Abstract
We describe the design and synthesis of two isatin-tethered quinolines series (Q6a–h and Q8a–h), in connection with our research interest in developing novel isatin-bearing anti-tubercular candidates. In a previous study, a series of small molecules bearing a quinoline-3-carbohydrazone moiety was developed as anti-tubercular agents, and compound IV disclosed the highest potency with MIC value equal to 6.24 µg/mL. In the current work, we adopted the bioisosteric replacement approach to replace the 3,4,5-trimethoxy-benzylidene moiety in the lead compound IV with the isatin motif, a privileged scaffold in the TB drug discovery, to furnish the first series of target molecules Q6a–h. Thereafter, the isatin motif was N-substituted with either a methyl or benzyl group to furnish the second series Q8a–h. All of the designed quinoilne-isatin conjugates Q6a–h and Q8a–h were synthesized and then biologically assessed for anti-tubercular actions towards drug-susceptible, MDR, and XDR strains. Superiorly, the N-benzyl-bearing compound Q8b possessed the best activities against the examined M. tuberculosis strains with MICs equal 0.06, 0.24, and 1.95 µg/mL, respectively.
1. Introduction
Tuberculosis (TB) remains one of the world’s most lethal infectious illnesses, killing an estimated 1.5 million people each year [1]. In 2018, the WHO reported a range of estimated incident cases between 9.0 and 11.1 million patients in addition to notifying 7.0 million new cases of TB [2]. The gap between the estimated incident cases and the new ones is obvious, showing the large global range of TB patients lacking the access to health care [3,4]. Furthermore, the situation worsened as a result of multiple aggravating factors, including difficulty in obtaining therapy, as well as the poor tolerability and efficacy of therapeutic regimens due to the administration of long-term drug combinations consisting of 1st-line medications (isoniazid, pyrazinamide, rifampicin, and ethambutol) alongside the more toxic, expensive, and less efficacious second-line agents (fluoroquinolones, aminoglycosides, and clofazimine). These drug protocols cause anti-tuberculosis drug-induced hepatotoxicity (ATDH), which causes significant morbidity and mortality while decreasing treatment effectiveness. Finally, incomplete treatment reduces effectiveness and eventually contributes to failure and relapse, which complicates disease resolution and results in serious TB drug resistance.
There is currently a substantial amount of information available regarding the spread of M. tuberculosis strains that are resistant to both first-line and second-line drugs for tuberculosis treatment. [5]. This resistance was studied and defined by the WHO into two types known as multi-drug-resistant tuberculosis (MDR-TB) that is considered to be resistant to rifampicin or isoniazid, and the extensively drug-resistant (XDR) tuberculosis that is able to resist also at least fluoroquinolone as well as either kanamycin or amikacin [6]. According to the World Health Organization (WHO), there were approximately 500,000 newly reported cases of rifampicin resistance (RR-TB) in 2018. Of these, 78% of the patients were MDR TB and 8.5% were XDR TB [2]. For patients suffering from XDR-TB, the efficiency of the standard treatments was quite limited. New hopes were provided through the approval of bedaquiline by the FDA. Rapidly, the failure of the clinically introduced drug by its first resistant M. tuberculosis isolate dramatically terminated the story [1]. As a result, the creation of novel anti-TB medications is urgently required. Novel drug candidates need to be able to shorten the therapeutic time period and simplify the actual treatment protocols, to enhance tolerance and to be effective towards resistant strains (MDR-TB and XDR-TB).
Quinoline, a fused benzo[b]pyridine heterocycle, stands out as one of the most useful and versatile scaffolds endowed with different antibacterial activities. Interestingly, several quinoline-based anti-infectives are currently in clinical use as the antibacterial drug ciprofloxacin I (Figure 1), in addition to the recently identified anti-tubercular medications bedaquiline II and mefloquine III (Figure 1) [7,8,9,10]. Moreover, several research teams have reported a different series of small molecules tethered with the quinoline scaffold as promising anti-tubercular candidates [11,12,13,14,15,16,17,18,19]. In a recent study, novel small molecules bearing a quinoline-3-carbohydrazone moiety were synthesized and examined for the anti-tubercular impact on the drug-susceptible M. tuberculosis strain. The developed molecules exhibited moderate anti-tubercular activity and a disclosed MIC range of 6.24–99.84 µg/mL. Within this series, quinoline-derivative IV (Figure 1) disclosed the highest activity with a MIC value equal to 6.24 µg/mL [20].
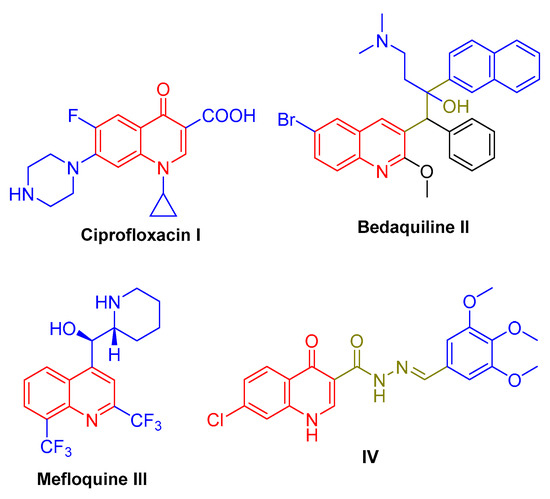
Figure 1.
Structure for certain quinoline-based anti-tubercular drugs I–III, as well as the lead anti-tubercular compound IV.
Isatin is an endogenous motif that has been identified in many organisms. Isatin is found in numerous naturally occurring substances, including marine natural products, alkaloids, and fungal metabolites, in addition to being endogenously present in mammalian tissues and fluids, including those of humans and other mammals [21]. Isatin nucleus represents a versatile scaffold for chemical modifications, where its derivatives disclosed diverse pharmacological actions for the treatment of diverse diseases and disorders [21,22,23,24,25,26,27]. Isatin-tethered molecules such as nintedanib, sunitinib, semaxanib, and orantinib have either received clinical approval (nintedanib and sunitinib) or are currently the subject of clinical trials (semaxanib and orantinib) [28,29]. Such successful clinical applications, as well as their broad-spectrum pharmacological activities and the ease of the structural modifications, inspired and paved the way for the researchers to create a lot of isatin derivatives with significant structural diversity. In the last two decades, the isatin motif has attracted the researchers’ attention as a promising privileged skeleton that could be exploited for the discovery and development of tuberculosis medications. Accordingly, a large number of isatin-based series were developed and biologically explored for their potential anti-tubercular actions towards drug-susceptible strains as well as towards drug-resistant TB strains [30,31,32,33,34,35,36].
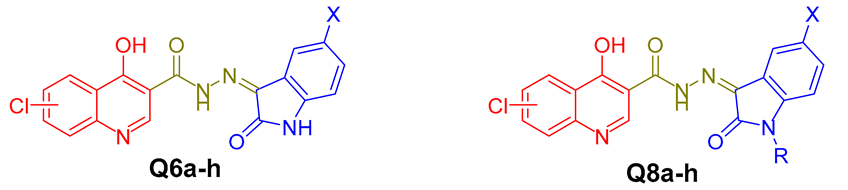
In light of the aforementioned findings, and following up on our earlier efforts to identify promising anti-tubercular candidates [35,36,37,38,39,40,41], we decided to broaden our investigation by bioisosterically replacing the 3,4,5-trimethoxy benzylidene moiety in lead compound IV with the privileged isatin motif to afford the first series of targeted molecules Q6a–h (Figure 2). Furthermore, the isatin motif in series Q6 was N-substituted with either a methyl or benzyl group to furnish the second series of target molecules Q8a–h (Figure 2). All of the designed quinoilne isatin conjugates Q6a–h and Q8a–h were synthesized and then biologically assessed for their anti-tubercular actions towards drug-susceptible MDR and XDR T.B. strains.

Figure 2.
Design for the target quinoline-based molecules Q6a–h and Q8a–h.
2. Results and Discussion
2.1. Synthestic Chemistry
The presented Scheme 1 illustrates the synthetic approach used to create the novel desired quinoline-isatin conjugates Q6a–h and Q8a–h. Ethyl anilinomethylene malonate 2a–b was prepared through the condensation of 3-chloroaniline 1a and 4-chloroaniline 1b with diethyl ethoxymethylenemalonate. Then, the cyclization of 2a–b in refluxing diphenyl ether produced the corresponding ethyl esters of 4-hydroxyquinoline-3-carboxylic acids 3a–b, which then interacted with hydrazine hydrate (NH2NH2.H2O, 99%) in refluxing ethanol to produce the acid hydrazides 4a–b. Finally, the target quinoline-isatin conjugates Q6a–h and Q8a–h were obtained by the condensation reaction of hydrazides 4a–b with diverse 5-substituted isatins (5a–d) or N-substituted isatins (7a–d), respectively, in glacial acetic acid under refluxing temperature.
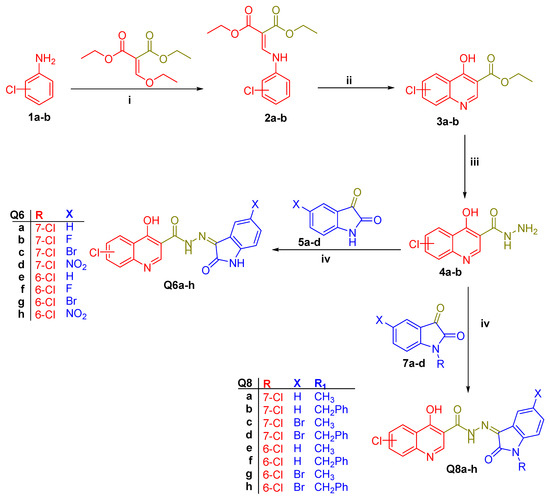
Scheme 1.
Synthetic route for target quinoline-isatin conjugates Q6a–h and Q8a–h; i: reflux 3 hours; ii: phenyl ether, reflux, 2 hours; iii: NH2NH2.H2O, reflux, 12 hours; and iv: glacial acetic acid, reflux, 4 hours.
The IR spectra for quinoline-isatin conjugates Q6a–h and Q8a–h revealed absorption bands for the carbonyl functionality within the region 1629–1715 cm−1, beside the absorption bands that assigned to the amino (NH) functionalities in the 3249–3413 cm−1 region. The 1H NMR spectra for conjugates Q6a–h and Q8a–h exhibited two D2O-exchangeable singlets corresponding to the –CONH proton of Z and E isomers within the region δ 10.55–11.74 ppm, whereas another two D2O exchangeable singlet signals at δ 13.71–15.19 ppm were assigned for the Z and E isomers of OH quinoline. In addition, the NH protons of the isatin motif for derivatives Q6a–h were identified around δ 8.88–9.39 ppm as a singlet signals. The 13C NMR spectrum for conjugate Q6b disclosed characteristic signals resonating around δ 161.51–176.14 ppm that were attributable to the Z and E isomers of carbonyl groups carbons, while compounds Q6f and Q6g showed one isomer only, and the carbons of carbonyl (C=O) functionality appeared in the region δ 161.54–175.46 ppm.
2.2. Biological Evaluation
2.2.1. Anti-Tubercular Effect for Quinolines Q6a–h and Q8a–h
The anti-tubercular activities for quinoline derivatives Q6a–h and Q8a–h towards RCMB 010126 M. tuberculosis were examined exploiting the microplate Alamar blue (MABA) protocol [42]. The used reference anti-tubercular drug was isoniazid. Table 1 summarises the anti-mycobacterial results, which are expressed as minimum inhibitory concentrations (MICs).

Table 1.
Anti-tubercular activity for the synthesized quinolines (Q6a–h) and (Q8a–h) against M. tuberculosis (MIC).
The outcomes demonstrated that the majority of the quinolines reported here had potent to moderate anti-M. tuberculosis activity, with the MICs range between 0.06 and 7.81 g/mL, with an exception for quinolines Q6c and Q6d (MIC = 31.25 and 15.63 µg/mL, respectively). Isatin-tethered derivative Q8b outperformed the lead compound IV in terms of growth inhibition towards M. tuberculosis, displaying a MIC of 0.06 g/mL, representing a 100-fold increase in activity (MIC for IV = 6.24 µg/mL). Moreover, compound Q8h also has potent anti-mycobacterial action with a MIC value of 0.12 µg/mL that equals the MIC for the standard isoniazide drug. In addition, quinolines Q6a, Q6b, Q8a, and Q8f exhibited potent anti-tubercular activity with MIC values in the range of 0.24–0.98 µg/mL, whereas quinolines Q6e–Q6h, Q8c, Q8e, and Q8g displayed moderate action with a range for the MIC values batween 1.95 and 7.81 µg/mL.
Studying the structural activity relationships of these quinoline-based hybrids revealed that their anti-tubercular actions have been influenced by two essential factors: the incorporation of a substituent at C-5 and N-1 of the oxindole motif, and the position of the chloro substituent (C-7 vs. C-6) at the quinoline moiety. With regard to the effect of the C-5 substitution within the oxindole motif within series Q6, it was found that grafting a 5-fluoro substituent resulted in an improved (for compound Q6b; MIC = 0.24 µg/mL) or maintained (for compound Q6f; MIC = 3.9 µg/mL) antitubercular impact in comparison to their unsubstituted analogues Q6a and Q6e (MICs = 0.48 and 3.9 µg/mL, respectively). The incorporation of 5-bromo (compounds Q6c and Q6g; MICs = 31.25 µg/mL and 7.81 µg/mL, respectively) or 5-nitro substituents (compounds Q6d and Q6h; MICs = 15.63 and 7.81 µg/mL, respectively), on the other hand, has resulted in a decreased anti-tubercular action in comparison to the unsubstituted analogues Q6a and Q6e.
Furthermore, upon investigation of the impact of the decoration of the oxindole motif with a 5-bromo substituent within series 8, we found that such substitution has led to a decreased activity for 7-chloroquinoline-bearing derivatives Q8c and Q8d (MICs = 0.98 and 15.63 µg/mL, respectively) with regard to their unsubstituted counterparts Q8a and Q8b (MICs = 0.24 and 0.06 µg/mL, respectively), whereas this substitution maintained or enhanced the anti-tubercular activity for the 6-chloroquinoline-bearing derivatives Q8g and Q8h (MICs = 1.95 and 0.12 µg/mL, respectively) in comparison to their unsubstituted analogues Q8e and Q8f (MICs = 1.95 and 0.98 µg/mL, respectively).
Thereafter, we explored the obtained results to assess the effect of the N-methylation and N-benzylation of the oxindole motif on the anti-tubercular activity of series 8. The results revealed that N-methylation enhanced the activity of both 7-chloroquinoline-bearing derivatives Q8a and Q8c (MICs = 0.24 and 0.98 µg/mL, respectively) and 6-chloroquinoline-bearing derivatives Q8e and Q8g (MICs = 1.95 µg/mL and 1.95 µg/mL, respectively). Similarly, the N-benzylation of the oxindole motif improved the activity of both the 7-chloroquinoline-bearing molecules Q8b and Q8d (MICs = 0.06 and 15.63 µg/mL, respectively) as well as the 6-chloroquinoline-bearing derivatives Q8f and Q8h (MICs = 0.98 µg/mL and 0.12 µg/mL, respectively). It is worth mentioning that the N-benzylated derivatives Q8b, Q8f, and Q8h (MICs = 0.06 µg/mL, 0.98 µg/mL, and 0.12 µg/mL) displayed better anti-tubercular activity than their corresponding N-methylated counterparts Q8a, Q8e, and Q8g (MICs = 0.24, 1.95, and 1.95 µg/mL).
Finally, upon comparing the anti-tubercular effect of 7-chloroquinoline-bearing derivatives Q6a–Q6d against their 7-chloroquinoline-bearing counterparts Q6e–Q6h, it was found that the introduction of the chloro substituent at C-7 is more beneficial for the activity of hybrids containing the un- and 5-F substituted oxindole motif (compounds Q6a and Q6b; MIC = 0.24 and 0.48 µg/mL, respectively), whereas grafting the chloro substituent at C-6 was found to be more advantageous than C-7 for hybrids endowed with the larger 5-Br and 5-NO2 substituents (compounds Q6g and Q6h; MIC = 7.81 µg/mL for both).
2.2.2. Anti-Tubercular Activities toward MDR and XDR T.B
The activity for the target quinolines Q6a–h and Q8a–h was assessed against the MDR strain (FJ05120) and the XDR strain (FJ05195). In Table 2, the assay outcomes are shown as minimum inhibitory concentration (MIC) values.

Table 2.
Anti-tubercular activities for the target quinolines (Q6a–h) and (Q8a–h) against MDR and XDR M. tuberculosis.
Compounds Q8b and Q8h showed superior activities against both MDR and XDR M. tuberculosis strains with MIC values equal to (0.24 µg/mL and 0.98 µg/mL) for MDR and (1.95 µg/mL, 3.9 µg/mL) for XDR, respectively. Additionally, quinolines Q6a, Q6b, Q8a, Q8c, Q8e, and Q8f exerted good activity against the MDR strain, with MIC values of 3.9, 3.9, 7.81, 3.9, 7.81, and 7.81 µg/mL, respectively (Table 2), whereas compounds Q6a, Q6b, Q8c, and Q8f displayed moderate anti-tubercular activity against the XDR strain, with a MIC range from 7.81 to 15.63 µg/mL.
2.2.3. Cytotoxicity against Non-Tumorous Human Cells
In this study, T. Mosmann’s MTT colorimetric test was used to evaluate the cytotoxic potential of isatin-tethered quinoline derivatives Q8b and Q8h towards the human non-tumorigenic WI-38 lung fibroblast cell line [43] (Table 3). Notably, both examined quinolines Q8b and Q8h had no cytotoxic effect on WI-38 cells, with IC50 values of 36.6 ± 0.9 and 42.8 ± 1.12 μM, respectively.

Table 3.
Impact of quinolines Q8b and Q8h toward the human non-tumorigenic lung fibroblast WI-38 cell line.
3. Conclusions
Briefly, two sets of chemically synthesized isatin-tethered quinolines Q6a–h and Q8a–h were assessed for their anti-tubercular activities towards a drug-susceptible strain, as well as towards the MDR and XDR strains. The design of these molecules is based on the bioisosteric replacement for the 3,4,5-trimethoxy-benzylidene motif in the lead compound IV with the privileged isatin motif to furnish series Q6. Then, the isatin motif was N-substituted with either a methyl or benzyl group to furnish the second series Q8. The results demonstrated that the majority of the quinolines that were evaluated had powerful to moderate potency against drug-susceptible M. tuberculosis, with MICs ranging from 0.06 to 7.81 g/mL. Among the investigated molecules, compound Q8b is the most potent counterpart with MIC = 0.06 µg/mL; it represents about 100-fold enhanced activity compared to the lead compound IV (MIC = 6.24 µg/mL). Furthermore, the assessment of the activities against the MDR and XDR strains revealed that quinolines Q8b and Q8h showed superior activities against both MDR and XDR strains with MIC values equal to 0.24 µg/mL and 0.98 µg/mL for MDR and 1.95 µg/mL and 3.9 µg/mL for XDR, respectively. In the cytotoxicity MTT assay, quinolines Q8b and Q8h disclosed non-significant activity (IC50 = 36.6 ± 0.96 µM and 42.8 ± 1.12 µM, respectively) toward non-tumorigenic human WI-38 lung fibroblast cells.
4. Experimental
4.1. Chemistry
4.1.1. General
Melting points (m.p.) were measured by a Stuart melting point apparatus and were uncorrected. The NMR spectra were recorded utilizing a Bruker 400 MHz FT-NMR spectrometer (Bruker, Billerica, MA, USA). Both 1H and 13C spectra were run at 400 MHz and 101 MHz, respectively, in DMSO-d6. Moreover, a Bruker FT-IR spectrophotometer was used to record the IR spectra.
4.1.2. Preparation of 4-Hydroxyquinoline-3-Carboxylic Acid Ethyl Esters 3a–b
A mixture of 3/4-chloroanilines 1a–b (1.9 g, 15 mmol) and diethyl ethoxymethylenemalonate (4.3 g, 20 mmol) was heated at 130 °C for three hours, where the produced ethyl alcohol was evaporated off. The crude mixture was filtered by a sintered funnel, washed with petroleum ether, and dried. The malonates 2a–b (3.6 g, 12 mol) were refluxed in diphenylether (40 mL) for 2 h. Upon cooling, the resultant solid was filtered off, dried, and recrystallized from acetonitrile to yield the corresponding hydroxyquinoline ethyl esters 3a–b [44].
4.1.3. Preparation of Quinoline-3-Carbohydrazide Derivatives 4a–b
After adding hydrazine hydrate 99% (1 mL, 20 mmol) to a solution of quinoline ethyl esters 3a–b (1 g, 4 mmol) in absolute ethanol (12 mL), the mixture was refluxed for 12 h. Before the mixture was added to the crushed ice, the excess solvent was removed. The solid that had precipitated was filtered through, washed with water, and then recrystallized from a mixture of DMF and water and dried to produce the corresponding hydroxyquinoline-3-carbohydrazides 4a–b [44].
4.1.4. Preparation of Target Quinoline-Isatin Conjugates Q6a–h and Q8a–h
The appropriate isatin derivative 5a–d or 7a–b (1.2 mmol) was added to a solution of quinoline-3-carbohydrazide derivatives 4a–b (0.35 g, 1.5 mmol) in acetic acid (15 mL). This mixture was reflux-heated for 4 h. The target isatin-tethered quinolines Q6a–h and Q8a–h were obtained in 72–87% yield by recrystallizing the precipitate from DMF/ethanol after it had been filtered off while still hot and washed with hot ethanol.
4.2. Biological Evaluation
4.2.1. Anti-Mycobacterial Activity
The MICs for the target isatin-tethered quinolines Q6a–h and Q8a–h against drug-susceptible, MDR, and XDR M. tuberculosis strains have been evaluated by the microplate alamar blue (MABA) assay, as described previously [36,45]. The detailed methodology was provided in the Supplementary Materials.
4.2.2. MTT Colorimetric Assay
The cytotoxic activity for isatin-tethered quinolines Q8b and Q8h against lung WI-38 cells was assessed using T. Mosmann’s MTT colorimetric assay [43,46]. The detailed methodology was provided in the Supplementary Materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27248807/s1, Chapter S1. Characterisation details (NMR, IR and elemental analysis) for the target quinolines (6a–h and 8a–h). Chapter S2. Microplate Alamar Blue Anti-Tubercular Assay. Chapter S3. MTT Cytotoxicity Assay. Figures S1–S19: The 1H, 13C NMR spectra for conjugates Q6a–h and Q8a–h. Figures S20–S23: The IR spectra for quinoline-isatin conjugates 6a,6d,6g,8h.
Author Contributions
Conceptualization, M.A.A., W.M.E. and M.A.S.; methodology, M.A.A., H.A., T.A.-W., M.M.A.-A. and T.A.M.; validation, M.A.A., H.A., T.A.-W. and T.A.M.; formal analysis, M.M.A.-A. and H.A.; investigation, T.A.-W., W.M.E. and M.A.S.; resources, M.M.A.-A. and M.A.S.; data curation, H.A. and M.A.S.; writing—original draft preparation, M.A.A., M.M.A.-A., W.M.E. and M.A.S.; writing—review and editing, M.A.A., T.A.M. and W.M.E.; supervision, W.M.E. and M.A.S.; project administration, W.M.E.; funding acquisition, T.A.-W. and T.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Small Groups Project under grant number (RGP.1/346/43). The authors extend their appreciation to the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R25), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Egyptian Russian University, Cairo, Egypt is highly appreciated for supporting this research. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Small Groups Project under grant number (RGP.1/346/43). The authors extend their appreciation to the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R25), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Coulibaly, S.; Cimino, M.; Ouattara, M.; Lecoutey, C.; Buchieri, M.V.; Alonso-Rodriguez, N.; Briffotaux, J.; Mornico, D.; Gicquel, B.; Rochais, C.; et al. Phenanthrolinic analogs of quinolones show antibacterial activity against M. tuberculosis. Eur. J. Med. Chem. 2020, 207, 112821. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Global Tuberculosis Report. 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-eng.pdf?sequence¼1&isAllowed¼y (accessed on 1 October 2022).
- World Health Organization Global Tuberculosis Report. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua¼1 (accessed on 1 October 2022).
- Ribeiro, R.C.; de Marins, D.B.; Di Leo, I.; Gomes, L.D.S.; de Moraes, M.G.; Abbadi, B.L.; Villela, A.D.; da Silva, W.F.; da Silva, L.C.R.; Machado, P.; et al. Anti-tubercular profile of new selenium-menadione conjugates against Mycobacterium tuberculosis H37Rv (ATCC 27294) strain and multidrug-resistant clinical isolates. Eur. J. Med. Chem. 2020, 209, 112859. [Google Scholar] [CrossRef] [PubMed]
- Ghiano, D.G.; Recio-Balsells, A.; Bortolotti, A.; Defelipe, L.A.; Turjanski, A.; Morbidoni, H.R.; Labadie, G.R. New one-pot synthesis of anti-tuberculosis compounds inspired on isoniazid. Eur. J. Med. Chem. 2020, 208, 112699. [Google Scholar] [CrossRef]
- Verma, S.K.; Verma, R.; Verma, S.; Vaishnav, Y.; Tiwari, S.; Rakesh, K. Anti-tuberculosis activity and its structure-activity relationship (SAR) studies of oxadiazole derivatives: A key review. Eur. J. Med. Chem. 2020, 209, 112886. [Google Scholar] [CrossRef] [PubMed]
- Matviiuk, T.; Madacki, J.; Mori, G.; Orena, B.S.; Menendez, C.; Kysil, A.; André-Barrès, C.; Rodriguez, F.; Korduláková, J.; Mallet-Ladeira, S.; et al. Pyrrolidinone and pyrrolidine derivatives: Evaluation as inhibitors of InhA and Mycobacterium tuberculosis. Eur. J. Med. Chem. 2016, 123, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, S.; Iso, Y.; Wan, B.; Franzblau, S.; Kozikowski, A.P. Design, Synthesis, and SAR Studies of Mefloquine-Based Ligands as Potential Antituberculosis Agents. Chemmedchem 2006, 1, 593–597. [Google Scholar] [CrossRef]
- Keri, R.S.; Patil, S.A. Quinoline: A promising antitubercular target. Biomed. Pharmacother. 2014, 68, 1161–1175. [Google Scholar] [CrossRef]
- Eswaran, S.; Adhikari, A.V.; Pal, N.K.; Chowdhury, I.H. Design and synthesis of some new quinoline-3-carbohydrazone derivatives as potential antimycobacterial agents. Bioorganic Med. Chem. Lett. 2010, 20, 1040–1044. [Google Scholar] [CrossRef]
- Briguglio, I.; Piras, S.; Corona, P.; Pirisi, M.; Jabes, D.; Carta, A. SAR and Anti-Mycobacterial Activity of Quinolones and Triazoloquinolones: An Update. Anti-Infect. Agents 2012, 11, 75–89. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, G.; Mangla, V.; Gupta, M.K. Quinoline and quinolones: Promising scaffolds for future antimycobacterial agents. J. Enzym. Inhib. Med. Chem. 2015, 30, 492–504. [Google Scholar] [CrossRef]
- Thomas, K.; Adhikari, A.V.; Telkar, S.; Chowdhury, I.H.; Mahmood, R.; Pal, N.K.; Row, G.; Sumesh, E. Design, synthesis and docking studies of new quinoline-3-carbohydrazide derivatives as antitubercular agents. Eur. J. Med. Chem. 2011, 46, 5283–5292. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-L.; Teng, F.; Xiong, L.; Li, X.; Gao, C.; Yu, L.-T. Discovery of quinolone derivatives as antimycobacterial agents. RSC Adv. 2021, 11, 24095–24115. [Google Scholar] [CrossRef] [PubMed]
- Imramovský, A.; Polanc, S.; Vinšová, J.; Kočevar, M.; Jampílek, J.; Rečková, Z.; Kaustová, J. A new modification of anti-tubercular active molecules. Bioorg. Med. Chem. 2007, 15, 2551–2559. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, A.; Malde, A.; Coutinho, E.; Jain, R. Synthesis, anti-tuberculosis activity, and 3D-QSAR study of ring-substituted-2/4-quinolinecarbaldehyde derivatives. Bioorg. Med. Chem. 2006, 14, 7302–7310. [Google Scholar] [CrossRef] [PubMed]
- Venugopala, K.N.; Uppar, V.; Chandrashekharappa, S.; Abdallah, H.H.; Pillay, M.; Deb, P.K.; Morsy, M.A.; Aldhubiab, B.E.; Attimarad, M.; Nair, A.B.; et al. Cytotoxicity and antimycobacterial properties of pyrrolo[1, 2-a] quinoline derivatives: Molecular target identification and molecular docking studies. Antibiotics 2020, 9, 233. [Google Scholar] [CrossRef]
- Insuasty, D.; Vidal, O.; Bernal, A.; Marquez, E.; Guzman, J.; Insuasty, B.; Quiroga, J.; Svetaz, L.; Zacchino, S.; Puerto, G.; et al. Antimicrobial Activity of Quinoline-Based Hydroxyimidazolium Hybrids. Antibiotics 2019, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Upadhayaya, R.S.; Vandavasi, J.K.; Kardile, R.A.; Lahore, S.V.; Dixit, S.S.; Deokar, H.S.; Shinde, P.D.; Sarmah, M.P.; Chattopadhyaya, J. Novel quinoline and naphthalene derivatives as potent antimycobacterial agents. Eur. J. Med. Chem. 2010, 45, 1854–1867. [Google Scholar] [CrossRef]
- Abdelrahman, M.A.; Salama, I.; Gomaa, M.S.; Elaasser, M.M.; Abdel-Aziz, M.M.; Soliman, D.H. Design, synthesis and 2D QSAR study of novel pyridine and quinolone hydrazone derivatives as potential antimicrobial and antitubercular agents. Eur. J. Med. Chem. 2017, 138, 698–714. [Google Scholar] [CrossRef]
- Cheke, R.S.; Patil, V.M.; Firke, S.D.; Ambhore, J.P.; Ansari, I.A.; Patel, H.M.; Shinde, S.D.; Pasupuleti, V.R.; Hassan, I.; Adnan, M.; et al. Therapeutic Outcomes of Isatin and Its Derivatives against Multiple Diseases: Recent Developments in Drug Discovery. Pharmaceuticals 2022, 15, 272. [Google Scholar] [CrossRef]
- Brandão, P.; Marques, C.; Burke, A.J.; Pineiro, M. The application of isatin-based multicomponent-reactions in the quest for new bioactive and druglike molecules. Eur. J. Med. Chem. 2020, 211, 113102. [Google Scholar] [CrossRef]
- Chowdhary, S.; Shalini; Arora, A.; Kumar, V. A Mini Review on Isatin, an Anticancer Scaffold with Potential Activities against Neglected Tropical Diseases (NTDs). Pharmaceuticals 2022, 15, 536. [Google Scholar] [CrossRef] [PubMed]
- Varun; Sonam; Kakkar, R. Isatin and its derivatives: A survey of recent syntheses, reactions, and applications. MedChemComm 2019, 10, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Cheke, R.S.; Firke, S.D.; Patil, R.R.; Bari, S.B. Isatin: New hope against convulsion. Cent. Nerv. Syst. Agents Med. Chem. (Former. Curr. Med. Chem.-Cent. Nerv. Syst. Agents) 2018, 18, 76–101. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.; Ghabbour, H.A.; Eldehna, W.M.; Qabeel, M.M.; Fun, H.K. Synthesis, crystal structure, and biological activity of cis/trans amide rotomers of (Z)-N′-(2-Oxoindolin-3-ylidene) formohydrazide. J. Chem. 2014, 2014, 760434. [Google Scholar] [CrossRef]
- Taghour, M.S.; Elkady, H.; Eldehna, W.M.; El-Deeb, N.M.; Kenawy, A.M.; Elkaeed, E.B.; Alsfouk, A.A.; Alesawy, M.S.; Metwaly, A.M.; Eissa, I.H. Design and synthesis of thiazolidine-2,4-diones hybrids with 1,2-dihydroquinolones and 2-oxindoles as potential VEGFR-2 inhibitors: In-vitro anticancer evaluation and in-silico studies. J. Enzym. Inhib. Med. Chem. 2022, 37, 1903–1917. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Al-Wabli, R.I.; Almutairi, M.S.; Keeton, A.B.; Piazza, G.A.; Abdel-Aziz, H.A.; Attia, M.I. Synthesis and biological evaluation of certain hydrazonoindolin-2-one derivatives as new potent anti-proliferative agents. J. Enzym. Inhib. Med. Chem. 2018, 33, 867–878. [Google Scholar]
- Eldehna, W.M.; Fares, M.; Ibrahim, H.S.; Alsherbiny, M.A.; Aly, M.H.; Ghabbour, H.A.; Abdel-Aziz, H.A. Synthesis and cytotoxic activity of biphenylurea derivatives containing indolin-2-one moieties. Molecules 2016, 21, 762. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, S.; Gao, C.; Fan, J.; Zhao, F.; Lv, Z.-S.; Feng, L.-S. Isatin hybrids and their anti-tuberculosis activity. Chin. Chem. Lett. 2016, 28, 159–167. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, G.-Q.; Liu, X.; Zhang, Z.; Feng, L.-S.; Liu, M.-L. Isatin Derivatives with Potential Antitubercular Activities. J. Heterocycl. Chem. 2018, 55, 1263–1279. [Google Scholar] [CrossRef]
- Karalı, N.; Gürsoy, A.; Kandemirli, F.; Shvets, N.; Kaynak, F.B.; Özbey, S.; Kovalishyn, V.; Dimoglo, A. Synthesis and structure–antituberculosis activity relationship of 1H-indole-2,3-dione derivatives. Bioorganic Med. Chem. 2007, 15, 5888–5904. [Google Scholar] [CrossRef]
- Shahlaei, M.; Fassihi, A.; Nezami, A. QSAR study of some 5-methyl/trifluoromethoxy-1H-indole-2,3-dione-3-thiosemicarbazone derivatives as anti-tubercular agents. Res. Pharm. Sci. 2009, 4, 123–131. [Google Scholar]
- Güzel, Ö.; Karalı, N.; Salman, A. Synthesis and antituberculosis activity of 5-methyl/trifluoromethoxy-1H-indole-2,3-dione 3-thiosemicarbazone derivatives. Bioorganic Med. Chem. 2008, 16, 8976–8987. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; El Hassab, M.A.; Abdelshafi, N.A.; Sayed, F.A.-Z.; Fares, M.; Al-Rashood, S.T.; Elsayed, Z.M.; Abdel-Aziz, M.M.; Elkaeed, E.B.; Elsabahy, M.; et al. Development of potent nanosized isatin-isonicotinohydrazide hybrid for management of Mycobacterium tuberculosis. Int. J. Pharm. 2021, 612, 121369. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, Z.M.; Eldehna, W.M.; Abdel-Aziz, M.M.; El Hassab, M.A.; Elkaeed, E.B.; Al-Warhi, T.; Abdel-Aziz, H.A.; Abou-Seri, S.M.; Mohammed, E.R. Development of novel isatin–nicotinohydrazide hybrids with potent activity against susceptible/resistant Mycobacterium tuberculosis and bronchitis causing–bacteria. J. Enzym. Inhib. Med. Chem. 2021, 36, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Fares, M.; Abdel-Aziz, M.M.; Abdel-Aziz, H.A. Design, Synthesis and Antitubercular Activity of Certain Nicotinic Acid Hydrazides. Molecules 2015, 20, 8800–8815. [Google Scholar] [CrossRef] [PubMed]
- Abo-Ashour, M.F.; Eldehna, W.M.; George, R.F.; Abdel-Aziz, M.M.; Elaasser, M.M.; Abou-Seri, S.M.; Gawad, N.M.A. Synthesis and biological evaluation of 2-aminothiazole-thiazolidinone conjugates as potential antitubercular agents. Futur. Med. Chem. 2018, 10, 1405–1419. [Google Scholar] [CrossRef] [PubMed]
- Soliman, D.H.; Eldehna, W.M.; Ghabbour, H.A.; Kabil, M.M.; Abdel-Aziz, M.M.; Abdel-Aziz, H.A. Novel 6-phenylnicotinohydrazide derivatives: Design, synthesis and biological evaluation as a novel class of antitubercular and antimicrobial agents. Biol. Pharm. Bull. 2017, 40, 1883–1893. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; Eldehna, W.M.; Fares, M.; Al-Rashood, S.T.A.; Al-Rashood, K.A.; Abdel-Aziz, M.M.; Soliman, D.H. Synthesis, biological evaluation and 2D-QSAR study of halophenyl bis-hydrazones as antimicrobial and antitubercular agents. Int. J. Mol. Sci. 2015, 16, 8719–8743. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.K.; Eldehna, W.M.; Fares, M.; Elsaman, T.; Abdel-Aziz, M.M.; Soliman, D.H. Synthesis, in vitro and in silico studies of some novel 5-nitrofuran-2-yl hydrazones as antimicrobial and antitubercular agents. Biol. Pharm. Bull. 2015, 38, 1617–1630. [Google Scholar] [CrossRef]
- Collins, L.; Franzblau, S.G. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 1997, 41, 1004–1009. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Srivatava, N.; Kumar, A. Synthesis of substituted-4-oxo-1,4-dihydro-3-[1-oxo-2-hydrazino-3-{p-toluenesulfon}] quinoline Derivatives and their Biological Activity Against Bacterial Infections. Orient. J. Chem. 2013, 29, 507–511. [Google Scholar] [CrossRef][Green Version]
- Franzblau, S.G.; Witzig, R.S.; McLaughlin, J.C.; Torres, P.; Madico, G.; Hernandez, A.; Degnan, M.T.; Cook, M.B.; Quenzer, V.K.; Ferguson, R.M.; et al. Rapid, Low-Technology MIC Determination with Clinical Mycobacterium tuberculosis Isolates by Using the Microplate Alamar Blue Assay. J. Clin. Microbiol. 1998, 36, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Hagras, M.M.A.; Deeb, E.; Elzahabi, H.S.A.; Elkaeed, E.B.; Mehany, A.B.M.; Eissa, I.H. Discovery of new quinolines as potent colchicine binding site inhibitors: Design, synthesis, docking studies, and anti-proliferative evaluation. J. Enzym. Inhib. Med. Chem. 2021, 36, 640–658. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).