossRef] [PubMed]
- Trabbic, C.J.; Overmeyer, J.H.; Alexander, E.M.; Crissman, E.J.; Kvale, H.M.; Smith, M.A.; Erhardt, P.W.; Maltese, W.A. Synthesis and biological evaluation of indolyl-pyridinyl-propenones having either methuosis or microtubule disruption activity. J. Med. Chem. 2015, 5, 2489–2512. [Google Scholar] [CrossRef]
- Trabbic, C.J.; George, S.M.; Alexander, E.M.; Du, S.; Offenbacher, J.M.; Crissman, E.J.; Overmeyer, J.H.; Maltese, W.A.; Erhardt, P.W. Synthesis and biological evaluation of isomeric methoxy substitutions on anti-cancer indolyl-pyridinyl-propenones: Effects on potency and mode of activity. Eur. J. Med. Chem. 2016, 122, 79–91. [Google Scholar] [CrossRef]
- Rheinheimer, J.; Rath, R.; Kulkarni, S.; Rosenbaum, C.; Wiebe, C.; Brahm, L.; Siepe, I.; Haden, E.; Roehl, F.; Khanna, S.; et al. Indole and Azaindole Compounds with Substitued 6-Membered Aryl and Heteroaryl Rings as Agrochemical Fungicides. Patent WO201957660, 28 March 2019. [Google Scholar]
- Wang, S.; Ran, X.; Zhao, Y.; Yang, C.-Y.; Liu, L.; Bai, L.; McEachern, D.; Stuckey, J.; Meagher, J.L.; Sun, D.; et al. Preparation of pyridoindole, pyridazinoindole and pyrimidinoindole compounds as BET bromodomain inhibitors. U.S. Patent 20140256706 A1, 11 September 2014. [Google Scholar]
- Baar, M.; Blechert, S. Graphitic Carbon Nitride Polymer as a Recyclable Photoredox Catalyst for Fluoroalkylation of Arenes. Chem. Eur. J. 2015, 21, 526–530. [Google Scholar] [CrossRef]
- Rao, V.R.; Patil, K.T.; Kumar, D.; Sebastian, S.; Gupta, M.K.; Shin, D.-S. Facile metal-free visible-light-mediated chlorotrifluoromethylation of terminal alkenes. Monatsh. Chem. 2022, 153, 495–500. [Google Scholar] [CrossRef]
- Ellis, G.P.; Romney-Alexander, T.M. Cyanation of aromatic halides. Chem. Rev. 1987, 87, 779–794. [Google Scholar] [CrossRef]
- Couture, C.; Paine, A.J. Mechanisms and models for homogeneous copper mediated ligand exchange reactions of the type: CuNu + ArX → ArNu + CuX. Can. J. Chem. 1985, 63, 111–120. [Google Scholar] [CrossRef]
- Connor, J.A.; Leeming, S.W.; Price, R.J. Influence of substrate structure on copper(I)-assisted cyanide substitution in aryl halides. Chem. Soc. Perkin Trans. 1 1990, 1127–1132. [Google Scholar] [CrossRef]
- Shimizu, R.; Egami, H.; Nagi, T.; Chae, J.; Hamashima, Y.; Sodeoka, M. Direct C2-trifluoromethylation of indole derivatives catalyzed by copper acetate. Tetrahedron Lett. 2010, 51, 5947–5949. [Google Scholar] [CrossRef]
- Yuan, H.; Guo, L.; Liu, F.; Miao, Z.; Feng, L.; Gao, H. Copper-Catalyzed Tandem O-Vinylation of Arylhydroxylamines/[3,3]-Rearrangement/Cyclization: Synthesis of Highly Substituted Indoles and Benzoindoles. ACS Catal. 2019, 9, 3906–3912. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzalevskiy, V.M.; Sizova, Z.A.; Nenajdenko, V.G. Synthesis and Reactions of 3-Halogenated 2-CF3-Indoles. Molecules 2022, 27, 8822. https://doi.org/10.3390/molecules27248822
Muzalevskiy VM, Sizova ZA, Nenajdenko VG. Synthesis and Reactions of 3-Halogenated 2-CF3-Indoles. Molecules. 2022; 27(24):8822. https://doi.org/10.3390/molecules27248822
Chicago/Turabian StyleMuzalevskiy, Vasiliy M., Zoia A. Sizova, and Valentine G. Nenajdenko. 2022. "Synthesis and Reactions of 3-Halogenated 2-CF3-Indoles" Molecules 27, no. 24: 8822. https://doi.org/10.3390/molecules27248822
APA StyleMuzalevskiy, V. M., Sizova, Z. A., & Nenajdenko, V. G. (2022). Synthesis and Reactions of 3-Halogenated 2-CF3-Indoles. Molecules, 27(24), 8822. https://doi.org/10.3390/molecules27248822






