Ratiometric Fluorescent Sensor Based on Tb(III) Functionalized Metal-Organic Framework for Formic Acid
Abstract
1. Introduction
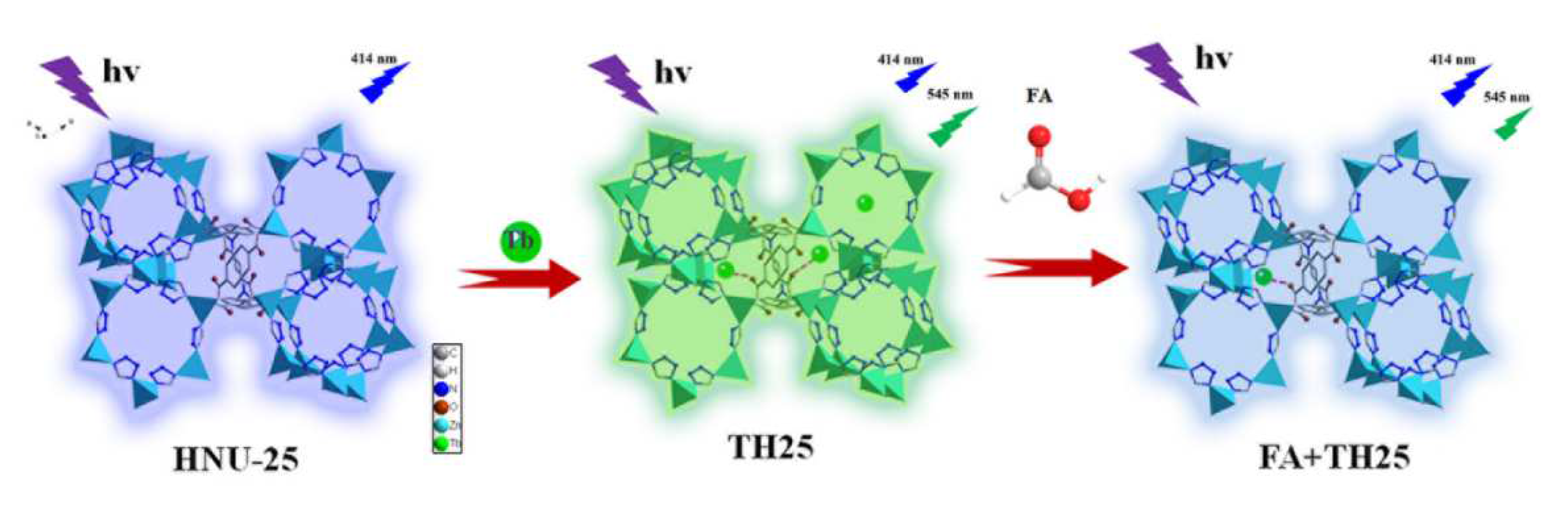
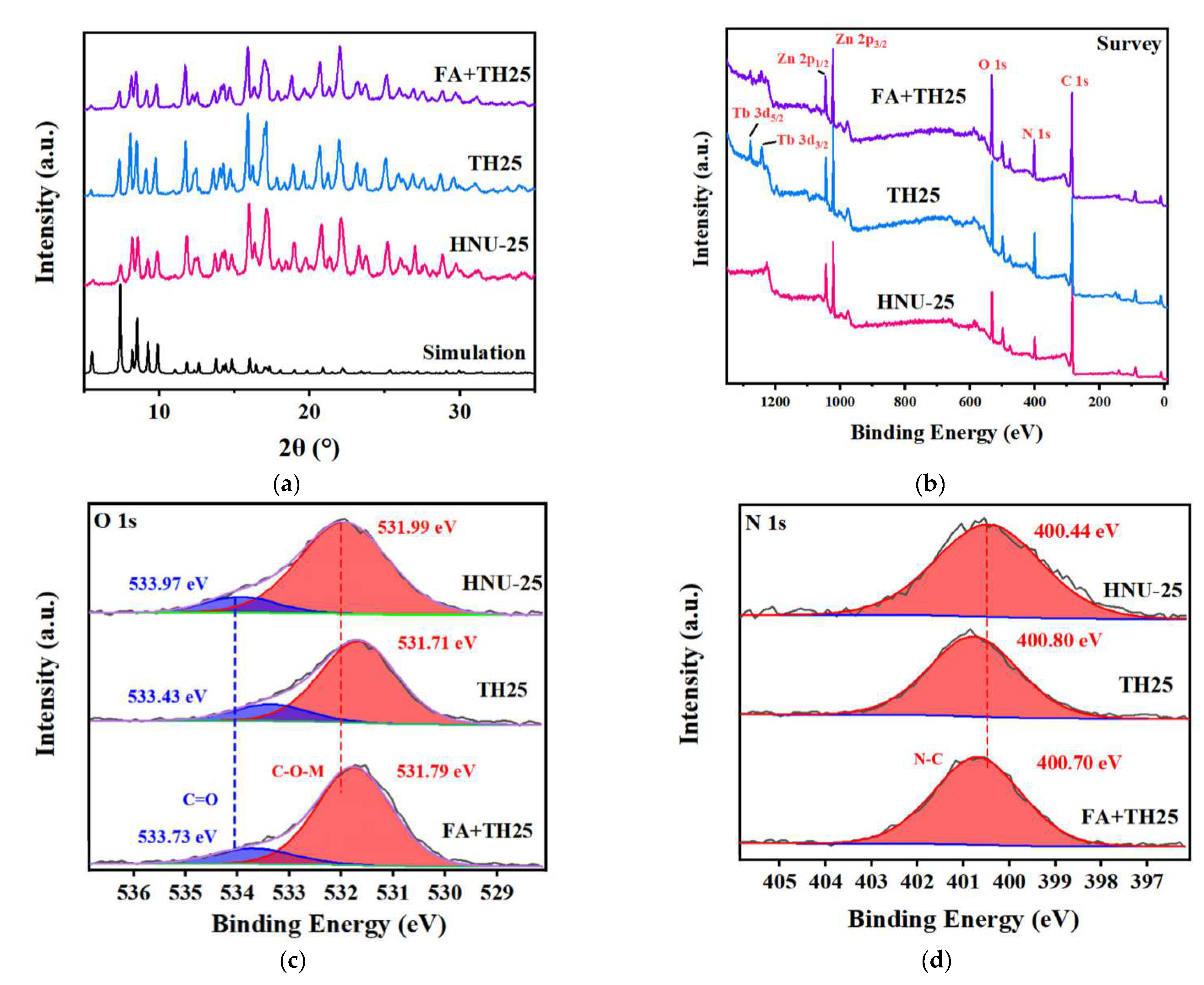
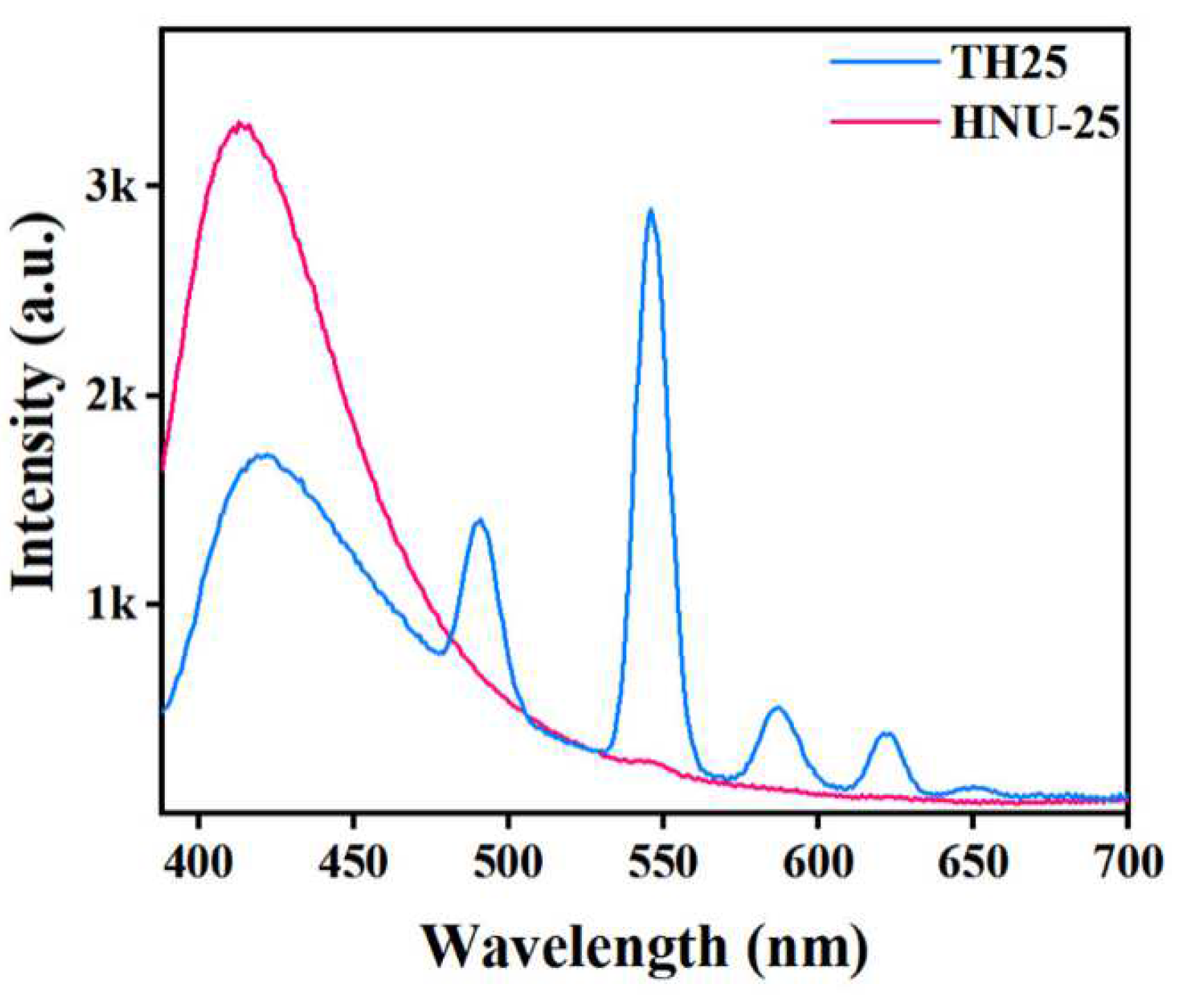
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Folkman, S.J.; Gonzalez-Cobos, J.; Giancola, S.; Sanchez-Molina, I.; Galan-Mascaros, J.R. Benchmarking Catalysts for Formic Acid/Formate Electrooxidation. Molecules 2021, 26, 4756. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-Y.; Tan, C.-H.; Li, Y.-P.; Guo, J.; Zhang, S.-Y. Formic acid–Formate blended solution: A new fuel system with high oxidation activity. Int. J. Hydrogen Energy 2012, 37, 3433–3437. [Google Scholar] [CrossRef]
- Onishi, N.; Kanega, R.; Kawanami, H.; Himeda, Y. Recent Progress in Homogeneous Catalytic Dehydrogenation of Formic Acid. Molecules 2022, 27, 455. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.; Ha, S.; Masela, R.I.; Waszczukb, P.; Wieckowskib, A.; Barnardb, T. Direct formic acid fuel cells. J. Power Sources 2002, 111, 83–89. [Google Scholar] [CrossRef]
- Vansco, M.F.; Zuraski, K.; Winiberg, F.A.F.; Au, K.; Trongsiriwat, N.; Walsh, P.J.; Osborn, D.L.; Percival, C.J.; Klippenstein, S.J.; Taatjes, C.A.; et al. Functionalized Hydroperoxide Formation from the Reaction of Methacrolein-Oxide, an Isoprene-Derived Criegee Intermediate, with Formic Acid: Experiment and Theory. Molecules 2021, 26, 3058. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Kouba, M.; Kos Durjava, M.; Lopez-Alonso, M.; Lopez Puente, S.; Marcon, F.; et al. Efficacy of sodium formate as a technological feed additive (hygiene condition enhancer) for all animal species. EFSA J. 2019, 17, e05645. [Google Scholar]
- Ricke, S.C.; Dittoe, D.K.; Richardson, K.E. Formic Acid as an Antimicrobial for Poultry Production: A Review. Front. Vet. Sci. 2020, 7, 563. [Google Scholar] [CrossRef]
- Rooke, J.A.; Greife, H.A.; Armstrong, D.G. The digestion by cattle of grass silages made with no additive or with the application of formic acid or formic acid and formaldehyde. Grass. Forage. Sci. 1983, 38, 301–310. [Google Scholar] [CrossRef]
- Liesivuori, J.; Savolainen, H. Methanol and Formic Acid Toxicity: Biochemical Mechanisms. Basic Clin. Pharmacol. Toxicol. 1991, 69, 157–163. [Google Scholar] [CrossRef]
- Vyata, V.; Durugu, S.; Jitta, S.R.; Khurana, S.; Jasti, J.R. An Atypical Presentation of Formic Acid Poisoning. Cureus 2020, 12, e7988. [Google Scholar] [CrossRef]
- Farahani, H.; Shokouhi, M.; Rahimi-Nasrabadi, M.; Zare-Dorabei, R. Green chemistry approach to analysis of formic acid and acetic acid in aquatic environment by headspace water-based liquid-phase microextraction and high-performance liquid chromatography. Toxicol. Environ. Chem. 2015, 98, 714–726. [Google Scholar] [CrossRef]
- Ueta, I.; Nakamura, Y.; Kawakubo, S.; Saito, Y. Determination of Aqueous Formic and Acetic Acids by Purge-and-Trap Analysis with a Needle-Type Extraction Device and Gas Chromatography Barrier Discharge Ionization Detector. Anal. Sci. 2018, 34, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Ueta, I.; Nakamura, Y.; Fujimura, K.; Kawakubo, S.; Saito, Y. Determination of Gaseous Formic and Acetic Acids by a Needle-Type Extraction Device coupled to a Gas Chromatography-Barrier Discharge Ionization Detector. Chromatographia 2016, 80, 151–156. [Google Scholar] [CrossRef]
- Kaewsiri, D.; Inyawilert, K.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Single-Nozzle Flame Synthesis of Spinel Zn₂SnO₄ Nanoparticles for Selective Detection of Formic Acid. IEEE Sens. J. 2020, 20, 6256–6262. [Google Scholar] [CrossRef]
- Lin, S.; Swager, T.M. Carbon Nanotube Formic Acid Sensors Using a Nickel Bis(ortho-diiminosemiquinonate) Selector. ACS Sens. 2018, 3, 569–573. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J.; Kong, Y.-R.; Luo, H.-B.; Liu, Y.; Ren, X.-M. Thin Films of an Ultrastable Metal–Organic Framework for Formic Acid Sensing with High Selectivity and Excellent Reproducibility. ACS Mater. Lett. 2021, 3, 1746–1751. [Google Scholar] [CrossRef]
- Liu, R.L.; Shi, Z.Q.; Wang, X.Y.; Li, Z.F.; Li, G. Two Highly Stable Proton Conductive Cobalt(II)-Organic Frameworks as Impedance Sensors for Formic Acid. Chem. A Eur. J. 2019, 25, 14108–14116. [Google Scholar] [CrossRef]
- Mattias Sandström, K.J.M.; Newman, J.; Sunesson, A.L.; Levin, J.O.; Turner, A.P.F. Amperometric biosensor for formic acid in air. Sens. Actuators B 2000, 70, 182–187. [Google Scholar] [CrossRef]
- Liu, R.L.; Qu, W.T.; Dou, B.H.; Li, Z.F.; Li, G. Proton-Conductive 3D Ln(III) Metal-Organic Frameworks for Formic Acid Impedance Sensing. Chem. Asian J. 2020, 15, 182–190. [Google Scholar] [CrossRef]
- Asiri, A.M.; Sobahi, T.R.; Al-Amari, M.M.; Asad, M.; Zayed, M.E.M.; Khan, S.A. Physicochemical Investigation of HDDP Azomethine Dye as Turn-On Fluorescent Chemosensor for High Selectivity and Sensitivity of Al3+ Ions. J. Solut. Chem. 2018, 47, 1711–1724. [Google Scholar] [CrossRef]
- Khan, S.A.; Ullah, Q.; Almalki, A.S.A.; Kumar, S.; Obaid, R.J.; Alsharif, M.A.; Alfaifi, S.Y.; Hashmi, A.A. Synthesis and photophysical investigation of (BTHN) Schiff base as off-on Cd2+ fluorescent chemosensor and its live cell imaging. J. Mol. Liq. 2021, 328, 115407. [Google Scholar] [CrossRef]
- Khan, S.A.; Ullah, Q.; Parveen, H.; Mukhtar, S.; Alzahrani, K.A.; Asad, M. Synthesis and photophysical investigation of novel imidazole derivative an efficient multimodal chemosensor for Cu(II) and fluoride ions. J. Photochem. Photobiol. A Chem. 2021, 406, 113022. [Google Scholar] [CrossRef]
- Cui, S.; Wang, B.; Teng, Y.; Wan, Z.; Zan, Y.; Chen, L.; Li, Y.; Yan, X. Highly sensitive sensing of polarity, temperature, and acid gases by a smart fluorescent molecule. Sens. Actuators B 2021, 344, 130120. [Google Scholar] [CrossRef]
- Moonrinta, S.; Kwon, B.; In, I.; Kladsomboon, S.; Sajomsang, W.; Paoprasert, P. Highly biocompatible yogurt-derived carbon dots as multipurpose sensors for detection of formic acid vapor and metal ions. Opt. Mater. 2018, 81, 93–101. [Google Scholar] [CrossRef]
- Wu, Y.; Hua, C.; Liu, Z.; Yang, J.; Huang, R.; Li, M.; Liu, K.; Miao, R.; Fang, Y. High-Performance Sensing of Formic Acid Vapor Enabled by a Newly Developed Nanofilm-Based Fluorescent Sensor. Anal. Chem. 2021, 93, 7094–7101. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Huang, R.; Tang, J.; Li, M.; Yang, J.; Wang, G.; Liu, K.; Fang, Y. Film Nanoarchitectonics of Pillar[5]arene for High-Performance Fluorescent Sensing: A Proof-of-Concept Study. ACS Appl. Mater. Interfaces 2021, 13, 54561–54569. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhao, Y.; Li, H.; Zhang, Q.; Yang, W.; Pan, Q. A Simple Colorimetric Probe for Sensitive Detection of Hg2+ Based on MnO2 Nanosheets and Monothioglycerol. ChemistrySelect 2020, 5, 13888–13894. [Google Scholar] [CrossRef]
- Li, M.; Gao, Y.; Yang, W.; Zhang, C.; Fang, Y.; Wang, C.; Song, S.; Pan, Q. Dye-Encapsulated Lanthanide-Based Metal-Organic Frameworks as a Dual-Emission Sensitization Platform for Alachlor Sensing. Inorg. Chem. 2022, 61, 9801–9807. [Google Scholar] [CrossRef]
- Wang, C.; Ren, G.; Zhou, S.; Yang, Y.; Sun, A.; Fang, Y.; Guo, D.Y.; Pan, Q. Heteronuclear metal–organic framework-based fluorescent sensor for the detection of tetracycline antibiotics. Appl. Organomet. Chem. 2022, 36, e6767. [Google Scholar] [CrossRef]
- Olorunyomi, J.F.; Sadiq, M.M.; Batten, M.; Konstas, K.; Chen, D.; Doherty, C.M.; Caruso, R.A. Advancing Metal-Organic Frameworks toward Smart Sensing: Enhanced Fluorescence by a Photonic Metal-Organic Framework for Organic Vapor Sensing. Adv. Opt. Mater. 2020, 8, 2000961. [Google Scholar] [CrossRef]
- Fang, Y.; Ren, G.; Li, M.; Yang, Y.; Guo, D.-Y.; Pan, Q. Sensitively liquid and gaseous detection of formaldehyde based on a supramolecular organic framework. Sens. Actuators B 2021, 349, 130726. [Google Scholar] [CrossRef]
- Yao, C.X.; Dong, L.; Yang, L.; Wang, J.; Li, S.J.; Lv, H.; Ji, X.M.; Liu, J.M.; Wang, S. Integration of Metal-Organic Frameworks with Bi-Nanoprobes as Dual-Emissive Ratiometric Sensors for Fast and Highly Sensitive Determination of Food Hazards. Molecules 2022, 27, 2356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, H.; Sun, L.; Yan, Y.; Wang, B.; Liang, Z.; Wang, L.; Li, J. Water Stable Metal–Organic Framework Based on Phosphono-containing Ligand as Highly Sensitive Luminescent Sensor toward Metal Ions. Cryst. Growth Des. 2018, 18, 7683–7689. [Google Scholar] [CrossRef]
- Han, L.-J.; Kong, Y.-J.; Zhang, X.-M.; Hou, G.-Z.; Chen, H.-C.; Zheng, H.-G. Fluorescence recognition of adenosine triphosphate and uric acid by two Eu-based metal–organic frameworks. J. Mater. Chem. C 2021, 9, 6051–6061. [Google Scholar] [CrossRef]
- Lian, Z.; Zhao, M.; Wang, J.; Yu, R.-C. Dual-emission ratiometric fluorescent sensor based molecularly imprinted nanoparticles for visual detection of okadaic acid in seawater and sediment. Sens. Actuators B 2021, 346, 130465. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Lu, H.; Yan, B. Dual-emission ratiometric fluorescent probe-based lanthanide-functionalized hydrogen-bonded organic framework for the visual detection of methylamine. J. Mater. Chem. C 2022, 10, 1212–1219. [Google Scholar] [CrossRef]
- Fan, Y.; Jiang, X.; Che, J.; Li, M.; Zhang, X.; Gao, D.; Bi, J.; Ning, Z. A Ratiometric Fluorescent Sensor Based on Dye/Tb (III) Functionalized UiO-66 for Highly Sensitive Detection of TDGA. Molecules 2022, 27, 6543. [Google Scholar] [CrossRef]
- Sun, N.N.; Yan, B. A fluorescent probe based on a Tb3+/Cu2+ co-functionalized MOF for urinary sarcosine detection. Analyst 2018, 143, 2349–2355. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Yan, B. Fabrication and application of a ratiometric and colorimetric fluorescent probe for Hg2+ based on dual-emissive metal–organic framework hybrids with carbon dots and Eu3+. J. Mater. Chem. C 2016, 4, 1543–1549. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Q.; Xia, T.; Zhang, J.; Yang, Y.; Cui, Y.; Chen, B.; Qian, G. Turn-on and Ratiometric Luminescent Sensing of Hydrogen Sulfide Based on Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2016, 8, 32259–32265. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, A.; Pan, J.; Xue, Z.; Li, J.; Wang, G. Metal–organic complex-derived 3D porous carbon-supported g-C3N4/TiO2 as photocatalysts for the efficient degradation of antibiotic. CrystEngComm 2021, 23, 4717–4723. [Google Scholar] [CrossRef]
- Chen, D.-M.; Sun, C.-X.; Peng, Y.; Zhang, N.-N.; Si, H.-H.; Liu, C.-S.; Du, M. Ratiometric fluorescence sensing and colorimetric decoding methanol by a bimetallic lanthanide-organic framework. Sens. Actuators B 2018, 265, 104–109. [Google Scholar] [CrossRef]
- Chu, S.; Wang, H.; Ling, X.; Yu, S.; Yang, L.; Jiang, C. A Portable Smartphone Platform Using a Ratiometric Fluorescent Paper Strip for Visual Quantitative Sensing. ACS Appl. Mater. Interfaces 2020, 12, 12962–12971. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, D.; Ye, Y.; Qiu, Y.; Liu, J.; Huang, L.; Liang, B.; Chen, B. A Fluorescent Metal-Organic Framework for Food Real-Time Visual Monitoring. Adv. Mater. 2021, 33, e2008020. [Google Scholar] [CrossRef] [PubMed]
- Sha, H.; Yan, B. A pH-responsive Eu(III) functionalized metal–organic framework hybrid luminescent film for amino acid sensing and anti-counterfeiting. J. Mater. Chem. C 2022, 10, 7633–7640. [Google Scholar] [CrossRef]
- Li, M.; Ren, G.; Wang, F.; Li, Z.; Yang, W.; Gu, D.; Wang, Y.; Zhu, G.; Pan, Q. Two metal–organic zeolites for highly sensitive and selective sensing of Tb3+. Inorg. Chem. Front. 2019, 6, 1129–1134. [Google Scholar] [CrossRef]
- Hou, L.; Song, Y.; Xiao, Y.; Wu, R.; Wang, L. Ratiometric fluorescence detection of dipicolinic acid based on Microporous Ln/melamine-terephthaladehyde schiff base networks complex. Talanta 2020, 209, 120534. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huang, X.; Xu, C.; Chen, C.; Yang, L.; Dou, W.; Chen, W.; Yang, H.; Liu, W. A Multi-responsive Regenerable Europium-Organic Framework Luminescent Sensor for Fe3+, CrVI Anions, and Picric Acid. Chem. A Eur. J. 2016, 22, 18769–18776. [Google Scholar] [CrossRef] [PubMed]
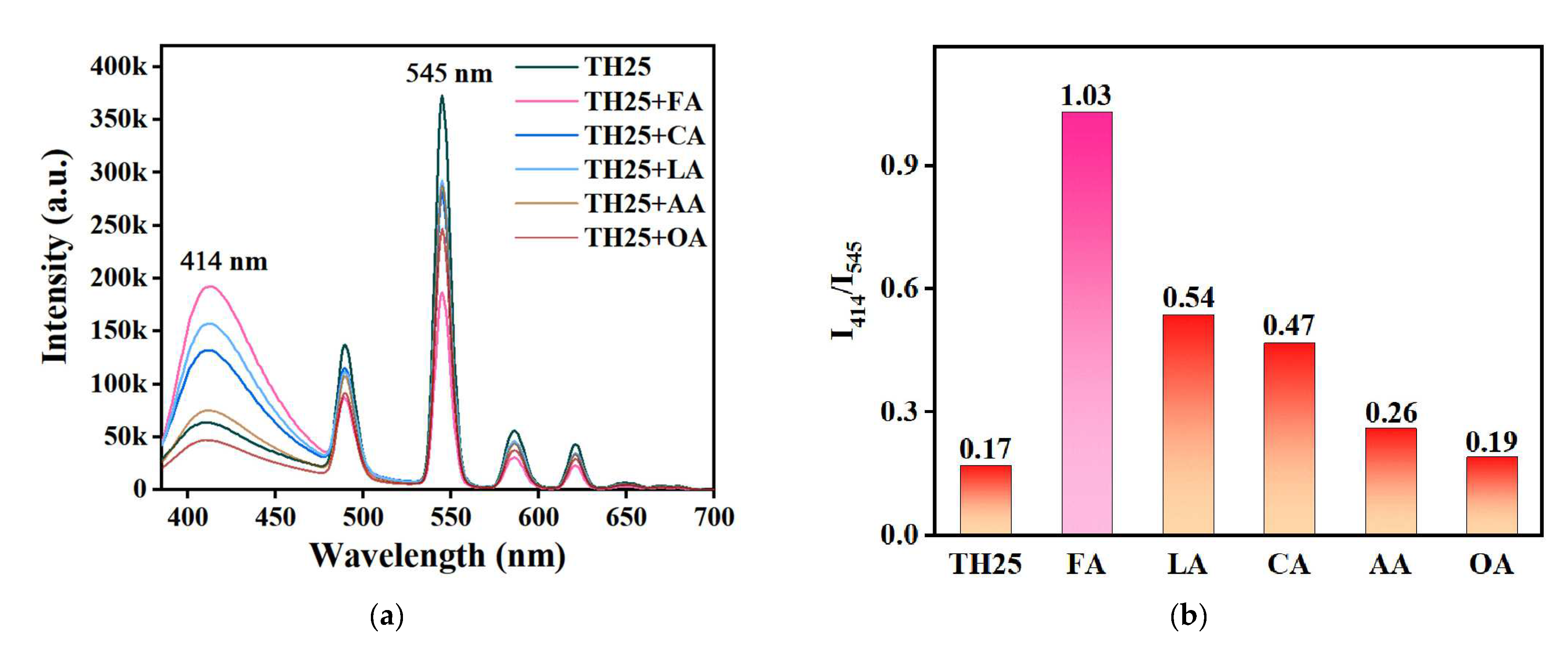
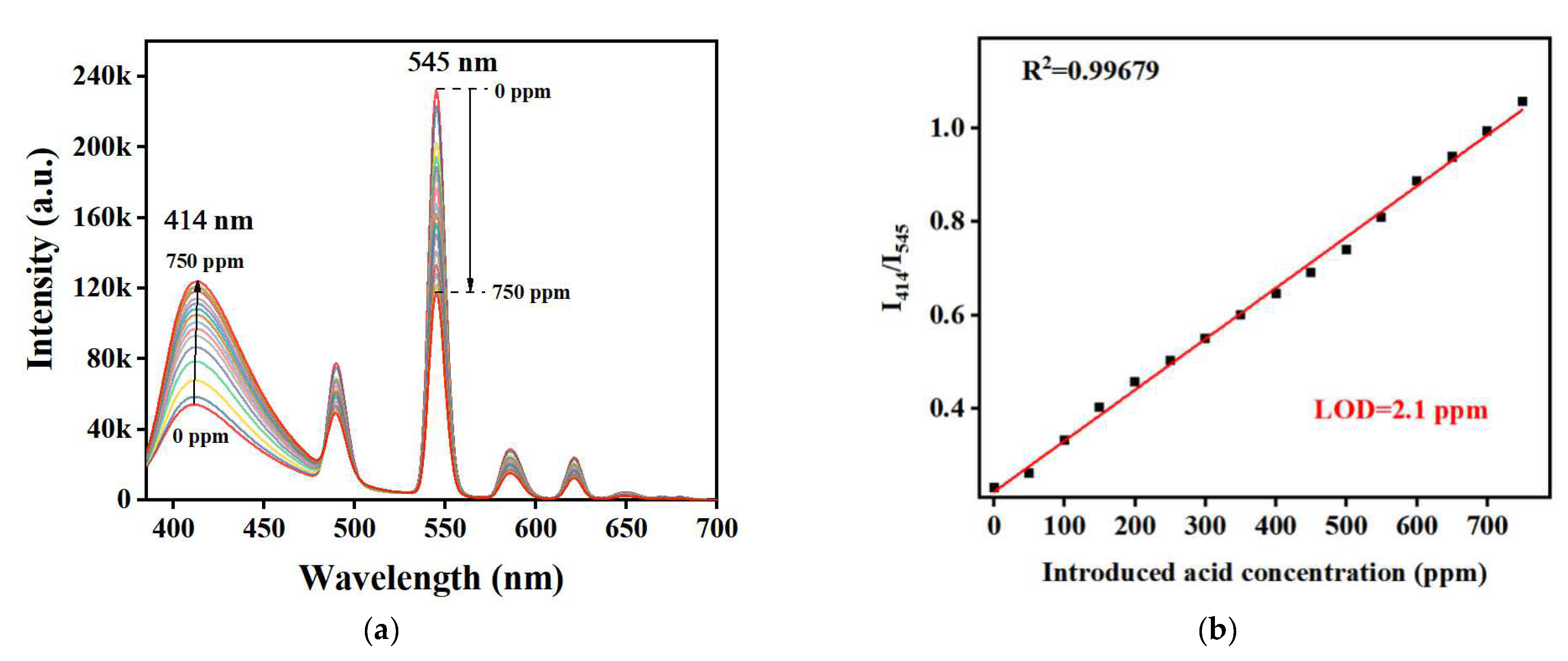
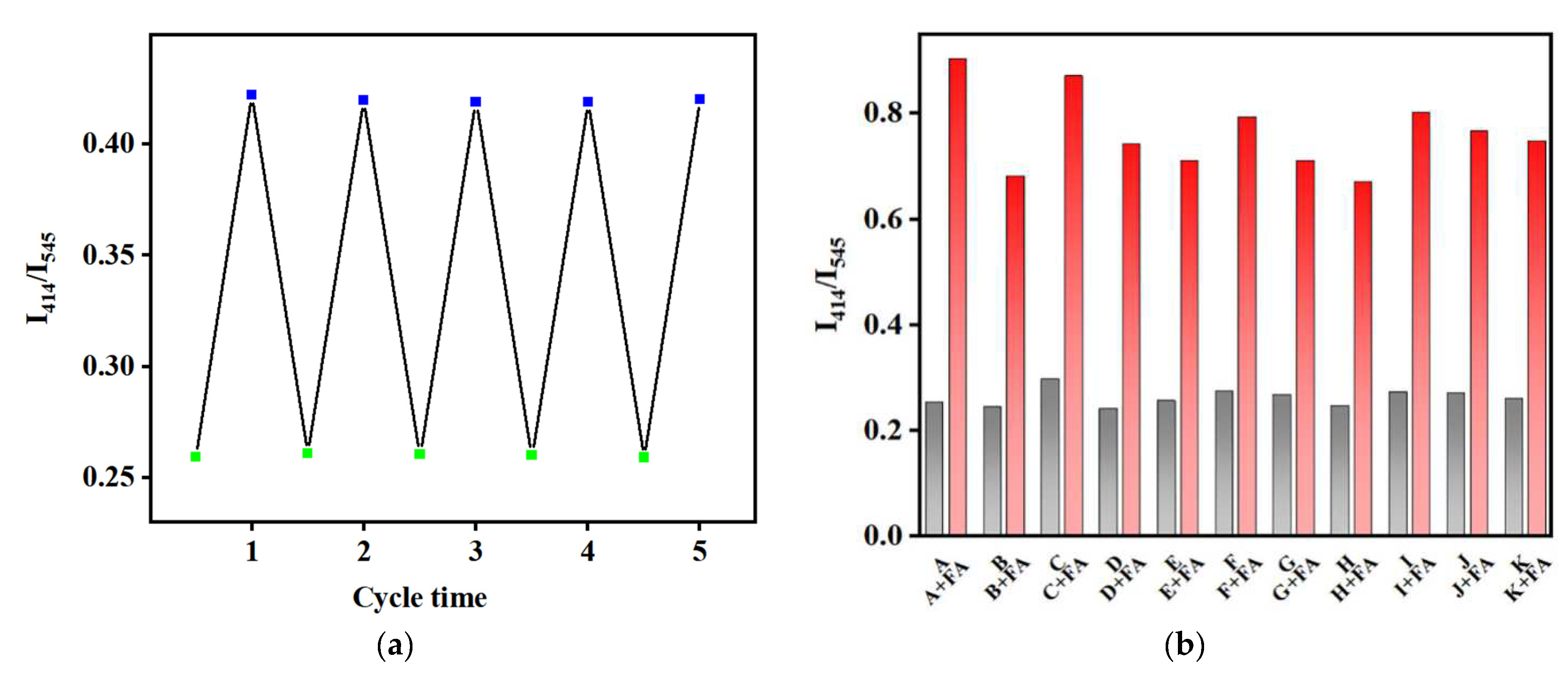
| Material | Phase State of FA | Detection Method | LOD (ppm) | Ref |
|---|---|---|---|---|
| TH25 | Liquid | Ratio fluorescence | 2.1 | this work |
| D-A fluorophore | Gas | 8.7 × 10−2 | [23] | |
| Carbon dots | 150 | [24] | ||
| MOF-802 | Liquid | Electrochemical analysis | 14.7 | [16] |
| Cobalt (II)-MOF 1 | 35.0 | [17] | ||
| ZZU-1 | 552 | [19] | ||
| - | Liquid | Microextraction—liquid chromatography | 3.0 × 10−4 | [11] |
| Added a (ppm) | Detected (ppm) | RSD (%) | Recovery (%) |
|---|---|---|---|
| 0 | 0 | - | - |
| 25 | 24.4 | 0.03 | 97.6 |
| 50 | 47.9 | 0.05 | 95.8 |
| 100 | 109 | 1.66 | 109 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.-W.; Li, M.-L.; Chen, Y.-D.; Zhou, Q.; Yang, W.-T. Ratiometric Fluorescent Sensor Based on Tb(III) Functionalized Metal-Organic Framework for Formic Acid. Molecules 2022, 27, 8702. https://doi.org/10.3390/molecules27248702
Zhang C-W, Li M-L, Chen Y-D, Zhou Q, Yang W-T. Ratiometric Fluorescent Sensor Based on Tb(III) Functionalized Metal-Organic Framework for Formic Acid. Molecules. 2022; 27(24):8702. https://doi.org/10.3390/molecules27248702
Chicago/Turabian StyleZhang, Chao-Wei, Mei-Ling Li, Yi-Duo Chen, Qi Zhou, and Wei-Ting Yang. 2022. "Ratiometric Fluorescent Sensor Based on Tb(III) Functionalized Metal-Organic Framework for Formic Acid" Molecules 27, no. 24: 8702. https://doi.org/10.3390/molecules27248702
APA StyleZhang, C.-W., Li, M.-L., Chen, Y.-D., Zhou, Q., & Yang, W.-T. (2022). Ratiometric Fluorescent Sensor Based on Tb(III) Functionalized Metal-Organic Framework for Formic Acid. Molecules, 27(24), 8702. https://doi.org/10.3390/molecules27248702