Cashew (Anacardium occidentale) Nut-Shell Liquid as Antioxidant in Bulk Soybean Oil
Abstract
1. Introduction
2. Results and Discussion
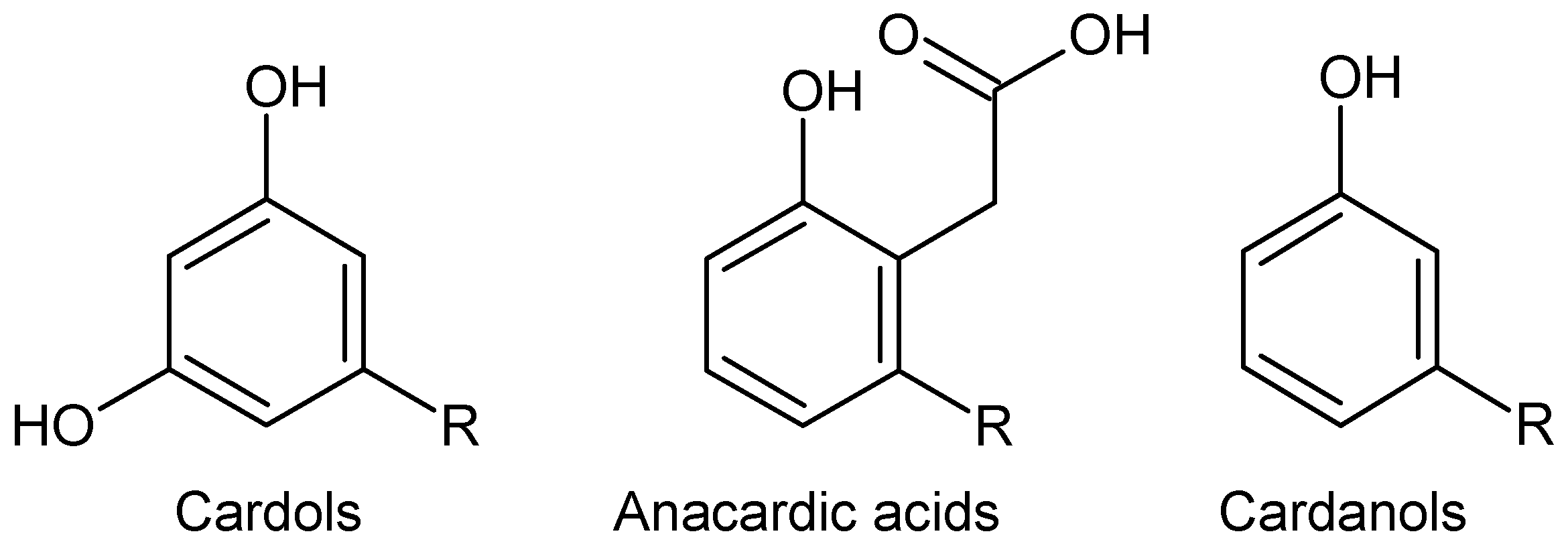
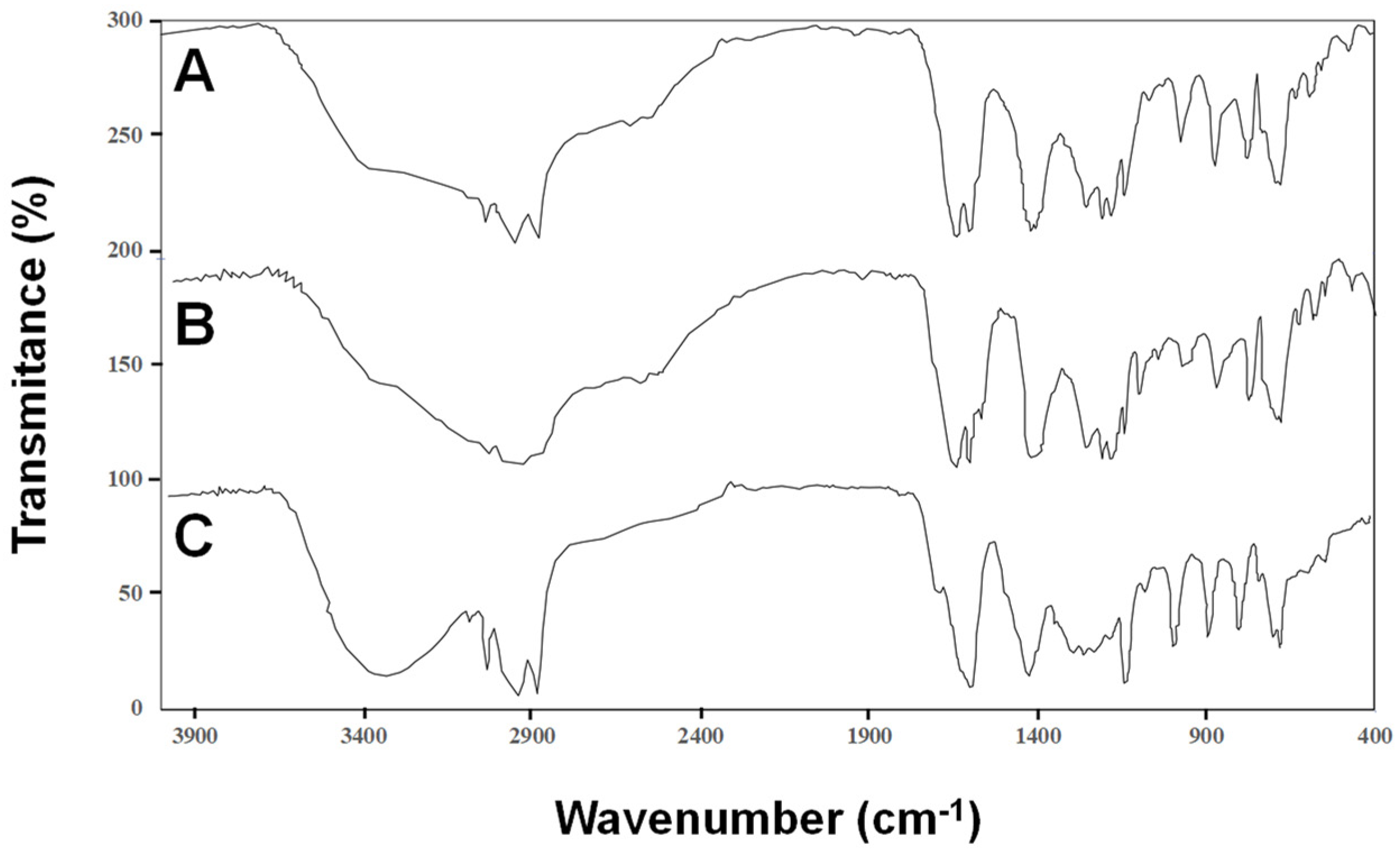
2.1. Composition of CNSL and Its Fractions
2.2. Accelerated Storage Experiments
3. Materials and Methods
3.1. Chemicals
3.2. Plant Materials
3.3. Cashew Nut-Shell Liquid (CNSL) Extraction
3.4. CNSL Fractionation
3.5. TLC and Fourier-Transform Infrared Spectroscopy (FTIR)
3.6. Accelerated Oxidation Assays
3.7. Peroxide Value
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Viana da Silva, M.; Santos, M.R.C.; Alves Silva, I.R.; Macedo Viana, E.B.; Dos Anjos, D.A.; Santos, I.A.; Lannes, S.C.D.S. Synthetic and natural antioxidants used in the oxidative stability of edible oils: An overview. Food Rev. Int. 2021, 1–24. [Google Scholar] [CrossRef]
- Park, J.; Gim, S.Y.; Jeon, J.Y.; Kim, M.J.; Choi, H.K.; Lee, J. Chemical profiles and antioxidant properties of roasted rice hull extracts in bulk oil and oil-in-water emulsion. Food Chem. 2019, 272, 242–250. [Google Scholar] [CrossRef]
- Fan, K.; Chen, L.; Wei, X.; He, J.; Yan, Z.; Yan, F. Antioxidative properties of rapeseed meal extract on soybean oil during accelerated storage. J. Food Process. Preserv. 2015, 39, 2455–2465. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations, FAO. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 November 2022).
- Hore, J.K.; Anitha, M. Product diversification of areca nut, cashew nut and oil palm. In Value Addition of Horticultural Crops: Recent Trends and Future Directions; Springer: New Delhi, India, 2015; pp. 167–178. [Google Scholar]
- Sharma, P.; Gaur, V.K.; Sirohi, R.; Larroche, C.; Kim, S.H.; Pandey, A. Valorization of cashew nut processing residues for industrial applications. Ind. Crops Prod. 2020, 152, 112550. [Google Scholar] [CrossRef]
- Paramashivappa, R.; Kumar, P.P.; Vithayathil, P.J.; Rao, A.S. Novel method for isolation of major phenolic constituents from cashew (Anacardium occidentale L.) nut shell liquid. J. Agric. Food Chem. 2001, 49, 2548–2551. [Google Scholar] [CrossRef]
- Tyman, J.H.P.; Johnson, R.A.; Muir, M.; Rokhgar, R. The extraction of natural cashew nut-shell liquid from the cashew nut (Anacardium occidentale). J. Am. Oil Chem. Soc. 1989, 66, 553–557. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, F.; He, F.; Ha, Y.M.; Li, Q.P.; Jin, J.; Deng, Z.X. The impact of technical cashew nut shell liquid on thermally-induced trans isomers in edible oils. J. Food Sci. Technol. 2016, 53, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Maia, F.J.N.; Ribeiro, F.W.P.; Rangel, J.H.G.; Lomonaco, D.; Luna, F.M.T.; de Lima-Neto, P.; Mazzetto, S.E. Evaluation of antioxidant action by electrochemical and accelerated oxidation experiments of phenolic compounds derived from cashew nut shell liquid. Ind. Crops Prod. 2015, 67, 281–286. [Google Scholar] [CrossRef]
- Oliveira, M.S.C.; de Morais, S.M.; Magalhães, D.V.; Batista, W.P.; Vieira, Í.G.P.; Craveiro, A.A.; de Manezes, J.E.S.A.; Carvalho, A.F.U.; de Lima, G.P.G. Antioxidant, larvicidal and antiacetylcholinesterase activities of cashew nut shell liquid constituents. Acta Trop. 2011, 117, 165–170. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque Mendes, M.K.; dos Santos Oliveira, C.B.; Veras, M.D.A.; Araújo, B.Q.; Dantas, C.; Chaves, M.H.; Vieira, E.C. Application of multivariate optimization for the selective extraction of phenolic compounds in cashew nuts (Anacardium occidentale L.). Talanta 2019, 205, 120100. [Google Scholar] [CrossRef]
- Mwaikambo, L.Y.; Ansell, M.P. Cure characteristics of alkali catalysed cashew nut shell liquid-formaldehyde resin. J. Mater. Sci. 2001, 36, 3693–3698. [Google Scholar] [CrossRef]
- Da Silva, A.C.; Jorge, N. Oxidative stability of soybean oil added to Lentinus edodes and Agaricus blazei mushrooms extracts in an accelerated storage test. Nutr. Food Sci. 2012, 42, 34–40. [Google Scholar] [CrossRef]
- Jalali Mousavi, S.R.; Niazmand, R.; Shahidi Noghabi, M. Antioxidant activity of purslane (Portulaca oleracea L.) seed hydro-alcoholic extract on the stability of soybean oil. J. Agric. Sci. Technol. 2015, 17, 1473–1480. [Google Scholar]
- Lee, J.; Lee, Y.; Choe, E. Temperature dependence of the autoxidation and antioxidants of soybean, sunflower, and olive oil. Eur. J. Lipid Sci. Technol. 2007, 226, 239–246. [Google Scholar] [CrossRef]
- Yang, Y.; Song, X.; Sui, X.; Qi, B.; Wang, Z.; Li, Y.; Jiang, L. Rosemary extract can be used as a synthetic antioxidant to improve vegetable oil oxidative stability. Ind. Crops Prod. 2016, 80, 141–147. [Google Scholar] [CrossRef]
- Farhoosh, R.; Hoseini-Yazdi, S.Z. Shelf-life prediction of olive oils using empirical models developed at low and high temperatures. Food Chem. 2013, 141, 557–565. [Google Scholar] [CrossRef]
- Chung, H.J.; Colakoglu, A.S.; Min, D.B. Relationship among headspace oxygen, peroxide value, and conjugated diene content of soybean oil oxidation. J. Food Sci. 2004, 69, fct83–fct88. [Google Scholar] [CrossRef]
- Colakoglu, A.S. Oxidation kinetics of soybean oil in the presence of monoolein, stearic acid and iron. Food Chem. 2007, 101, 724–728. [Google Scholar] [CrossRef]
- Choe, E.O.; Lee, J.; Min, D.B. Chemistry for oxidative stability of edible oils. In Healthful Lipids; Akoh, C.C., Lai, O.M., Eds.; AOCS Press: Champaign, IL, USA, 2005; pp. 569–574. [Google Scholar]
- Kamal-Eldin, A. Effect of fatty acids and tocopherols on the oxidative stability of vegetable oils. Eur. J. Lipid Sci. Technol. 2006, 108, 1051–1061. [Google Scholar] [CrossRef]
- Dos Santos, T.D.J.A.; Araújo, B.Q.; Citó, A.M.D.G.L.; da Silva, J.; Saffi, J.; Richter, M.F.; Ferraz, A.D.B.F. Antioxidant properties and chemical composition of technical cashew nut shell liquid (tCNSL). Food Chem. 2011, 126, 1044–1048. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Umeda, W.M.; Jorge, N. Oxidative stability of soybean oil added of purple onion (Allium cepa L.) peel extract during accelerated storage conditions. Food Control. 2021, 127, 108130. [Google Scholar] [CrossRef]
- Rahmati, S.; Bazargani-Gilani, B.; Aghajani, N. Effect of extraction methods on the efficiency of sumac (Rhus coriaria L.) fruit extract in soybean oil quality during accelerated conditions. Food Sci. Nutr. 2022, 10, 3302–3313. [Google Scholar] [CrossRef] [PubMed]
- Phani Kumar, P.; Paramashivappa, R.; Vithayathil, P.J.; Subba Rao, P.V.; Srinivasa Rao, A. Process for isolation of cardanol from technical cashew (Anacardium occidentale L.) nut shell liquid. J. Agric. Food Chem. 2002, 50, 4705–4708. [Google Scholar] [CrossRef]
- Wanasundara, U.N.; Shahidi, F. Antioxidant and pro-oxidant activity of green tea extracts in marine oils. Food Chem. 1998, 63, 335–342. [Google Scholar] [CrossRef]
- Yeo, J.; Park, J.; Lee, J. Evaluation of antioxidant capacity of sesamol and free radical scavengers at different heating temperatures. Eur. J. Lipid Sci. Technol. 2011, 113, 910–915. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 4th ed.; Firestone, D., Ed.; American Oil Chemists’ Society: Champaign, IL, USA, 1990. [Google Scholar]
 ; CCF fraction,
; CCF fraction,  ; Cashew nut-shell liquid,
; Cashew nut-shell liquid,  ; Control without antioxidant,
; Control without antioxidant,  ; FA Fraction,
; FA Fraction,  . Data are presented as mean (n = 3) ± SD. Only the linear part, after the induction phase was finished, was linearized. r2 values range from 0.996 to 0.999.
. Data are presented as mean (n = 3) ± SD. Only the linear part, after the induction phase was finished, was linearized. r2 values range from 0.996 to 0.999.
 ; CCF fraction,
; CCF fraction,  ; Cashew nut-shell liquid,
; Cashew nut-shell liquid,  ; Control without antioxidant,
; Control without antioxidant,  ; FA Fraction,
; FA Fraction,  . Data are presented as mean (n = 3) ± SD. Only the linear part, after the induction phase was finished, was linearized. r2 values range from 0.996 to 0.999.
. Data are presented as mean (n = 3) ± SD. Only the linear part, after the induction phase was finished, was linearized. r2 values range from 0.996 to 0.999.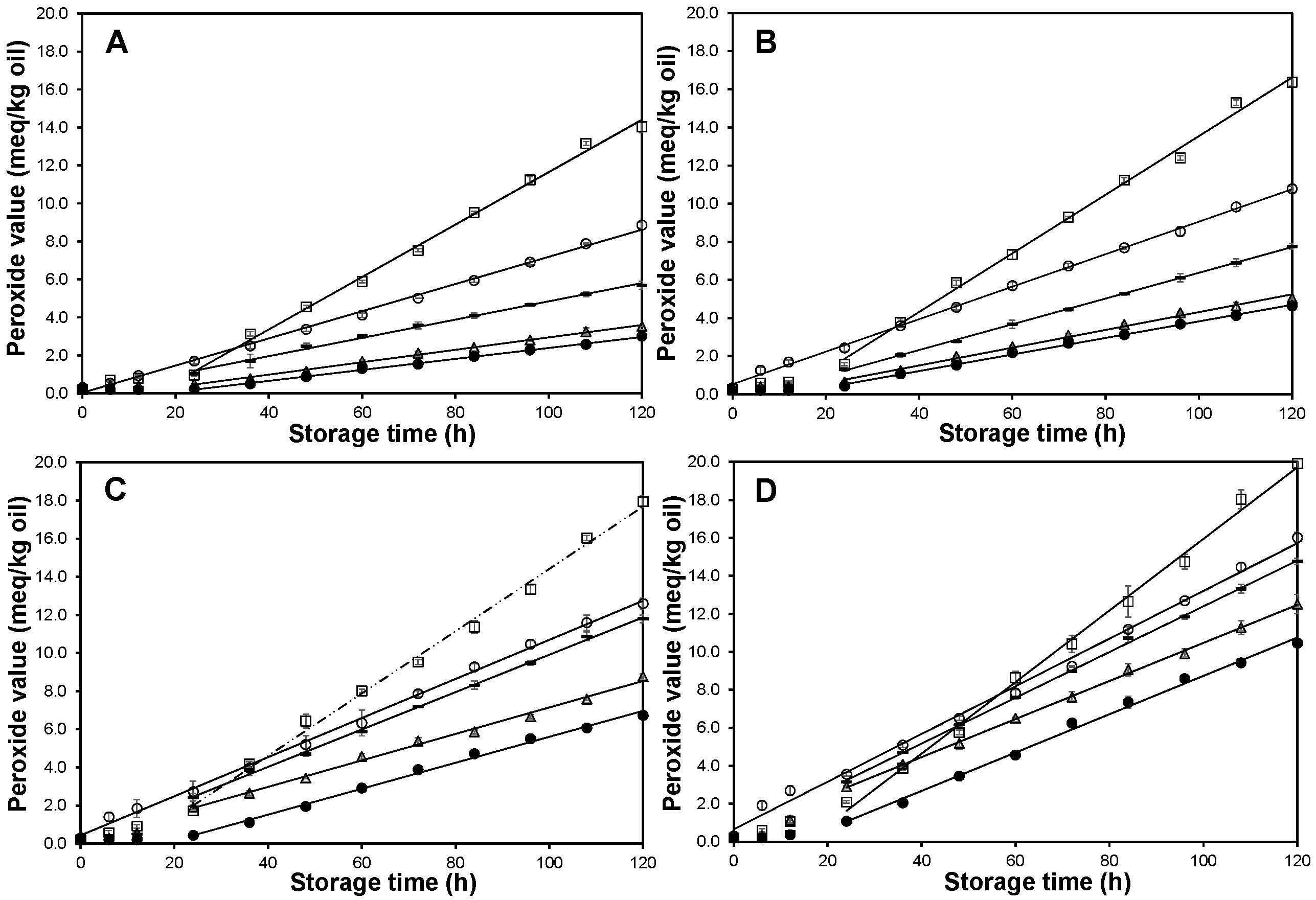
 ; CCF fraction,
; CCF fraction,  ; Cashew nut-shell liquid,
; Cashew nut-shell liquid,  ; Control without antioxidant,
; Control without antioxidant,  ; FA Fraction,
; FA Fraction,  . Data are presented as mean (n = 3) ± SD. r2 values range from 0.973 to 0.999. Each slope represents Ea/R.
. Data are presented as mean (n = 3) ± SD. r2 values range from 0.973 to 0.999. Each slope represents Ea/R.
 ; CCF fraction,
; CCF fraction,  ; Cashew nut-shell liquid,
; Cashew nut-shell liquid,  ; Control without antioxidant,
; Control without antioxidant,  ; FA Fraction,
; FA Fraction,  . Data are presented as mean (n = 3) ± SD. r2 values range from 0.973 to 0.999. Each slope represents Ea/R.
. Data are presented as mean (n = 3) ± SD. r2 values range from 0.973 to 0.999. Each slope represents Ea/R.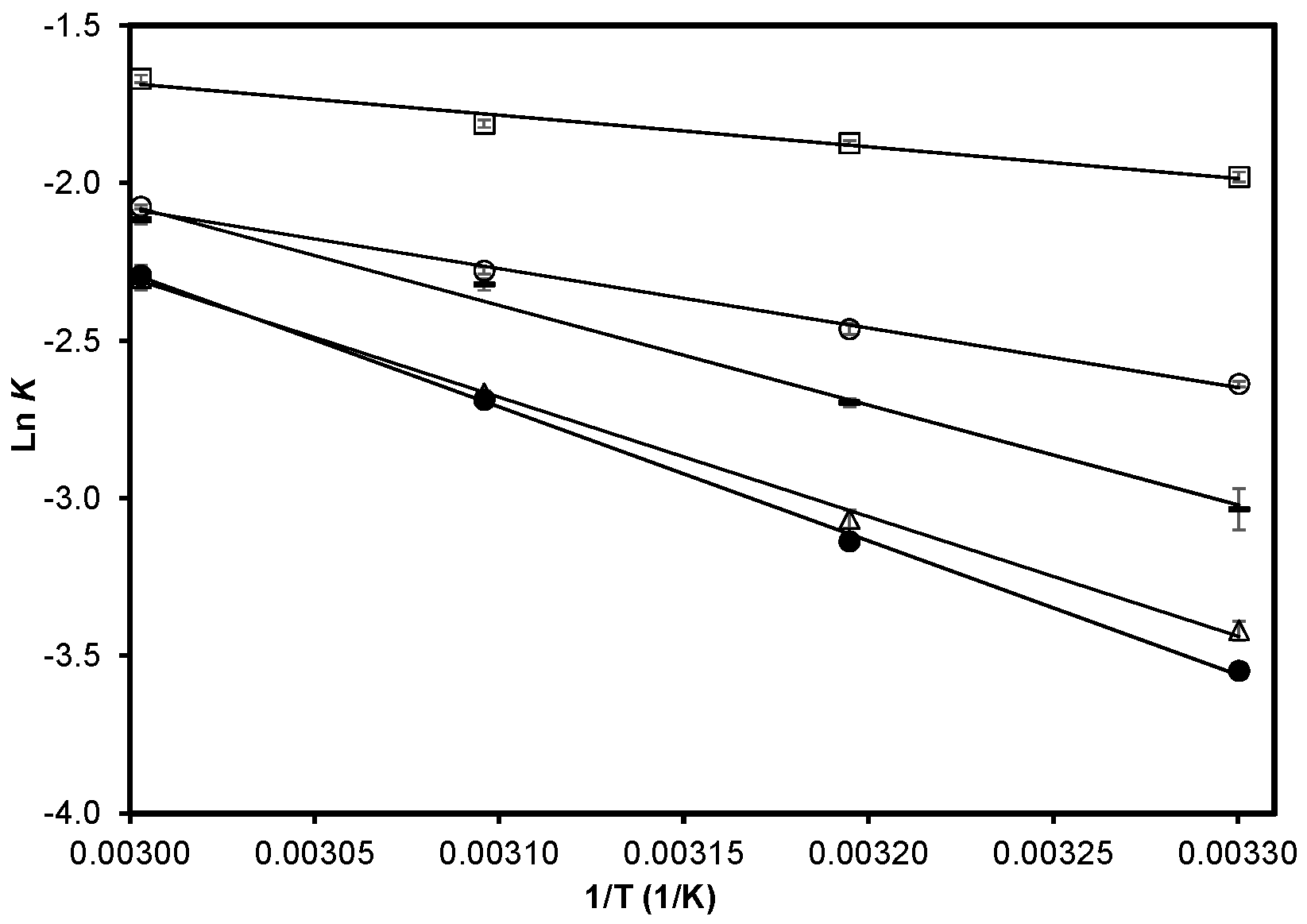
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaitán-Jiménez, S.-Y.; Restrepo-Sánchez, L.-P.; Parada-Alfonso, F.; Narváez-Cuenca, C.-E. Cashew (Anacardium occidentale) Nut-Shell Liquid as Antioxidant in Bulk Soybean Oil. Molecules 2022, 27, 8733. https://doi.org/10.3390/molecules27248733
Gaitán-Jiménez S-Y, Restrepo-Sánchez L-P, Parada-Alfonso F, Narváez-Cuenca C-E. Cashew (Anacardium occidentale) Nut-Shell Liquid as Antioxidant in Bulk Soybean Oil. Molecules. 2022; 27(24):8733. https://doi.org/10.3390/molecules27248733
Chicago/Turabian StyleGaitán-Jiménez, Sandra-Yaneth, Luz-Patricia Restrepo-Sánchez, Fabián Parada-Alfonso, and Carlos-Eduardo Narváez-Cuenca. 2022. "Cashew (Anacardium occidentale) Nut-Shell Liquid as Antioxidant in Bulk Soybean Oil" Molecules 27, no. 24: 8733. https://doi.org/10.3390/molecules27248733
APA StyleGaitán-Jiménez, S.-Y., Restrepo-Sánchez, L.-P., Parada-Alfonso, F., & Narváez-Cuenca, C.-E. (2022). Cashew (Anacardium occidentale) Nut-Shell Liquid as Antioxidant in Bulk Soybean Oil. Molecules, 27(24), 8733. https://doi.org/10.3390/molecules27248733







