Design of a New Chiral Deep Eutectic Solvent Based on 3-Amino-1,2-propanediol and Its Application in Organolithium Chemistry
Abstract
1. Introduction
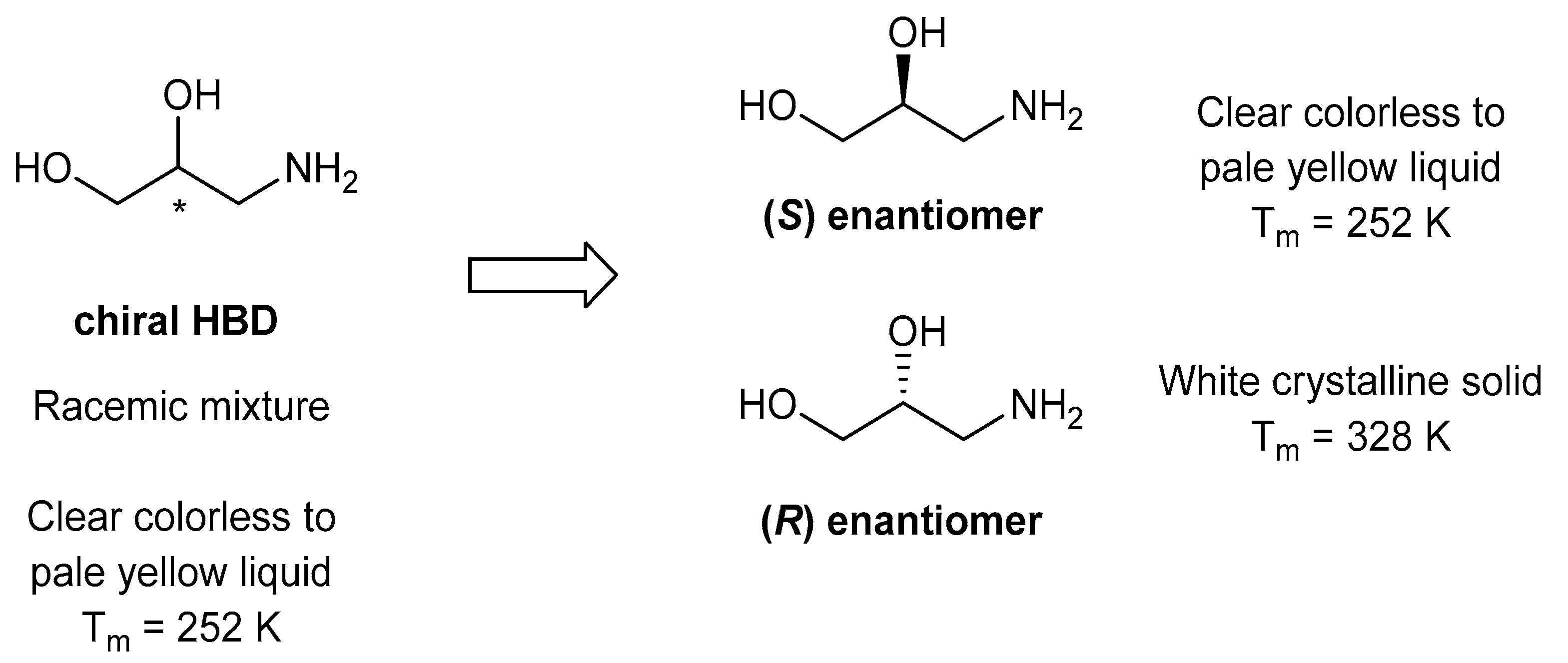
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.epa.gov/indoor-air-quality-iaq/technical-overview-volatile-organic-compounds (accessed on 24 September 2022).
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 39, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y. Deep Eutectic Solvents; Springer International Publishing: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F.; Looi, C.Y. Emerging frontiers of deep eutectic solvents in drug discovery and drug delivery systems. J. Control. Release 2019, 316, 168–195. [Google Scholar] [CrossRef]
- Emami, S.; Shayanfar, A. Deep eutectic solvents for pharmaceutical formulation and drug delivery applications. Pharm. Dev. Technol. 2020, 25, 779–796. [Google Scholar] [CrossRef]
- Trombino, S.; Siciliano, C.; Procopio, D.; Curcio, F.; Laganà, A.S.; Di Gioia, M.L.; Cassano, R. Deep Eutectic Solvents for Improving the Solubilization and Delivery of Dapsone. Pharmaceutics 2022, 14, 333. [Google Scholar] [CrossRef]
- Nkuku, C.A.; LeSuer, R.J. Electrochemistry in Deep Eutectic Solvents. J. Phys. Chem. B 2007, 111, 13271–13277. [Google Scholar] [CrossRef]
- Brett, C.M.A. Deep eutectic solvents and applications in electrochemical sensing. Curr. Opin. Electrochem. 2018, 10, 143–148. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, J.; Li, G.; Zhao, W.; Zhao, X.; Mu, T. The electrochemical stability of ionic liquids and deep eutectic solvents. Sci. China Ser. B Chem. 2016, 59, 571–577. [Google Scholar] [CrossRef]
- Fuchs, D.; Bayer, B.; Gupta, T.; Szabo, G.L.; Wilhelm, R.A.; Eder, D.; Meyer, J.; Steiner, S.; Gollas, B. The Electrochemical Behavior of Graphene in a Deep Eutectic Solvent. ACS Appl. Mater. Interfaces 2020, 12, 40937–40948. [Google Scholar] [CrossRef] [PubMed]
- Tomé, L.I.N.; Baião, V.; da Silva, W.; Brett, C.M.A. Deep eutectic solvents for the production and application of new materials. Appl. Mater. Today 2018, 10, 30–50. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. TrAC Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep Eutectic Solvents: The Organic Reaction Medium of the Century. Eur. J. Org. Chem. 2016, 2016, 612–632. [Google Scholar] [CrossRef]
- Ghinato, S.; De Nardi, F.; Bolzoni, P.; Antenucci, A.; Blangetti, M.; Prandi, C. Chemo- and Regioselective Anionic Fries Rearrangement Promoted by Lithium Amides under Aerobic Conditions in Sustainable Reaction Media. Chem.-A Eur. J. 2022, 2, e202201154. [Google Scholar] [CrossRef]
- Körner, S.; Albert, J.; Held, C. Catalytic Low-Temperature Dehydration of Fructose to 5-Hydroxymethylfurfural Using Acidic Deep Eutectic Solvents and Polyoxometalate Catalysts. Front. Chem. 2019, 7, 661. [Google Scholar] [CrossRef]
- Lima, F.; Dietz, C.H.J.T.; Silvestre, A.J.D.; Branco, L.C.; Lopes, J.C.; Gallucci, F.; Shimizu, K.; Held, C.; Marrucho, I.M. Vapor Pressure Assessment of Sulfolane-Based Eutectic Solvents: Experimental, PC-SAFT, and Molecular Dynamics. J. Phys. Chem. B 2020, 124, 10386–10397. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Sazonova, A.Y.; Frolkova, A.K.; Zaitsau, D.; Prikhodko, I.; Held, C. Separation Performance of BioRenewable Deep Eutectic Solvents. Ind. Eng. Chem. Res. 2015, 54, 3498–3504. [Google Scholar] [CrossRef]
- Vidal, C.; García-Álvarez, J.; Hernán-Gómez, A.; Kennedy, A.R.; Hevia, E. Introducing Deep Eutectic Solvents to Polar Organometallic Chemistry: Chemoselective Addition of Organolithium and Grignard Reagents to Ketones in Air. Angew. Chem. Int. Ed. 2014, 53, 5969–5973. [Google Scholar] [CrossRef]
- Ghinato, S.; Dilauro, G.; Perna, F.M.; Capriati, V.; Blangetti, M.; Prandi, C. Directed ortho-metalation–nucleophilic acyl substitution strategies in deep eutectic solvents: The organolithium base dictates the chemoselectivity. Chem. Commun. 2019, 55, 7741–7744. [Google Scholar] [CrossRef] [PubMed]
- Arnodo, D.; Ghinato, S.; Nejrotti, S.; Blangetti, M.; Prandi, C. Lateral lithiation in deep eutectic solvents: Regioselective functionalization of substituted toluene derivatives. Chem. Commun. 2020, 56, 2391–2394. [Google Scholar] [CrossRef] [PubMed]
- Ghinato, S.; Territo, D.; Maranzana, A.; Capriati, V.; Blangetti, M.; Prandi, C. A Fast and General Route to Ketones from Amides and Organolithium Compounds under Aerobic Conditions: Synthetic and Mechanistic Aspects. Chem.-A Eur. J. 2020, 27, 2868–2874. [Google Scholar] [CrossRef] [PubMed]
- Gontrani, L.; Plechkova, N.V.; Bonomo, M. In-Depth Physico-Chemical and Structural Investigation of a Dicarboxylic Acid/Choline Chloride Natural Deep Eutectic Solvent (NADES): A Spotlight on the Importance of a Rigorous Preparation Procedure. ACS Sustain. Chem. Eng. 2019, 7, 12536–12543. [Google Scholar] [CrossRef]
- Bonomo, M.; Gontrani, L.; Capocefalo, A.; Sarra, A.; Nucara, A.; Carbone, M.; Postorino, P.; Dini, D. A combined electrochemical, infrared and EDXD tool to disclose Deep Eutectic Solvents formation when one precursor is liquid: Glyceline as case study. J. Mol. Liq. 2020, 319, 114292. [Google Scholar] [CrossRef]
- Claudy, P.; Commerçon, J.; Lètoffé, J. Quasi-static study of the glass transition of glycerol by DSC. Thermochim. Acta 1988, 128, 251–260. [Google Scholar] [CrossRef]
- Cappelluti, F.; Mariani, A.; Bonomo, M.; Damin, A.; Bencivenni, L.; Passerini, S.; Carbone, M.; Gontrani, L. Stepping away from serendipity in Deep Eutectic Solvent formation: Prediction from precursors ratio. J. Mol. Liq. 2022, 367, 120443. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Hayyan, M.; Alsaadi, M.A.; Akib, S.; Hayyan, A.; Hashim, M.A. Glycerol-based deep eutectic solvents: Physical properties. J. Mol. Liq. 2016, 215, 98–103. [Google Scholar] [CrossRef]
- Pitrè, D.; Fedeli, F. Optical Resolution of 1-Amino-2,3-Propanediol. Arch. Pharm. 1986, 319, 193–195. [Google Scholar] [CrossRef]
- Quivelli, A.F.; D’Addato, G.; Vitale, P.; García-Álvarez, J.; Perna, F.M.; Capriati, V. Expeditious and practical synthesis of tertiary alcohols from esters enabled by highly polarized organometallic compounds under aerobic conditions in Deep Eutectic Solvents or bulk water. Tetrahedron 2021, 81, 131898. [Google Scholar] [CrossRef]
- Palomba, T.; Ciancaleoni, G.; Del Giacco, T.; Germani, R.; Ianni, F.; Tiecco, M. Deep Eutectic Solvents formed by chiral components as chiral reaction media and studies of their structural properties. J. Mol. Liq. 2018, 262, 285–294. [Google Scholar] [CrossRef]
- Tiecco, M.; Alonso, D.A.; Ñíguez, D.R.; Ciancaleoni, G.; Guillena, G.; Ramón, D.J.; Bonillo, A.A.; Germani, R. Assessment of the organocatalytic activity of chiral l-Proline-based Deep Eutectic Solvents based on their structural features. J. Mol. Liq. 2020, 313, 113573. [Google Scholar] [CrossRef]
- Gilman, H.; Haubein, A.H. The Quantitative Analysis of Alkyllithium Compounds1. J. Am. Chem. Soc. 1944, 66, 1515–1516. [Google Scholar] [CrossRef]
- Bachmann, W.E. Syntheses of Phenanthrene Derivatives. I. Reactions of 9-Phenanthrylmagnesium Bromide. J. Am. Chem. Soc. 1934, 56, 1363–1367. [Google Scholar] [CrossRef]
- Converso, A.; Saaidi, P.-L.; Sharpless, A.K.B.; Finn, M.G. Nucleophilic Substitution by Grignard Reagents on Sulfur Mustards. J. Org. Chem. 2004, 69, 7336–7339. [Google Scholar] [CrossRef]
- Kupracz, L.; Kirschning, A. Multiple Organolithium Generation in the Continuous Flow Synthesis of Amitriptyline. Adv. Synth. Catal. 2013, 355, 3375–3380. [Google Scholar] [CrossRef]
- Bassindale, A.R.; Eaborn, C.; Walton, D.R.M. A novel synthesis of neopentylbenzenes. J. Chem. Soc. C Org. 1969, 19, 2505–2506. [Google Scholar] [CrossRef]
- Pryor, W.A.; Tang, F.Y.; Tang, R.H.; Church, D.F. Chemistry of the tert-butyl radical: Polar character, .rho. value for reaction with toluenes, and the effect of radical polarity on the ratio of benzylic hydrogen abstraction to addition to aromatic rings. J. Am. Chem. Soc. 1982, 104, 2885–2891. [Google Scholar] [CrossRef]
- Wurtz, A. Sur une nouvelle classe de radicaux organiques. Ann. Chim. Phys. 1855, 44, 275–312. [Google Scholar] [CrossRef]
- Wurtz, A. Ueber eine neue Klasse organischer Radicale. Eur. J. Org. Chem. 1855, 96, 364–375. [Google Scholar] [CrossRef]
- Antenucci, A.; Bonomo, M.; Ghigo, G.; Gontrani, L.; Barolo, C.; Dughera, S. How do arenediazonium salts behave in deep eutectic solvents? A combined experimental and computational approach. J. Mol. Liq. 2021, 339, 116743. [Google Scholar] [CrossRef]
- Ghigo, G.; Bonomo, M.; Antenucci, A.; Reviglio, C.; Dughera, S. Copper-Free Halodediazoniation of Arenediazonium Tetrafluoroborates in Deep Eutectic Solvents-like Mixtures. Molecules 2022, 27, 1909. [Google Scholar] [CrossRef] [PubMed]
- Ghigo, G.; Bonomo, M.; Antenucci, A.; Damin, A.; Dughera, S. Ullmann homocoupling of arenediazonium salts in a deep eutectic solvent. Synthetic and mechanistic aspects. RSC Adv. 2022, 12, 26640–26647. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, W.; Zheng, Z.; Zou, X. One-pot α-nucleophilic fluorination of acetophenones in a deep eutectic solvent. J. Fluor. Chem. 2010, 131, 340–344. [Google Scholar] [CrossRef]
- Di Carmine, G.; Abbott, A.P.; D’Agostino, C. Deep eutectic solvents: Alternative reaction media for organic oxidation reactions. React. Chem. Eng. 2021, 6, 582–598. [Google Scholar] [CrossRef]
- Vitale, P.; Cicco, L.; Perna, F.M.; Capriati, V. Introducing deep eutectic solvents in enolate chemistry: Synthesis of 1-arylpropan-2-ones under aerobic conditions. React. Chem. Eng. 2021, 6, 1796–1800. [Google Scholar] [CrossRef]
- Saito, Y.; Yamanoue, K.; Segawa, Y.; Itami, K. Selective Transformation of Strychnine and 1,2-Disubstituted Benzenes by C–H Borylation. Chem 2020, 6, 985–993. [Google Scholar] [CrossRef]
- Maekawa, T.; Sekizawa, H.; Itami, K. Controlled Alcohol-Carbonyl Interconversion by Nickel Catalysis. Angew. Chem. Int. Ed. 2011, 50, 7022–7026. [Google Scholar] [CrossRef]
- Condon, F.E.; West, D.L. Cyclization-rearrangement of alkylstyrenes. 1. A. 1-Phenyl-1-pentene and homologs. B. A short synthesis of calamenene. J. Org. Chem. 1980, 45, 2006–2009. [Google Scholar] [CrossRef]
- Ardiansah, B.; Tanimoto, H.; Tomohiro, T.; Morimoto, T.; Kakiuchi, K. Sulfonium ion-promoted traceless Schmidt reaction of alkyl azides. Chem. Commun. 2021, 57, 8738–8741. [Google Scholar] [CrossRef]
- Matsui, M.; Tsuge, M.; Shibata, K.; Muramatsu, H. Photochromism of 1,1-Diaryl-1-alkanols. Bull. Chem. Soc. Jpn. 1994, 67, 1753–1755. [Google Scholar] [CrossRef]
- Napolitano, E.; Giovani, E.; Ceccarelli, N.; Pelosi, P. Tertiary Alcohols with Earthy Odor. J. Agric. Food Chem. 1996, 44, 2806–2809. [Google Scholar] [CrossRef]
- Gao, F.; Deng, X.-J.; Tang, Y.; Tang, J.-P.; Yang, J.; Zhang, Y.-M. A simple and efficient copper oxide-catalyzed Barbier–Grignard reaction of unactivated aryl or alkyl bromides with ester. Tetrahedron Lett. 2014, 55, 880–883. [Google Scholar] [CrossRef]
- Le Bailly, B.A.F.; Greenhalgh, M.D.; Thomas, S.P. Iron-catalysed, hydride-mediated reductive cross-coupling of vinyl halides and Grignard reagents. Chem. Commun. 2011, 48, 1580–1582. [Google Scholar] [CrossRef][Green Version]
- Maeda, H.; Kikui, T.; Nakatsuji, Y.; Okahara, M. Synthesis of aminomethyl crown ethers. J. Org. Chem. 1982, 47, 5167–5171. [Google Scholar] [CrossRef]
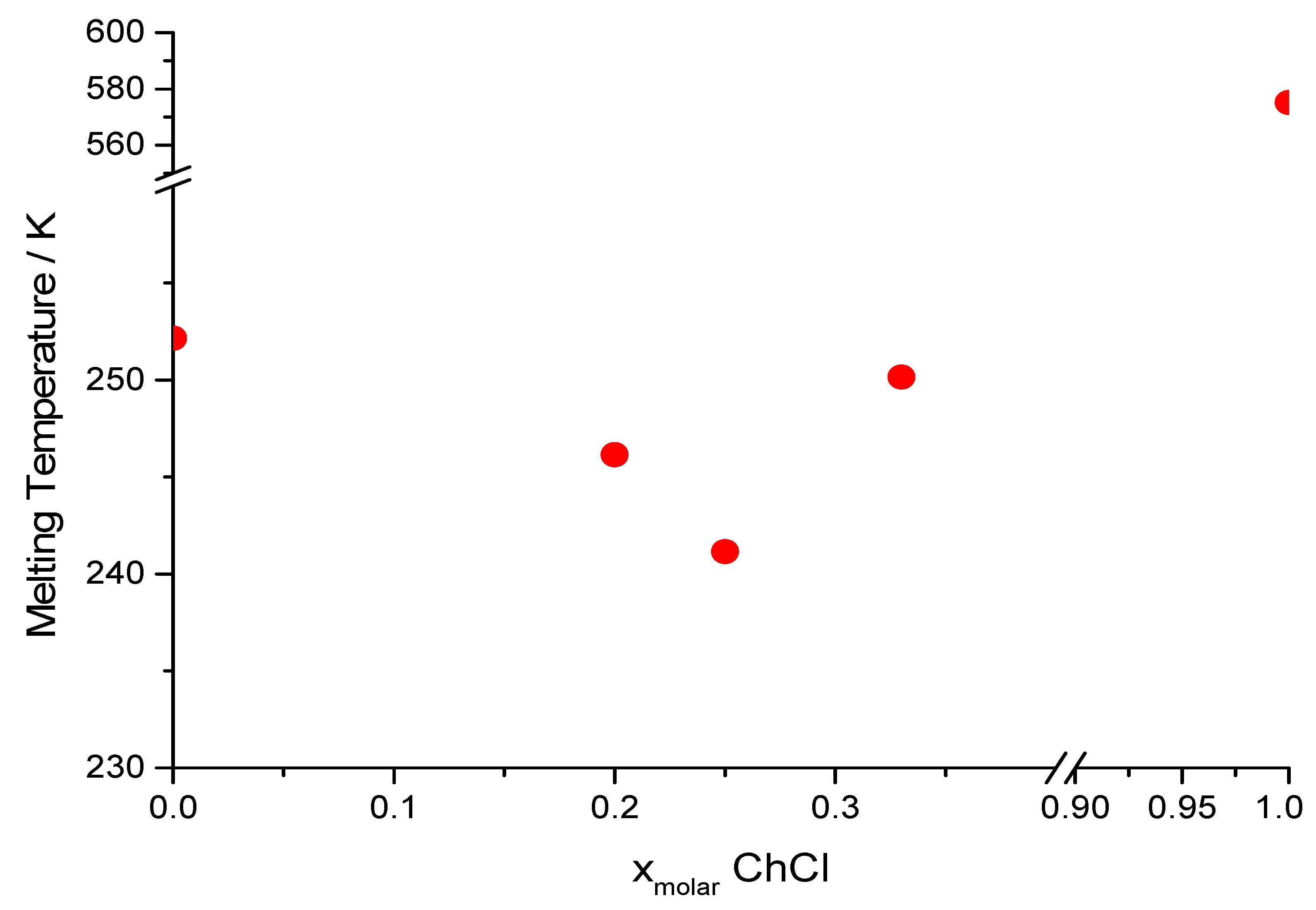
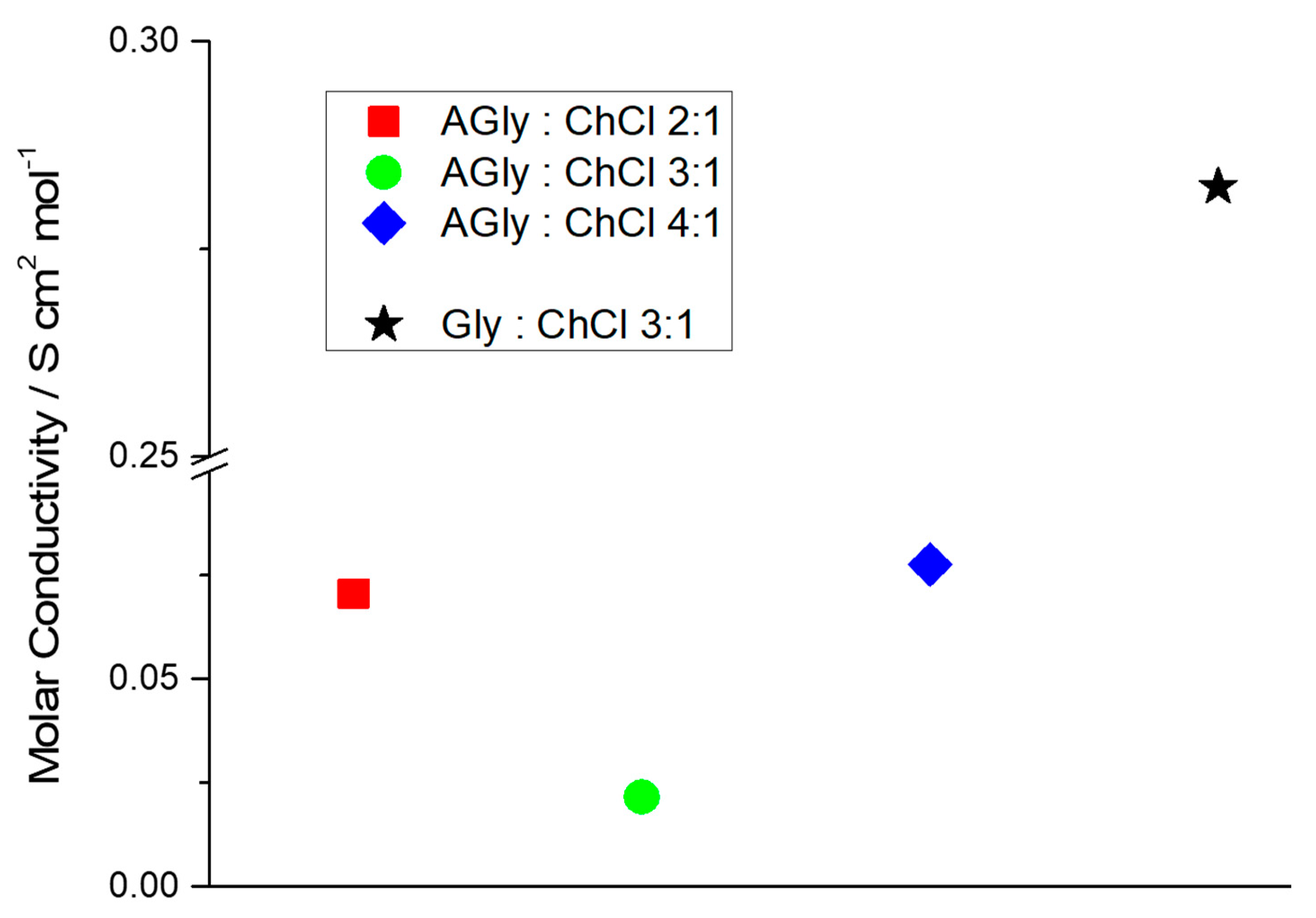
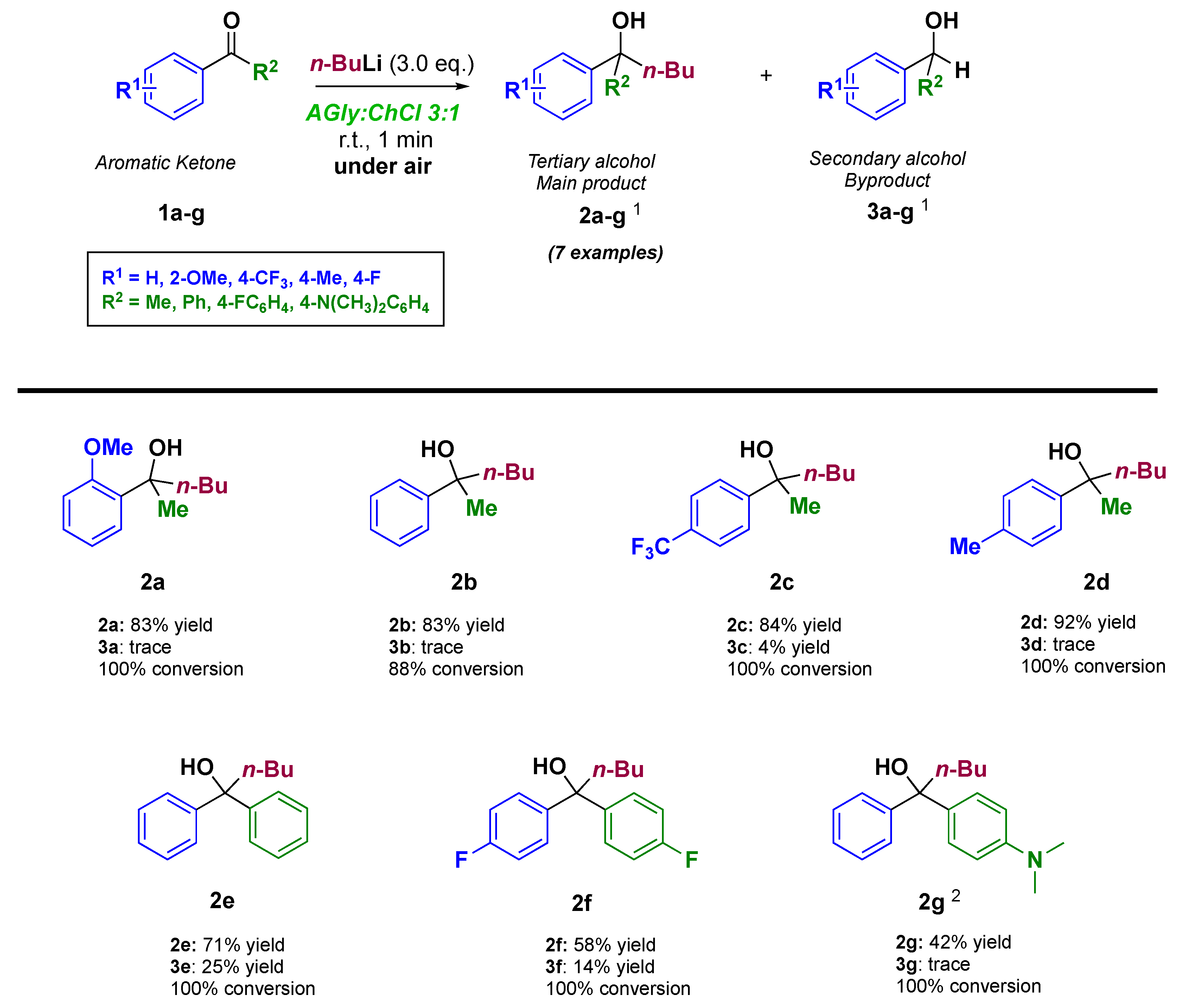
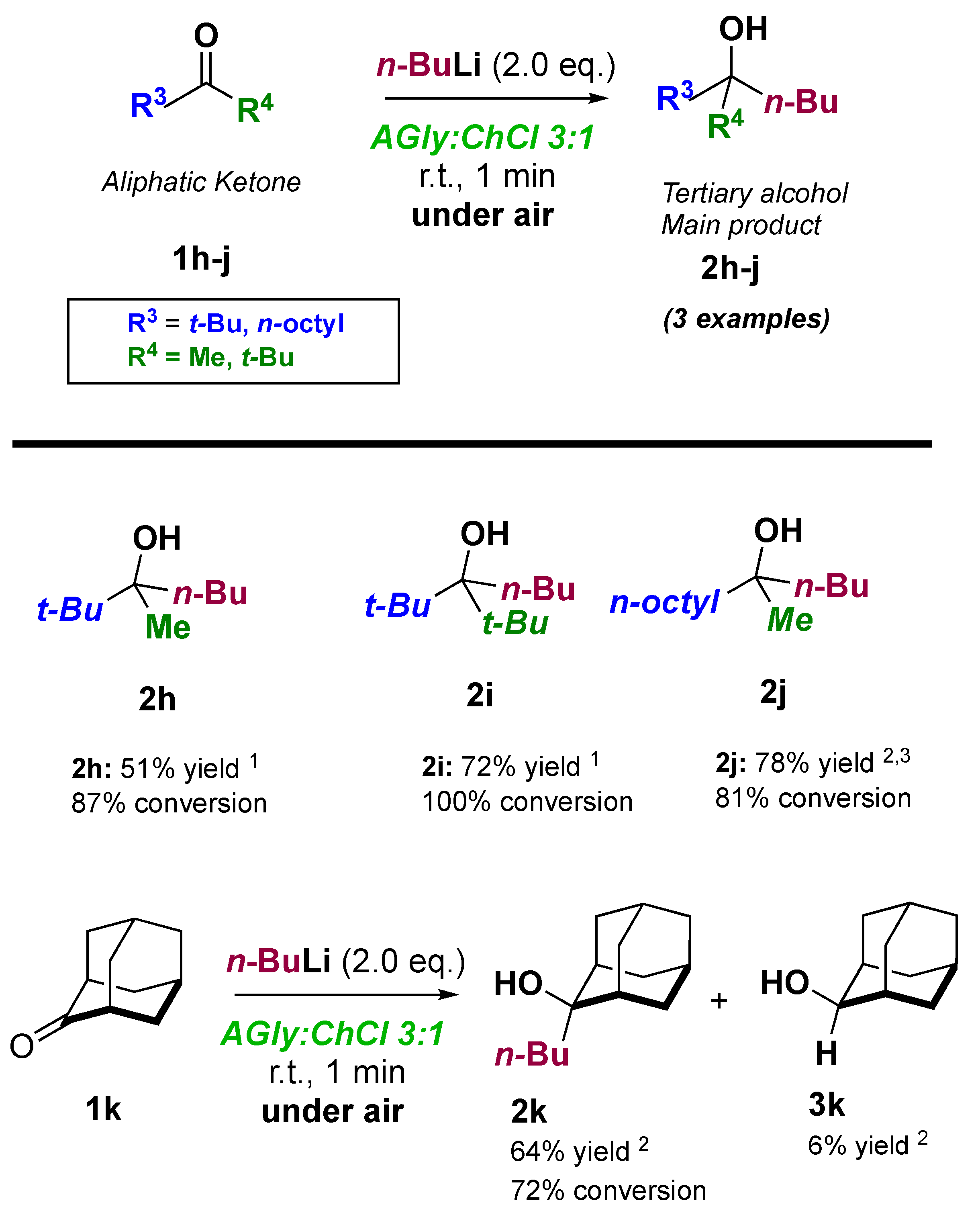
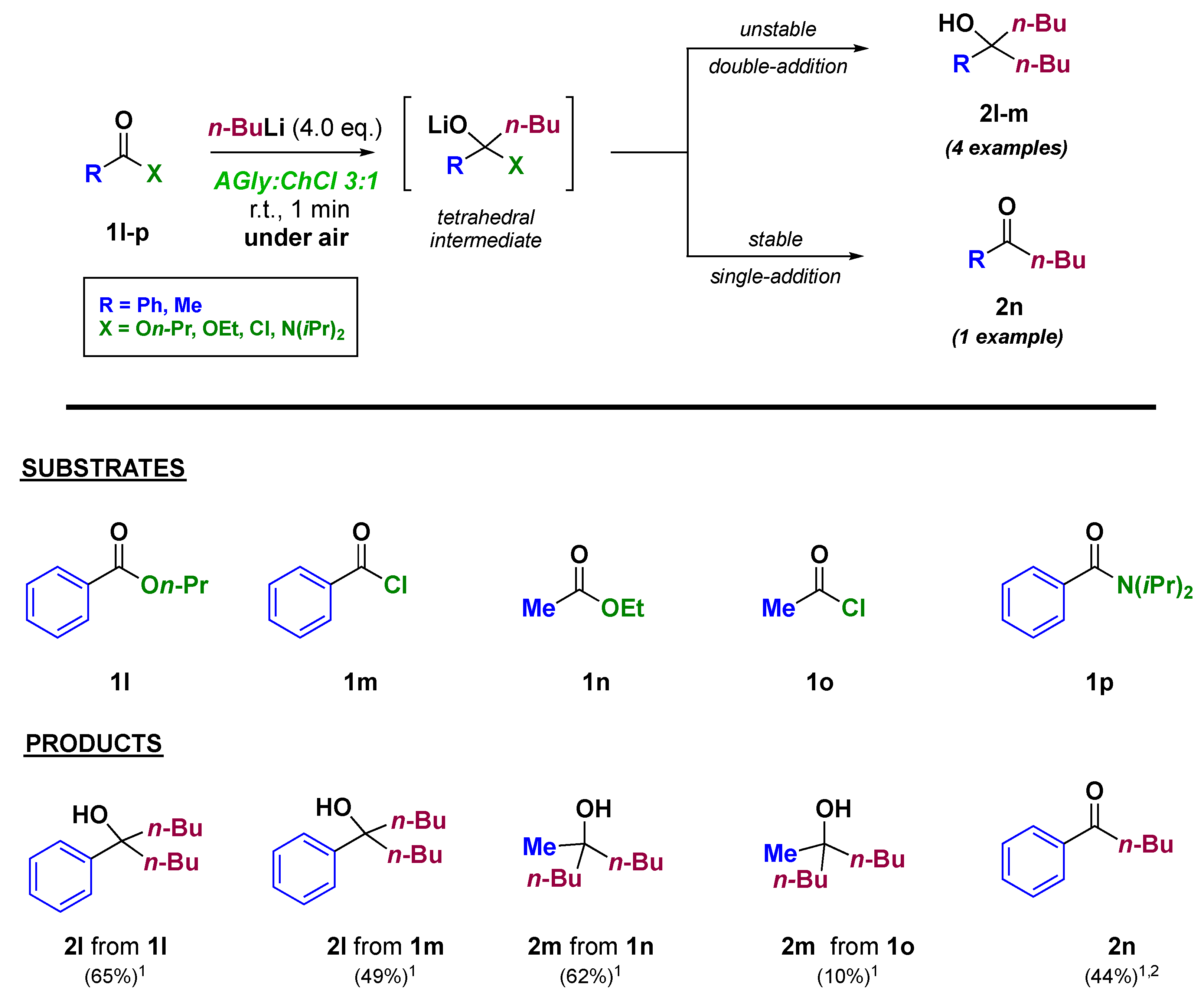
| AGly:ChCl Ratio | AGly (g) | ChCl (g) | Physical State at RT | Tm (K) 1 | h/P | L (mS cm−1 M−1) |
|---|---|---|---|---|---|---|
| 1:1 | 6.226 | 9.526 | Solid | >353 | n.m. | n.m. |
| 2:1 | 6.008 | 4.600 | Unstable liquid | n.d. (250) | 315.41 | 71 ± 5 |
| 3:1 | 6.144 | 3.138 | Liquid | 241 (243) | 93.51 | 22 ± 3 |
| 4:1 | 6.939 | 2.660 | Liquid | 246 (248) | 11.27 | 77 ± 7 |
 | |||
|---|---|---|---|
| Entry | DES | n-BuLi eq. | Yield 2a (%) |
| 1 | Gly:ChCl 2:1 | 2.0 | 67 |
| 2 | AGly:ChCl 3:1 | 2.0 | 67 |
| 3 | AGly:ChCl 3:1 | 3.0 | 83 |
| 4 | AGly | 3.0 | 48 1 |
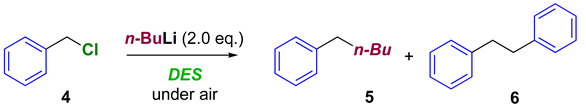 | |||
|---|---|---|---|
| Entry | DES | Yield 5 (%) 1 | Yield 6 (%) 1 |
| 1 | Gly:ChCl 2:1 | 28 | 3 |
| 2 | Gly:ChCl 3:1 | 70 | 12 |
| 3 | Urea:ChCl 2:1 | 61 | 7 |
| 4 | AGly:ChCl 3:1 | 85 | 11 |
| 5 | LA:ChCl 2:1 | 32 | 2 |
| 6 | Gly:KF 6:1 | 74 | 7 |
| 7 | H2O:ChCl 2:1 | - | - |
| 8 | EG:ChCl 2:1 | 34 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antenucci, A.; Bonomo, M.; Ghinato, S.; Blangetti, M.; Dughera, S. Design of a New Chiral Deep Eutectic Solvent Based on 3-Amino-1,2-propanediol and Its Application in Organolithium Chemistry. Molecules 2022, 27, 8566. https://doi.org/10.3390/molecules27238566
Antenucci A, Bonomo M, Ghinato S, Blangetti M, Dughera S. Design of a New Chiral Deep Eutectic Solvent Based on 3-Amino-1,2-propanediol and Its Application in Organolithium Chemistry. Molecules. 2022; 27(23):8566. https://doi.org/10.3390/molecules27238566
Chicago/Turabian StyleAntenucci, Achille, Matteo Bonomo, Simone Ghinato, Marco Blangetti, and Stefano Dughera. 2022. "Design of a New Chiral Deep Eutectic Solvent Based on 3-Amino-1,2-propanediol and Its Application in Organolithium Chemistry" Molecules 27, no. 23: 8566. https://doi.org/10.3390/molecules27238566
APA StyleAntenucci, A., Bonomo, M., Ghinato, S., Blangetti, M., & Dughera, S. (2022). Design of a New Chiral Deep Eutectic Solvent Based on 3-Amino-1,2-propanediol and Its Application in Organolithium Chemistry. Molecules, 27(23), 8566. https://doi.org/10.3390/molecules27238566