Abstract
Tacca leontopetaloides (T. leontopetaloides) contain a number of active compounds such as flavonoids, tannins, phenolics, steroids, and alkaloids. The active compounds from plants have been shown to reduce the risk of cardiovascular disease by lowering cholesterol levels by inhibiting the enzyme 3-hydroxy-3-methylglutaryl-coenzym A (HMG-CoA) reductase activity. This study aims to investigate the potential active compounds in the ethanolic extract of Tacca tubers (T. leontopetaloides) from the Banyak Islands, Aceh Singkil Regency, Aceh Province both in vitro and in silico. Tacca tubers contain secondary metabolites including flavonoids, phenolics, tannins, steroids and saponins, according to phytochemical screening. In vitro investigation of ethanolic extract of Tacca tuber revealed inhibitory activity of HMG Co-A reductase with an IC50 value of 4.92 ppm. Based on the in silico study, active compound from the extract, namely Stigmasterol with the highest binding affinities with HMG Co-A reductase (−7.2 kcal/mol). As a comparison, the inhibition of HMG Co-A reductase activity by simvastatin with an IC50 4.62 ppm and binding affinity −8.0 Kcal/mol. Our findings suggest that the ethanolic extract of Tacca tuber (T. leontopetaloides) from Banyak Islands, Aceh Province has the potential to inhibit the activity of HMG Co-A reductase.
1. Introduction
Cardiovascular diseases (CVDs) are the leading cause of death worldwide, with an estimated 17.9 million people dying from CVDs in 2019 [1]. According to the 2018 Basic Health Research (Riskesdas) data, incidences of heart and blood vessel disease are increasing year after year, with 15 out of 1000 persons suffering from cardiovascular disease. Hypercholesterolemia is a disorder in which total cholesterol and LDL cholesterol (low density lipoprotein) levels in the blood are increased, and incidentally, high cholesterol levels are a major risk factor for cardiovascular disease [2]. Hypercholesterolemia can raise the chance of developing atherosclerosis [3]. Statins or hydroxymethylglutaryl Co-A reductase inhibitors are routinely used to treat hypercholesterolemia. The enzyme 3-hydroxy-3-methylglutaryl-coenzym A (HMG-CoA) reductase is important in the production of cholesterol in the liver. HMG Co-A reductase catalyzes the conversion of HMG Co-A to mevalonic acid, the initial step in the production of cholesterol [4]. Inhibiting HMG Co-A reductase activity has been shown to lower cholesterol levels in both humans and animals [5]. HMG Co-A reductase inhibitors can lower intracellular cholesterol biosynthesis by inhibiting the conversion of HMG-CoA to mevalonate [6]. HMG Co-A reductase inhibitors, also known as statins, can lower total cholesterol, LDL cholesterol, and triglycerides while increasing HDL cholesterol [7]. As an effort of adding modality in treating hypercholesterolemia, researchers have explored the use of natural products. Plants produce a variety of phytochemical substances, or secondary metabolites, that are beneficial to health [8]. It was also reported that many medicinal plants have high antioxidant activity [9]. Phytochemical compounds found naturally in plants are powerful effectors of biological processes that can reduce disease risk via complimentary pathways. Moreover, in vitro studies have demonstrated that plant extract bioactive components have a hypocholesterolemic effect [5]. Flavonoids, for example, have demonstrated the ability to reduce the activity of the HMG Co-A reductase enzyme and hence prevent the synthesis of cholesterol [10]. Natural components such as medicinal herbs and nutraceuticals have the ability to limit the activity of the HMG Co-A reductase enzyme, and hence prevent cardiovascular disease and dyslipidemia. Additionally, they are easily available and cost-effective [11].
Tacca leontopetaloides, or Tacca, is a plant in the Taccaceae family that thrives in coastal locations less than 200 m above sea level (masl) [12]. The Tacca plant is thought to have originated in Southeast Asia and has since spread to tropical areas such as Africa, Asia, Australia and Oceania. It is also known as East Indian arrowroot, Polynesian arrowroot, Arrowroot, Fiji arrowroot, Tacca, Williams arrowroot and Tahiti arrowroot [13,14]. Tacca plants are generally eaten as food, but in some places, they are also used to treat various maladies [15,16]. Several earlier investigations have found glycosides, flavonoids, phenols, alkaloids, tannins, coumarins, polysaccharides, glycosides, gums, terpenes, terpenoids and Taccalins in Tacca tubers [12,16]. Ndouyang et al. [17] discovered that Tacca tubers have a good effect on fat metabolism and that administering unprocessed tubers in modest doses can lower LDL (low density lipoprotein) cholesterol levels in rats. Assatou et al. [18] discovered that Tacca tuber water extract had an antihyperlipidemic and a hypolipidemic effect in hyperlipidemic rats. However, research on the potential of Tacca tubers (T. leontopetaloides) in the treatment of hypercholesterolemia is still relatively limited, and no information is available on the inhibitory mechanism of HMG Co-A reductase enzyme activity by active chemicals found in Tacca tubers. The Tacca plant (T. leontopetaloides) grows wild in the Banyak Islands in Aceh Singkil Regency, and its use is still limited. The tuber section of the Tacca plant is often turned into starch and then used to make traditional cakes in Pulau Banyak, especially around Eid al-Fitr. There is currently no information on the active chemical content of Tacca tubers grown in the Banyak Islands of Aceh Singkil Regency. The purpose of this work is to determine the active compounds of ethanolic extract from Tacca tubers in the Banyak Islands, Aceh Singkil Regency, as well as the potential of these active compounds as HMG Co-A reductase inhibitors, both in vitro and in silico.
2. Results and Discussion
2.1. Plant Determination
The results of the identification of Tacca plants (Figure 1) and their taxonomic classification are shown in Table 1.

Figure 1.
Tacca Plant (T. leontopetaloides).

Table 1.
Taxonomic Classification of Tacca Plant.
2.2. Extract Yield
The extraction yield was used as an indicator of the effect of the extraction condition [19], where the yield extract indicates the amount of active compound in the extract [20]. This study revealed that the percentage yield value of ethanolic extract from Tacca tuber was 9.19%.
2.3. Phytochemical Analysis
Several phytochemical substances have been found to promote health by decreasing cholesterol levels and avoiding lipid oxidation, whereas others have anti-inflammatory and antiplatelet properties [21]. Low-density lipoprotein (LDL) cholesterol levels have been demonstrated to be reduced by phytochemical compounds, indicating that phytochemical compounds are effective in decreasing blood cholesterol levels and preventing the formation of undesirable cholesterol [22]. Several studies have found that active chemicals such as flavonoids, phytosterols, phenolics and alkaloids have an essential role in human health and the prevention of chronic diseases by decreasing cholesterol [23,24,25].
The identification of phytochemical substances from the extract of Tacca tuber revealed that phenolics compounds, tannins, flavonoids, steroids and saponins were detected in ethanolic extracts from Tacca tubers, unlike alkaloid compounds, which were not found in the ethanolic extract. The environmental conditions affect the synthesis and accumulation of the active compounds from the plant sample [26]. The geographical conditions, climate, genetic variation, agronomy and storage of plants are several factors that affect the active compounds of plants [27], as well as several other environmental factors, such as lighting, temperature, groundwater, soil fertility and salinity [28,29]. Some previous studies stated that Tacca tubers contain a variety of active chemicals, or secondary metabolites, including flavonoids (rutin, diosmin), saponins (chlorogenic acid, quercetin), phenols, alkaloids, tannins, coumarins, polysaccharides, glycosides, gums, terpenes, terpenoids and taccalin [30,31]. The Alkaloids, phenolics, terpenoids and tannins are phytochemical compounds that play an essential role in disease prevention, with some phytochemical substances having an effect on cholesterol metabolism, which can lower cholesterol levels [32]. Several studies have found that flavonoids can help avoid cardiovascular disease [33,34]. Tresserra-Rimbau et al. [35] found that flavonoids can reduce total cholesterol, triglycerides, low density lipoprotein cholesterol (LDL-cholesterol) and apolipoprotein B (apoB) levels while increasing HDL cholesterol and acid secretion. Catabolism of bile and lipids saponin active substances can directly block cholesterol absorption in the small intestine and indirectly impede bile acid reabsorption to lower plasma cholesterol [22]. Flavonoids, tannins, saponins, alkaloids and terpenoids have biological action as antioxidants, anti-inflammatory, anti-diarrheal, anti-ulcer and anticancer substances [36].
2.4. Analysis of GC-MS
Gas chromatography–mass spectrometry (GC-MS) is a technique for determining and identifying phytochemical substances in plant samples [37]. The GC-MS analysis revealed a number of active compounds, particularly fatty acids, steroids and triterpenoids in the ethanolic extract from Tacca tuber. The active compounds from the fatty acid group (i.e., hexadecanoic acid and 9,12-octadecadienoic acid) and the steroids group (i.e., campesterol, gamma sitosterol and stigmasterol) were found with varying retention times and percentages of content.
The GC-MS chromatogram of ethanolic extract from Tacca tuber revealed a total of 35 peaks with different retention times (Figure 2), however there were 20 active compounds identified due to the repetition of several compounds (Table 2). The ethanolic extract from Tacca tuber contains several active compounds including 1,2-benzenedicarboxylic acid, dinonyl ester (44.39%), 9,12-octadecadienoic acid (7.0%), hexadecanoic acid (3.98%), 1-isopropyl -2-methoxycarbonyl-1-aza-cyclopropane (3.33%), phthalic acid, bis (7-methyloctyl) ester (2.74%), furancarboxaldehyde and 5-hydroxymethyl (2.12%). The ethanolic extract from Tacca tubers also contains less active compounds from the steroid group, namely campesterol (0.62%), stigmasterol (0.78%) and gamma sitosterol (1.53%).
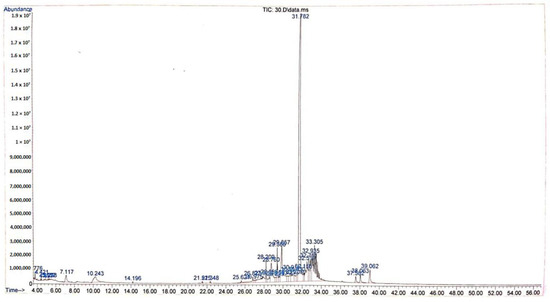
Figure 2.
Chromatogram of Tacca tuber ethanolic extract.

Table 2.
The active compounds in ethanolic extract from Tacca tuber (T.leontopetaloides) by GC-MS.
1,2-Benzenedicaboxylic acid, butyl octyl ester is a plasticizer compound that has antimicrobial, antifouling, antioxidant and hypocholesterolemic activity [38]. The active compounds 9,12-octadecadienoic acid (linoleic acid) and hexadecanoic acid (palmitic acid) are antioxidants, anti-inflammatory, hypocholesterolemic, nemasida, hemolytic and 5-alpha reductase inhibitors [39]. Gamma-sitosterol has hypolipidemic agent [40] and antihyperglycemic [41], while campesterol has hypocholesterolemic, antidiabetic, anticancer and anti-inflammatory activity [42].
2.5. Molecular Docking (In Silico)
Molecular docking is a computational approach used to determine the interaction of proteins with ligands through the formation of supramolecular complexes or assemblies that can enhance or inhibit biological functions [43], inhibition of the enzyme HMG-CoA reductase by the active compound of Tacca tuber ethanolic extract and to get an idea of the mechanism that occurs between the protein target and the active compound. Molecular docking, or an in-silico test, was carried out by docking 17 active compounds of ethanolic extract from Tacca tuber (T. leontopetaloides) obtained by GC-MS and simvastatin as a control for the HMG-CoA reductase target protein and visualization using the Discovery Studio Visualizer 2021.
The initial stage of this research was carried out by downloading the molecular structure of the HMG-CoA reductase receptor in 3D format (.pdb) which was obtained from the Protein Data Bank, specifically the website www.rcsb.org, accessed on 3 January 2022. The molecular structure of the HMG-CoA reductase receptor used in this study is PDB ID: 2R4F [44].
The in silico study in this research demonstrates that all active compounds in the ethanolic extract from Tacca tubers have a larger free bond energy than simvastatin, indicating that all active compounds in the ethanolic extract from Tacca tubers have a lower binding affinity to the binding site of the HMG-CoA reductase enzyme when compared to simvastatin (Table 3).

Table 3.
Molecular docking of the active compounds in the ethanolic extract from Tacca tuber and simvastatin.
The docking studies also revealed active components in the ethanolic extract from Tacca tuber with the highest binding affinity, namely stigmasterol (−7.2 kcal/mol). Interaction of stigmasterol with HMG-CoA reductase (PDB ID: 2R4F) showed the presence of van der Waals forces and hydrophobic interactions, namely alkyl and π-alkyl (Figure 3). Stigmasterol (C29H48O) is a steroid derivative characterized by the presence of a hydroxyl group in position C-3 of the steroid skeleton, as well as unsaturated bonds at positions 5–6 of the B ring and 22–23 in the alkyl substituent (Stigmasterol|C29H48O—PubChem (nih.gov, accessed on 3 January 2022) Stigmasterol is a phytosterol that has been demonstrated to reduce cholesterol absorption and the risk of cardiovascular disease [45]. According to Batta et al. [46], injection of stigmasterol can lower blood cholesterol levels and block the formation of liver cholesterol and bile acids in Wistar rats. The ethanolic extract of tacca tuber is known to contain steroid derivative compounds such as campesterol (C28H48O), Stigmasterol (C29H48O) and Gamma sitosterol (C29H50O). The presence of these compounds can help reduce of cholesterol levels. Intake of phytosterols is advised as a complementary treatment for hypercholesterolemia, and plant sterols can lower cholesterol absorption at the intestinal lumen via the Niemann-Pick C1 Like 1 (NPC1L1) transporter pathway by competitively solubilizing in mixed micelles [47].
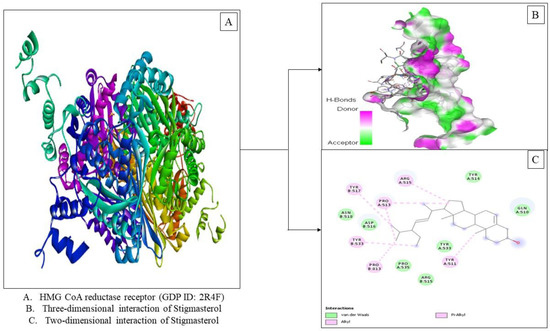
Figure 3.
Visualization of the HMG-CoA reductase receptor docking (PDB ID: 2R4F) with stigmasterol.
Based on the results of molecular docking in Table 3, Simvastatin has a binding affinity (−8.0 Kcal/mol) in the presence of three interactions, namely Van der Waals forces, hydrogen bonds and hydrophobic alkyl interactions (Figure 4). Visualization of the interaction between simvastatin and stigmasterol in the HMG-CoA reductase enzyme shows similarities in amino acid residues such as van der Waals forces, namely Aspartate: 516, Arginine: 515 and Tyrosine: 514 and hydrophobic interactions, namely Tyrosine: 511, Tyrosine: 517 and Proline: 513. The lower binding affinity value of stigmasterol could be due to the absence of hydrogen bonding as occurs in simvastatin.
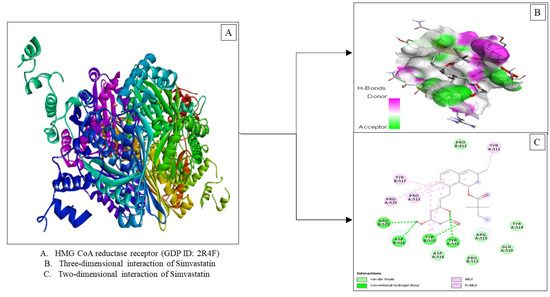
Figure 4.
Visualization of the HMG-CoA reductase receptor docking (PDB ID: 2R4F) with simvastatin.
2.6. In Vitro Potential of HMG-CoA Inhibitor
The IC50 value indicates the potential for inhibition of the HMG-CoA reductase enzyme, where the smaller the IC50 value, the greater the inhibition potential for the activity of the HMG-CoA reductase enzyme [48]. In vitro testing using the HMG-CoA reductase assay kit showed that the ethanol extract from Tacca tubers acted as an inhibitor of HMG-CoA reductase with an IC50 value of 4.92 ppm, which was close to the IC50 value of simvastatin by comparison (4.62 ppm). The results of this study indicate that the higher the concentration, the greater the inhibition of HMG-CoA reductase, as shown in Figure 5. Tacca tubers, the ethanol extract from Tacca tubers and simvastatin have inhibitory activities against HMG-CoA reductase of 79%, 88% and 94%, respectively, at a concentration of 20 ppm. The inhibitory activity of the HMG-CoA reductase enzyme from the ethanol extract of Tacca tubers was lower than simvastatin, therefore it is concluded that the ethanol extract from Tacca tubers has the potential to inhibit cholesterol synthesis, although not as well as simvastatin. Carbonell & Freire [49] stated that plant extracts capable of inhibiting the activity of the HMG-CoA reductase enzyme may function as hypocholesterolemic agents.
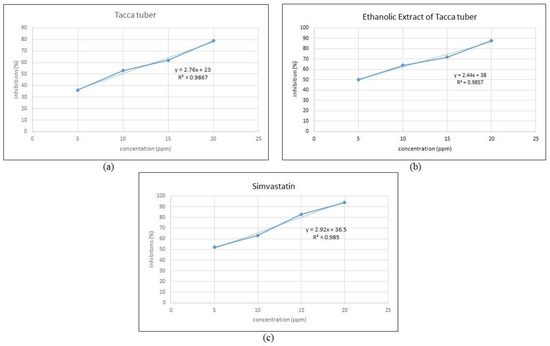
Figure 5.
Inhibitory activity of HMG-CoA reductase of Tacca tuber (a), ethanolic extract of Tacca tuber (b) and simvastatin (c).
3. Materials and Methods
3.1. Materials
The plant materials, namely Tacca tubers (T. leontopetaloides), were collected from Teluk Nibung village, Ujung Batu Island, Banyak Islands, Aceh Singkil, Aceh Province. The sampling location was 2°20′54″ N 97°23′08″ E (Figure 6). The materials used in this study were distilled water, ethanol 96%, Dragendorff reagent, Wagner reagent, Meyer reagent, FeCl3 (Merck & Co, Rahway, NJ, USA), magnesium powder, HCl (Merck), chloroform (Merck), anhydrous acetic acid, H2SO4 (Merck), Folin–Ciocalteau reagent (Merck), Na2CO3 (Merck), NaNO2 (Merck), AlCl3 (Merck) and NaOH (Merck). The 3D structure of the HMG-CoA reductase enzyme (PDB ID: 2R4F) and the 3D structure of the ligand were downloaded from the Protein Data Bank (PDB).
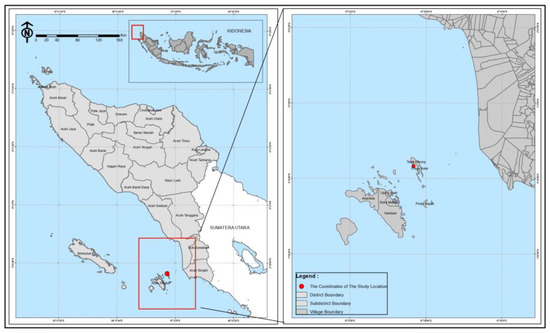
Figure 6.
Sampling location map.
This research utilized gas chromatography–mass spectrometry (GC-MS), a computer (hardware specifications: Intel Celeron 4205U processor Dual core 1.8 GHz base frequency, 4GB RAM, Windows 10 Home ×64 operating system) and the web-based software Autodock 4.0 on a docking server (http://www.dockingserver.com, accessed on 3 January 2022).
3.2. Methods
3.2.1. Preparation of Tuber Extract
Samples of the Tacca plant (T. leontopetaloides) were determinations in the Department of Biology, Faculty of Mathematics and Natural Sciences, University of Syiah Kuala (USK) Banda Aceh. The samples in this study were Tacca tubers with weights of ±300 g (Figure 7). Samples of Tacca tubers were peeled and cut into pieces before the extraction process. Then the samples were dried using an oven at a temperature of 35–40 °C. The extraction process was carried out by the maceration method 3 × 24 h using ethanol 96%, with a ratio of 1:10. The samples in the form of mashed Tacca tubers (simplicia) were put into maceration bottles (macerator). Solvent was then added until all the ingredients were submerged and allowed to stand for 3 days in a place protected from sunlight while stirring occasionally. After 3 days, filtering was carried out using filter paper so that the macerated sample and pulp were obtained, which were then re-maceration twice. The resulting macerated sample was then concentrated using a Rotary Evaporator (Buchi Rotavapor® R-300, Flawil, Switzerland) to produce a concentrated extract [50].

Figure 7.
Tuber of Tacca leontopetaloides.
3.2.2. Phytochemical Test
Qualitative testing of the phytochemical compounds from the ethanolic extract of Tacca tuber includes testing for alkaloids, flavonoids, steroids, terpenoids, phenolics, tannins and saponins, following the general instructions by Harbone [51]. The saponin test was carried out by checking the stale foam after shaking using distilled water. The steroid and terpenoid test was conducted using the Liberman-Burchard reagent, whereas for the alkaloid test, we used Mayer, Dragendorff and Wagner reagents. The phenolic test used FeCl3, the flavonoid test used Mg powder and the tannin test used gelatin and sulfuric acid [52].
3.2.3. Identification of Active Compounds Using Gas Chromatography-Mass Spectrometry (GC-MS)
A total of 5 µL of ethanolic extract from a Tacca tuber was injected into the GC-MS instrument (Agilents Technologies 7890GC/5975MS) using an HP Ultra 2 capillary column with a length of 30 m, inner diameter of 0.20 mm and film thickness of 0.11 µm, and helium as the carrier gas with a constant column flow of 1.2 mL/min and split ratio of 8:1. The oven temperature was initially set to a temperature of 80 °C (held for 0 min), then increased at 3 °C/min to 150 °C (held for 1 min) and then finally increased at 20 °C/min to 280 °C (held for 26 min). The injection site temperature was 250 °C, the ion source temperature was 230 °C and the interface temperature was 280 °C. The eluted component was detected on the mass detector. The mass spectrum fragmentation pattern was compared with that stored in the spectrometer database, which uses the W8N08 Mass Spectral library. The percentage of each component was calculated from the relative peak area of each component in the chromatogram [53].
3.2.4. Molecular Docking (In Silico)
Molecular docking was carried out in several stages, namely, protein preparation, ligand preparation, docking and analysis [54,55,56,57].
- Preparation of target proteins (receptors)
The target protein used was HMG-CoA reductase. The 3D structure of the HMG-CoA reductase enzyme can be downloaded from the Protein Data Bank (PDB) at www.rcsb.org, accessed on 3 January 2022. The downloaded protein was removed the water molecule and optimized. We then determined the active site of the HMG-CoA reductase enzyme. The target protein was then prepared using BIOVIA Discovery Studio software 2021 and saved in the PDB format.
- 2.
- Preparation of ligands
The active compounds selected as test ligands to be attached to the target protein. The chosen control ligand is the simvastatin one of the HMG-CoA reductase enzyme. Ligands can be downloaded through the PubChem database, prepared with OPEN BABEL Sketch and then saved in PDB format.
- 3.
- Determination of the active site
The active side is where the ligand and compound will bind. Determination of the active side is done with Autodock 4.0.
- 4.
- Molecular docking
At this stage, the docking between the target protein (HMG-CoA) with the ligand and the comparison compound simvastatin is carried out. The docking process is carried out using AutoDock Vina software with PyRx emulator software. The ligand molecule will interact on the active site of the receptor and will then inhibit the function of the receptor. Finally, the ligand is able to act as a drug.
- 5.
- Analysis
The next stage is the analysis or interpretation of the results by looking at the type of molecular bond interactions that occur between the protein and the ligand.
3.2.5. In Vitro HMG Co-A Reductase Inhibitory Activity [58,59]
HMG-CoA reductase inhibitory activity was analyzed using the HMG-CoA reductase (HMGCR) enzymatic assay kit (Sigma Aldrich, Catalog No. CS1090). The kit consists of an assay buffer, NADPH, HMG-CoA as the substrate solution, HMG-CoA reductase (HMGCR) as the catalytic domain (0.5–0.7 mg/mL) and pravastatin as an inhibitor solution. The inhibitory activity of the ethanolic extract on HMG-CoA reductase (HMGCR) activity is monitored by comparing it to pravastatin (kit inhibitor) and simvastatin (commercial inhibitor), which serve as references. The Tacca tuber, ethanolic extract and simvastatin were tested at four concentrations: 5, 10, 15 and 20 ppm. The assay reaction was prepared by mixing 1× assay buffer, pravastatin, NADPH, HMG-CoA and HMGCR, according to the procedure of the assay kit. The activity of the HMG-CoA enzyme was measured immediately with a Micro-plate/ELISA Reader UV/Vis spectrophotometer every 10 s for 10 min at 37 °C and λ = 340 nm. Enzyme activity is expressed in units/mg of protein and calculated by Equation (1).
where:
A340 = absorbance
TV = total volume (mL)
V = volume of enzyme used
LP = light path (cm)
0.6 = enzyme concentration in mg protein/mL
12.44 = NADPH requirement during reaction (coefficient for NADPH at 340 nm is 6.22/mM cm)
The percentage of inhibition of HMGCR was calculated using the equation:
The IC50 value is the inhibitor concentration required to achieve 50% HMG-CoA reductase inhibition. Determination of the IC50 value was completed using linear regression to get the slope value of the variable, or non-linear regression using the sigmoidal equation, where the independent variable is the concentration of the inhibitor and the dependent variable is the percentage of inhibition.
4. Conclusions
The HMG-CoA reductase activity of the ethanolic extract of Tacca tuber was inhibited by in vitro, with an IC50 value of 4.92 ppm. The stigmasterol (−7.2 Kcal/mol) component of the phytosterols is the substance from GCMS of the ethanolic extract of the tacca tuber that shows inhibitory activity against HMG-CoA reductase (PDB ID: 2R4F). Simvastatin is used as a positive control, and it has a binding affinity of −8.0 Kcal/mol and an IC50 value of 4.62 ppm. Based on the results, it can be concluded that the ethanolic extract from Tacca tubers has the potential to inhibit cholesterol by inhibiting the activity of HMG-CoA reductase.
Author Contributions
Conceptualization, D.D., E.S. and N.B.M.; Data curation, R.R. and E.S.; Formal analysis, R.R., D.D. and E.S.; Methodology, R.I. and D.D.; Resources, R.I. and E.S.; Software, R.I. and N.B.M.; Supervision, R.I.; Validation, D.D.; Visualization, N.B.M.; Writing—original draft, R.R., R.I. and N.B.M.; Writing—review and editing, R.R., R.I., D.D., E.S. and N.B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Direktorat Riset, Teknologi, dan Pengabdian kepada Masyarakat—Direktorat Jenderal Pendidikan Tinggi, Riset, dan Teknologi—Kementerian Pendidikan, Kebudayaan, Riset, dan Teknologi, grant number 145/E5/PG.02.00.PT/2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data in this study are available upon request to the author.
Acknowledgments
The authors would like to thank the LPPM Universitas Syiah Kuala for supporting this research.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- World Health Organization (WHO). Cardiovascular Disease (CVDs); WHO: Geneva, Switzerland, 2022.
- Johnston, T.P.; Korolenko, T.A.; Pirro, M.; Sahebkar, A. Preventing Cardiovascular Heart Disease: Promising Nutraceutical and Non-Nutraceutical Treatments for Cholesterol Management. Pharmacol. Res. 2017, 120, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Cuchel, M.; Bruckert, E.; Ginsberg, H.N.; Raal, F.J.; Santos, R.D.; Hegele, R.A.; Kuivenhoven, J.A.; Nordestgaard, B.G.; Descamps, O.S.; Steinhagen-Thiessen, E.; et al. Homozygous Familial Hypercholesterolaemia: New Insights and Guidance for Clinicians to Improve Detection and Clinical Management. A Position Paper Fromthe Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur. Heart J. 2014, 35, 2146–2157. [Google Scholar] [CrossRef] [PubMed]
- Gesto, D.S.; Pereira, C.M.S.; Cerqueira, N.M.F.S.; Sousa, S.F. An Atomic-Level Perspective of HMG-CoA-Reductase: The Target Enzyme to Treat Hypercholesterolemia. Molecules 2020, 25, 3891. [Google Scholar] [CrossRef]
- Baskaran, G.; Salvamani, S.; Ahmad, S.A.; Shaharuddin, N.A.; Pattiram, P.D. HMG-CoA Reductase Inhibitory Activity and Phytocomponent Investigation of Basella Alba Leaf Extract as a Treatment for Hypercholesterolemia. Drug Des. Devel. Ther. 2015, 9, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Lennernäs, H.; Fager, G. Pharmacodynamics and Pharmacokinetics of the HMG-CoA Reductase Inhibitors. Similarities and Differences. Clin. Pharmacokinet. 1997, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.B.; Cassagnol, M. HMG-CoA Reductase Inhibitors; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Patel, D.K. Plants as a Source of Medicine. Med. Aromat. Plants 2015, s3, 1. [Google Scholar] [CrossRef]
- Imelda, E.; Idroes, R.; Khairan, K.; Lubis, R.R.; Abas, A.H.; Nursalim, A.J.; Rafi, M.; Tallei, T.E. Natural Antioxidant Activities of Plants in Preventing Cataractogenesis. Antioxidants 2022, 11, 1285. [Google Scholar] [CrossRef] [PubMed]
- Son, K.H.; Lee, J.Y.; Lee, J.S.; Kang, S.S.; Sohn, H.Y.; Kwon, C.S. Screening of Flavonoid Compounds with HMG-CoA Reductase Inhibitory Activities. J. Sci. 2018, 28, 247–256. [Google Scholar]
- Mahdavi, A.; Bagherniya, M.; Fakheran, O.; Reiner, Z.; Xu, S.; Sahebkar, A. Medicinal Plants and Bioactive Natural Compounds as Inhibitors of HMG-CoA Reductase: A Literature Review. BioFactors 2020, 46, 1–19. [Google Scholar] [CrossRef]
- LIPI. Taka (Tacca Leontopetaloides) Untuk Kemandirian Pangan; Erlinawati, I., Lestari, P., Ruqayah, Ermayanti, T.M., Eds.; LIPI Press: Jakarta, Indonesia, 2018; ISBN 9789797999865.
- Jukema, J.; Paisooksantivatana, Y. Plant Resources of South-East Asia; Flach, M., Rumawas, F., Eds.; Backhuys Publisher: Leiden, The Netherlands, 1996; ISBN 907334851X. [Google Scholar]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2016; Volume 12, ISBN 9783319260648. [Google Scholar]
- Keardrit, K.; Rujjanawate, C.; Amornierdpison, D. Analgesic, Antipyretic and Anti-Inflammatory Effects of Tacca Chantrieri Andre. J. Med. Plants Res. 2010, 4, 1991–1995. [Google Scholar] [CrossRef]
- Pradeepika, C.; Selvakumar, R.; Krishnakumar, T.; Nabi, S.U.; Sajeev, M.S. Pharmacology and Phytochemistry of Underexploited Tuber Crops: A Review. J. Pharmacogn. Phytochem. 2018, 7, 1007–1019. [Google Scholar]
- Ndouyang, C.; Nguimbou, R.; Njintang, Y.; Scher, J.; Facho, B.; Mbofung, C.; Cmf, M. In Vivo Assessment of the Nutritional and Subchronic Toxicity of Tacca Leontopetaloides (L.). Tubers. Sch. J. Agric. Sci. 2014, 4, 5–13. [Google Scholar]
- Aïssatou, D.S.; Metsagang, J.T.N.; Sokeng, D.; Njintang, N.Y. Antihyperlipidemic and Hypolipidemic Properties of Tacca Leontopetaloides (L) Kuntze ( Dioscoreales: Dioscoreaceae ) Tuber’ s Aqueous Extracts in the Rats. Brazilian J. Biol. Sci. 2017, 4, 67–80. [Google Scholar] [CrossRef]
- Dhanani, T.; Shah, S.; Gajbhiye, N.A.; Kumar, S. Effect of Extraction Methods on Yield, Phytochemical Constituents and Antioxidant Activity of Withania Somnifera. Arab. J. Chem. 2017, 10, S1193–S1199. [Google Scholar] [CrossRef]
- Seriana, I.; Akmal, M.; Darusman; Wahyuni, S.; Khairan, K.; Sugito. Phytochemicals Characterizations of Neem (Azadirachta Indica a. Juss) Leaves Ethanolic Extract: An Important Medicinal Plant as Male Contraceptive Candidate. Rasayan J. Chem. 2021, 14, 343–350. [Google Scholar] [CrossRef]
- Upadhyay, S.; Dixit, M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxid. Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, V. Phytochemicals Effective in Lowering Low-Density Lipoproteins. J. Biol. Eng. Res. Rev. 2020, 7, 16–23. [Google Scholar]
- Ma, C.; Yin, Z.; Zhu, P.; Luo, J.; Shi, X.; Gao, X. Blood Cholesterol in Late-Life and Cognitive Decline: A Longitudinal Study of the Chinese Elderly. Mol. Neurodegener. 2017, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and Their Derivatives: Structural Diversity, Distribution, Metabolism, Analysis, and Health-Promoting Uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Oilseed Crop Sunflower (Helianthus Annuus) as a Source of Food: Nutritional and Health Benefits. Food Sci. Nutr. 2020, 8, 4666–4684. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Shukla, S. Impact of Various Factors Responsible for Fluctuation in Plant Secondary Metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Gahukar, R.T. Factors Affecting Content and Bioefficacy of Neem (Azadirachta Indica A. Juss.) Phytochemicals Used in Agricultural Pest Control: A Review. Crop Prot. 2014, 62, 93–99. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil Salinity: A Serious Environmental Issue and Plant Growth Promoting Bacteria as One of the Tools for Its Alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Caddick, L.R.; Wilkin, P.; Rudall, P.J.; Hedderson, T.A.J.; Chase, W.; Caddick, L.R.; Wilkini, P.; Rudalll, P.J.; Hedderson, T.A.J.; Mark, W. Yams Reclassified: A Recircumscription of Dioscoreaceae and Dioscoreales. Taxon 2002, 51, 103–114. [Google Scholar] [CrossRef]
- Jagtap, S.; Satpute, R. Chemical Fingerprinting of Flavonoids in Tuber Extracts of Tacca Leontopetaloides (L.) O. Ktze. J. Acad. Ind. Res. 2015, 3, 485–489. [Google Scholar]
- Cao, Y.; Bei, W.; Hu, Y.; Cao, L.; Huang, L.; Wang, L.; Luo, D.; Chen, Y.; Yao, X.; He, W.; et al. Hypocholesterolemia of Rhizoma Coptidis Alkaloids Is Related to the Bile Acid by Up-Regulated CYP7A1 in Hyperlipidemic Rats. Phytomedicine 2012, 19, 686–692. [Google Scholar] [CrossRef]
- Kim, K.; Vance, T.M.; Chun, O.K. Greater Flavonoid Intake Is Associated with Improved CVD Risk Factors in US Adults. Br. J. Nutr. 2016, 115, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.H.; Yang, H.M.; Wang, Y.L.; Chen, Y.G. Phytochemical and Pharmacological Studies of the Genus Tacca: A Review. Trop. J. Pharm. Res. 2014, 13, 63–648. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse Association between Habitual Polyphenol Intake and Incidence of Cardiovascular Events in the PREDIMED Study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef]
- Starlin, T.; Saravana Prabha, P.; Thayakumar, B.K.A.; Gopalakrishnan, V.K. Screening and GC-MS Profiling of Ethanolic Extract of Tylophora Pauciflora. Bioinformation 2019, 15, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Uma, G.; Balasubramaniam, V. GC-MS Analysis of Nothapodytes Nimmoniana [J. Graham] Mabberly Leaves. J. Chem. Pharm. Res. 2012, 4, 4417–4419. [Google Scholar]
- Sathish, S.S. Phytochemical Analysis of Vitex Altissima L. Using UV-VIS, FTIR and GC-MS. Int. J. Pharm. Sci. Drug Res. 2012, 4, 56–62. [Google Scholar]
- Ben, W. Dr. Duke’s Phytochemical and Ethnobotanical Databases. Choice Rev. Online 2001, 38, 38–3317. [Google Scholar] [CrossRef]
- Balamurugan, R.; Stalin, A.; Aravinthan, A.; Kim, J.H. γ-Sitosterol a Potent Hypolipidemic Agent: In Silico Docking Analysis. Med. Chem. Res. 2015, 24, 124–130. [Google Scholar] [CrossRef]
- Sirikhansaeng, P.; Tanee, T.; Sudmoon, R.; Chaveerach, A. Major Phytochemical as γ -Sitosterol Disclosing and Toxicity Testing in Lagerstroemia Species. Evidence-based Complement. Altern. Med. 2017, 2017, 7209851. [Google Scholar] [CrossRef]
- Santas, J.; Rafecas, M.; Codony, R. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Springer: Berlin, Germany, 2013; pp. 1–4242. [Google Scholar] [CrossRef]
- De Vivo, M.; Masetti, M.; Bottegoni, G.; Cavalli, A. Role of Molecular Dynamics and Related Methods in Drug Discovery. J. Med. Chem. 2016, 59, 4035–4061. [Google Scholar] [CrossRef] [PubMed]
- Pfefferkorn, J.A.; Choi, C.; Larsen, S.D.; Auerbach, B.; Hutchings, R.; Park, W.; Askew, V.; Dillon, L.; Hanselman, J.C.; Lin, Z.; et al. Substituted Pyrazoles as Hepatoselective HMG-CoA Reductase Inhibitors: Discovery of (3R,5R)-7-[2-(4-Fluoro-Phenyl)-4-Isopropyl-5-(4-Methyl- Benzylcarbamoyl)-2H-Pyrazol-3-Yl]-3,5-Dihydroxyheptanoic Acid (PF-3052334) as a Candidate for the Treatment of Hype. J. Med. Chem. 2008, 51, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Vahouny, G.V.; Connor, W.E.; Subramaniam, S.; Lin, D.S.; Gallo, L.L. Comparative Lymphatic Absorption of Sitosterol, Stigmasterol, and Fucosterol and Differential Inhibition of Cholesterol Absorption. Am. J. Clin. Nutr. 1983, 37, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Batta, A.K.; Xu, G.; Honda, A.; Miyazaki, T.; Salen, G. Stigmasterol Reduces Plasma Cholesterol Levels and Inhibits Hepatic Synthesis and Intestinal Absorption in the Rat. Metabolism 2006, 55, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Izar, M.C.; Tegani, D.M.; Kasmas, S.H.; Fonseca, F.A. Phytosterols and Phytosterolemia: Gene-Diet Interactions. Genes Nutr. 2011, 6, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Yunarto, N.; Aini, N.; Oktoberia, I.S.; Sulistyowati, I.; Kurniatri, A.A. Aktivitas Antioksidan Serta Penghambatan HMG CoA Dan Lipase Dari Kombinasi Ekstrak Daun Binahong-Rimpang Temu Lawak. J. Kefarmasian Indones. 2019, 9, 89–96. [Google Scholar] [CrossRef]
- Carbonell, T.; Freire, E. Binding Thermodynamics of Statins to HMG-CoA Reductase. Biochemistry 2005, 44, 11741–11748. [Google Scholar] [CrossRef]
- Departemen Kesehatan, R.I. Paramater Standar Umum Ekstrak Tumbuhan Obat; Departemen Kesehatan RI: Kota Palembang, Indonesia, 2000.
- Harbone, J. Phytochemical Methodes; Chapman Hall: London, UK, 1991. [Google Scholar]
- Kemala, P.; Idroes, R.; Khairan, K.; Ramli, M.; Jalil, Z.; Idroes, G.M.; Tallei, T.E.; Helwani, Z.; Safitri, E.; Iqhrammullah, M.; et al. Green Synthesis and Antimicrobial Activities of Silver Nanoparticles Using Calotropis Gigantea from Ie Seu-Um Geothermal Area, Aceh Province, Indonesia. Molecules 2022, 27, 5310. [Google Scholar] [CrossRef]
- Dineshkumar, G.; Rajakumar, R. Gc-Ms Evaluation of Bioactive Molecules from The Methanolic Leaf Extract of Azadirachta Indica (A. Juss). Asian J. Pharm. Sci. Technol. 2015, 5, 64–69. [Google Scholar]
- Jasmine, J.M.; Vanaja, R. In Silico Analysis of Phytochemical Compounds for Optimizing the Inhibitors of HMG CoA Reductase. J. Appl. Pharm. Sci. Vol. 2013, 3, 43–47. [Google Scholar] [CrossRef][Green Version]
- Lin, S.H.; Huang, K.J.; Weng, C.F.; Shiuan, D. Exploration of Natural Product Ingredients as Inhibitors of Human HMg-Coareductase through Structure-Based Virtual Screening. Drug Des. Devel. Ther. 2015, 9, 3313–3324. [Google Scholar]
- Tallei, T.E.; Tumilaar, S.G.; Niode, N.J.; Fatimawali, F.; Kepel, B.J.; Idroes, R.; Effendi, Y. Potential of Plant Bioactive Compounds as SARS-CoV-2 Main Protease (Mpro) and Spike (S) Glycoprotein Inhibitors: A Molecular Docking Study. Scientifica 2020, 2020, 6307457. [Google Scholar] [CrossRef]
- Maulydia, N.B.; Tallei, T.E.; Ginting, B.; Idroes, R.; Illian, D.N.; Faradilla, M. Analysis of Flavonoid Compounds of Orange (Citrus sp.) Peel as Anti-Main Protease of SARS-CoV-2: A Molecular Docking Study. IOP Conf. Ser. Earth Environ. Sci. 2022, 951, 012078. [Google Scholar] [CrossRef]
- Sulistiyani; Sari, R.K.; Triwahyuni, W. Eksplorasi Tumbuhan Obat Hutan Berkhasiat Inhibitor HMG-KoA Reduktase (Exploration of Medicinal Forest Plants with HMG-CoA Reductase Inhibitory Activity. J. Ilmu Teknol. Kayu Trop. 2017, 15, 141–154. [Google Scholar]
- Pangestika, I.; Oksal, E.; Sifzizul, T.; Muhammad, T.; Amir, H.; Syamsumir, D. Inhibitory Effects of Tangeretin and Trans-Ethyl Caffeate on the HMG-CoA Reductase Activity. Potential agents reducing Cholest levels. Saudi J. Biol. 2020, 27, 1947–1960. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).