Challenges in Preparation of Albumin Nanoparticle-Based Radiopharmaceuticals
Abstract
1. Background
1.1. Albumin Nanoparticle-Based Pharmaceuticals
1.2. Colloids as Radiopharmaceuticals
1.3. Objectives
2. Design Challenges
2.1. Physical Characteristics: Particle Size and Surface Charge
2.2. Targeting: Passive or Active
| Product (Manufacturer) | Specification or Measurement | Method * | References |
|---|---|---|---|
| Nanocoll (Sorin/GE Healthcare) | ≥95% of particles < 80 nm in diameter | Not stated | Monograph |
| >85% of particles 7–15 nm in diameter | PCS | [20] | |
| Mean diameter 12 nm | PCS | [20] | |
| Mean diameter 6.3 nm | PCS | [28] | |
| Mean diameter 8 nm | PCS | [29] | |
| Mean diameter 56 nm | ACS | [17] | |
| Mean diameter 9 nm | DLS | [18] | |
| Nanotop (Rotop) | ≥95% of particles ≤ 80 nm in diameter | Filtration | Monograph |
| Mean diameter 7 nm | DLS | [18] | |
| Nanoscan (Medi-Radiopharma) | ≥95% of particles ≤ 80 nm in diameter | Not stated | Monograph |
| Nanoalbumon (Medi-Radiopharma) | >80% of particles < 100 nm in diameter | Not stated | Monograph |
| Mean diameter 18 nm | DLS | [18] | |
| SentiScint (Medi-Radiopharma) | >80% of particles 100–600 nm in diameter | PCS | Monograph |
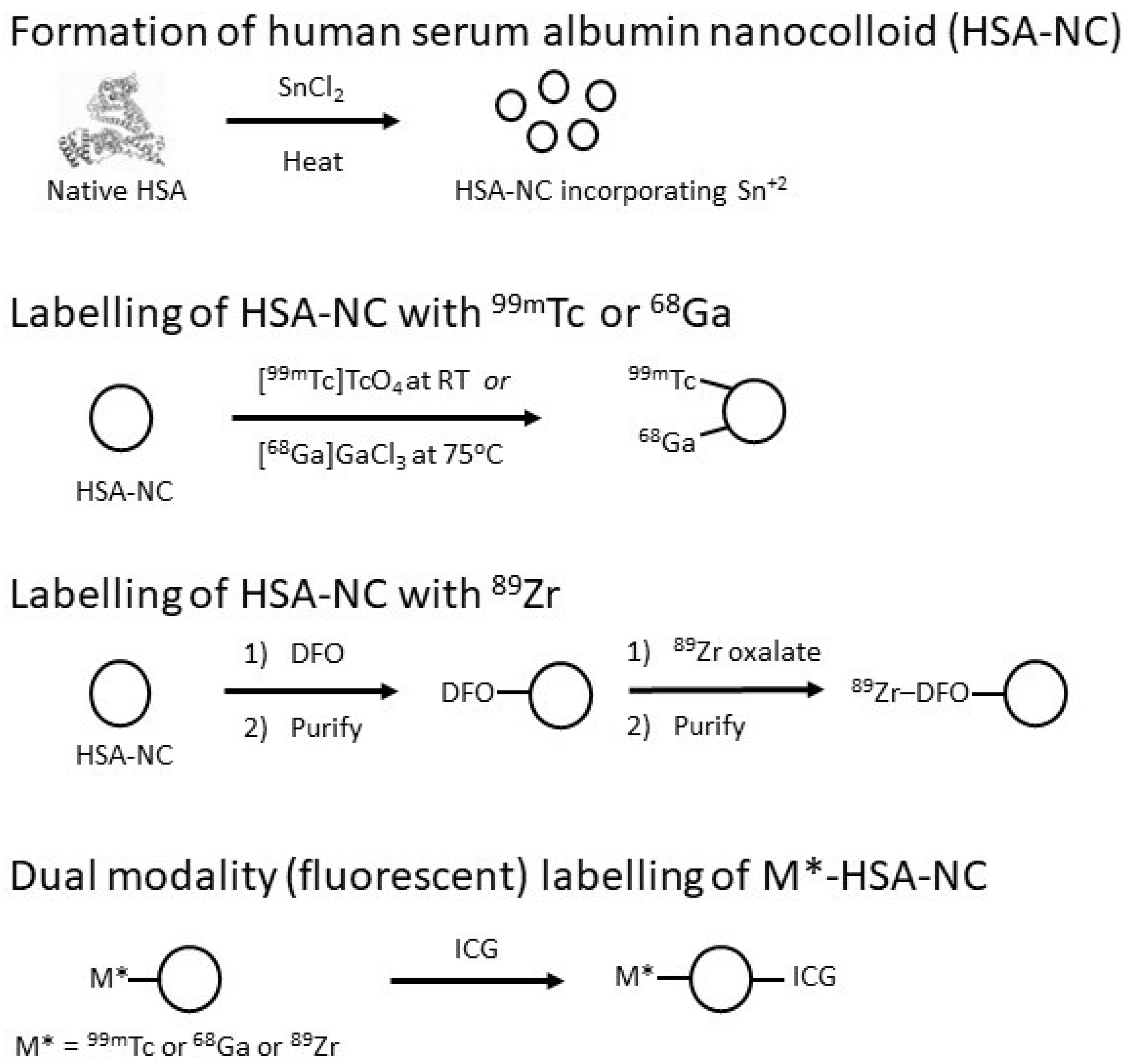
2.3. Derivatisation for Radiolabelling
2.4. Derivatisation for Dual-Modality Studies
2.5. Demonstration That Radiolabelling Does Not Affect Targeting
3. Preparation Challenges
3.1. Radiolabelling and Purification
3.2. Maintenance of Sterility and Apyrogenicity
3.3. Quality Assurance
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, X.; Liu, X.; Li, T.; Chen, Y.; Chen, Y.; Wang, P.; Zheng, L.; Yang, H.; Wu, C.; Deng, S.; et al. Recent Advancements in Serum Albumin-Based Nanovehicles toward Potential Cancer Diagnosis and Therapy. Front. Chem. 2021, 9, 746646. [Google Scholar] [CrossRef]
- Hornok, V. Serum Albumin Nanoparticles: Problems and Prospects. Polymers 2021, 13, 3759. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, N.; Wang, P.; Zhu, H.; Zhang, Z.; Yang, Z.; Zhang, W.; Guo, H.; Lin, J. Nanoalbumin–Prodrug Conjugates Prepared via a Thiolation-and-Conjugation Method Improve Cancer Chemotherapy and Immune Checkpoint Blockade Therapy by Promoting CD8+ T-Cell Infiltration. Bioeng. Transl. Med. 2022; e10377early view. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Tjulandin, S.; Davidson, N.; Shaw, H.; Desai, N.; Bhar, P.; Hawkins, M.; O’Shaughnessy, J. Phase III Trial of Nanoparticle Albumin-Bound Paclitaxel Compared with Polyethylated Castor Oil-Based Paclitaxel in Women with Breast Cancer. J. Clin. Oncol. 2005, 23, 7794–7803. [Google Scholar] [CrossRef] [PubMed]
- Sofias, A.M.; Dunne, M.; Storm, G.; Allen, C. The Battle of “Nano” Paclitaxel. Adv. Drug Deliv. Rev. 2017, 122, 20–30. [Google Scholar] [CrossRef]
- Barkat, M.A.; Beg, S.; Pottoo, F.H.; Ahmad, F.J. Nanopaclitaxel Therapy: An Evidence Based Review on the Battle for next-Generation Formulation Challenges. Nanomedicine 2019, 14, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Hahn, P.F. Treatment of Neoplasms by the Direct Infiltration of Radioactive Colloidal Metallic Gold. Am. J. Med. 1948, 4, 458. [Google Scholar] [CrossRef]
- Loken, M.K.; Staab, E.V.; Shea, A.W. 131-I Colloidal Albumin as an Agent for Scanning Liver and Spleen. An Experimental Study in Animals and Man. Investig. Radiol. 1966, 1, 295–300. [Google Scholar] [CrossRef]
- Dworkin, H.J.; Nelis, A.; Dowse, L. Rectilinear Liver Scanning with Technetium 99m Sulfide Colloid. Am. J. Roentgenol. Radium. Ther. Nucl. Med. 1967, 101, 557–560. [Google Scholar] [CrossRef]
- Mazzeo, F.; Accurso, A.; Petrella, G.; Capuano, S.; Maurelli, L.; Celentano, L.; Squame, G.; Salvatore, M. Pre-Operative Axillary Lymphoscintigraphy in Breast Cancer: Experience with Sub-Areolar Injection of 99Tcm-Nanocolloidal Albumin. Nucl. Med. Commun. 1986, 7, 5–16. [Google Scholar] [CrossRef]
- Gould, E.A.; Winship, T.; Philbin, P.H.; Kerr, H.H. Observations on a “Sentinel Node” in Cancer of the Parotid. Cancer 1960, 13, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.L.; Wen, D.R.; Wong, J.H.; Economou, J.S.; Cagle, L.A.; Storm, F.K.; Foshag, L.J.; Cochran, A.J. Technical Details of Intraoperative Lymphatic Mapping for Early Stage Melanoma. Arch. Surg. 1992, 127, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, K.; Vandamme, M.; Bormans, G.; Cleeren, F. Design and Challenges of Radiopharmaceuticals. Semin. Nucl. Med. 2019, 49, 339–356. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, C.; Cremonesi, M.; Luini, A.; Bartolomei, M.; Grana, C.; Prisco, G.; Galimberti, V.; Calza, P.; Viale, G.; Veronesi, U.; et al. Lymphoscintigraphy and Radioguided Biopsy of the Sentinel Axillary Node in Breast Cancer. J. Nucl. Med. 1998, 39, 2080–2084. [Google Scholar]
- Mariani, G.; Moresco, L.; Viale, G.; Villa, G.; Bagnasco, M.; Canavese, G.; Buscombe, J.; Strauss, H.W.; Paganelli, G. Radioguided Sentinel Lymph Node Biopsy in Breast Cancer Surgery. J. Nucl. Med. 2001, 42, 1198–1215. [Google Scholar]
- Bergqvist, L.; Strand, S.E.; Persson, B.R. Particle Sizing and Biokinetics of Interstitial Lymphoscintigraphic Agents. Semin. Nucl. Med. 1983, 13, 9–19. [Google Scholar] [CrossRef]
- Bergqvist, L.; Strand, S.E. Autocorrelation Spectroscopy for Particle Sizing and Stability Tests of Radiolabelled Colloids. Eur. J. Nucl. Med. 1989, 15, 641–645. [Google Scholar] [CrossRef]
- Persico, M.G.; Lodola, L.; Buroni, F.E.; Morandotti, M.; Pallavicini, P.; Aprile, C. 99mTc-Human Serum Albumin Nanocolloids: Particle Sizing and Radioactivity Distribution. J. Labelled. Comp. Radiopharm. 2015, 58, 376–382. [Google Scholar] [CrossRef]
- Ballinger, J. The Use of Protein Based Radiocolloids for Sentinel Node Localisation. Clin. Transl. Imaging 2015, 3, 179–186. [Google Scholar] [CrossRef]
- Gommans, G.M.; van Dongen, A.; van der Schors, T.G.; Gommans, E.; Visser, J.F.; Clarijs, W.W.; de Waard, J.W.; van de Bos, J.; Boer, R.O. Further Optimisation of 99mTc-Nanocoll Sentinel Node Localisation in Carcinoma of the Breast by Improved Labelling. Eur. J. Nucl. Med. 2001, 28, 1450–1455. [Google Scholar] [CrossRef]
- Alkureishi, L.W.T.; Burak, Z.; Alvarez, J.A.; Ballinger, J.; Bilde, A.; Britten, A.J.; Calabrese, L.; Chiesa, C.; Chiti, A.; de Bree, R.; et al. Joint Practice Guidelines for Radionuclide Lymphoscintigraphy for Sentinel Node Localization in Oral/Oropharyngeal Squamous Cell Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1915–1936. [Google Scholar] [CrossRef] [PubMed]
- Chakera, A.H.; Hesse, B.; Burak, Z.; Ballinger, J.R.; Britten, A.; Caraco, C.; Cochran, A.J.; Cook, M.G.; Drzewiecki, K.T.; Essner, R.; et al. EANM-EORTC General Recommendations for Sentinel Node Diagnostics in Melanoma. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1713–1742. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-Based Nanoparticles as Potential Controlled Release Drug Delivery Systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.G.; Thakur, M.L. Radiopharmaceuticals for Spleen and Bone Marrow Studies. Semin. Nucl. Med. 1985, 15, 229–238. [Google Scholar] [CrossRef]
- Rychlik, A.; Zalewski, K. Tracers and Corresponding Detection Devices: Technetium Colloids, Blue Dyes & NIR Fluorescence. Chin. Clin. Oncol. 2021, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Ellner, S.J.; Hoh, C.K.; Vera, D.R.; Darrah, D.D.; Schulteis, G.; Wallace, A.M. Dose-Dependent Biodistribution of [99mTc]DTPA-Mannosyl-Dextran for Breast Cancer Sentinel Lymph Node Mapping. Nucl. Med. Biol 2003, 30, 805–810. [Google Scholar] [CrossRef]
- Sondak, V.K.; King, D.W.; Zager, J.S.; Schneebaum, S.; Kim, J.; Leong, S.P.L.; Faries, M.B.; Averbook, B.J.; Martinez, S.R.; Puleo, C.A.; et al. Combined Analysis of Phase III Trials Evaluating [99mTc]Tilmanocept and Vital Blue Dye for Identification of Sentinel Lymph Nodes in Clinically Node-Negative Cutaneous Melanoma. Ann. Surg. Oncol. 2013, 20, 680–688. [Google Scholar] [CrossRef]
- Millar, A.M.; O’Brien, L.; Beattie, L.; Craig, F.; McDade, J. Validation of an Extended Shelf-Life for 99mTc Albumin Nanocolloid Injection. Nucl. Med. Commun. 2007, 28, A15. [Google Scholar] [CrossRef]
- Jimenez, I.R.; Roca, M.; Vega, E.; García, M.L.; Benitez, A.; Bajén, M.; Martín-Comín, J. Particle Sizes of Colloids to Be Used in Sentinel Lymph Node Radiolocalization. Nucl. Med. Commun. 2008, 29, 166–172. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Lozza, I.; Torres-Suárez, A.I. Actively Targeted Nanomedicines in Breast Cancer: From Pre-Clinical Investigation to Clinic. Cancers 2022, 14, 1198. [Google Scholar] [CrossRef] [PubMed]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The Entry of Nanoparticles into Solid Tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hou, S.; Mao, S.; Wei, D.; Song, X.; Lu, Y. Uptake of Folate-Conjugated Albumin Nanoparticles to the SKOV3 Cells. Int. J. Pharm. 2004, 287, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.K.; Singodia, D.; Verma, R.K.; Vyas, S.P. RGD Modified Albumin Nanospheres for Tumour Vasculature Targeting. J. Pharm. Pharmacol. 2011, 63, 33–40. [Google Scholar] [CrossRef]
- Cai, Z.; Chattopadhyay, N.; Yang, K.; Kwon, Y.L.; Yook, S.; Pignol, J.-P.; Reilly, R.M. 111In-Labeled Trastuzumab-Modified Gold Nanoparticles Are Cytotoxic In Vitro to HER2-Positive Breast Cancer Cells and Arrest Tumor Growth In Vivo in Athymic Mice after Intratumoral Injection. Nucl. Med. Biol. 2016, 43, 818–826. [Google Scholar] [CrossRef]
- Kouchakzadeh, H.; Shojaosadati, S.A.; Maghsoudi, A.; Vasheghani Farahani, E. Optimization of PEGylation Conditions for BSA Nanoparticles Using Response Surface Methodology. AAPS PharmSciTech 2010, 11, 1206–1211. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Technetium-99m Radiopharmaceuticals: Manufacture of Kits; Technical Report Series; International Atomic Energy Agency: Vienna, Austria, 2008. [Google Scholar]
- Sadkin, V.; Sкuridin, V.; Nesterov, E.; Stasyuk, E.; Rogov, A.; Varlamova, N.; Zelchan, R. 99mTc-Labeled Nanocolloid Drugs: Development Methods. Sci. Rep. 2020, 10, 14013. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Nova, P.; Ancira-Cortez, A.; Ferro-Flores, G.; Ocampo-García, B.; Gibbens-Bandala, B. Controlled-Release Nanosystems with a Dual Function of Targeted Therapy and Radiotherapy in Colorectal Cancer. Pharmaceutics 2022, 14, 1095. [Google Scholar] [CrossRef]
- Persico, M.G.; Marenco, M.; De Matteis, G.; Manfrinato, G.; Cavenaghi, G.; Sgarella, A.; Aprile, C.; Lodola, L. 99mTc-68Ga-ICG-Labelled Macroaggregates and Nanocolloids of Human Serum Albumin: Synthesis Procedures of a Trimodal Imaging Agent Using Commercial Kits. Contrast. Media. Mol. Imaging 2020, 2020, 3629705. [Google Scholar] [CrossRef]
- Marenco, M.; Canziani, L.; De Matteis, G.; Cavenaghi, G.; Aprile, C.; Lodola, L. Chemical and Physical Characterisation of Human Serum Albumin Nanocolloids: Kinetics, Strength and Specificity of Bonds with 99mTc and 68Ga. Nanomaterials 2021, 11, 1776. [Google Scholar] [CrossRef]
- Doughton, J.A.; Hofman, M.S.; Eu, P.; Hicks, R.J.; Williams, S. A First-in-Human Study of 68Ga-Nanocolloid PET/CT Sentinel Lymph Node Imaging in Prostate Cancer Demonstrates Aberrant Lymphatic Drainage Pathways. J. Nucl. Med. 2018, 59, 1837–1842. [Google Scholar] [CrossRef]
- Heuveling, D.A.; Visser, G.W.M.; Baclayon, M.; Roos, W.H.; Wuite, G.J.L.; Hoekstra, O.S.; Leemans, C.R.; de Bree, R.; van Dongen, G.A.M.S. 89Zr-Nanocolloidal Albumin-Based PET/CT Lymphoscintigraphy for Sentinel Node Detection in Head and Neck Cancer: Preclinical Results. J. Nucl. Med. 2011, 52, 1580–1584. [Google Scholar] [CrossRef]
- Heuveling, D.A.; van Schie, A.; Vugts, D.J.; Hendrikse, N.H.; Yaqub, M.; Hoekstra, O.S.; Karagozoglu, K.H.; Leemans, C.R.; van Dongen, G.A.M.S.; de Bree, R. Pilot Study on the Feasibility of PET/CT Lymphoscintigraphy with 89Zr-Nanocolloidal Albumin for Sentinel Node Identification in Oral Cancer Patients. J. Nucl. Med. 2013, 54, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Heuveling, D.A.; Karagozoglu, K.H.; Van Lingen, A.; Hoekstra, O.S.; Van Dongen, G.A.M.S.; De Bree, R. Feasibility of Intraoperative Detection of Sentinel Lymph Nodes with 89-Zirconium-Labelled Nanocolloidal Albumin PET-CT and a Handheld High-Energy Gamma Probe. EJNMMI Res. 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, O.R.; Klop, W.M.C.; Buckle, T.; Vermeeren, L.; van den Brekel, M.W.M.; Balm, A.J.M.; Nieweg, O.E.; Valdés Olmos, R.A.; van Leeuwen, F.W.B. Feasibility of Sentinel Node Biopsy in Head and Neck Melanoma Using a Hybrid Radioactive and Fluorescent Tracer. Ann. Surg. Oncol. 2012, 19, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, F.W.B.; Schottelius, M.; Brouwer, O.R.; Vidal-Sicart, S.; Achilefu, S.; Klode, J.; Wester, H.-J.; Buckle, T. Trending: Radioactive and Fluorescent Bimodal/Hybrid Tracers as Multiplexing Solutions for Surgical Guidance. J. Nucl. Med. 2020, 61, 13–19. [Google Scholar] [CrossRef]
- Heuveling, D.A.; Visser, G.W.M.; de Groot, M.; de Boer, J.F.; Baclayon, M.; Roos, W.H.; Wuite, G.J.L.; Leemans, C.R.; de Bree, R.; van Dongen, G.A.M.S. Nanocolloidal Albumin-IRDye 800CW: A near-Infrared Fluorescent Tracer with Optimal Retention in the Sentinel Lymph Node. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1161–1168. [Google Scholar] [CrossRef]
- van Leeuwen, F.W.B.; Cornelissen, B.; Caobelli, F.; Evangelista, L.; Rbah-Vidal, L.; Del Vecchio, S.; Xavier, C.; Barbet, J.; de Jong, M. Generation of Fluorescently Labeled Tracers—Which Features Influence the Translational Potential? EJNMMI Radiopharm. Chem. 2017, 2, 15. [Google Scholar] [CrossRef]
- Hernandez, R.; Heskamp, S.; Rijpkema, M.; Bos, D.L.; Goldenberg, D.M.; McBride, W.J.; Morgenstern, A.; Bruchertseifer, F.; Cai, W.; Boerman, O.C. Preventing Radiobleaching of Cyanine Fluorophores Enhances Stability of Nuclear/NIRF Multimodality Imaging Agents. Theranostics 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Baeten, I.G.T.; Hoogendam, J.P.; Jeremiasse, B.; Braat, A.J.A.T.; Veldhuis, W.B.; Jonges, G.N.; Jürgenliemk-Schulz, I.M.; van Gils, C.H.; Zweemer, R.P.; Gerestein, C.G. Indocyanine Green versus Technetium-99m with Blue Dye for Sentinel Lymph Node Detection in Early-Stage Cervical Cancer: A Systematic Review and Meta-Analysis. Cancer Rep. 2022, 5, e1401. [Google Scholar] [CrossRef]
- Bargon, C.A.; Huibers, A.; Young-Afat, D.A.; Jansen, B.A.M.; Lavalaye, J.; van Slooten, H.-J.; Verkooijen, H.M.; van Swol, C.F.P.; Doeksen, A. Sentinel Lymph Node Mapping in Breast Cancer Patients through Fluorescent Imaging Using Indocyanine Green—The INFLUENCE Trial. Ann. Surg. 2022, 276, 913–920. [Google Scholar] [CrossRef]
- KleinJan, G.H.; Bunschoten, A.; van den Berg, N.S.; Olmos, R.A.V.; Klop, W.M.C.; Horenblas, S.; van der Poel, H.G.; Wester, H.-J.; van Leeuwen, F.W.B. Fluorescence Guided Surgery and Tracer-Dose, Fact or Fiction? Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- van der Poel, H.G.; Buckle, T.; Brouwer, O.R.; Valdés Olmos, R.A.; van Leeuwen, F.W.B. Intraoperative Laparoscopic Fluorescence Guidance to the Sentinel Lymph Node in Prostate Cancer Patients: Clinical Proof of Concept of an Integrated Functional Imaging Approach Using a Multimodal Tracer. Eur. Urol. 2011, 60, 826–833. [Google Scholar] [CrossRef]
- Gillings, N.; Hjelstuen, O.; Ballinger, J.; Behe, M.; Decristoforo, C.; Elsinga, P.; Ferrari, V.; Peitl, P.K.; Koziorowski, J.; Laverman, P.; et al. Guideline on Current Good Radiopharmacy Practice (CGRPP) for the Small-Scale Preparation of Radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2021, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Olsen, H.; Andersen, A.; Nordbø, A.; Kongsgaard, U.E.; Børmer, O.P. Pharmaceutical-Grade Albumin: Impaired Drug-Binding Capacity In Vitro. BMC Clin. Pharmacol. 2004, 4, 4. [Google Scholar] [CrossRef]
- Taguchi, K.; Chuang, V.T.G.; Maruyama, T.; Otagiri, M. Pharmaceutical Aspects of the Recombinant Human Serum Albumin Dimer: Structural Characteristics, Biological Properties, and Medical Applications. J. Pharm. Sci. 2012, 101, 3033–3046. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.P.; Frier, M.; Johnson, R.A.; Berezenko, S.; Perkins, A.C. Preparation of Tc-99m-Macroaggregated Albumin from Recombinant Human Albumin for Lung Perfusion Imaging. Eur. J. Pharm. Biopharm. 2006, 62, 26–31. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Quality Control in the Production of Radiopharmaceuticals; IAEA Technical Documents; International Atomic Energy Agency: Vienna, Austria, 2018. [Google Scholar]
- Ballinger, J.R.; Blower, P.J. Radiochemical Purity Testing of 99mTc-Labelled Radiopharmaceuticals: How Much Is Enough? Nucl. Med. Commun. 2011, 32, 761–763. [Google Scholar] [CrossRef]
- Cusnir, R.; Leresche, M.; Pilloud, C.; Straub, M. An Investigation of Aspects of Radiochemical Purity of 99mTc-Labelled Human Serum Albumin Nanocolloid. EJNMMI Radiopharm. Chem. 2021, 6, 35. [Google Scholar] [CrossRef]
| Radionuclide | Half-Life | Emission (Abundance), Mean Energy | Route of Production |
|---|---|---|---|
| Technetium-99m, 99mTc | 6 h | Gamma (89%), 140 keV | Generator (or cyclotron) |
| Gallium-68, 68Ga | 1.1 h | Positron (89%), 0.89 MeV | Generator or cyclotron |
| Zirconium-89, 89Zr | 78 h | Positron (23%), 0.39 MeV | Cyclotron |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballinger, J.R. Challenges in Preparation of Albumin Nanoparticle-Based Radiopharmaceuticals. Molecules 2022, 27, 8596. https://doi.org/10.3390/molecules27238596
Ballinger JR. Challenges in Preparation of Albumin Nanoparticle-Based Radiopharmaceuticals. Molecules. 2022; 27(23):8596. https://doi.org/10.3390/molecules27238596
Chicago/Turabian StyleBallinger, James R. 2022. "Challenges in Preparation of Albumin Nanoparticle-Based Radiopharmaceuticals" Molecules 27, no. 23: 8596. https://doi.org/10.3390/molecules27238596
APA StyleBallinger, J. R. (2022). Challenges in Preparation of Albumin Nanoparticle-Based Radiopharmaceuticals. Molecules, 27(23), 8596. https://doi.org/10.3390/molecules27238596






