Interactions of Apigenin and Safranal with the 5HT1A and 5HT2A Receptors and Behavioral Effects in Depression and Anxiety: A Molecular Docking, Lipid-Mediated Molecular Dynamics, and In Vivo Analysis
Abstract
1. Introduction
2. Results
2.1. Domain Architecture Analysis and In Silico Computations
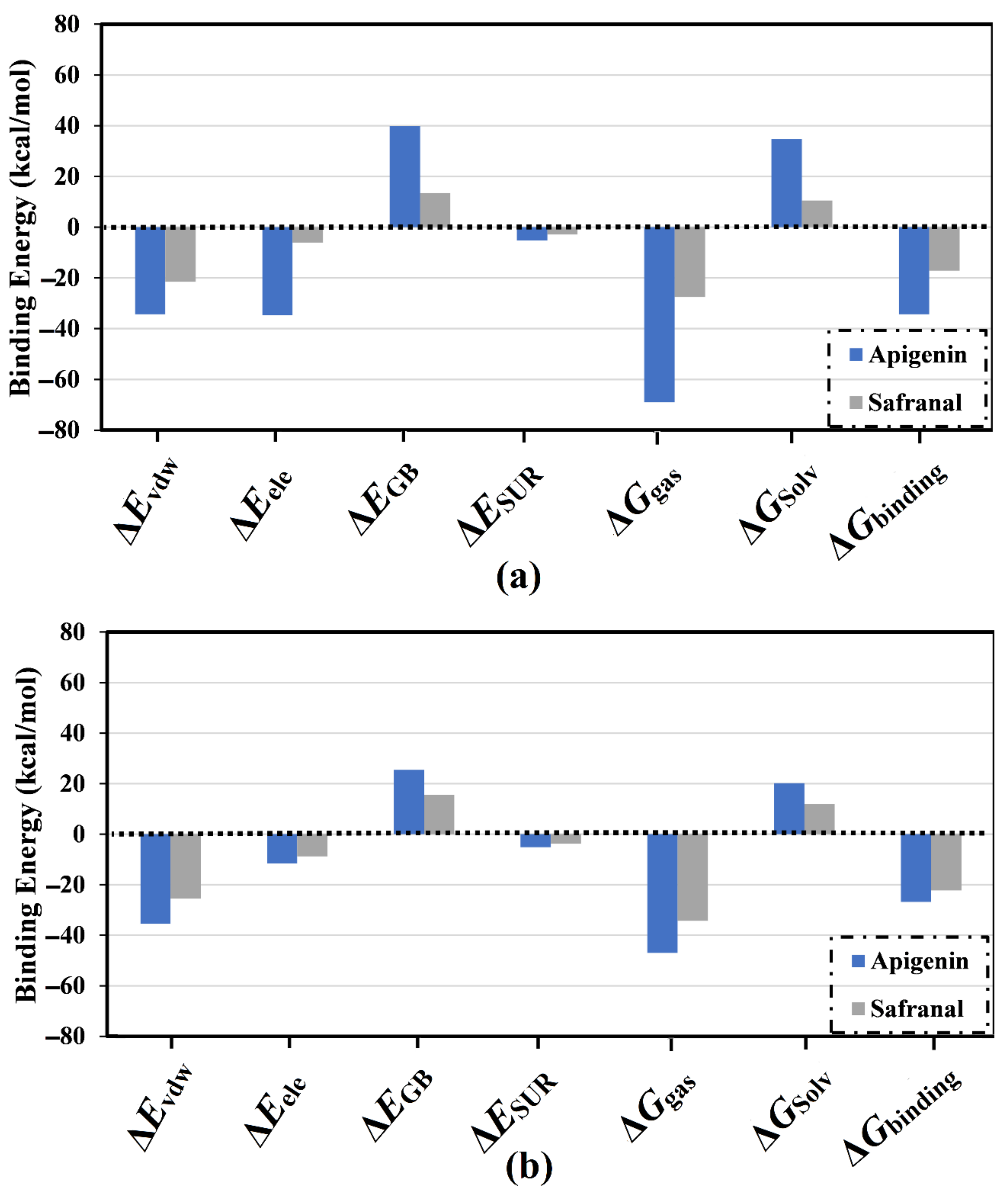
2.2. Analysis of Binding Affinity Partitioning
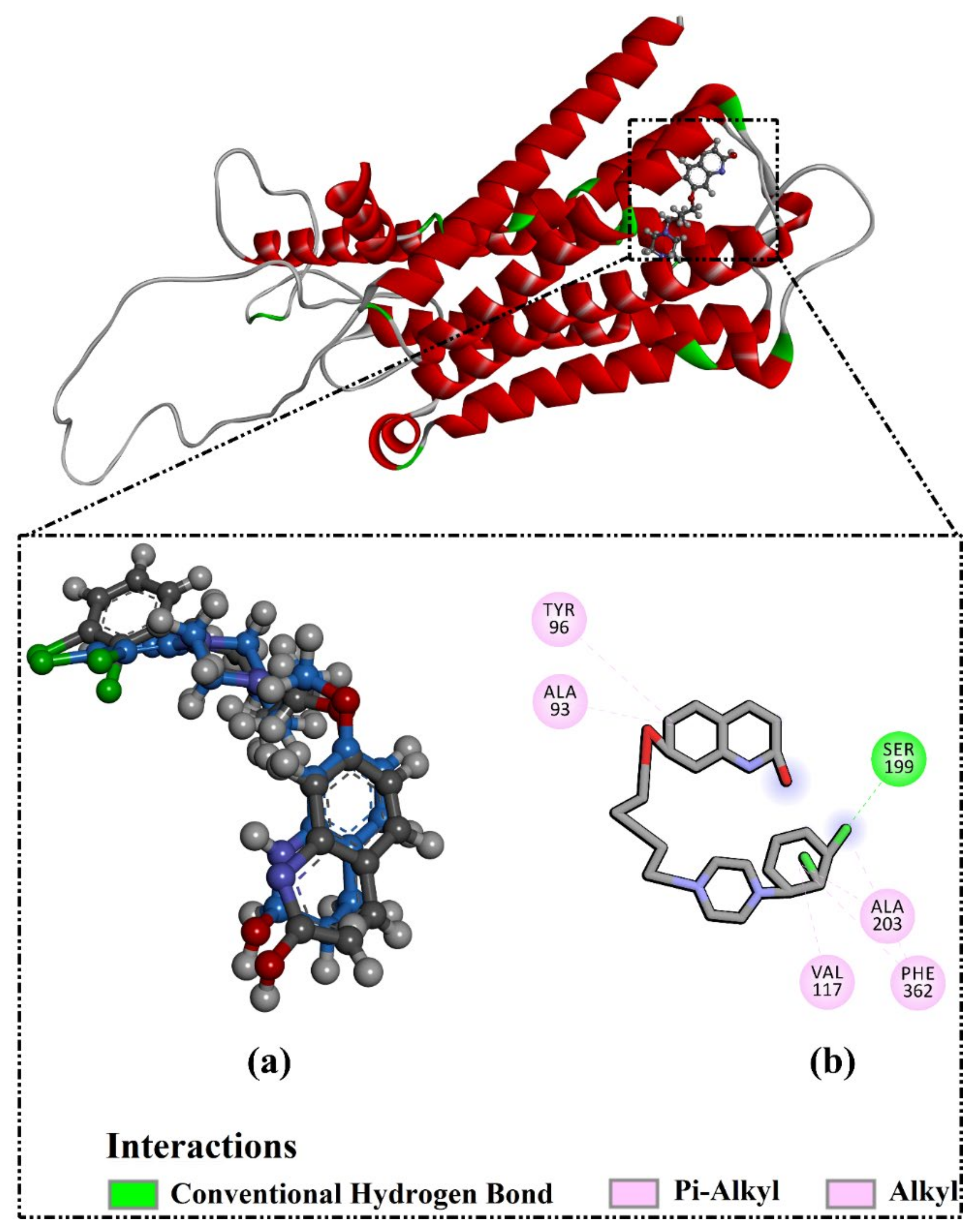
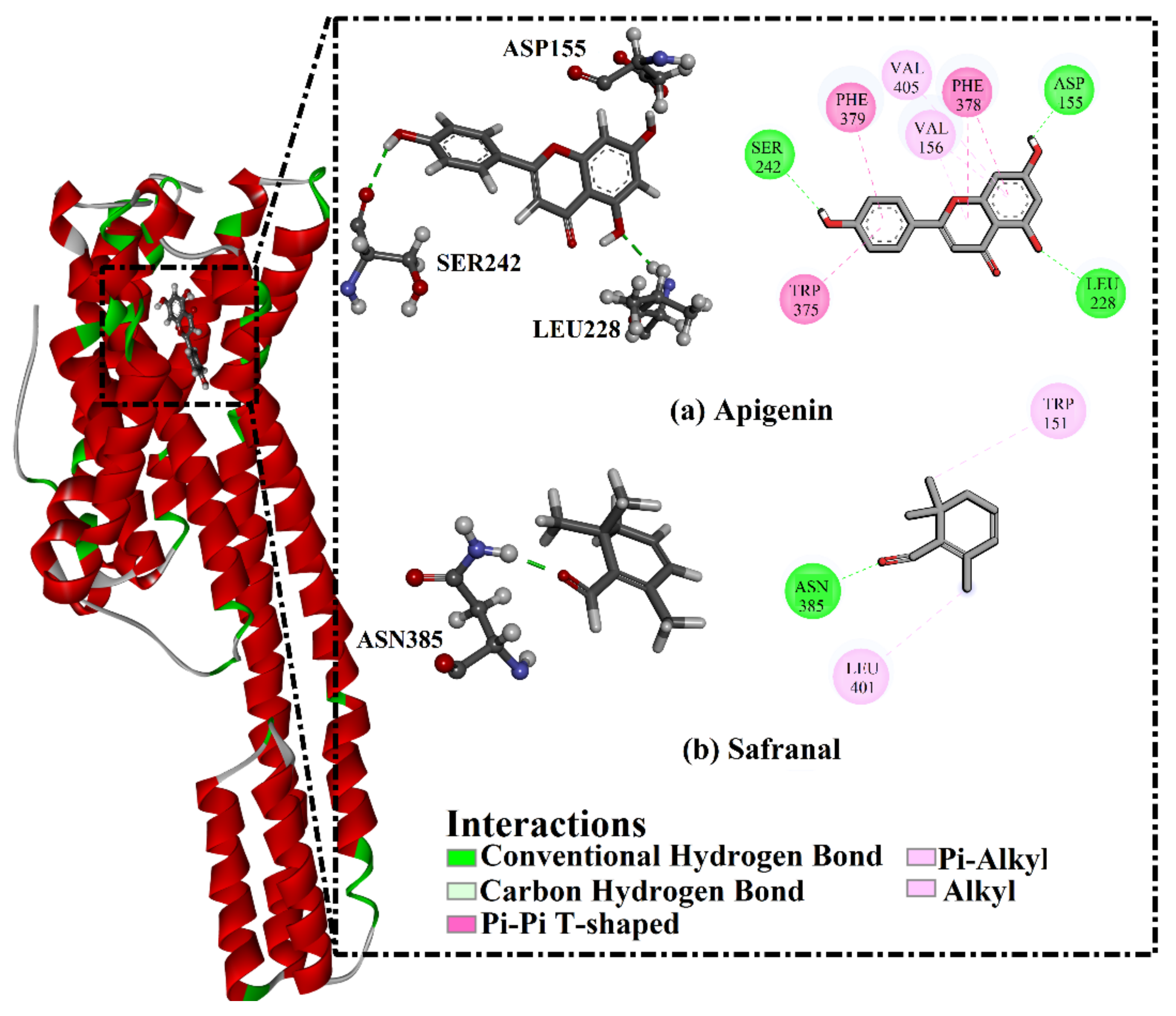
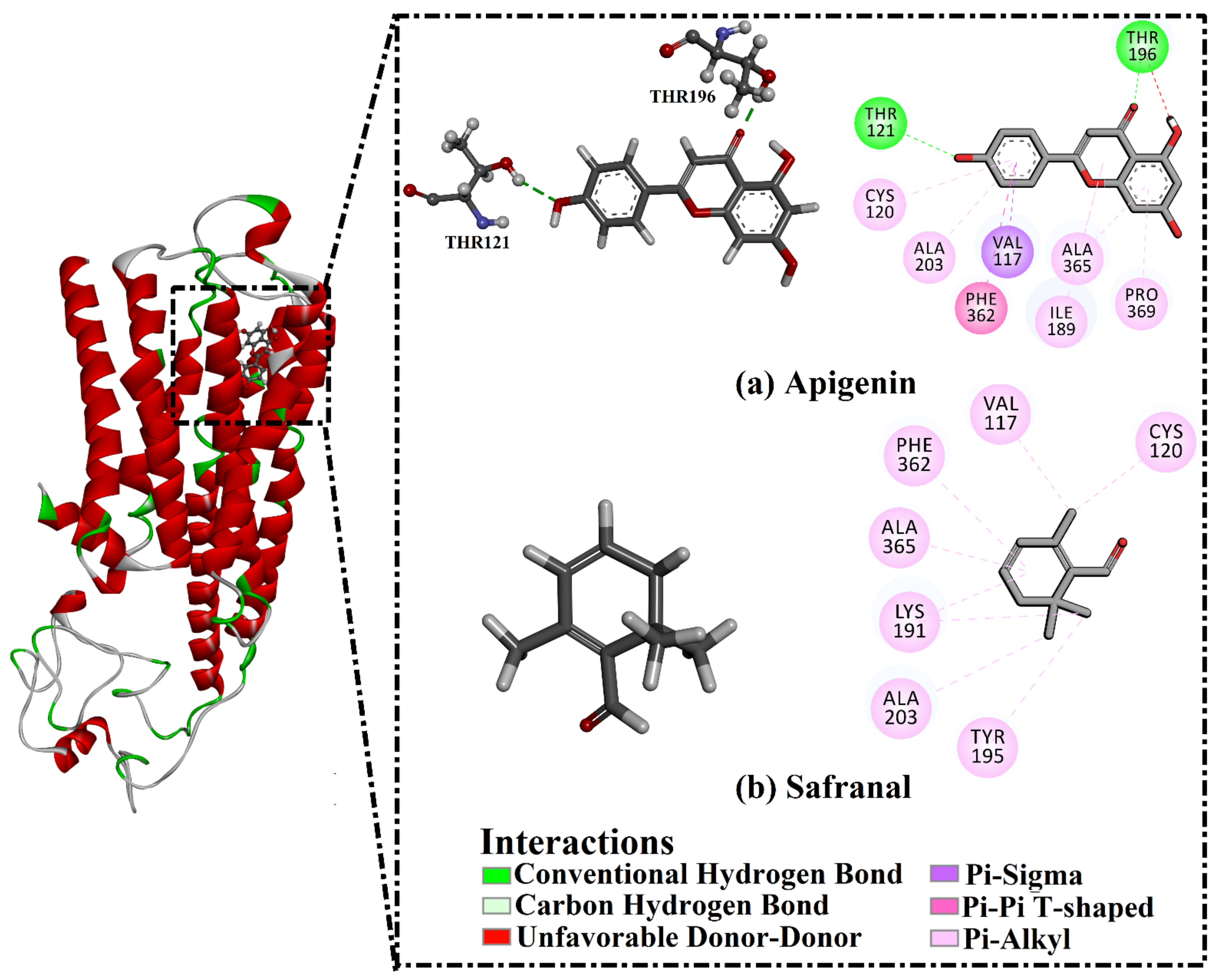
2.3. Analysis of the Key Amino Acids Involved in Drug–Protein Interactions
2.4. Post-Molecular Dynamics Analyses
2.4.1. Binding Affinity per Frame
2.4.2. Center of Mass Distance
2.4.3. Root Mean Square Deviation
2.4.4. Protein-Lipid System MD Simulations
2.5. Drug-Likeness Evaluation
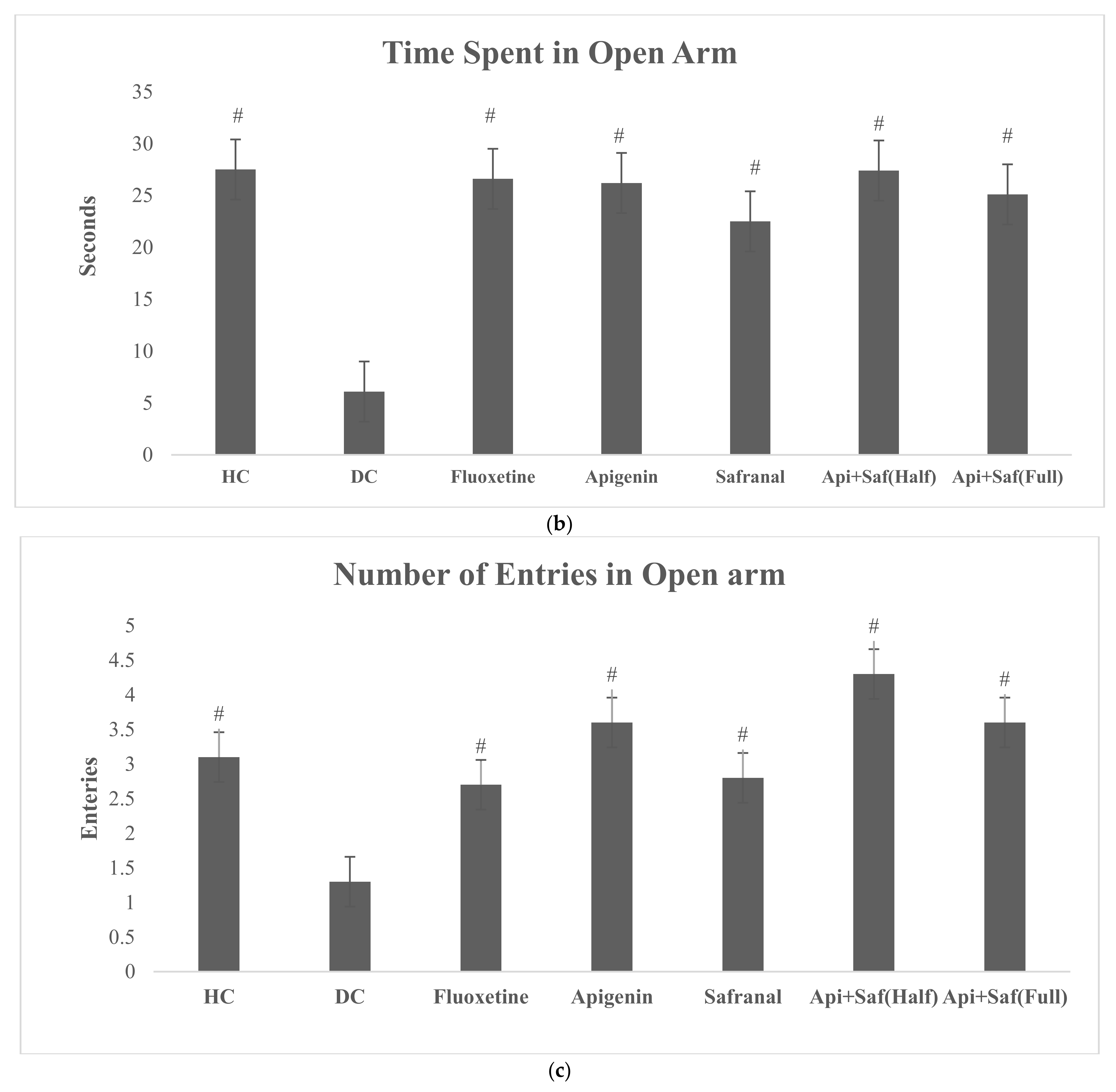
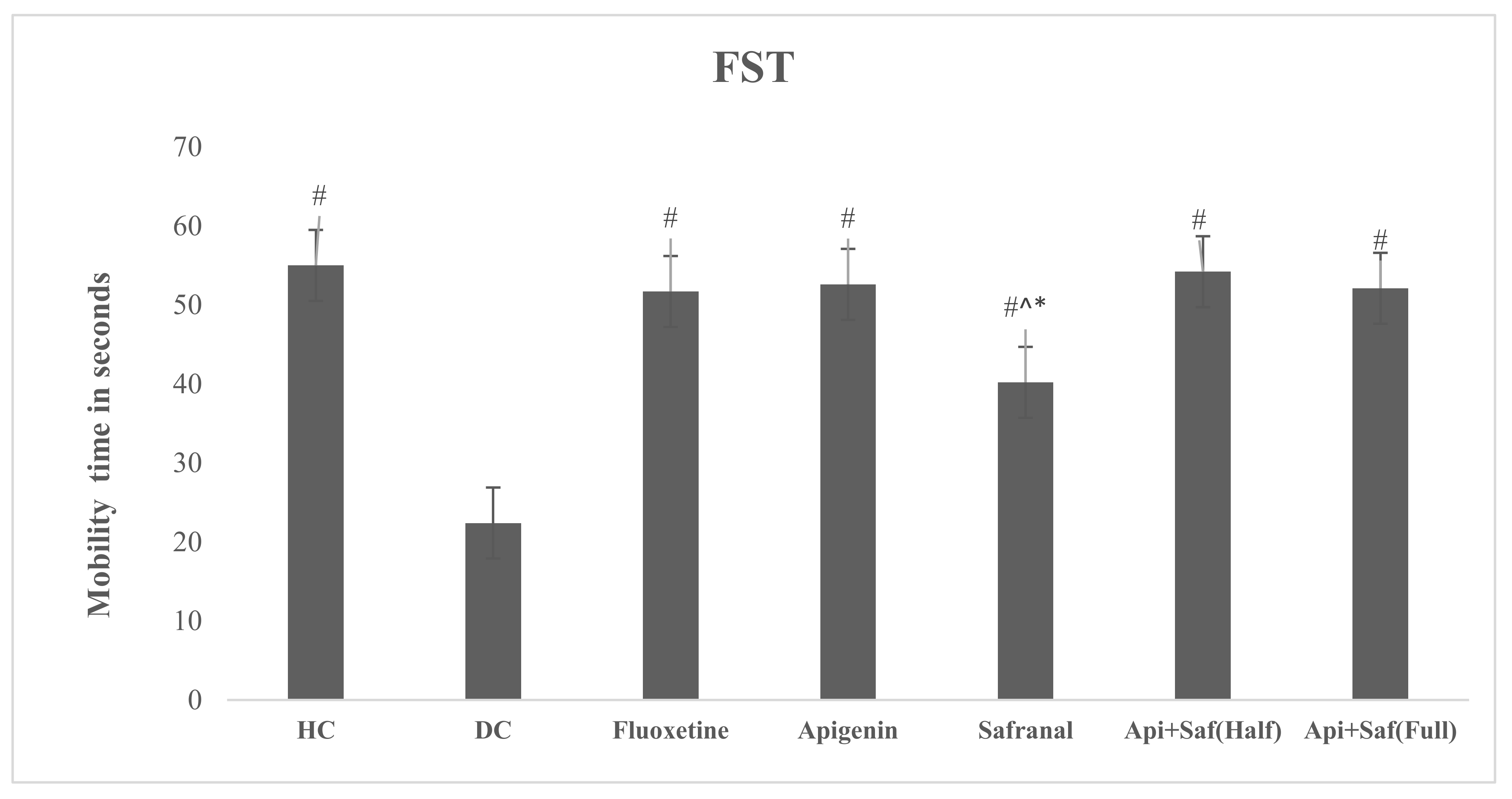
2.6. EPMT and FST for Analyzing Behavioral Effects of Apigenin and Safranal in the Rodent Model of Depression
3. Discussion
4. Materials and Methods
4.1. Molecular Structures of Safranal and Apigenin and the Protein Structures of the 5HT2AR and 5HT1AR
4.2. Molecular Docking
4.3. Molecular Dynamics
4.3.1. Protein-Lipid Complex Construction
4.3.2. Binding Energy Computations
4.4. Prediction of the Physicochemical Features of Apigenin and Safranal
4.4.1. In Vivo Rodent Model for Analysis of the Behavioral Effects of Apigenin and Safranal on Depression and Anxiety
4.4.2. Diabetes Induction in Rats
4.4.3. Administration of Drugs
4.4.4. Elevated Plus Maze Test (EPMT)
4.4.5. Forced Swim Test (FST)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Smith, K. Mental health: A world of depression. Nature 2014, 515, 181. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.; Siguelbaum, S.A.; Hudspeth, A.J. Principles of Neural Science; McGraw-Hill: New York, NY, USA, 2013. [Google Scholar]
- Hoyer, D. Targeting the 5-HT system: Potential side effects. Neuropharmacology 2020, 179, 108233. [Google Scholar] [CrossRef]
- Celada, P.; Puig, M.; Amargos-Bosch, M.; Adell, A.; Artigas, F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J. Psychiatry Neurosci. 2004, 29, 252–265. [Google Scholar] [PubMed]
- Beliveau, V.; Ganz, M.; Feng, L.; Ozenne, B.; Hojgaard, L.; Fisher, P.M.; Svarer, C.; Greve, D.N.; Knudsen, G.M. A High-Resolution In Vivo Atlas of the Human Brain’s Serotonin System. J. Neurosci. 2017, 37, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Nutt, D.J. Serotonin and brain function: A tale of two receptors. J. Psychopharmacol. 2017, 31, 1091–1120. [Google Scholar] [CrossRef]
- Yoshimura, R.; Okamoto, N. A Case of Major Depression Comorbid with Social Phobia Treated Successfully with Vortioxetine. Psychiatr. Danub. 2020, 32, 441. [Google Scholar]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Focus 2018, 16, 420–429. [Google Scholar] [CrossRef]
- Citrome, L. Vortioxetine for major depressive disorder: An indirect comparison with duloxetine, escitalopram, levomilnacipran, sertraline, venlafaxine, and vilazodone, using number needed to treat, number needed to harm, and likelihood to be helped or harmed. J. Affect. Disord. 2016, 196, 225–233. [Google Scholar] [CrossRef]
- Croft, H.A.; Pomara, N.; Gommoll, C.; Chen, D.; Nunez, R.; Mathews, M. Efficacy and safety of vilazodone in major depressive disorder: A randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatry 2014, 75, 6228. [Google Scholar] [CrossRef]
- Sagud, M.; Mihaljevic-Peles, A.; Muck-Seler, D.; Jakovljevic, M.; Pivac, N. Quetiapine augmentation in treatment-resistant depression: A naturalistic study. Psychopharmacology 2006, 187, 511–514. [Google Scholar] [CrossRef][Green Version]
- Papakostas, G.I.; Shelton, R.C.; Smith, J.; Fava, M. Augmentation of antidepressants with atypical antipsychotic medications for treatment-resistant major depressive disorder: A meta-analysis. J. Clin. Psychiatry 2007, 68, 826–831. [Google Scholar] [CrossRef]
- Shelton, R.C.; Papakostas, G.I. Augmentation of antidepressants with atypical antipsychotics for treatment-resistant major depressive disorder. Acta Psychiatr. Scand. 2008, 117, 253–259. [Google Scholar] [CrossRef]
- Thomas, K.; Saadabadi, A.; Olanzapine. StatPearls Publishing. [Updated 2020 August 29]. 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532903/ (accessed on 22 April 2021).
- Mathews, J.; Garcia, K.S.; Mintun, M.A.; Sheline, Y.I. Antidepressant efficacy of olanzapine as monotherapy in major depressive disorder, without psychosis: A pilot study. Psychiatry Res. Neuroimaging 2006, 146, 149–155. [Google Scholar] [CrossRef]
- Zmudzka, E.; Salaciak, K.; Sapa, J.; Pytka, K. Serotonin receptors in depression and anxiety: Insights from animal studies. Life Sci. 2018, 210, 106–124. [Google Scholar] [CrossRef]
- Dulawa, S.C.; Holick, K.A.; Gundersen, B.; Hen, R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 2004, 29, 1321–1330. [Google Scholar] [CrossRef]
- Rush, A.J.; Thase, M.E.; Dubé, S. Research issues in the study of difficult-to-treat depression. Biol. Psychiatry 2003, 53, 743–753. [Google Scholar] [CrossRef]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR* D report. Focus 2008, 6, 128–142. [Google Scholar] [CrossRef]
- Ventriglio, A.; Bhugra, D.; Sampogna, G.; Luciano, M.; De Berardis, D.; Sani, G.; Fiorillo, A. From dysthymia to treatment-resistant depression: Evolution of a psychopathological construct. Int. Rev. Psychiatry 2020, 32, 471–476. [Google Scholar] [CrossRef]
- Gaynes, B.N.; Lux, L.; Gartlehner, G.; Asher, G.; Forman-Hoffman, V.; Green, J.; Boland, E.; Weber, R.P.; Randolph, C.; Bann, C.; et al. Defining treatment-resistant depression. Depress. Anxiety 2020, 37, 134–145. [Google Scholar] [CrossRef]
- Siddiqui, M.J.; Saleh, M.S.M.; Basharuddin, S.N.B.B.; Zamri, S.H.B.; Najib, M.H.B.; Ibrahim, M.Z.B.; Noor, A.B.M.; Mazha, H.N.B.; Hassan, N.M.; Khatib, A. Saffron (Crocus sativus L.): As an Antidepressant. J. Pharm. Bioallied Sci. 2018, 10, 173–180. [Google Scholar] [CrossRef]
- Srivastava, R.; Ahmed, H.; Dixit, R.K.; Dharamveer Saraf, S.A. Crocus sativus L.: A comprehensive review. Pharmacogn. Rev. 2010, 4, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, J.D.; Shults, J.; Soeller, I.; Mao, J.J.; Rockwell, K.; Newberg, A.B. Chamomile (Matricaria recutita) may have antidepressant activity in anxious depressed humans-an exploratory study. Altern. Ther. Health Med. 2012, 18, 44. [Google Scholar] [PubMed]
- Yi, L.T.; Li, J.M.; Li, Y.C.; Pan, Y.; Xu, Q.; Kong, L.D. Antidepressant-like behavioral and neurochemical effects of the citrus-associated chemical apigenin. Life Sci. 2008, 82, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.I.; Liu, C.Y.; Yang, C.H. Persistent depressive disorder has long-term negative impacts on depression, anxiety, and somatic symptoms at 10-year follow-up among patients with major depressive disorder. J. Affect. Disord. 2019, 243, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-J.; Zou, W.; Yuan, J.; Zhang, P.; Tian, Y.; Xiao, Z.-F.; Li, M.-H.; Wei, H.-J.; Tang, X.-Q. Antidepressant-like and anxiolytic-like effects of hydrogen sulfide in streptozotocin-induced diabetic rats through inhibition of hippocampal oxidative stress. Behav. Pharmacol. 2015, 26, 427–435. [Google Scholar] [CrossRef]
- Tabatabaei, S.R.F.; Ghaderi, S.; Bahrami-Tapehebur, M.; Farbood, Y.; Rashno, M. Aloe vera gel improves behavioral deficits and oxidative status in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2017, 96, 279–290. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.-M.; Zhang, M.-Y.; Chen, Y.-J.; Liu, Y.-W. Hesperetin ameliorates diabetes-associated anxiety and depression-like behaviors in rats via activating Nrf2/ARE pathway. Metab. Brain Dis. 2021, 36, 1969–1983. [Google Scholar] [CrossRef]
- Li, H.; Zuo, Y.; Lei, Y.; Xu, R.; Wang, Y. Modeling of diabetes mellitus-related depression. Neurophysiology 2014, 46, 71–78. [Google Scholar] [CrossRef]
- Maes, M.; Leonard, B.; Myint, A.; Kubera, M.; Verkerk, R. The new ‘5-HT’hypothesis of depression: Cell-mediated immune activation induces indoleamine 2, 3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 702–721. [Google Scholar]
- Wigner, P.; Czarny, P.; Galecki, P.; Su, K.-P.; Sliwinski, T. The molecular aspects of oxidative & nitrosative stress and the tryptophan catabolites pathway (TRYCATs) as potential causes of depression. Psychiatry Res. 2018, 262, 566–574. [Google Scholar]
- Unluturk, U.; Erbas, T. Diabetes and tryptophan metabolism. In Tryptophan Metabolism: Implications for Biological Processes, Health and Disease; Humana Press: Cham, Switzerland, 2015; pp. 147–171. [Google Scholar]
- Hagen, J.M.; Sutterland, A.L.; Sousa, P.A.D.F.P.D.; Schirmbeck, F.; Cohn, D.M.; Lok, A.; Tan, H.L.; Zwinderman, A.H.; de Haan, L. Association between skin autofluorescence of advanced glycation end products and affective disorders in the lifelines cohort study. J. Affect. Disord. 2020, 275, 230–237. [Google Scholar] [CrossRef]
- Caviedes, A.; Lafourcade, C.; Soto, C.; Wyneken, U. BDNF/NF-κB signaling in the neurobiology of depression. Curr. Pharm. Des. 2017, 23, 3154–3163. [Google Scholar] [CrossRef]
- Nitta, A.; Murai, R.; Suzuki, N.; Ito, H.; Nomoto, H.; Katoh, G.; Furukawa, Y. Diabetic neuropathies in brain are induced by deficiency of BDNF. Neurotoxicol. Teratol. 2002, 24, 695–701. [Google Scholar] [CrossRef]
- Patel, S.; Santani, D. Role of NF-κB in the pathogenesis of diabetes and its associated complications. Pharmacol. Rep. 2009, 61, 595–603. [Google Scholar] [CrossRef]
- Hilakivi-Clarke, L.; Wozniak, K.; Durcan, M.J.; Linnoila, M. Behavior of streptozotocin-diabetic mice in tests of exploration, locomotion, anxiety, depression and aggression. Physiol. Behav. 1990, 48, 429–433. [Google Scholar] [CrossRef]
- Rao, V.S.; Srinivas, K. Modern drug discovery process: An in silico approach. J. Bioinform. Seq. Anal. 2011, 2, 89–94. [Google Scholar]
- Latorraca, N.R.; Venkatakrishnan, A.; Dror, R.O. GPCR dynamics: Structures in motion. Chem. Rev. 2017, 117, 139–155. [Google Scholar] [CrossRef]
- Kimura, K.; Asada, H.; Inoue, A.; Kadji, F.M.N.; Im, D.; Mori, C.; Arakawa, T.; Hirata, K.; Nomura, Y.; Nomura, N.; et al. Structures of the 5-HT2A receptor in complex with the antipsychotics risperidone and zotepine. Nat. Struct. Mol. Biol. 2019, 26, 121–128. [Google Scholar] [CrossRef]
- Xu, P.; Huang, S.; Zhang, H.; Mao, C.; Zhou, X.E.; Cheng, X.; Simon, I.A.; Shen, D.-D.; Yen, H.-Y.; Robinson, C.V.; et al. Structural insights into the lipid and ligand regulation of serotonin receptors. Nature 2021, 592, 469–473. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Nakazawa, T.; Yasuda, T.; Ueda, J.; Ohsawa, K. Antidepressant-like effects of apigenin and 2,4,5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol. Pharm. Bull. 2003, 26, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H., Karimi, G., Niapoor, M., Eds.; Antidepressant effect of Crocus sativus L. stigma extracts and their constituents, crocin and safranal, in mice. In Proceedings of the International Symposium on Saffron Biology and Biotechnology, Albacete, Spain, 22–25 October 2003; p. 650. [Google Scholar]
- Lampropoulos, P.; Lambropoulou, M.; Papalois, A.; Basios, N.; Manousi, M.; Simopoulos, C.; Tsaroucha, A.K. The role of apigenin in an experimental model of acute pancreatitis. J. Surg. Res. 2013, 183, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Guo, X.; Li, Y.; Yang, X.; Han, Y. Apigenin reverses depression-like behavior induced by chronic corticosterone treatment in mice. Eur. J. Pharm. 2016, 774, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Berge, L.I.; Riise, T. Comorbidity between Type 2 Diabetes and Depression in the Adult Population: Directions of the Association and Its Possible Pathophysiological Mechanisms. Int. J. Endocrinol. 2015, 2015, 164760. [Google Scholar] [CrossRef] [PubMed]
- Mackay, G.M.; Forrest, C.M.; Christofides, J.; Bridel, M.A.; Mitchell, S.; Cowlard, R.; Stone, T.W.; Darlington, L.G. Kynurenine metabolites and inflammation markers in depressed patients treated with fluoxetine or counselling. Clin. Exp. Pharm. Physiol. 2009, 36, 425–435. [Google Scholar] [CrossRef]
- Santoso, H.B.; Rahmi, R.A.; Kartikasari, D. (Eds.) Blood glucose level of white rats (Rattus norvegicus) after giving catfish biscuit (Pangasius hypothalmus). BIO Web Conf. 2020, 20, 04005. [Google Scholar] [CrossRef]
- Kolanos, R.; Stice, S.A. German Chamomile. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 757–772. [Google Scholar]
- Ghaderi, A.; Asbaghi, O.; Reiner, Ž.; Kolahdooz, F.; Amirani, E.; Mirzaei, H.; Banafshe, H.R.; Dana, P.M.; Asemi, Z. The effects of saffron (Crocus sativus L.) on mental health parameters and C-reactive protein: A meta-analysis of randomized clinical trials. Complement. Med. 2020, 48, 102250. [Google Scholar] [CrossRef]
- Nanda, S.; Madan, K.J.H. The role of safranal and saffron stigma extracts in oxidative stress, diseases and photoaging: A systematic review. Heliyon 2021, 7, e06117. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Shakib, S.S.; Sameni, A.K.; Taghiabadi, E. Acute and subacute toxicity of safranal, a constituent of saffron, in mice and rats. Iran. J. Pharm. Res. 2013, 12, 93. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Karimi, G.; Niapoor, M. Antidepressant effects of Crocus sativus stigma extracts and its constituents, crocin and safranal, in mice. J. Med. Plants 2004, 3, 48–58. [Google Scholar]
- Hosseinzadeh, H.; Noraei, N.B. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 768–774. [Google Scholar]
- Samarghandian, S.; Borji, A.; Delkhosh, M.B.; Samini, F. Safranal treatment improves hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats. J. Pharm. Pharm. Sci. 2013, 16, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jiang, Y.; Ma, J.; Wu, H.; Wacker, D.; Katritch, V.; Han, G.W.; Liu, W.; Huang, X.-P.; Vardy, E.; et al. Structural basis for molecular recognition at serotonin receptors. Science 2013, 340, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- Kukić, P.; Nielsen, J.E. Electrostatics in proteins and protein–ligand complexes. Future Med. Chem. 2010, 2, 647–666. [Google Scholar] [CrossRef]
- Zanoli, P.; Avallone, R.; Baraldi, M. Behavioral characterisation of the flavonoids apigenin and chrysin. Fitoterapia 2000, 71 (Suppl. 1), S117–S123. [Google Scholar] [CrossRef]
- Yang, Y.; Bai, L.; Li, X.; Xiong, J.; Xu, P.; Guo, C.; Xue, M. Transport of active flavonoids, based on cytotoxicity and lipophilicity: An evaluation using the blood-brain barrier cell and Caco-2 cell models. Toxicol. Vitr. 2014, 28, 388–396. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharm. 1977, 229, 327–336. [Google Scholar]
- Handley, S.L.; Mithani, S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1984, 327, 1–5. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Guo, H.-R.; Tsai, W.-C.; Yang, K.-L.; Lin, L.-C.; Cheng, T.-J.; Chuu, J.-J. Subchronic Arsenic Exposure Induces Anxiety-Like Behaviors in Normal Mice and Enhances Depression-Like Behaviors in the Chemically Induced Mouse Model of Depression. Biomed Res. Int. 2015, 2015, 159015. [Google Scholar] [CrossRef]
- Mbiantcha, M.; Khalid, R.; Dawe, A.; Mehreen, A.; Atsamo, D.A.; Ateufack, G.; Hamza, D.; Nana, W.Y.; Bomba, F.T.D.; Naeem, R.U.; et al. Antihypernociceptive and neuroprotective effects of Combretin A and Combretin B on streptozotocin-induced diabetic neuropathy in mice. Naunyn Schmiedebergs Arch. Pharm. 2019, 392, 697–713. [Google Scholar] [CrossRef]
- Rebai, R.; Jasmin, L.; Boudah, A. The antidepressant effect of melatonin and fluoxetine in diabetic rats is associated with a reduction of the oxidative stress in the prefrontal and hippocampal cortices. Brain Res. Bull. 2017, 134, 142–150. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Boskabady, M.H.; Hosseini, M.; Rezaee, R.; Tsatsakis, A.M. The effects of Crocus sativus (saffron) and its constituents on nervous system: A review. Avicenna J. Phytomed. 2015, 5, 376. [Google Scholar]
- Manzoor, M.; Dhar, M.K. A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomed. Pharmacother. 2018, 98, 733–745. [Google Scholar]
- Sharma, P.; Sharma, S.; Singh, D. Apigenin reverses behavioural impairments and cognitive decline in kindled mice via CREB-BDNF upregulation in the hippocampus. Nutr. Neurosci. 2020, 23, 118–127. [Google Scholar] [CrossRef]
- Qiu, Z.K.; Zhong, D.S.; He, J.L.; Liu, X.; Chen, J.S.; Nie, H. The anxiolytic-like effects of puerarin are associated with the changes of monoaminergic neurotransmitters and biosynthesis of allopregnanolone in the brain. Metab. Brain Dis. 2018, 33, 167–175. [Google Scholar] [CrossRef]
- Miura, H.; Naoi, M.; Nakahara, D.; Ohta, T.; Nagatsu, T. Changes in monoamine levels in mouse brain elicited by forced-swimming stress, and the protective effect of a new monoamine oxidase inhibitor, RS-8359. J. Neural Transm. Gen. Sect. 1993, 94, 175–187. [Google Scholar] [CrossRef]
- Cryan, J.F.; Page, M.E.; Lucki, I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology 2005, 182, 335–344. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Marti-Renom, M.A.; Stuart, A.C.; Fiser, A.; Sanchez, R.; Melo, F.; Sali, A. Comparative protein structure modeling of genes and genomes. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 291–325. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.C.; Myers, J.B.; Folta, T.; Shoja, V.; Heath, L.S.; Onufriev, A. H++: A server for estimating pKas and adding missing hydrogens to macromolecules. Nucleic Acids Res. 2005, 33, W368–W371. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Dassault Systèmes BIOVIA, B.D.S.V.; Version 2019; Dassault Systèmes BIOVIA: San Diego, CA, USA, 2019.
- Case, D.A.; Betz, R.M.; Cerutti, D.S.; Cheatham, T.E.; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; Homeyer, N.; et al. AMBER 2016; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Bayly, C.I.; Cieplak, P.; Cornell, W.D.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges—The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Frisch, M.J.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision E01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kollman, P.A. Settle—An Analytical Version of the Shake and Rattle Algorithm for Rigid Water Models. J. Comput. Chem. 1992, 13, 952–962. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Massova, I.; Kollman, P.A. Combined molecular mechanical and continuum solvent approach (MM-PBSA/GBSA) to predict ligand binding. Perspect. Drug Discov. 2000, 18, 113–135. [Google Scholar] [CrossRef]
- Onufriev, A.; Bashford, D.; Case, D.A. Exploring protein native states and large-scale conformational changes with a modified generalized born model. Proteins 2004, 55, 383–394. [Google Scholar] [CrossRef]
- Ho, N.; Balu, D.T.; Hilario, M.R.; Blendy, J.A.; Lucki, I. Depressive phenotypes evoked by experimental diabetes are reversed by insulin. Physiol. Behav. 2012, 105, 702–708. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, L.; Xu, W.; Lin, Q.; Zhou, Y.; Huang, X.; Ye, X.; He, J.; Bai, G.; Yan, Z.; et al. Metabolic Changes Associated with a Rat Model of Diabetic Depression Detected by Ex Vivo 1H Nuclear Magnetic Resonance Spectroscopy in the Prefrontal Cortex, Hippocampus, and Hypothalamus. J. Neural Plast. 2018, 2018, 6473728. [Google Scholar] [CrossRef]
- Pawluski, J.L.; Van Donkelaar, E.; Abrams, Z.; Houbart, V.; Fillet, M.; Steinbusch, H.W.M.; Charlier, T.D. Fluoxetine dose and administration method differentially affect hippocampal plasticity in adult female rats. Neural Plast. 2014, 2014, 123026. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Mohamadi-Zarch, S.M.; Roghani, M. Safranal, an active ingredient of saffron, attenuates cognitive deficits in amyloid beta-induced rat model of Alzheimer’s disease: Underlying mechanisms. Metab. Brain Dis. 2019, 34, 1747–1759. [Google Scholar] [CrossRef]
- Nikbakht, F.; Khadem, Y.; Haghani, S.; Hoseininia, H.; Moein Sadat, A.; Heshemi, P.; Jamali, N. Protective Role of Apigenin Against Abeta 25-35 Toxicity Via Inhibition of Mitochondrial Cytochrome c Release. Basic Clin. Neurosci. 2019, 10, 557–566. [Google Scholar]
- Haider, S.; Naqvi, F.; Batool, Z.; Tabassum, S.; Sadir, S.; Liaquat, L.; Naqvi, F.; Zuberi, N.A.; Shakeel, H.; Perveen, T. Pretreatment with curcumin attenuates anxiety while strengthens memory performance after one short stress experience in male rats. Brain Res. Bull. 2015, 115, 1–8. [Google Scholar] [CrossRef]
- Naqvi, F.; Saleem, S.; Naqvi, F.; Batool, Z.; Sadir, S.; Tabassum, S.; Ahmed, S.; Liaquat, L.; Haider, S. Curcumin lessens unpredictable chronic mild stress-induced depression and memory deficits by modulating oxidative stress and cholinergic activity. Pak. J. Pharm. Sci. 2019, 32, 1893–1900. [Google Scholar] [PubMed]

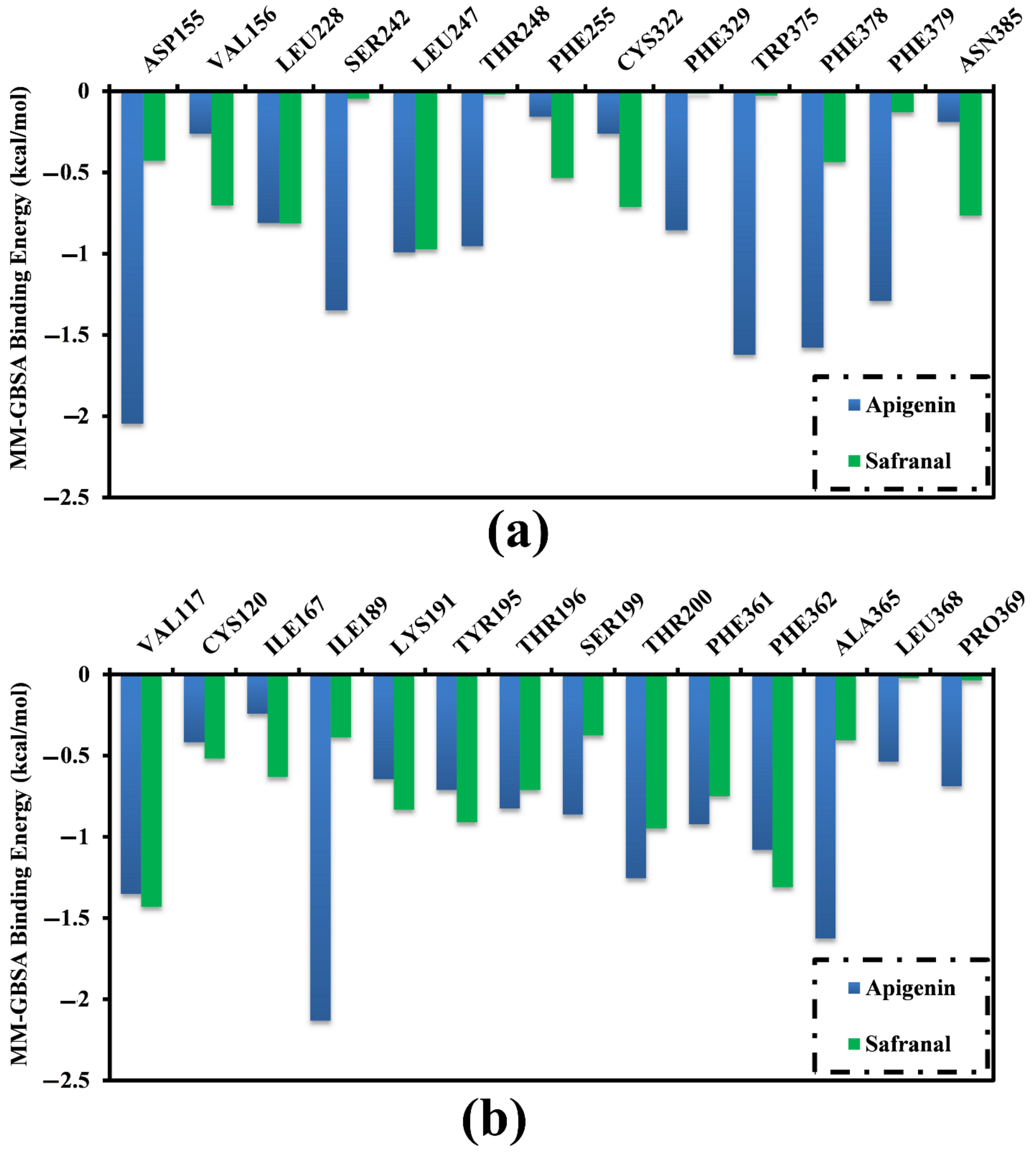
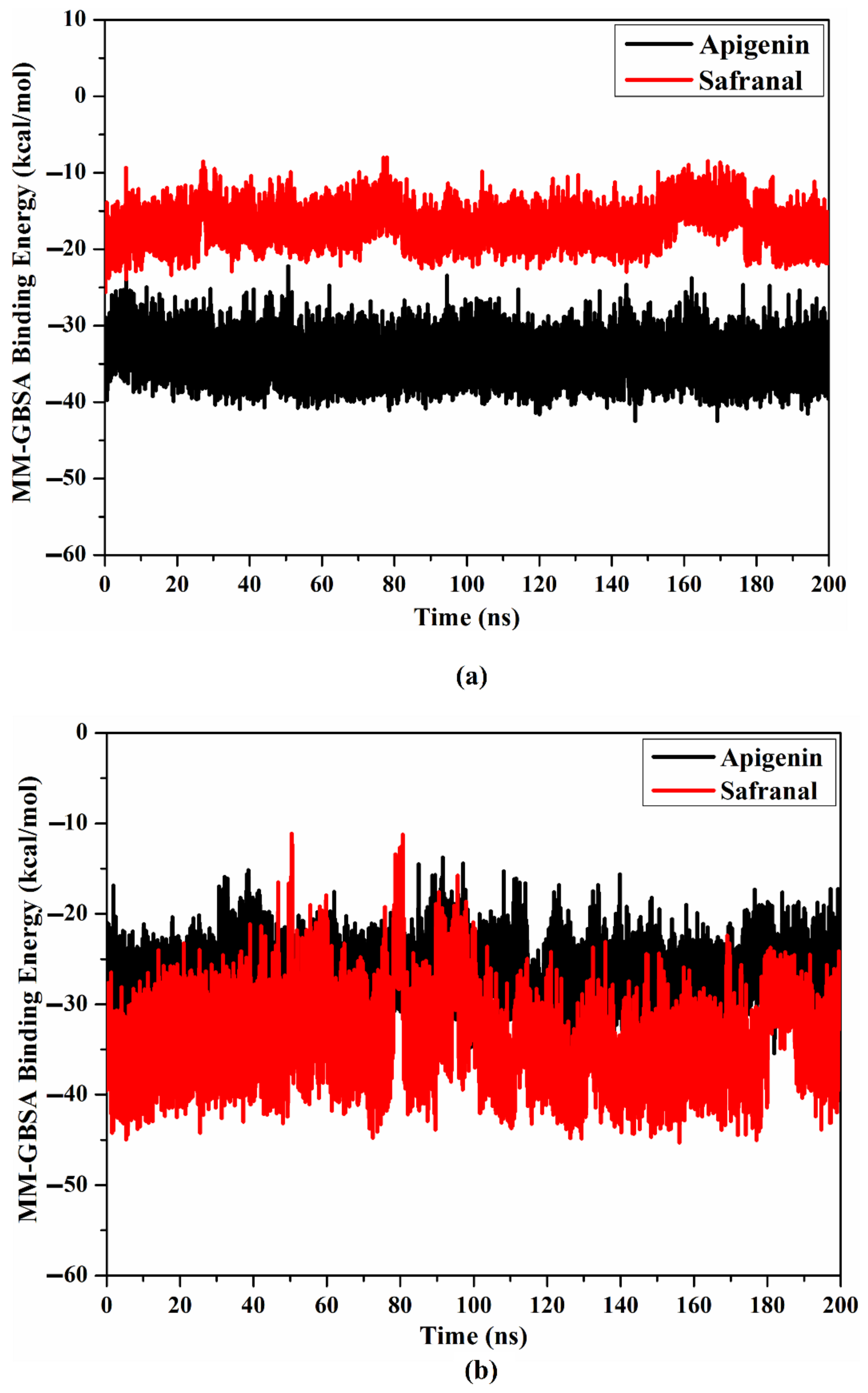
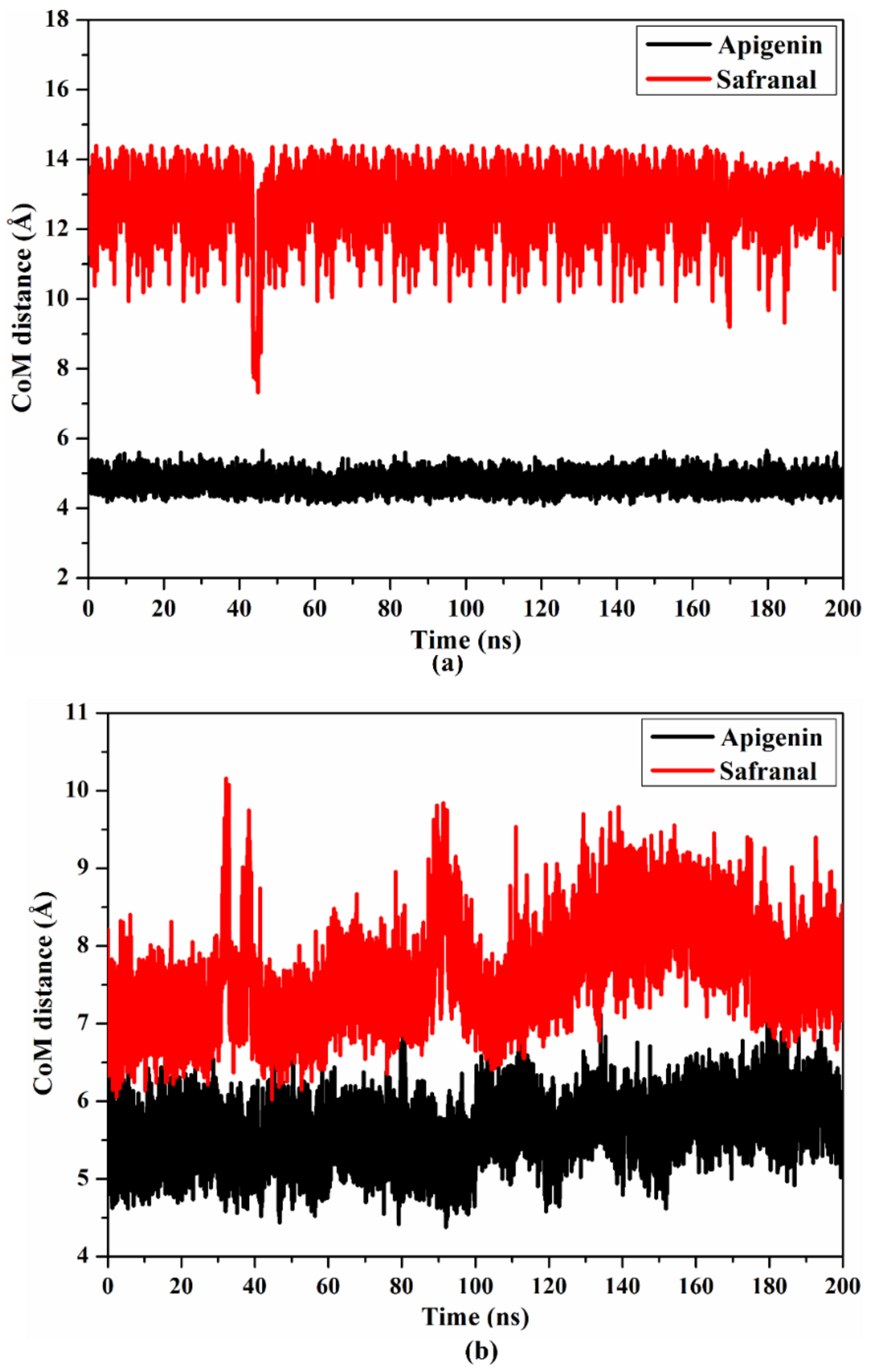
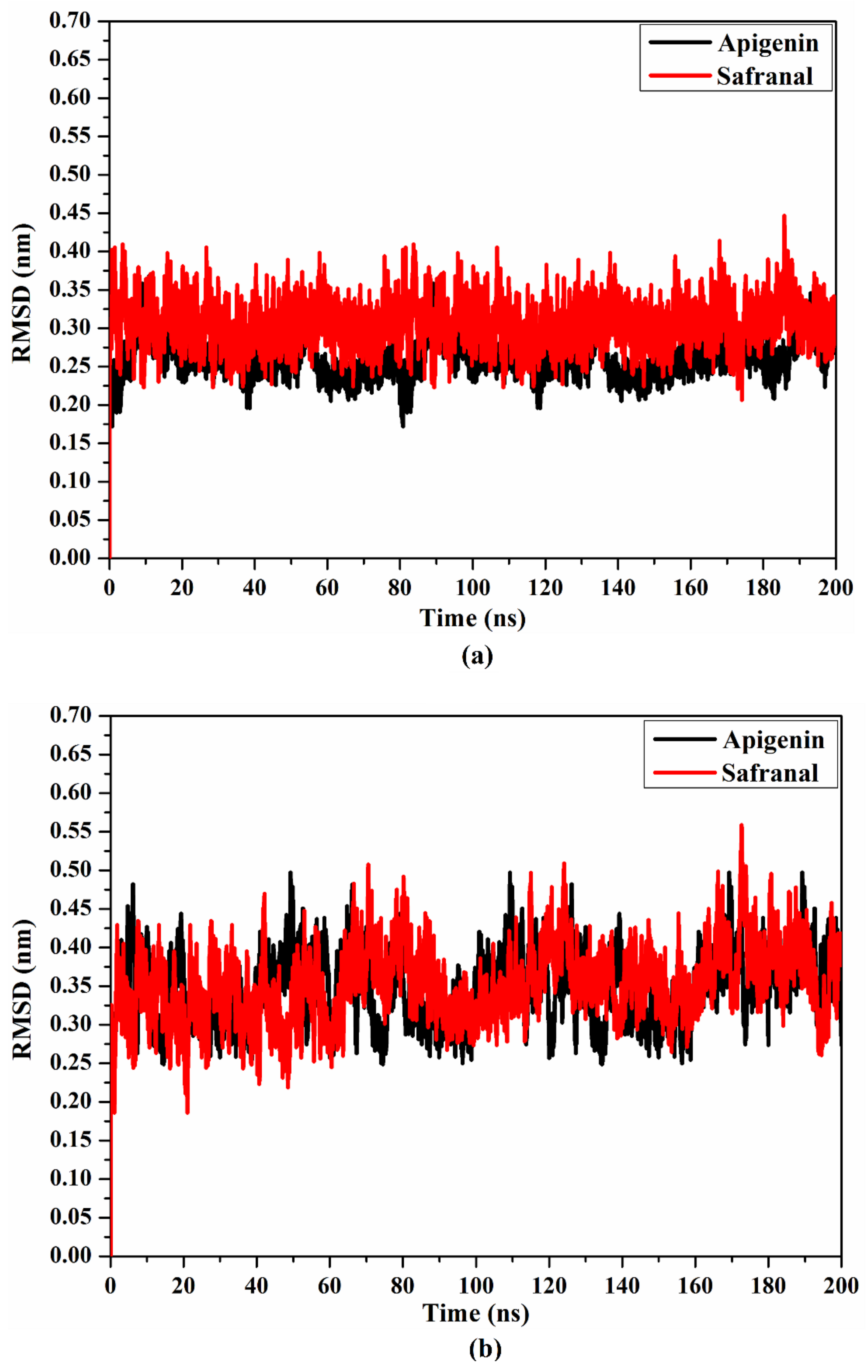


| Receptor | Compound Name | Docking Score (kcal/mol) | Binding Features a,b | MM-GBSA Binding Energy (kcal/mol) |
|---|---|---|---|---|
| 5HT2A | Apigenin | −8.9 | ASP155 (1.69 Å) | −34.3 |
| LEU228 (2.32 Å) | ||||
| SER242 (2.45 Å) | ||||
| Safranal | −6.8 | ASN385 (1.90 Å) | −17.1 | |
| 5HT1A | Apigenin | −8.4 | THR121 (2.57 Å) | −26.8 |
| THR196 (1.88 Å) | ||||
| Safranal | −6.0 | ----- c | −22.3 |
| Compound Name | miLogP | TPSA | nON | nOHNH | Nrotb | MVol | MWt | %ABS |
|---|---|---|---|---|---|---|---|---|
| Apigenin | 2.5 | 90.9 | 5 | 3 | 1 | 224.1 | 270.2 | 77.6% |
| Safranal | 3.0 | 17.1 | 1 | 0 | 1 | 158.6 | 150.2 | 103.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, F.; Ibrahim, M.A.A.; Rizwan-ul-Hasan, S.; Khaliq, S.; Gabr, G.A.; Muhammad; Khan, A.; Sidhom, P.A.; Tikmani, P.; Shawky, A.M.; et al. Interactions of Apigenin and Safranal with the 5HT1A and 5HT2A Receptors and Behavioral Effects in Depression and Anxiety: A Molecular Docking, Lipid-Mediated Molecular Dynamics, and In Vivo Analysis. Molecules 2022, 27, 8658. https://doi.org/10.3390/molecules27248658
Amin F, Ibrahim MAA, Rizwan-ul-Hasan S, Khaliq S, Gabr GA, Muhammad, Khan A, Sidhom PA, Tikmani P, Shawky AM, et al. Interactions of Apigenin and Safranal with the 5HT1A and 5HT2A Receptors and Behavioral Effects in Depression and Anxiety: A Molecular Docking, Lipid-Mediated Molecular Dynamics, and In Vivo Analysis. Molecules. 2022; 27(24):8658. https://doi.org/10.3390/molecules27248658
Chicago/Turabian StyleAmin, Faiq, Mahmoud A. A. Ibrahim, Syed Rizwan-ul-Hasan, Saima Khaliq, Gamal A. Gabr, Muhammad, Asra Khan, Peter A. Sidhom, Prashant Tikmani, Ahmed M. Shawky, and et al. 2022. "Interactions of Apigenin and Safranal with the 5HT1A and 5HT2A Receptors and Behavioral Effects in Depression and Anxiety: A Molecular Docking, Lipid-Mediated Molecular Dynamics, and In Vivo Analysis" Molecules 27, no. 24: 8658. https://doi.org/10.3390/molecules27248658
APA StyleAmin, F., Ibrahim, M. A. A., Rizwan-ul-Hasan, S., Khaliq, S., Gabr, G. A., Muhammad, Khan, A., Sidhom, P. A., Tikmani, P., Shawky, A. M., Ahmad, S., & Abidi, S. H. (2022). Interactions of Apigenin and Safranal with the 5HT1A and 5HT2A Receptors and Behavioral Effects in Depression and Anxiety: A Molecular Docking, Lipid-Mediated Molecular Dynamics, and In Vivo Analysis. Molecules, 27(24), 8658. https://doi.org/10.3390/molecules27248658







