Maslinic Acid: A New Compound for the Treatment of Multiple Organ Diseases
Abstract
1. Introduction
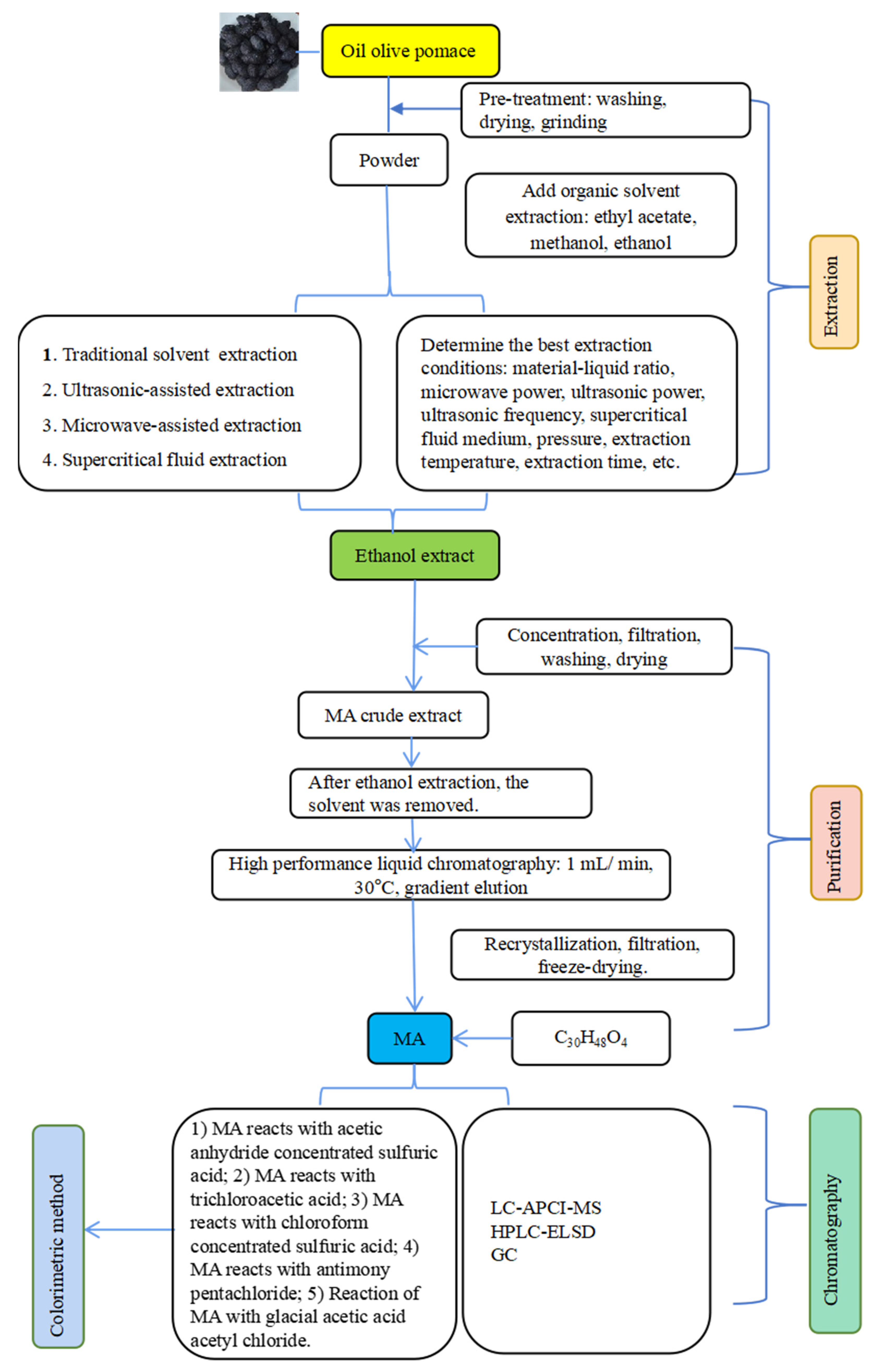
2. Extraction of MA
2.1. Extraction
2.2. Purification and Separation of MA
2.3. Identification of MA
2.3.1. Colorimetric Method
2.3.2. Chromatography
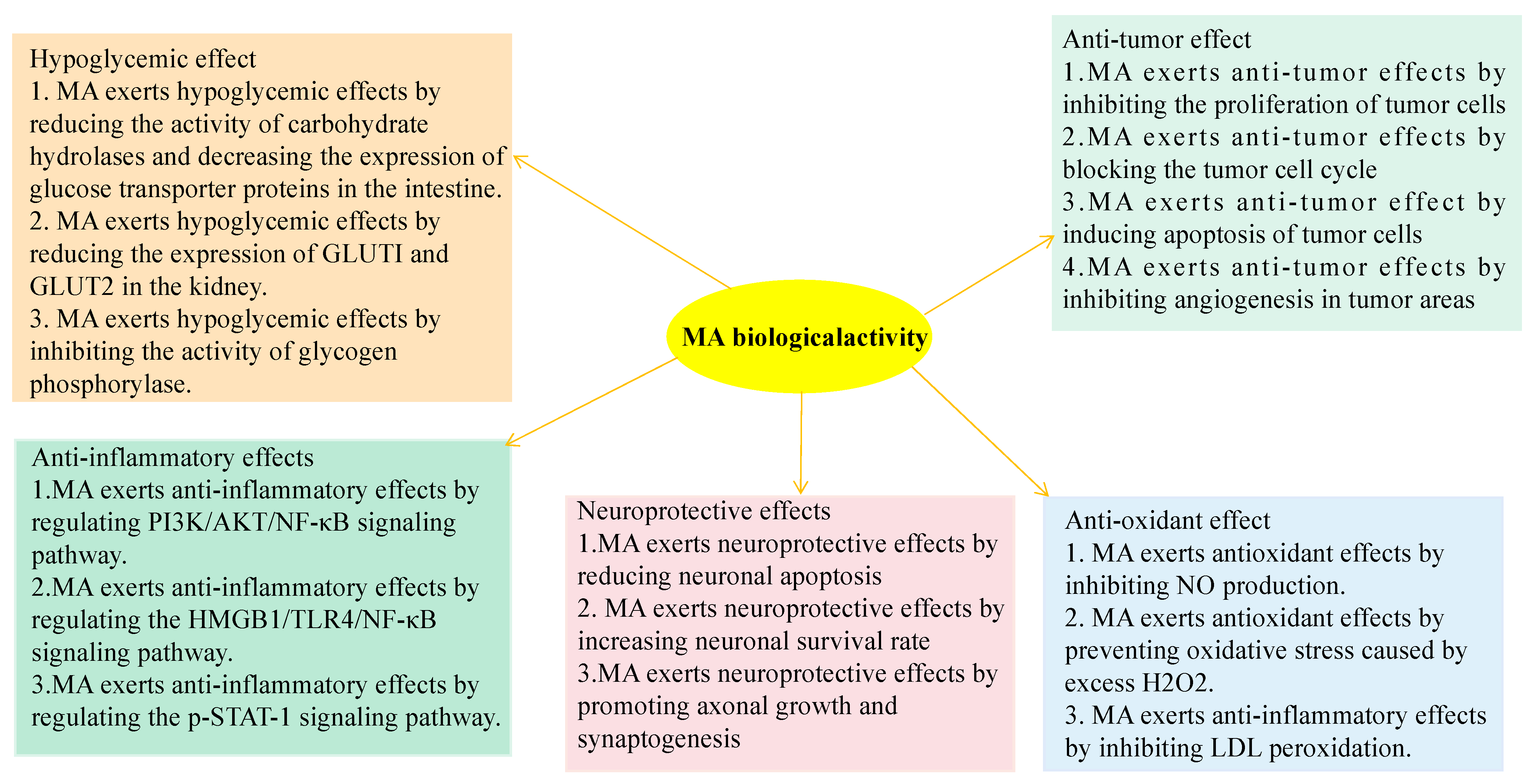
3. Biological Activity of MA
3.1. Hypoglycemic Effect
3.2. Antioxidant Effect
3.3. Neuroprotective Effect
3.4. Anti-Inflammatory Effect
3.5. Anti-Tumor Effect
4. Bioavailability of MA
5. Therapeutic Effects of MA
5.1. MA Treatment of Brain Diseases
5.1.1. MA Treatment of Alzheimer’s Disease
5.1.2. MA Treatment of Epilepsy
5.1.3. MA Treatment of Ischemic Stroke
5.1.4. MA Treatment of Malignant Astrocytoma
5.2. MA Treatment of Lung Diseases
5.2.1. MA Treatment of Lung Cancer
5.2.2. MA Treatment of Lung System Damage
5.3. MA Treatment of Heart Disease
5.3.1. MA Treatment of Pathological Cardiac Hypertrophy
5.3.2. MA Treatment of Acute Myocardial Infarction
5.3.3. MA Treatment of Diabetic Heart Disease
5.4. MA Treatment of Liver Diseases
5.4.1. MA Treatment of Acute Liver Injury
5.4.2. MA Treatment of Liver Cancer
5.4.3. MA Treatment of Nonalcoholic Fatty Liver Disease
5.5. MA Treatment of Gastric Diseases
5.5.1. MA Treatment of Gastric Ulcer
5.5.2. MA Treatment of Gastric Cancer
5.6. MA Treatment of Intestinal Diseases
MA Treatment of Colorectal Cancer
5.7. MA Treatment of Kidney Disease
5.7.1. MA Treatment of Diabetic Nephropathy
5.7.2. MA Treatment of Acute Kidney Injury
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stiti, N.; Triki, S.; Hartmann, M.A. Formation of triterpenoids throughout Olea europaea fruit ontogeny. Lipids 2007, 42, 55–67. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Brenes, M.; Dobarganes, M.; Romero, C.; Ruiz-Méndez, M. Enrichment of pomace olive oil in triterpenic acids during storage of “Alpeorujo” olive paste. Eur. J. Lipid Sci. Technol. 2008, 110, 1136–1141. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Z.; Zuo, L.; Wang, Z.; Zhou, L.; Shi, Y.; Kang, J.; Zhu, Z.; Zhang, X. Qualitative and quantitative determination of YiXinShu Tablet using ultra high performance liquid chromatography with Q Exactive hybrid quadrupole orbitrap high-resolution accurate mass spectrometry. J. Sep. Sci. 2017, 40, 4453–4466. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.; Lin, W.; Kitanaka, S.; Chang, C.; Wu, J. Analysis of bioactive triterpenes in Eriobotrya japonica Lindl. by high-performance liquid chromatography. Yao Wu Shi Pin Fen Xi = J. Food Drug Anal. 2020, 16, 5. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.A.; Tang, Y.P.; Yang, N.Y.; Qian, D.W.; Su, S.L.; Shang, E.X. Characterization of triterpenic acids in fruits of ziziphus species by HPLC-ELSD-MS. J. Agric. Food Chem. 2010, 58, 6285–6289. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Duan, J.A.; Tang, Y.; Qian, Y.; Zhao, J.; Qian, D.; Su, S.; Shang, E. Simultaneous qualitative and quantitative analysis of triterpenic acids, saponins and flavonoids in the leaves of two Ziziphus species by HPLC-PDA-MS/ELSD. J. Pharm. Biomed. Anal. 2011, 56, 264–270. [Google Scholar] [CrossRef]
- Savina, A.; Sokol’skaya, T.; Fesenko, D. Maslinic acid from the leaves of Eucalyptus viminalis. Chem. Nat. Compd. 1983, 19, 114–115. [Google Scholar] [CrossRef]
- Zong, W.; Xia, W.; Cui, B. Determination of corosolic and maslinic acids in Lagerstroemia speciosa leaves by TLC/HPLC method. Pharm. Chem. J. 2007, 41, 222–224. [Google Scholar] [CrossRef]
- Pan, Z.H.; Wang, Y.Y.; Li, M.M.; Xu, G.; Peng, L.Y.; He, J.; Zhao, Y.; Li, Y.; Zhao, Q.S. Terpenoids from Salvia trijuga. J. Nat. Prod. 2010, 73, 1146–1150. [Google Scholar] [CrossRef]
- Tarvainen, M.; Suomela, J.; Kallio, H.; Yang, B. Triterpene Acids in Plantago major: Identification, Quantification and Comparison of Different Extraction Methods. Chromatographia 2010, 71, 279–284. [Google Scholar] [CrossRef]
- Lee, I.K.; Kim, D.H.; Lee, S.Y.; Kim, K.R.; Choi, S.U.; Hong, J.K.; Lee, J.H.; Park, Y.H.; Lee, K.R. Triterpenoic acids of Prunella vulgaris var. lilacina and their cytotoxic activities in vitro. Arch. Pharmacal Res. 2008, 31, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Guinda, A.; Rada, M.; Delgado, T.; Castellano, J. Pentacyclic triterpenic acids from Argania spinosa. Eur. J. Lipid Sci. Technol. 2011, 113, 231–237. [Google Scholar] [CrossRef]
- Pérez-Camino, M.C.; Cert, A. Quantitative determination of hydroxy pentacyclic triterpene acids in vegetable oils. J. Agric. Food Chem. 1999, 47, 1558–1562. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, M.; Lozano-Mena, G.; Juan, M.E.; García-Granados, A.; Planas, J.M. Assessment of the safety of maslinic acid, a bioactive compound from Olea europaea L. Mol. Nutr. Food Res. 2013, 57, 339–346. [Google Scholar] [CrossRef]
- Lozano-Mena, G.; Sánchez-González, M.; Juan, M.; Planas, J. Maslinic acid, a natural phytoalexin-type triterpene from olives—A promising nutraceutical? Molecules 2014, 19, 11538–11559. [Google Scholar] [CrossRef]
- Jing, Z.; Rui, W.; Ruihua, L.; Hao, Y.; Hengtong, F. Review of the Biological Activity of Maslinic Acid. Curr. Drug Targets 2021, 22, 1496–1506. [Google Scholar] [CrossRef]
- Yap, W.; Lim, Y. Mechanistic Perspectives of Maslinic Acid in Targeting Inflammation. Biochem. Res. Int. 2015, 2015, 279356. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.; Reyes-Zurita, F.; Fuentes-Almagro, C.; de la Higuera, M.; Lupiáñez, J.; Peragón, J. Proteomics in the liver of gilthead sea bream (Sparus aurata) to elucidate the cellular response induced by the intake of maslinic acid. Proteomics 2011, 11, 3312–3325. [Google Scholar] [CrossRef]
- Fernández-Navarro, M.; Peragón, J.; Esteban, F.; de la Higuera, M.; Lupiáñez, J. Maslinic acid as a feed additive to stimulate growth and hepatic protein-turnover rates in rainbow trout (Onchorhynchus mykiss). Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2006, 144, 130–140. [Google Scholar] [CrossRef]
- Xie, P.; Huang, L.; Zhang, C.; Deng, Y.; Wang, X.; Cheng, J. Enhanced extraction of hydroxytyrosol, maslinic acid and oleanolic acid from olive pomace: Process parameters, kinetics and thermodynamics, and greenness assessment. Food Chem. 2019, 276, 662–674. [Google Scholar] [CrossRef]
- Guinda, A.; Rada, M.; Delgado, T.; Gutiérrez-Adánez, P.; Castellano, J.M. Pentacyclic triterpenoids from olive fruit and leaf. J. Agric. Food Chem. 2010, 58, 9685–9691. [Google Scholar] [CrossRef]
- Giménez, E.; Juan, M.E.; Calvo-Melià, S.; Planas, J.M. A sensitive liquid chromatography-mass spectrometry method for the simultaneous determination in plasma of pentacyclic triterpenes of Olea europaea L. Food Chem. 2017, 229, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Hernández, A.; Martinez, A.; Rivas, F.; García-Mesa, J.A.; Parra, A. Effect of the solvent and the sample preparation on the determination of triterpene compounds in two-phase olive-mill-waste samples. J. Agric. Food Chem. 2015, 63, 4269–4275. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Pastor, I.; Fernandez-Hernandez, A.; Perez-Criado, S.; Rivas, F.; Martinez, A.; Garcia-Granados, A.; Parra, A. Microwave-assisted extraction versus Soxhlet extraction to determine triterpene acids in olive skins. J. Sep. Sci. 2017, 40, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, G.; Liu, S.; Liu, D.; Chen, G.; Hu, N.; Suo, Y.; You, J. Simultaneous determination of six triterpenic acids in some Chinese medicinal herbs using ultrasound-assisted dispersive liquid-liquid microextraction and high-performance liquid chromatography with fluorescence detection. J. Pharm. Biomed. Anal. 2015, 107, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cruz, I.; Contreras, M.D.M.; Romero, I.; Castro, E. Sequential Extraction of Hydroxytyrosol, Mannitol and Triterpenic Acids Using a Green Optimized Procedure Based on Ultrasound. Antioxidants 2021, 10, 1781. [Google Scholar] [CrossRef]
- Song, L.; Zhang, L.; Xu, L.; Ma, Y.; Lian, W.; Liu, Y.; Wang, Y. Optimized Extraction of Total Triterpenoids from Jujube (Ziziphus jujuba Mill.) and Comprehensive Analysis of Triterpenic Acids in Different Cultivars. Plants 2020, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- Goulas, V.; Manganaris, G.A. Towards an efficient protocol for the determination of triterpenic acids in olive fruit: A comparative study of drying and extraction methods. Phytochem. Anal. 2012, 23, 444–449. [Google Scholar] [CrossRef] [PubMed]
- De Melo, M.; Silvestre, A.; Silva, C. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Benavides, A.; Martín-Álvarez, P.; Vázquez, L.; Reglero, G.; Señoráns, F.; Ibáñez, E. Optimization of Countercurrent Supercritical Fluid Extraction of Minor Components from Olive Oil. Curr. Anal. Chem. 2013, 10, 78–85. [Google Scholar] [CrossRef]
- Xynos, N.; Papaefstathiou, G.; Psychis, M.; Argyropoulou, A.; Aligiannis, N.; Skaltsounis, A. Development of a green extraction procedure with super/subcritical fluids to produce extracts enriched in oleuropein from olive leaves. J. Supercrit. Fluids 2012, 67, 89–93. [Google Scholar] [CrossRef]
- Gómez-Cruz, I.; Contreras, M.D.M.; Romero, I.; Castro, E. Optimization of Microwave-Assisted Water Extraction to Obtain High Value-Added Compounds from Exhausted Olive Pomace in a Biorefinery Context. Foods 2022, 11, 2002. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Nile, A.; Liu, J.; Kim, D.H.; Kai, G. Exploitation of apple pomace towards extraction of triterpenic acids, antioxidant potential, cytotoxic effects, and inhibition of clinically important enzymes. Food Chem. Toxicol. 2019, 131, 110563. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.S. Triterpenoid Chemistry; Chemical Industry Press: Beijing, China, 2008; p. 75. [Google Scholar]
- Wu, L.J. Natural Pharmaceutical Chemistry, 4th ed.; People’s Medical Publishing House: Beijing, China, 2004; pp. 292–293. [Google Scholar]
- Yang, J.; Hu, Y.-J.; Yu, B.-Y.; Qi, J. Integrating qualitative and quantitative characterization of Prunellae Spica by HPLC-QTOF/MS and HPLC-ELSD. Chin. J. Nat. Med. 2016, 14, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, M.; Lozano-Mena, G.; Juan, M.E.; García-Granados, A.; Planas, J.M. Liquid chromatography-mass spectrometry determination in plasma of maslinic acid, a bioactive compound from Olea europaea L. Food Chem. 2013, 141, 4375–4381. [Google Scholar] [CrossRef]
- Akinnuga, A.M.; Siboto, A.; Khumalo, B.; Sibiya, N.H.; Ngubane, P.; Khathi, A. Bredemolic Acid Ameliorates Selected Liver Function Biomarkers in a Diet-Induced Prediabetic Rat Model. Can. J. Gastroenterol. Hepatol. 2020, 2020, 2475301. [Google Scholar] [CrossRef]
- Mkhwanazi, B.N.; Serumula, M.R.; Myburg, R.B.; Van Heerden, F.R.; Musabayane, C.T. Antioxidant effects of maslinic acid in livers, hearts and kidneys of streptozotocin-induced diabetic rats: Effects on kidney function. Ren. Fail. 2014, 36, 419–431. [Google Scholar] [CrossRef]
- Liu, J.; Sun, H.; Duan, W.; Mu, D.; Zhang, L. Maslinic acid reduces blood glucose in KK-Ay mice. Biol. Pharm. Bull. 2007, 30, 2075–2078. [Google Scholar] [CrossRef]
- Khathi, A.; Serumula, M.R.; Myburg, R.B.; Van Heerden, F.R.; Musabayane, C.T. Effects of Syzygium aromaticum-derived triterpenes on postprandial blood glucose in streptozotocin-induced diabetic rats following carbohydrate challenge. PLoS ONE 2013, 8, e81632. [Google Scholar] [CrossRef]
- Gao, H.; Wu, H. Maslinic acid activates renal AMPK/SIRT1 signaling pathway and protects against diabetic nephropathy in mice. BMC Endocr. Disord. 2022, 22, 25. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Zhang, Z.; Xu, J.; Xie, Z.; Slavin, M.; Gao, X. In vitro and in vivo antioxidant activity of a fructan from the roots of Arctium lappa L. Int. J. Biol. Macromol. 2014, 65, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, R. Oleanolic acid and related triterpenoids from olives on vascular function: Molecular mechanisms and therapeutic perspectives. Curr. Med. Chem. 2015, 22, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Zeng, F.Q.; Wan, M.; Sim, K.Y. Anti-HIV triterpene acids from Geum japonicum. J. Nat. Prod. 1996, 59, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Márquez Martín, A.; de la Puerta Vázquez, R.; Fernández-Arche, A.; Ruiz-Gutiérrez, V. Supressive effect of maslinic acid from pomace olive oil on oxidative stress and cytokine production in stimulated murine macrophages. Free. Radic. Res. 2006, 40, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Bast, A.; Haenen, G.R. Ten misconceptions about antioxidants. Trends Pharmacol. Sci. 2013, 34, 430–436. [Google Scholar] [CrossRef]
- Mokhtari, K.; Rufino-Palomares, E.E.; Pérez-Jiménez, A.; Reyes-Zurita, F.J.; Figuera, C.; García-Salguero, L.; Medina, P.P.; Peragón, J.; Lupiáñez, J.A. Maslinic Acid, a Triterpene from Olive, Affects the Antioxidant and Mitochondrial Status of B16F10 Melanoma Cells Grown under Stressful Conditions. Evid. Based Complementary Altern. Med. 2015, 2015, 272457. [Google Scholar] [CrossRef]
- Huang, L.; Guan, T.; Qian, Y.; Huang, M.; Tang, X.; Li, Y.; Sun, H. Anti-inflammatory effects of maslinic acid, a natural triterpene, in cultured cortical astrocytes via suppression of nuclear factor-kappa B. Eur. J. Pharmacol. 2011, 672, 169–174. [Google Scholar] [CrossRef]
- Qian, Y.; Guan, T.; Tang, X.; Huang, L.; Huang, M.; Li, Y.; Sun, H.; Yu, R.; Zhang, F. Astrocytic glutamate transporter-dependent neuroprotection against glutamate toxicity: An in vitro study of maslinic acid. Eur. J. Pharmacol. 2011, 651, 59–65. [Google Scholar] [CrossRef]
- Serhan, C.N. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. FASEB J. 2017, 31, 1273–1288. [Google Scholar] [CrossRef]
- Chen, Y.L.; Yan, D.Y.; Wu, C.Y.; Xuan, J.W.; Jin, C.Q.; Hu, X.L.; Bao, G.D.; Bian, Y.J.; Hu, Z.C.; Shen, Z.H.; et al. Maslinic acid prevents IL-1β-induced inflammatory response in osteoarthritis via PI3K/AKT/NF-κB pathways. J. Cell. Physiol. 2021, 236, 1939–1949. [Google Scholar] [CrossRef]
- Li, Q.; Xu, M.; Li, Z.; Li, T.; Wang, Y.; Chen, Q.; Wang, Y.; Feng, J.; Yin, X.; Lu, C. Maslinic Acid Attenuates Ischemia/Reperfusion Injury-Induced Myocardial Inflammation and Apoptosis by Regulating HMGB1-TLR4 Axis. Front. Cardiovasc. Med. 2021, 8, 768947. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, J.; Park, E.K.; Bae, J.S. Maslinic Acid Ameliorates Inflammation via the Downregulation of NF-κB and STAT-1. Antioxidants 2020, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Zurita, F.J.; Pachón-Peña, G.; Lizárraga, D.; Rufino-Palomares, E.E.; Cascante, M.; Lupiáñez, J.A. The natural triterpene maslinic acid induces apoptosis in HT29 colon cancer cells by a JNK-p53-dependent mechanism. BMC cancer 2011, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; Medina, P.P.; Leticia García-Salguero, E.; Peragón, J.; Cascante, M.; Lupiáñez, J.A. Antitumour activity on extrinsic apoptotic targets of the triterpenoid maslinic acid in p53-deficient Caco-2 adenocarcinoma cells. Biochimie 2013, 95, 2157–2167. [Google Scholar] [CrossRef]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; García-Salguero, L.; Peragón, J.; Medina, P.P.; Parra, A.; Cascante, M.; Lupiáñez, J.A. Maslinic Acid, a Natural Triterpene, Induces a Death Receptor-Mediated Apoptotic Mechanism in Caco-2 p53-Deficient Colon Adenocarcinoma Cells. PLoS ONE 2016, 11, e0146178. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.E.; Reyes-Zurita, F.J.; García-Salguero, L.; Mokhtari, K.; Medina, P.P.; Lupiáñez, J.A.; Peragón, J. Maslinic acid, a triterpenic anti-tumoural agent, interferes with cytoskeleton protein expression in HT29 human colon-cancer cells. J. Proteom. 2013, 83, 15–25. [Google Scholar] [CrossRef]
- Hsia, T.C.; Liu, W.H.; Qiu, W.W.; Luo, J.; Yin, M.C. Maslinic acid induces mitochondrial apoptosis and suppresses HIF-1α expression in A549 lung cancer cells under normoxic and hypoxic conditions. Molecules 2014, 19, 19892–19906. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, Y.; Jiang, H.; Yang, P.; Li, H.; Zhang, Y.; He, P. Effects of maslinic acid on the proliferation and apoptosis of A549 lung cancer cells. Mol. Med. Rep. 2016, 13, 117–122. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, B.; Li, P.; Wen, X.; Yang, J. Maslinic Acid Inhibits Colon Tumorigenesis by the AMPK-mTOR Signaling Pathway. J. Agric. Food Chem. 2019, 67, 4259–4272. [Google Scholar] [CrossRef]
- Juan, M.E.; Lozano-Mena, G.; Sánchez-González, M.; Planas, J.M. Reduction of Preneoplastic Lesions Induced by 1,2-Dimethylhydrazine in Rat Colon by Maslinic Acid, a Pentacyclic Triterpene from Olea europaea L. Molecules 2019, 24, 1266. [Google Scholar] [CrossRef]
- Li, C.; Yang, Z.; Zhai, C.; Qiu, W.; Li, D.; Yi, Z.; Wang, L.; Tang, J.; Qian, M.; Luo, J.; et al. Maslinic acid potentiates the anti-tumor activity of tumor necrosis factor alpha by inhibiting NF-kappaB signaling pathway. Mol. Cancer 2010, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, H.; Farooq, A.A.; Nie, B.; Chen, X.; Su, S.; Yuan, R.; Qiao, G.; Li, C.; Li, X.; et al. Maslinic acid induces autophagy by down-regulating HSPA8 in pancreatic cancer cells. Phytother. Res. 2018, 32, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Thakor, P.; Song, W.; Subramanian, R.B.; Thakkar, V.R.; Vesey, D.A.; Gobe, G.C. Maslinic Acid Inhibits Proliferation of Renal Cell Carcinoma Cell Lines and Suppresses Angiogenesis of Endothelial Cells. J. Kidney Cancer VHL 2017, 4, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Nho, C.W.; Kwon, D.Y.; Kang, Y.H.; Lee, K.W.; Park, J.H. Maslinic acid inhibits the metastatic capacity of DU145 human prostate cancer cells: Possible mediation via hypoxia-inducible factor-1α signalling. Br. J. Nutr. 2013, 109, 210–222. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, D.; Zhang, X.; Shan, L.; Liu, Z. Maslinic acid induced apoptosis in bladder cancer cells through activating p38 MAPK signaling pathway. Mol. Cell. Biochem. 2014, 392, 281–287. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Xia, N.; Li, B.; Jiang, X. Maslinic acid potentiates the antitumor activities of gemcitabine in vitro and in vivo by inhibiting NF-κB-mediated survival signaling pathways in human gallbladder cancer cells. Oncol. Rep. 2015, 33, 1683–1690. [Google Scholar] [CrossRef]
- Cho, J.; Rho, O.; Junco, J.; Carbajal, S.; Siegel, D.; Slaga, T.J.; DiGiovanni, J. Effect of Combined Treatment with Ursolic Acid and Resveratrol on Skin Tumor Promotion by 12-O-Tetradecanoylphorbol-13-Acetate. Cancer Prev. Res. 2015, 8, 817–825. [Google Scholar] [CrossRef]
- Wang, D.; Tang, S.; Zhang, Q. Maslinic acid suppresses the growth of human gastric cells by inducing apoptosis via inhibition of the interleukin-6 mediated Janus kinase/signal transducer and activator of transcription 3 signaling pathway. Oncol. Lett. 2017, 13, 4875–4881. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Dong, Q.; Hao, X.; Qiao, L. Maslinic acid induces anticancer effects in human neuroblastoma cells mediated via apoptosis induction and caspase activation, inhibition of cell migration and invasion and targeting MAPK/ERK signaling pathway. AMB Express. 2020, 10, 104. [Google Scholar] [CrossRef]
- Chang, T.; Li, X.; Chen, X.; Zhang, L.; Yang, F.; Li, Z.; Li, J. Maslinic acid activates mitochondria-dependent apoptotic pathway in cardiac carcinoma. Clin. Investig. Med. 2014, 37, E217–E224. [Google Scholar] [CrossRef]
- Yap, W.H.; Khoo, K.S.; Lim, S.H.; Yeo, C.C.; Lim, Y.M. Proteomic analysis of the molecular response of Raji cells to maslinic acid treatment. Phytomedicine 2012, 19, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Yan, S.; Yin, M. Corrigendum to “Inhibitory effects of maslinic acid upon human esophagus, stomach and pancreatic cancer cells” [J. Funct. Foods 11 (2014) 581–588]. J. Funct. Foods 2017, 33, 446. [Google Scholar] [CrossRef]
- De la Torre, R.; Carbó, M.; Pujadas, M.; Biel, S.; Mesa, M.D.; Covas, M.I.; Expósito, M.; Espejo, J.A.; Sanchez-Rodriguez, E.; Díaz-Pellicer, P.; et al. Pharmacokinetics of maslinic and oleanolic acids from olive oil—Effects on endothelial function in healthy adults. A randomized, controlled, dose-response study. Food Chem. 2020, 322, 126676. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.C.; Lin, M.C.; Mong, M.C.; Lin, C.Y. Bioavailability, distribution, and antioxidative effects of selected triterpenes in mice. J. Agric. Food Chem. 2012, 60, 7697–7701. [Google Scholar] [CrossRef]
- Martín, R.; Carvalho-Tavares, J.; Carvalho, J.; Ibeas, E.; Hernández, M.; Ruiz-Gutierrez, V.; Nieto, M.L. Acidic triterpenes compromise growth and survival of astrocytoma cell lines by regulating reactive oxygen species accumulation. Cancer Res. 2007, 67, 3741–3751. [Google Scholar] [CrossRef][Green Version]
- Jerónimo-Santos, A.; Fonseca-Gomes, J.; Guimarães, D.A.; Tanqueiro, S.R.; Ramalho, R.M.; Ribeiro, J.A.; Sebastião, A.M.; Diógenes, M.J. Brain-derived neurotrophic factor mediates neuroprotection against Aβ-induced toxicity through a mechanism independent on adenosine 2A receptor activation. Growth Factors 2015, 33, 298–308. [Google Scholar] [CrossRef]
- Ismail, N.A.; Leong Abdullah, M.F.I.; Hami, R.; Ahmad Yusof, H. A narrative review of brain-derived neurotrophic factor (BDNF) on cognitive performance in Alzheimer’s disease. Growth Factors 2020, 38, 210–225. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, Y.; Lee, H.E.; Park, S.J.; Jeon, S.J.; Jeon, S.J.; Cheong, J.H.; Shin, C.Y.; Son, K.H.; Ryu, J.H. Oroxylin A enhances memory consolidation through the brain-derived neurotrophic factor in mice. Brain Res. Bull. 2014, 108, 67–73. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, J.M.; Park, S.J.; Lee, S.; Shin, C.Y.; Cheong, J.H.; Ryu, J.H. Hippocampal extracellular signal-regulated kinase signaling has a role in passive avoidance memory retrieval induced by GABAA Receptor modulation in mice. Neuropsychopharmacology 2012, 37, 1234–1244. [Google Scholar] [CrossRef][Green Version]
- Bae, H.J.; Kim, J.; Kim, J.; Goo, N.; Cai, M.; Cho, K.; Jung, S.Y.; Kwon, H.; Kim, D.H.; Jang, D.S.; et al. The effect of maslinic acid on cognitive dysfunction induced by cholinergic blockade in mice. Br. J. Pharmacol. 2020, 177, 3197–3209. [Google Scholar] [CrossRef]
- Friedman, A.; Dingledine, R. Molecular cascades that mediate the influence of inflamation on epilepsy. Epilepsia 2011, 52 (Suppl. S3), 33–39. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Lin, C.C.; Jeng, K.C.; Yao, P.W.; Chuang, L.T.; Kuo, S.L.; Hou, C.W. Fresh green tea and gallic acid ameliorate oxidative stress in kainic acid-induced status epilepticus. J. Agric. Food Chem. 2012, 60, 2328–2336. [Google Scholar] [CrossRef] [PubMed]
- Teocchi, M.A.; Ferreira, A.; da Luz de Oliveira, E.P.; Tedeschi, H.; D’Souza-Li, L. Hippocampal gene expression dysregulation of Klotho, nuclear factor kappa B and tumor necrosis factor in temporal lobe epilepsy patients. J. Neuroinflammation 2013, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Kovac, S.; Domijan, A.M.; Walker, M.C.; Abramov, A.Y. Seizure activity results in calcium- and mitochondria-independent ROS production via NADPH and xanthine oxidase activation. Cell Death Dis. 2014, 5, e1442. [Google Scholar] [CrossRef]
- Ravizza, T.; Balosso, S.; Vezzani, A. Inflammation and prevention of epileptogenesis. Neurosci. Lett. 2011, 497, 223–230. [Google Scholar] [CrossRef]
- Wang, Z.H.; Mong, M.C.; Yang, Y.C.; Yin, M.C. Asiatic acid and maslinic acid attenuated kainic acid-induced seizure through decreasing hippocampal inflammatory and oxidative stress. Epilepsy Res. 2018, 139, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Guan, T.; Tang, X.; Huang, L.; Huang, M.; Li, Y.; Sun, H. Maslinic acid, a natural triterpenoid compound from Olea europaea, protects cortical neurons against oxygen-glucose deprivation-induced injury. Eur. J. Pharmacol. 2011, 670, 148–153. [Google Scholar] [CrossRef]
- Guan, T.; Qian, Y.; Tang, X.; Huang, M.; Huang, L.; Li, Y.; Sun, H. Maslinic acid, a natural inhibitor of glycogen phosphorylase, reduces cerebral ischemic injury in hyperglycemic rats by GLT-1 up-regulation. J. Neurosci. Res. 2011, 89, 1829–1839. [Google Scholar] [CrossRef]
- Qian, Y.; Tang, X.; Guan, T.; Li, Y.; Sun, H. Neuroprotection by Combined Administration with Maslinic Acid, a Natural Product from Olea europaea, and MK-801 in the Cerebral Ischemia Model. Molecules 2016, 21, 1093. [Google Scholar] [CrossRef]
- Qian, Y.; Huang, M.; Guan, T.; Chen, L.; Cao, L.; Han, X.J.; Huang, L.; Tang, X.; Li, Y.; Sun, H. Maslinic acid promotes synaptogenesis and axon growth via Akt/GSK-3β activation in cerebral ischemia model. Eur. J. Pharmacol. 2015, 764, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Kim, J.; Park, E.K.; Baek, M.C.; Bae, J.S. Inhibitory functions of maslinic acid on particulate matter-induced lung injury through TLR4-mTOR-autophagy pathways. Environ. Res. 2020, 183, 109230. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Guo, S.; Wu, H.K.; Lv, F.; Jin, L.; Zhang, M.; Xie, P.; Wang, Y.; Song, Y.; Wu, F.; et al. Cardiac Ischemic Preconditioning Promotes MG53 Secretion through H2O2-Activated Protein Kinase C-δ Signaling. Circulation 2020, 142, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, C.; Liu, Q.; Xu, W.; Zhou, X. Molecular biomarkers in cardiac hypertrophy. J. Cell. Mol. Med. 2019, 23, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef]
- Cao, G.; Li, H.B.; Yin, Z.; Flavell, R.A. Recent advances in dynamic m6A RNA modification. Open Biol. 2016, 6, 160003. [Google Scholar] [CrossRef]
- Dorn, L.E.; Lasman, L.; Chen, J.; Xu, X.; Hund, T.J.; Medvedovic, M.; Hanna, J.H.; van Berlo, J.H.; Accornero, F. The N6-Methyladenosine mRNA Methylase METTL3 Controls Cardiac Homeostasis and Hypertrophy. Circulation 2019, 139, 533–545. [Google Scholar] [CrossRef]
- Qin, Y.; Li, L.; Luo, E.; Hou, J.; Yan, G.; Wang, D.; Qiao, Y.; Tang, C. Role of m6A RNA methylation in cardiovascular disease (Review). Int. J. Mol. Med. 2020, 46, 1958–1972. [Google Scholar] [CrossRef]
- Ma, Z.G.; Yuan, Y.P.; Zhang, X.; Xu, S.C.; Wang, S.S.; Tang, Q.Z. Piperine Attenuates Pathological Cardiac Fibrosis Via PPAR-γ/AKT Pathways. EBioMedicine 2017, 18, 179–187. [Google Scholar] [CrossRef]
- Ma, Z.G.; Dai, J.; Zhang, W.B.; Yuan, Y.; Liao, H.H.; Zhang, N.; Bian, Z.Y.; Tang, Q.Z. Protection against cardiac hypertrophy by geniposide involves the GLP-1 receptor/AMPKα signalling pathway. Br. J. Pharmacol. 2016, 173, 1502–1516. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Deng, J.; Zhou, Q.; Hu, Z.; Yang, L. Maslinic acid protects against pressure-overload-induced cardiac hypertrophy by blocking METTL3-mediated m6A methylation. Aging 2022, 14, 2548–2557. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Kong, C.Y.; Song, P.; Zhou, H.; Zhao, X.S.; Tang, Q.Z. Maslinic acid protects against pressure overload-induced cardiac hypertrophy in mice. J. Pharmacol. Sci. 2018, 138, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Cervellati, C.; Vigna, G.B.; Trentini, A.; Sanz, J.M.; Zimetti, F.; Dalla Nora, E.; Morieri, M.L.; Zuliani, G.; Passaro, A. Paraoxonase-1 activities in individuals with different HDL circulating levels: Implication in reverse cholesterol transport and early vascular damage. Atherosclerosis 2019, 285, 64–70. [Google Scholar] [CrossRef]
- Sagor, M.A.; Tabassum, N.; Potol, M.A.; Alam, M.A. Xanthine Oxidase Inhibitor, Allopurinol, Prevented Oxidative Stress, Fibrosis, and Myocardial Damage in Isoproterenol Induced Aged Rats. Oxidative Med. Cell. Longev. 2015, 2015, 478039. [Google Scholar] [CrossRef]
- Hussain Shaik, A.; Rasool, S.N.; Abdul Kareem, M.; Krushna, G.S.; Akhtar, P.M.; Devi, K.L. Maslinic acid protects against isoproterenol-induced cardiotoxicity in albino Wistar rats. J. Med. Food 2012, 15, 741–746. [Google Scholar] [CrossRef]
- Shaik, A.H.; Shaik, S.R.; Shaik, A.S.; Daoud, A.; Salim, M.; Kodidhela, L.D. Analysis of maslinic acid and gallic acid compounds as xanthine oxidase inhibitors in isoprenaline administered myocardial necrotic rats. Saudi J. Biol. Sci. 2021, 28, 2575–2580. [Google Scholar] [CrossRef]
- Huang, D.; Refaat, M.; Mohammedi, K.; Jayyousi, A.; Al Suwaidi, J.; Abi Khalil, C. Macrovascular Complications in Patients with Diabetes and Prediabetes. BioMed Res. Int. 2017, 2017, 7839101. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: Preclinical and clinical evidence. Nat. Rev. Cardiol. 2020, 17, 585–607. [Google Scholar] [CrossRef]
- Hung, Y.C.; Yang, H.T.; Yin, M.C. Asiatic acid and maslinic acid protected heart via anti-glycative and anti-coagulatory activities in diabetic mice. Food Funct. 2015, 6, 2967–2974. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.J.; Dai, Y.W.; Wang, C.L.; Fang, L.W.; Huang, W.C. Maslinic acid protects against obesity-induced nonalcoholic fatty liver disease in mice through regulation of the Sirt1/AMPK signaling pathway. FASEB J. 2019, 33, 11791–11803. [Google Scholar] [CrossRef] [PubMed]
- Guillen, N.; Acín, S.; Surra, J.C.; Arnal, C.; Godino, J.; García-Granados, A.; Muniesa, P.; Ruiz-Gutiérrez, V.; Osada, J. Apolipoprotein E determines the hepatic transcriptional profile of dietary maslinic acid in mice. J. Nutr. Biochem. 2009, 20, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Huang, C.Y.; Mong, M.C.; Chan, C.Y.; Yin, M.C. Antiangiogenic potential of three triterpenic acids in human liver cancer cells. J. Agric. Food Chem. 2011, 59, 755–762. [Google Scholar] [CrossRef]
- Mohr, A.M.; Lavery, R.F.; Barone, A.; Bahramipour, P.; Magnotti, L.J.; Osband, A.J.; Sifri, Z.; Livingston, D.H. Angiographic embolization for liver injuries: Low mortality, high morbidity. J. Trauma-Inj. Infect. Crit. Care 2003, 55, 1077–1081; discussion 1081–1802. [Google Scholar] [CrossRef]
- Andrade, R.; Lucena, M.; Fernández, M.; Pelaez, G.; Pachkoria, K.; García-Ruiz, E.; García-Muñoz, B.; González-Grande, R.; Pizarro, A.; Durán, J.; et al. Drug-Induced Liver Injury: An Analysis of 461 Incidences Submitted to the Spanish Registry over a 10-Year Period. Gastroenterology 2005, 129, 512–521. [Google Scholar] [CrossRef]
- Mahler, H.; Pasi, A.; Kramer, J.M.; Schulte, P.; Scoging, A.C.; Bär, W.; Krähenbühl, S. Fulminant liver failure in association with the emetic toxin of Bacillus cereus. N. Engl. J. Med. 1997, 336, 1142–1148. [Google Scholar] [CrossRef]
- Su, G.L. Lipopolysaccharides in liver injury: Molecular mechanisms of Kupffer cell activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G256–G265. [Google Scholar] [CrossRef]
- Koch, O.R.; Pani, G.; Borrello, S.; Colavitti, R.; Cravero, A.; Farrè, S.; Galeotti, T. Oxidative stress and antioxidant defenses in ethanol-induced cell injury. Mol. Asp. Med. 2004, 25, 191–198. [Google Scholar] [CrossRef]
- Lluis, J.M.; Colell, A.; García-Ruiz, C.; Kaplowitz, N.; Fernández-Checa, J.C. Acetaldehyde impairs mitochondrial glutathione transport in HepG2 cells through endoplasmic reticulum stress. Gastroenterology 2003, 124, 708–724. [Google Scholar] [CrossRef]
- McClain, C.J.; Song, Z.; Barve, S.S.; Hill, D.B.; Deaciuc, I. Recent advances in alcoholic liver disease. IV. Dysregulated cytokine metabolism in alcoholic liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G497–G502. [Google Scholar] [CrossRef] [PubMed]
- Naveau, S.; Balian, A.; Capron, F.; Raynard, B.; Fallik, D.; Agostini, H.; Grangeot-Keros, L.; Portier, A.; Galanaud, P.; Chaput, J.C.; et al. Balance between pro and anti-inflammatory cytokines in patients with acute alcoholic hepatitis. Gastroenterol. Clin. Biol. 2005, 29, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Tipoe, G.L.; Leung, T.M.; Liong, E.C.; Lau, T.Y.; Fung, M.L.; Nanji, A.A. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology 2010, 273, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.L.; Yang, H.T.; Lee, H.L.; Yin, M.C. Corrigendum to “Protective effects of maslinic acid against alcohol-induced acute liver injury in mice” [Food and Chem. Toxicol. 74 (2014 Dec) 149–55]. Food Chem. Toxicol. 2017, 106 Pt A, 570. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Diao, B.Z.; Zhong, L.H.; Lu, B.L.; Cheng, Y.; Yu, L.; Zhu, L.Y. Maslinic acid protects against lipopolysaccharide/d-galactosamine-induced acute liver injury in mice. Microb. Pathog. 2018, 119, 49–53. [Google Scholar] [CrossRef]
- Pang, R.; Poon, R.T. Angiogenesis and antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett. 2006, 242, 151–167. [Google Scholar] [CrossRef]
- Finn, R.S.; Zhu, A.X. Targeting angiogenesis in hepatocellular carcinoma: Focus on VEGF and bevacizumab. Expert Rev. Anticancer. Ther. 2009, 9, 503–509. [Google Scholar] [CrossRef]
- Andreasen, P.A.; Kjøller, L.; Christensen, L.; Duffy, M.J. The urokinase-type plasminogen activator system in cancer metastasis: A review. Int. J. Cancer 1997, 72, 1–22. [Google Scholar] [CrossRef]
- Morbidelli, L.; Donnini, S.; Ziche, M. Role of nitric oxide in the modulation of angiogenesis. Curr. Pharm. Des. 2003, 9, 521–530. [Google Scholar] [CrossRef]
- Wu, W.S. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006, 25, 695–705. [Google Scholar] [CrossRef]
- Kitade, H.; Chen, G.; Ni, Y.; Ota, T. Nonalcoholic Fatty Liver Disease and Insulin Resistance: New Insights and Potential New Treatments. Nutrients 2017, 9, 387. [Google Scholar] [CrossRef]
- Hazlehurst, J.M.; Woods, C.; Marjot, T.; Cobbold, J.F.; Tomlinson, J.W. Non-alcoholic fatty liver disease and diabetes. Metab. Clin. Exp. 2016, 65, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Donatini, R.S.; Diaz, I.E.; Yoshida, M.; Bacchi, E.M.; Kato, E.T. Evaluation of gastroprotective activity of Plinia edulis (Vell.) Sobral (Myrtaceae) leaves in rats. J. Ethnopharmacol. 2008, 118, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, R.L.; Nesello, L.; Mariano, L.N.B.; Somensi, L.B.; Campos, A.; Pinheiro, A.M.; Costa, S.; Rial, M.; Tozzo, M.; Cechinel-Filho, V.; et al. Gastroprotective activity of the methanol extract from peels of Plinia edulis (Vell.) Sobral fruits and its isolated triterpenes: Maslinic and ursolic acids. Naunyn-Schmiedebergs Arch. Pharmacol. 2018, 391, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Laine, L.; Takeuchi, K.; Tarnawski, A. Gastric mucosal defense and cytoprotection: Bench to bedside. Gastroenterology 2008, 135, 41–60. [Google Scholar] [CrossRef]
- Rozza, A.L.; Cesar, D.A.; Pieroni, L.G.; Saldanha, L.L.; Dokkedal, A.L.; De-Faria, F.M.; Souza-Brito, A.R.; Vilegas, W.; Takahira, R.K.; Pellizzon, C.H. Antiulcerogenic Activity and Toxicity of Bauhinia holophylla Hydroalcoholic Extract. Evid. Based Complementary Altern. Med. 2015, 2015, 439506. [Google Scholar] [CrossRef]
- Njor, S.H.; Friis-Hansen, L.; Andersen, B.; Søndergaard, B.; Linnemann, D.; Jørgensen, J.C.R.; Roikjær, O.; Rasmussen, M. Three years of colorectal cancer screening in Denmark. Cancer Epidemiol. 2018, 57, 39–44. [Google Scholar] [CrossRef]
- Eng, C. Toxic effects and their management: Daily clinical challenges in the treatment of colorectal cancer. Nat. Rev. Clin. Oncol. 2009, 6, 207–218. [Google Scholar] [CrossRef]
- Reyes, F.J.; Centelles, J.J.; Lupiáñez, J.A.; Cascante, M. (2Alpha,3beta)-2,3-dihydroxyolean-12-en-28-oic acid, a new natural triterpene from Olea europea, induces caspase dependent apoptosis selectively in colon adenocarcinoma cells. FEBS Lett. 2006, 580, 6302–6310. [Google Scholar] [CrossRef]
- Sánchez-Tena, S.; Reyes-Zurita, F.J.; Díaz-Moralli, S.; Vinardell, M.P.; Reed, M.; García-García, F.; Dopazo, J.; Lupiáñez, J.A.; Günther, U.; Cascante, M. Maslinic acid-enriched diet decreases intestinal tumorigenesis in Apc(Min/+) mice through transcriptomic and metabolomic reprogramming. PLoS ONE 2013, 8, e59392. [Google Scholar] [CrossRef]
- Lozano-Mena, G.; Sánchez-González, M.; Parra, A.; Juan, M.E.; Planas, J.M. Identification of gut-derived metabolites of maslinic acid, a bioactive compound from Olea europaea L. Mol. Nutr. Food Res. 2016, 60, 2053–2064. [Google Scholar] [CrossRef]
- Sánchez-González, M.; Colom, H.; Lozano-Mena, G.; Juan, M.E.; Planas, J.M. Population pharmacokinetics of maslinic acid, a triterpene from olives, after intravenous and oral administration in rats. Mol. Nutr. Food Res. 2014, 58, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Furuichi, K.; Shimizu, M.; Okada, H.; Narita, I.; Wada, T. Clinico-pathological features of kidney disease in diabetic cases. Clin. Exp. Nephrol. 2018, 22, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Flemming, N.B.; Gallo, L.A.; Forbes, J.M. Mitochondrial Dysfunction and Signaling in Diabetic Kidney Disease: Oxidative Stress and Beyond. Semin. Nephrol. 2018, 38, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Matsui, M.; Samejima, K.; Kanki, T.; Nishimoto, M.; Tanabe, K.; Murashima, M.; Eriguchi, M.; Akai, Y.; Iwano, M.; et al. Renal arteriolar hyalinosis, not intimal thickening in large arteries, is associated with cardiovascular events in people with biopsy-proven diabetic nephropathy. Diabet. Med. 2020, 37, 2143–2152. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, J.B.; Selvin, E. Prediabetes and What It Means: The Epidemiological Evidence. Annu. Rev. Public Health 2021, 42, 59–77. [Google Scholar] [CrossRef]
- De Nicola, L.; Conte, G.; Minutolo, R. Prediabetes as a Precursor to Diabetic Kidney Disease. Am. J. Kidney Dis. 2016, 67, 817–819. [Google Scholar] [CrossRef]
- Akinnuga, A.M.; Siboto, A.; Khumalo, B.; Sibiya, N.H.; Ngubane, P.; Khathi, A. Ameliorative Effects of Bredemolic Acid on Markers Associated with Renal Dysfunction in a Diet-Induced Prediabetic Rat Model. Oxidative Med. Cell. Longev. 2020, 2020, 2978340. [Google Scholar] [CrossRef]
- Mkhwanazi, B.N.; van Heerden, F.R.; Mavondo, G.A.; Mabandla, M.V.; Musabayane, C.T. Triterpene derivative improves the renal function of streptozotocin-induced diabetic rats: A follow-up study on maslinic acid. Ren. Fail. 2019, 41, 547–554. [Google Scholar] [CrossRef]
- Andreucci, M.; Faga, T.; Pisani, A.; Sabbatini, M.; Russo, D.; Michael, A. Prevention of contrast-induced nephropathy through a knowledge of its pathogenesis and risk factors. Sci. World J. 2014, 2014, 823169. [Google Scholar] [CrossRef]
- Cooper, J.E.; Wiseman, A.C. Acute kidney injury in kidney transplantation. Curr. Opin. Nephrol. Hypertens. 2013, 22, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, Q.; Wen, J.; Chen, T.; He, L.; Wang, Y.; Yin, J.; Wu, R.; Xue, R.; Li, S.; et al. Ischemic Duration and Frequency Determines AKI-to-CKD Progression Monitored by Dynamic Changes of Tubular Biomarkers in IRI Mice. Front. Physiol. 2019, 10, 153. [Google Scholar] [CrossRef]
- Han, S.J.; Lee, H.T. Mechanisms and therapeutic targets of ischemic acute kidney injury. Kidney Res. Clin. Pract. 2019, 38, 427–440. [Google Scholar] [CrossRef]
- Sun, W.; Choi, H.S.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. Maslinic Acid Attenuates Ischemia/Reperfusion-Induced Acute Kidney Injury by Suppressing Inflammation and Apoptosis through Inhibiting NF-κB and MAPK Signaling Pathway. Front. Pharmacol. 2022, 13, 807452. [Google Scholar] [CrossRef] [PubMed]
- Dellepiane, S.; Marengo, M.; Cantaluppi, V. Detrimental cross-talk between sepsis and acute kidney injury: New pathogenic mechanisms, early biomarkers and targeted therapies. Crit. Care 2016, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Hwang, I.G.; Ji, J.H.; Oh, S.Y.; Yi, J.H.; Lim, D.H.; Lim, H.Y.; Lee, S.J.; Park, S.H. Intrinsic resistance to sunitinib in patients with metastatic renal cell carcinoma. Asia-Pac. J. Clin. Oncol. 2017, 13, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Small, E.J. Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. J. Clin. Oncol. 2005, 23, 1028–1043. [Google Scholar] [CrossRef]

| Organs | In Vivo/In Vitro | Diseases | Treatment Mechanism | References |
|---|---|---|---|---|
| Brain | ||||
| In vivo | AD | MA promotes the expression of BDNF, reduces the apoptosis of neurons, improves the memory and cognitive impairment of mice caused by cholinergic system damage, and enhances the cognitive function of mice | [82] | |
| In vivo | Epilepsy | MA can reduce the production of inflammatory factors, reduce the level of glutamate in the hippocampus, improve the antioxidant capacity of the hippocampus and thus improve the production of epileptic behavior | [89] | |
| In vitro | Ischemic stroke | MA can block the cell necrosis induced by hypoxia, reduce the necrosis of neurons, effectively prevent the damage of cell bodies and neurites, and increase the survival rate of neurons | [90] | |
| In vivo | Ischemic stroke | MA prolonged the therapeutic time window of MK-801 from 1 h to 3 h. MA and MK-801 jointly increased the level of glutamate transporter GLT-1 in astrocytes and promoted astrocytes to regulate glutamate excitotoxicity, thus playing a therapeutic role in ischemia | [92] | |
| In vivo | Ischemic stroke | MA can significantly prevent axon injury, promote axon regeneration and increase the expression of synaptophysin after 7 days of ischemia | [93] | |
| In vivo | Ischemic stroke | MA treatment can enhance the expression of glial glutamate transporter GLT-1 at the protein and mRNA levels, leaving extracellular glutamate at a low concentration, thus playing a protective role in nerve cells during stroke ischemia | [91] | |
| In vitro | Astrocytoma (1321N1 cells) | MA can induce apoptosis of 1321N1 cell line | [77] | |
| Lung | ||||
| In vitro | Lung cancer (A549 cells) | MA treatment mediates mitochondrial apoptosis pathway and HIF-1 α pathway induced apoptosis of A549 cells | [59] | |
| In vitro | Lung cancer (A549 cells) | MA can promote the expression of caspase-3, caspase-8 and caspase-9 by regulating the expression of Smac and reducing the expression of c-IAP1, c-IAP2, XIAP and survivin, thereby inducing apoptosis of A549 cells | [60] | |
| In vivo | Lung damage | MA antagonizes lung injury caused by diesel PM2.5 by regulating TLR4-MyD88 and mTOR autophagy pathway | [94] | |
| In vivo | Lung injury | MA exerts anti-inflammatory effects by down-regulating NF-κB and p-STAT-1 to regulate iNOS | [54] | |
| Heart | ||||
| In vitro | Myocardial hypertrophy (NMCMs, H9C2 cells) | MA treatment significantly inhibited Ang-II-induced hypertrophy of NMCMs, and the dose did not affect the cell viability of H9C2 and NCMCs | [104] | |
| In vivo | Myocardial hypertrophy | MA can significantly improve myocardial hypertrophy, myocardial fibrosis and cardiac function, probably through the METTL3-mediated m 6A methylation pathway | [104] | |
| In vivo | Myocardial hypertrophy | MA reduces stress-overload-induced cardiac hypertrophy in vivo by reducing phosphorylation of AKT and ERK signaling pathways | [105] | |
| In vivo | Myocardial infarction | MA provides cardioprotection by increasing PON activity, reducing LDL-C levels and inhibiting lipid peroxidation (LPO) | [109] | |
| In vivo | Myocardial infarction | MA can inhibit the enzyme xanthine oxidase XO to relieve myocardial infarction | [110] | |
| Liver | ||||
| In vivo | Acute liver injury | MA inhibits CYP2E1, NF-κB and MAPK pathways, reducing the production of downstream oxidative and inflammatory factors (such as NO, TNF-α and PGE2), ultimately reducing alcohol-induced hepatotoxicity | [126] | |
| In vivo | Acute liver injury | MA exerts anti-inflammatory and antioxidant effects by inhibiting NF-κB and activating the Nrf2 signaling pathway, thereby providing protection against LPS/D-gal-induced liver injury | [127] | |
| In vitro | Liver cancer (hepatocellular carcinoma Hep3B, Huh7 and HA22T cells) | MA significantly inhibits angiogenesis and delays the metastasis and invasion of liver cancer cells | [116] | |
| In vitro | Fatty liver disease | MA can reduce hepatic fat infiltration, restore liver glycogen levels and reduce triglyceride and total cholesterol levels by inhibiting the expression of genes involved in hepatic fat formation | [114] | |
| Stomach | ||||
| In vivo | Gastric ulcer | MA pretreatment effectively reduces the area of gastric damage, inhibits H[+] and K[+]-ATPase activity, and provides gastroprotection | [136] | |
| In vivo | Gastric cancer | MA was able to inhibit IL-6 expression, induce JAK and STAT3 phosphorylation, and down-regulate STAT3-mediated protein Bad, Bcl-2 and Bax expression to treat gastric cancer | [70] | |
| Intestine | ||||
| In vitro | Colorectal cancer (HCT116, SW480 cells) | MA mainly induces apoptosis of colorectal cancer cells and inhibits proliferation and migration of colorectal tumors, and induces apoptosis to play an anti-tumor role | [61] | |
| Kidney | ||||
| In vivo | Diabetic nephropathy | MA activation of renal AMPK/SIRT1 signaling pathway improves diabetic nephropathy | [42] | |
| In vivo | Diabetic nephropathy | MA increases renal excretion of Na+ and can also lower blood glucose values | [151] | |
| In vivo | Renal cell carcinoma | MA inhibited the proliferation of cancer cells by reducing nuclear antigen expression, anti-proliferation and anti-colony production in proliferating cells, and down-regulating VEGF in vascular endothelial cells and PCNA in RCC to inhibit angiogenesis and proliferation | [65] | |
| In vivo | Acute kidney injury | MA inhibits IRI-induced AKI injury via NF-κB and MAPK signaling pathways | [156] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Wang, Y.; Yang, K.; Jiao, J.; Zhan, H.; Yang, Y.; Lv, D.; Li, W.; Ding, W. Maslinic Acid: A New Compound for the Treatment of Multiple Organ Diseases. Molecules 2022, 27, 8732. https://doi.org/10.3390/molecules27248732
He Y, Wang Y, Yang K, Jiao J, Zhan H, Yang Y, Lv D, Li W, Ding W. Maslinic Acid: A New Compound for the Treatment of Multiple Organ Diseases. Molecules. 2022; 27(24):8732. https://doi.org/10.3390/molecules27248732
Chicago/Turabian StyleHe, Yan, Yi Wang, Kun Yang, Jia Jiao, Hong Zhan, Youjun Yang, De Lv, Weihong Li, and Weijun Ding. 2022. "Maslinic Acid: A New Compound for the Treatment of Multiple Organ Diseases" Molecules 27, no. 24: 8732. https://doi.org/10.3390/molecules27248732
APA StyleHe, Y., Wang, Y., Yang, K., Jiao, J., Zhan, H., Yang, Y., Lv, D., Li, W., & Ding, W. (2022). Maslinic Acid: A New Compound for the Treatment of Multiple Organ Diseases. Molecules, 27(24), 8732. https://doi.org/10.3390/molecules27248732









