[MII(H2dapsc)]-[Cr(CN)6] (M = Mn, Co) Chain and Trimer Complexes: Synthesis, Crystal Structure, Non-Covalent Interactions and Magnetic Properties
Abstract
1. Introduction
2. Results and Discussion
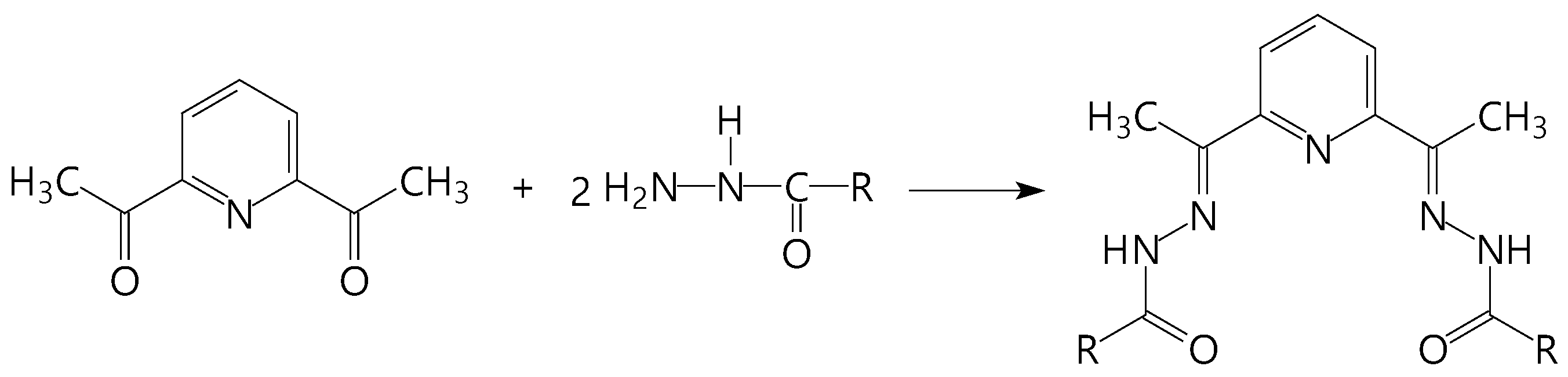
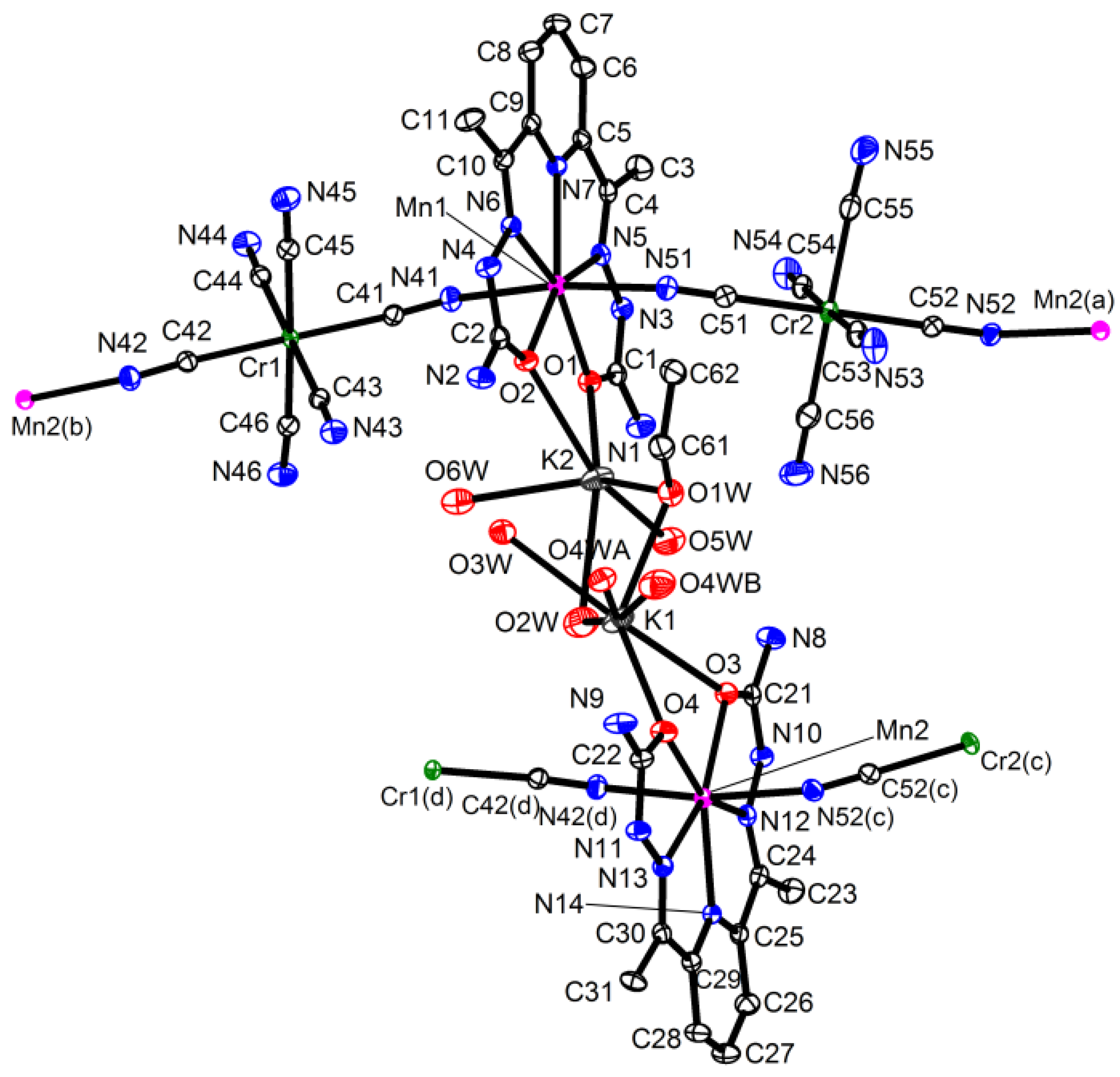
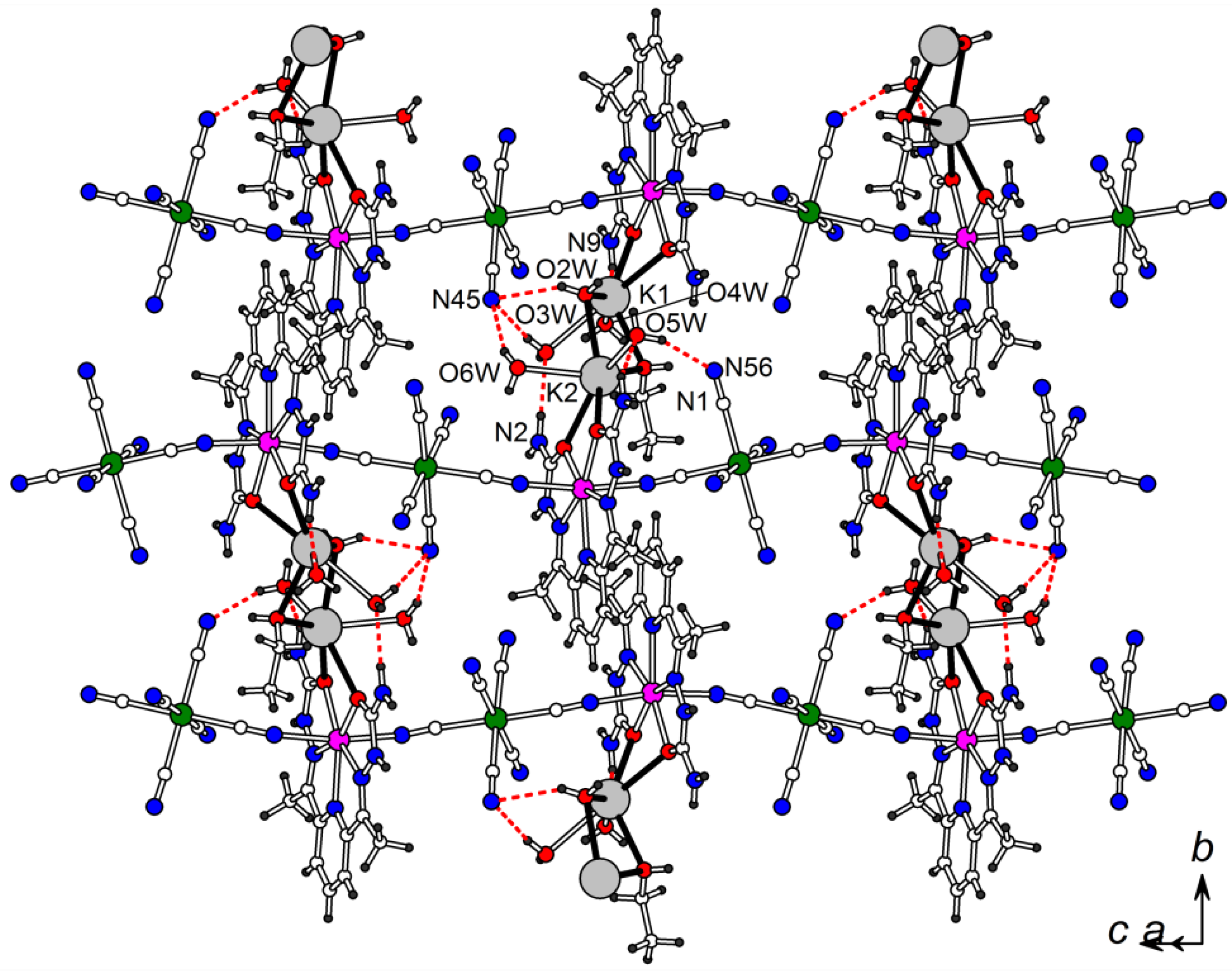
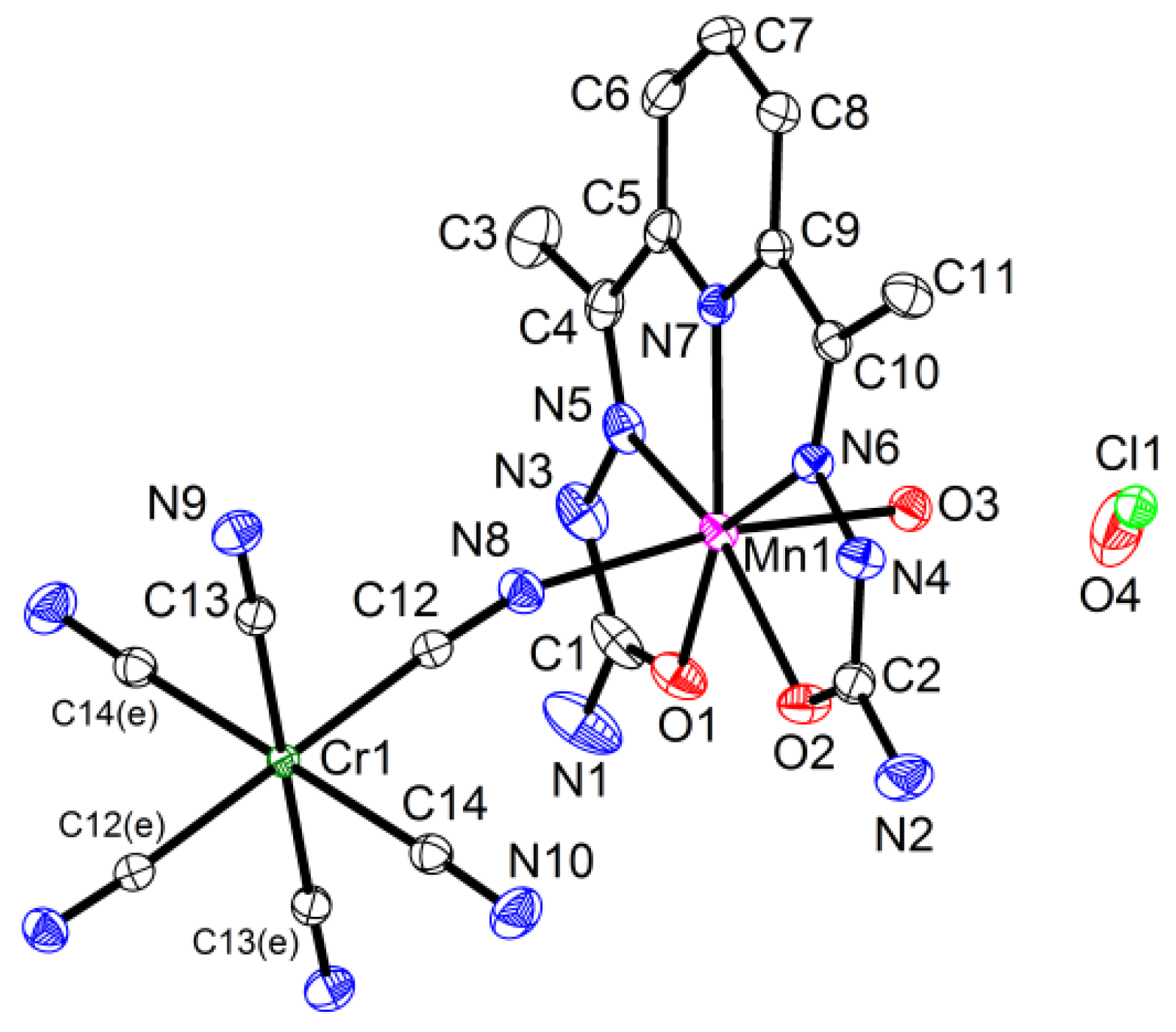
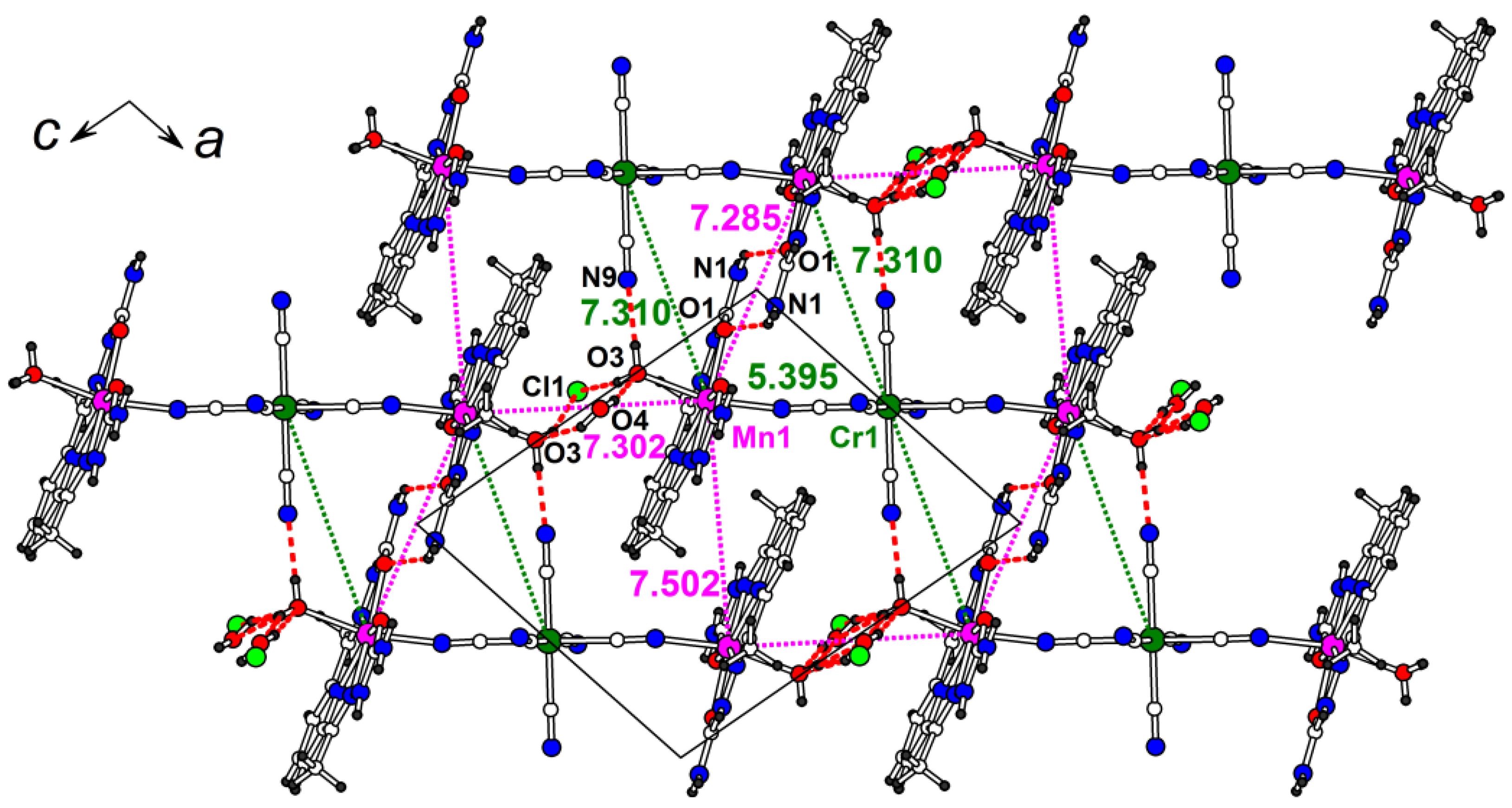
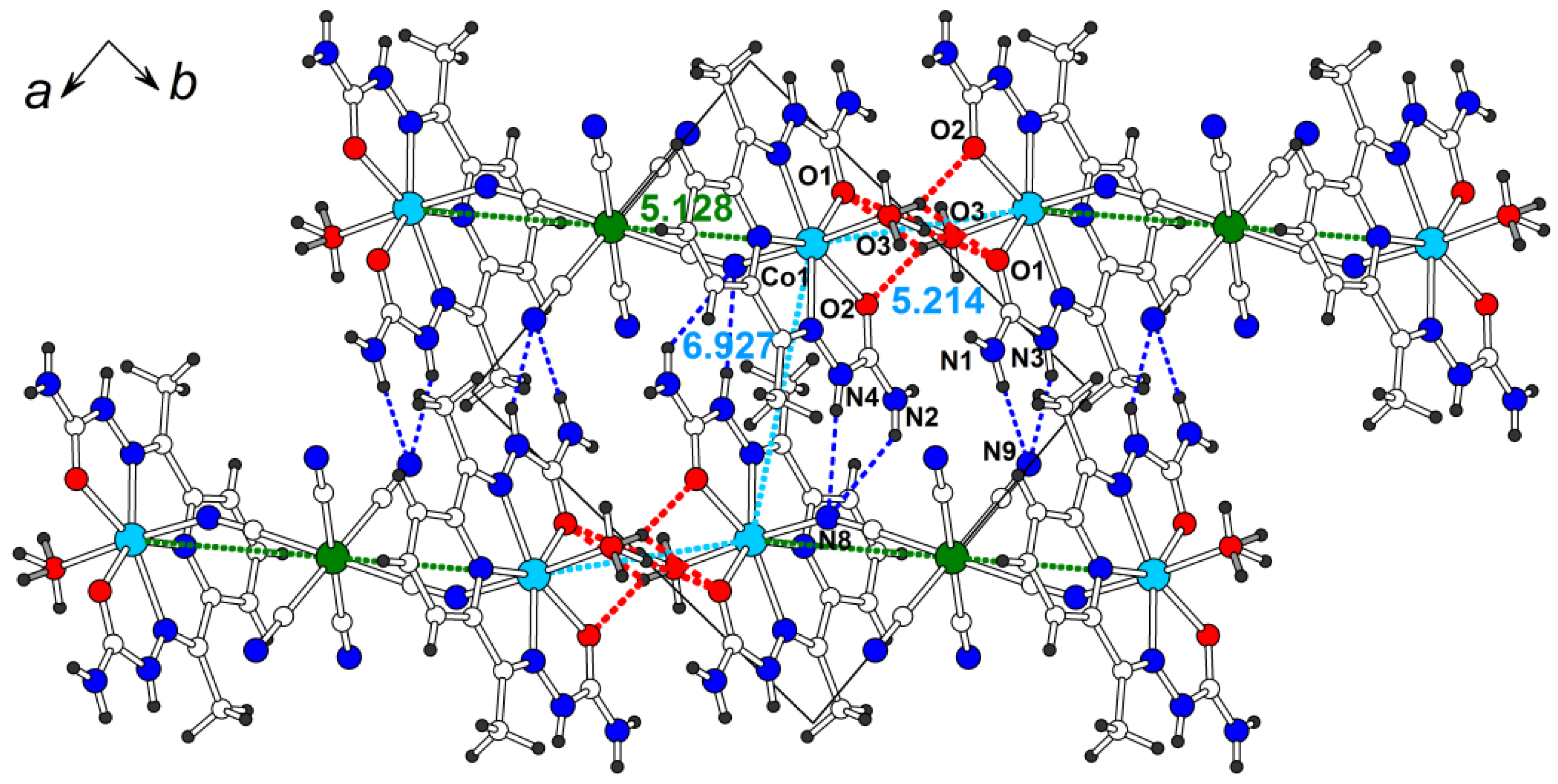
2.1. Synthesis and Crystal Structure
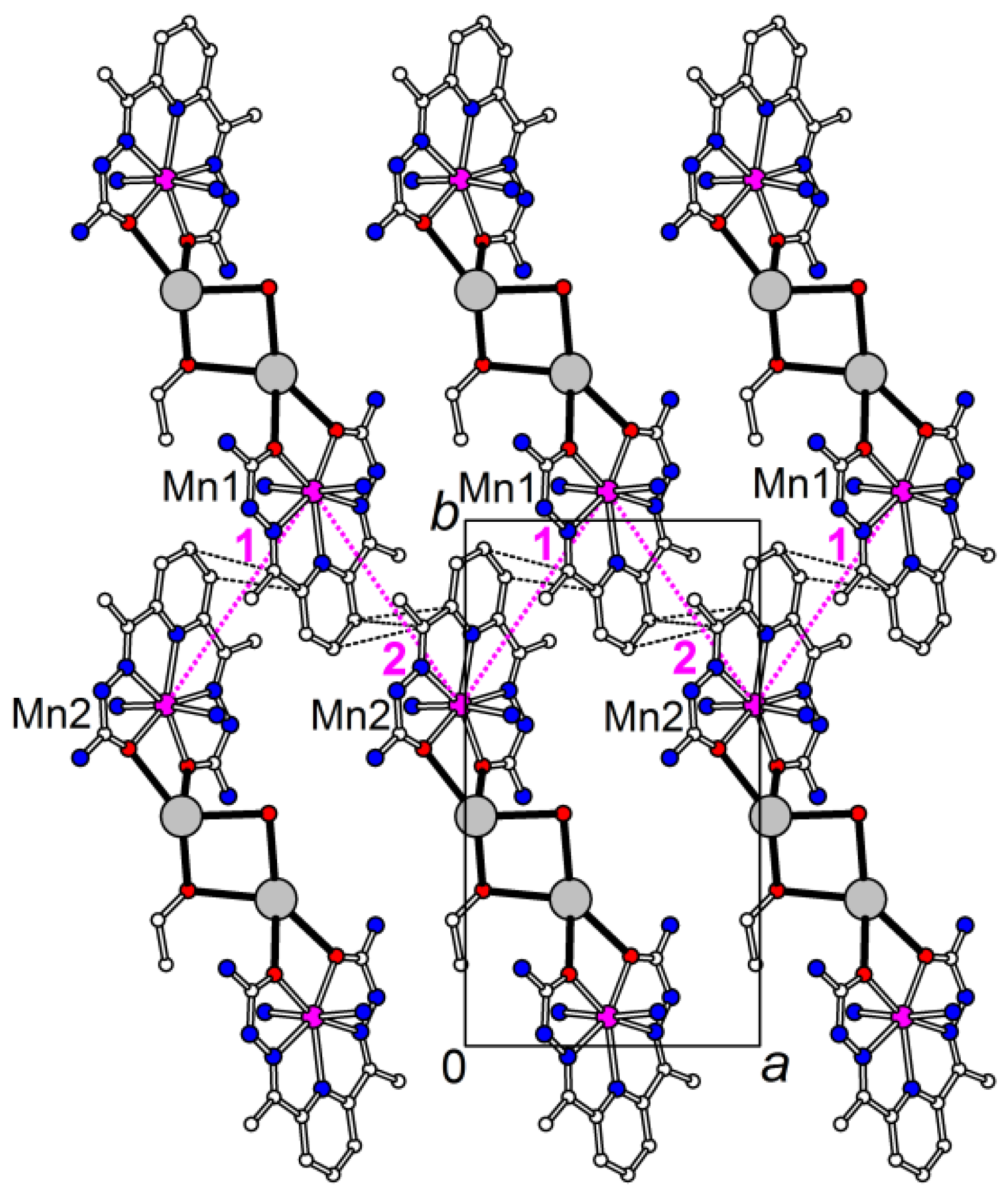
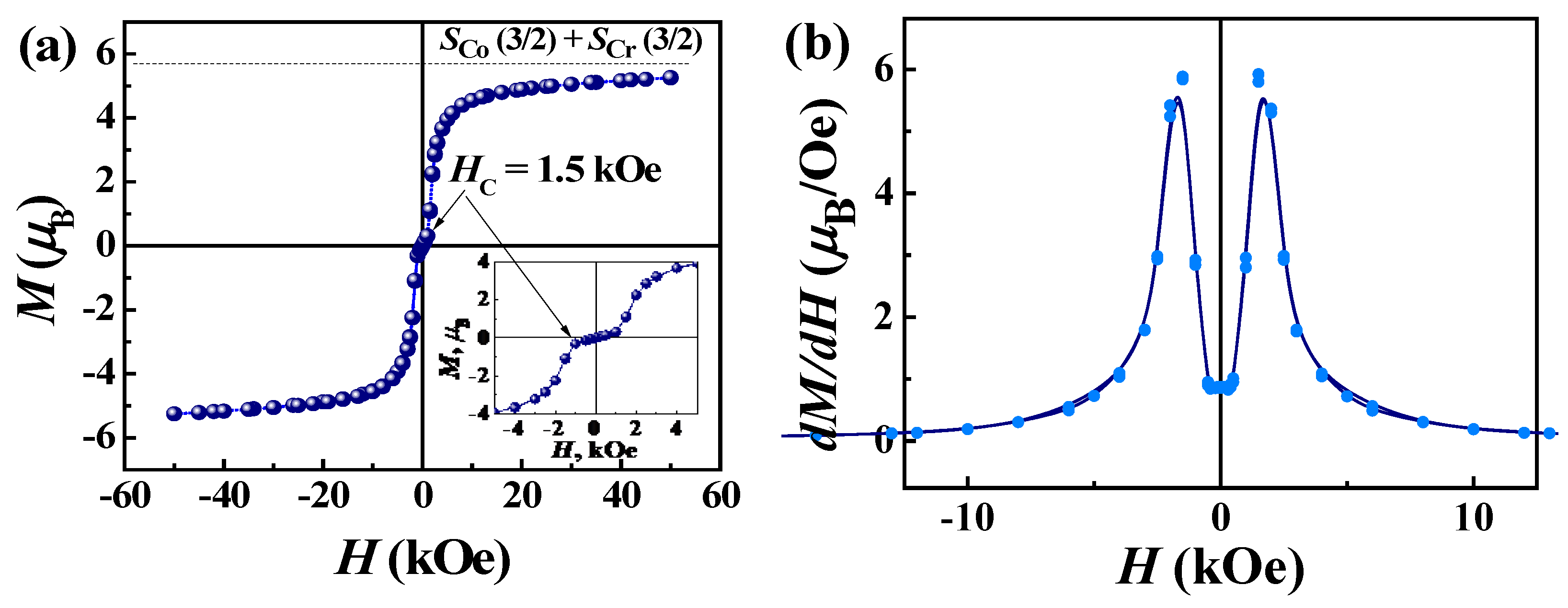
2.2. Magnetic Properties
2.2.1. Static (dc) Magnetic Properties
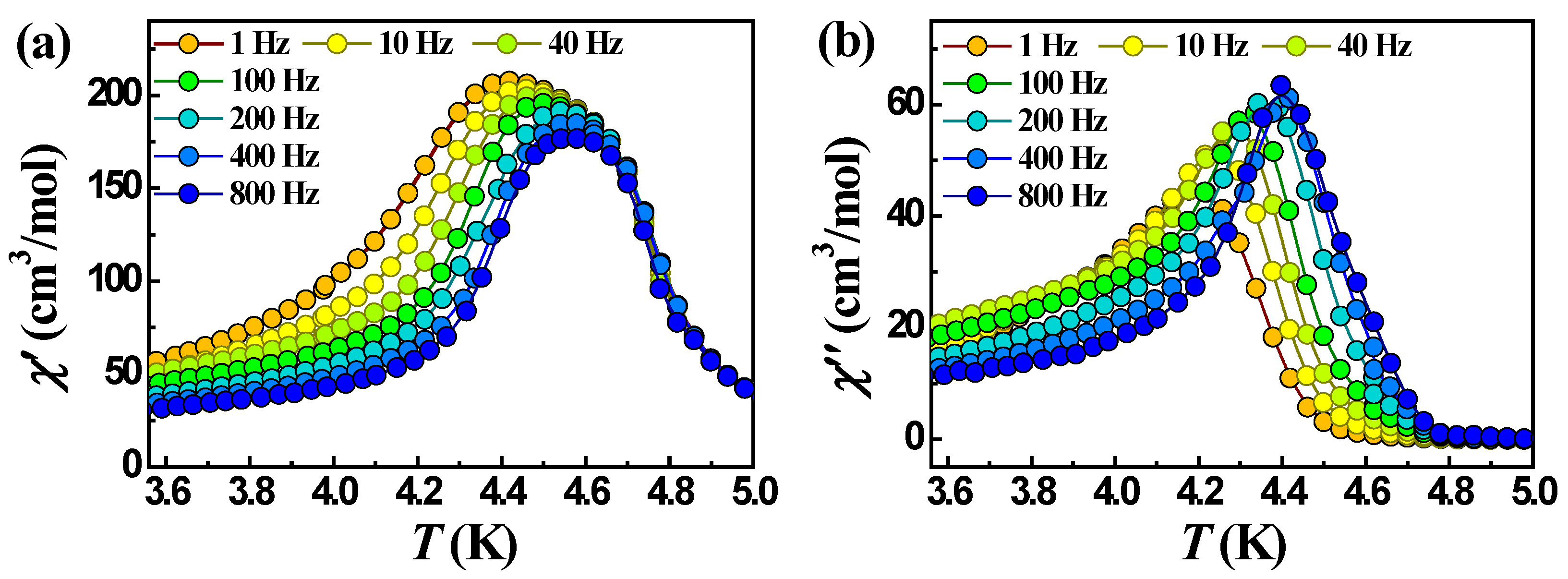
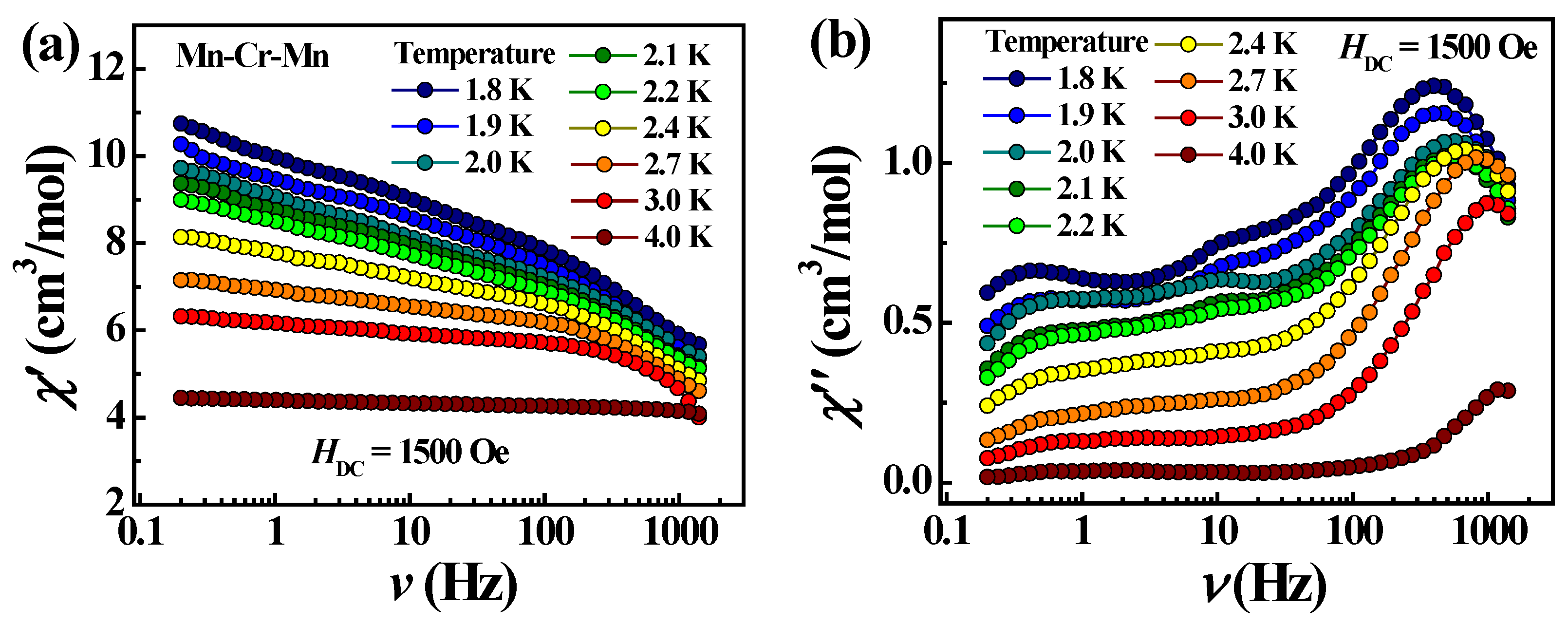
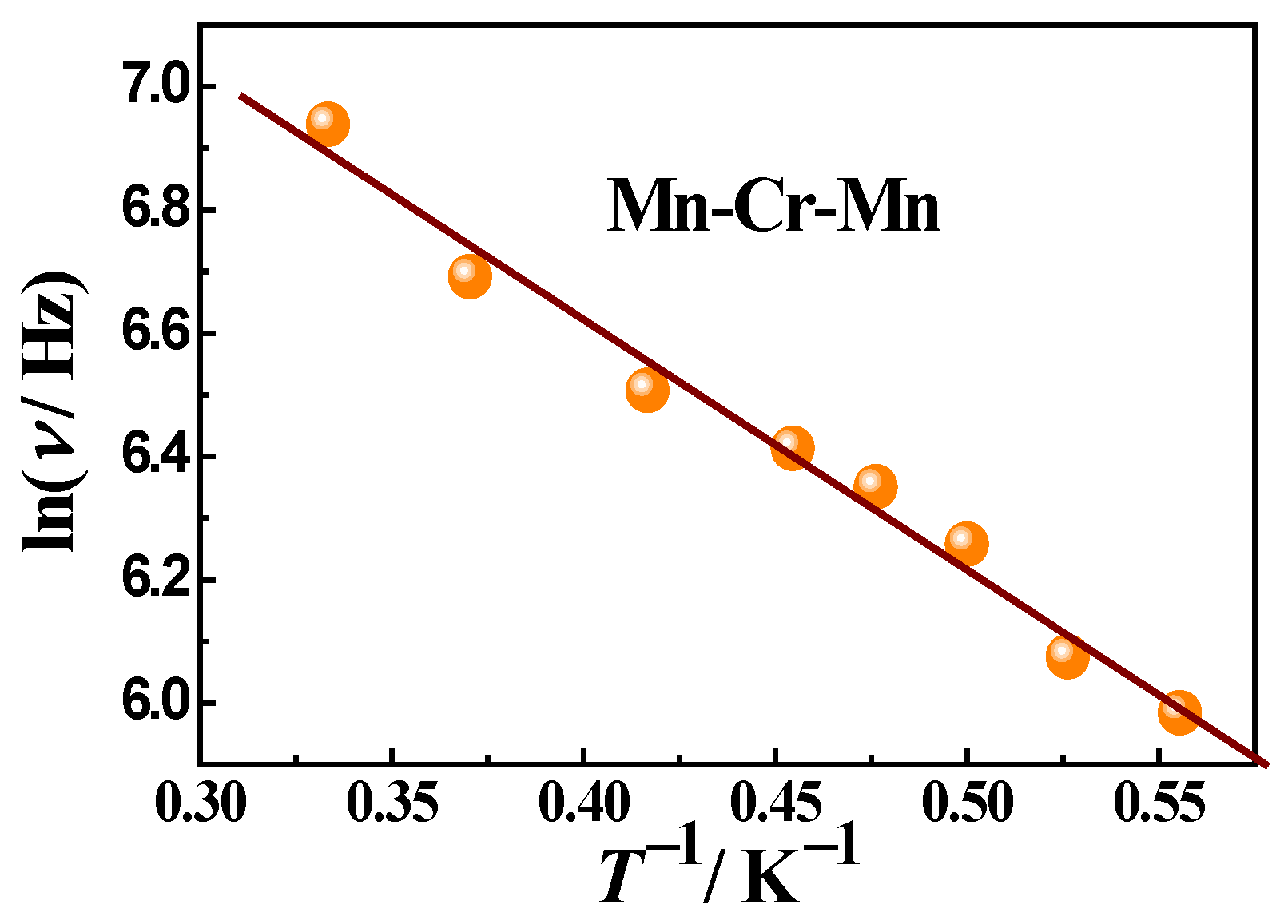
2.2.2. Dynamic (ac) Magnetic Properties
3. Materials and Methods
3.1. Synthesis
3.1.1. {[Mn(H2dapsc)]CrIII(CN)6K(H2O)2.5(EtOH)0.5}n·1.2n(H2O) (1)
3.1.2. {[Co(H2dapsc)]CrIII(CN)6K(H2O)2.5(EtOH)0.5}n·1.2n(H2O) (2)
3.1.3. {[Mn(H2dapsc)]2Cr(CN)6(H2O)2}Cl·H2O (3)
3.1.4. {[Co(H2dapsc)]2Cr(CN)6(H2O)2}Cl·2EtOH·3H2O (4)
3.2. Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| 1 | |||
|---|---|---|---|
| Mn(1)-O(1) | 2.244(2) | Mn(2)-O(3) | 2.272(2) |
| Mn(1)-O(2) | 2.255(2) | Mn(2)-O(4) | 2.218(2) |
| Mn(1)-N(5) | 2.291(2) | Mn(2)-N(12) | 2.274(2) |
| Mn(1)-N(6) | 2.311(2) | Mn(2)-N(13) | 2.310(2) |
| Mn(1)-N(7) | 2.306(3) | Mn(2)-N(14) | 2.282(3) |
| Mn(1)-N(41) | 2.210(3) | Mn(2)-N(42) d | 2.227(3) |
| Mn(1)-N(51) | 2.221(2) | Mn(2)-N(52) c | 2.214(3) |
| Cr(1)-CCN | 2.063(3)–2.080(3) | Cr(2)-CCN | 2.070(3)–2.076(3) |
| O(1)-Mn(1)-O(2) | 84.21(7) | O(3)-Mn(2)-O(4) | 83.08(7) |
| O(1)-Mn(1)-N(5) | 70.68(8) | O(3)-Mn(2)-N(12) | 70.41(8) |
| O(2)-Mn(1)-N(6) | 69.53(8) | O(4)-Mn(2)-N(13) | 69.76(8) |
| N(5)-Mn(1)-N(7) | 68.14(9) | N(12)-Mn(2)-N(14) | 68.77(9) |
| N(6)-Mn(1)-N(7) | 67.46(8) | N(13)-Mn(2)-N(14) | 67.98(9) |
| N(41)-Mn(1)-N(51) | 171.0(1) | N(42)d-Mn(2)-N(52) c | 170.0(1) |
| Mn(1)-N(41)-C(41) | 152.3(2) | Mn(2)-N(42)d-C(42) d | 151.8(2) |
| Mn(1)-N(51)-C(51) | 151.4(2) | Mn(2)-N(52)c-C(52)c | 149.6(2) |
| Cr(1)-C(41)-N(41) | 173.3(3) | Cr(1)-C(42)-N(42) | 173.1(3) |
| Cr(2)-C(51)-N(51) | 175.2(2) | Cr(2)-C(52)-N(52) | 175.9(2) |
| 2 | |||
| Co(1)-O(1) | 2.208(2) | Co(2)-O(3) | 2.243(2) |
| Co(1)-O(2) | 2.225(2) | Co(2)-O(4) | 2.189(2) |
| Co(1)-N(5) | 2.204(3) | Co(2)-N(12) | 2.185(3) |
| Co(1)-N(6) | 2.220(3) | Co(2)-N(13) | 2.218(3) |
| Co(1)-N(7) | 2.205(3) | Co(2)-N(14) | 2.178(3) |
| Co(1)-N(41) | 2.091(3) | Co(2)-N(42)d | 2.097(3) |
| Co(1)-N(51) | 2.099(3) | Co(2)-N(52)c | 2.098(3) |
| Cr(1)-CCN | 2.068(3)–2.081(4) | Cr(2)-CCN | 2.063(4)–2.079(4) |
| O(1)-Co(1)-O(2) | 77.5(1) | O(3)-Co(2)-O(4) | 76.4(1) |
| O(1)-Co(1)-N(5) | 71.9(1) | O(3)-Co(2)-N(12) | 71.6(1) |
| O(2)-Co(1)-N(6) | 70.8(1) | O(4)-Co(2)-N(13) | 71.2(1) |
| N(5)-Co(1)-N(7) | 70.3(1) | N(12)-Co(2)-N(14) | 70.8(1) |
| N(6)-Co(1)-N(7) | 69.5(1) | N(13)-Co(2)-N(14) | 70.0(1) |
| N(41)-Co(1)-N(51) | 170.2(1) | N(42)d-Co(2)-N(52) c | 170.6(1) |
| Co(1)-N(41)-C(41) | 158.3(3) | Co(2)-N(42)d-C(42) d | 160.9(3) |
| Co(1)-N(51)-C(51) | 155.2(3) | Co(2)-N(52)c-C(52)c | 154.9(3) |
| Cr(1)-C(41)-N(41) | 171.1(3) | Cr(1)-C(42)-N(42) | 170.4(3) |
| Cr(2)-C(51)-N(51) | 173.4(3) | Cr(2)-C(52)-N(52) | 173.3(3) |
| 3 | 4 | ||
| Mn(1)-O(1) | 2.205(1) | Co(1)-O(1) | 2.157(4) |
| Mn(1)-O(2) | 2.272(1) | Co(1)-O(2) | 2.168(3) |
| Mn(1)-N(5) | 2.301(1) | Co(1)-N(5) | 2.182(4) |
| Mn(1)-N(6) | 2.285(1) | Co(1)-N(6) | 2.181(4) |
| Mn(1)-N(7) | 2.323(1) | Co(1)-N(7) | 2.171(4) |
| Mn(1)-N(8) | 2.226(1) | Co(1)-N(8) | 2.106(4) |
| Mn(1)-O(3) | 2.265(1) | Co(1)-O(3) | 2.164(3) |
| Cr(1)-CCN | 2.069(1)–2.087(1) | Cr(1)-CCN | 2.058(5)–2.072(6) |
| O(1)-Mn(1)-O(2) | 85.12(4) | O(1)-Co(1)-O(2) | 74.8(1) |
| O(1)-Mn(1)-N(5) | 70.34(4) | O(1)-Co(1)-N(5) | 71.6(1) |
| O(2)-Mn(1)-N(6) | 69.26(4) | O(2)-Co(1)-N(6) | 72.3(1) |
| N(5)-Mn(1)-N(7) | 67.21(4) | N(5)-Co(1)-N(7) | 70.9(2) |
| N(6)-Mn(1)-N(7) | 67.75(4) | N(6)-Co(1)-N(7) | 70.5(1) |
| N(8)-Mn(1)-O(3) | 165.80(4) | N(8)-Co(1)-O(3) | 176.4(1) |
| Mn(1)-N(8)-C(12) | 164.7(1) | Co(1)-N(8)-C(12) | 151.3(4) |
| Cr(1)-C(12)-N(8) | 174.1(1) | Cr(1)-C(12)-N(8) | 176.9(4) |
References
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Benelli, C.; Gatteschi, D. Introduction to Molecular Magnetism; Wiley-VCH Verlag & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar]
- Dey, A.; Kalita, P.; Chandrasekhar, V. Lanthanide(III)-based single-ion magnets. ACS Omega 2018, 3, 9462–9475. [Google Scholar] [CrossRef] [PubMed]
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Aromí, G.; Aguilà, D.; Gamez, P.; Luis, F.; Roubeau, O. Design of magnetic coordination complexes for quantum computing. Chem. Soc. Rev. 2012, 41, 537–546. [Google Scholar] [CrossRef]
- Bartolomé, S.J.; Luis, F.; Fernández, J.F. (Eds.) Molecular Magnets: Physics and Applications; Springer: New York, NY, USA, 2014. [Google Scholar]
- Goodwin, C.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature 2017, 548, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhao, C.; Feng, T.; Liu, X.; Ying, X.; Li, X.-L.; Zhang, Y.-Q.; Tang, J. Air-stable chiral single-molecule magnets with record anisotropy barrier exceeding 1800 K. J. Am. Chem. Soc. 2021, 143, 10077–10082. [Google Scholar] [CrossRef]
- Mironov, V.; Bazhenova, T.; Manakin, Y.; Yagubskii, E. Pentagonal-bipyramidal 4d and 5d complexes with unquenched orbital angular momentum as a unique platform for advanced single-molecule magnets: Current state and perspectives. Dalton Trans. 2022, in press. [Google Scholar]
- Craig, G.A.; Murrie, M. 3d single-ion magnets. Chem. Soc. Rev. 2015, 44, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Avendaño, C.; Dunbar, K.R. Molecular magnetic materials based on 4d and 5d transition metals. Chem. Soc. Rev. 2011, 40, 3213–3238 and references therein. [Google Scholar] [CrossRef]
- Layfield, R.A.; Murugesu, M. Lanthanides and Actinides in Molecular Magnetism; Wiley-VCH Verlag & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar]
- Bar, A.K.; Pichon, C.; Sutter, J.-P. Magnetic anisotropy in two- to eight-coordinated transition-metal complexes: Recent developments in molecular magnetism. Coord. Chem. Rev. 2016, 308, 346–380. [Google Scholar] [CrossRef]
- Boča, R. Zero-field splitting in metal complexes. Coord. Chem. Rev. 2004, 248, 757–815. [Google Scholar] [CrossRef]
- Rebilly, J.-N.; Charron, G.; Rivière, E.; Guillot, R.; Barra, A.-L.; Serrano, M.D.; van Slageren, J.; Mallah, T. Large magnetic anisotropy in pentacoordinate NiII complexes. Chem. Eur. J. 2008, 14, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Wang, B.-W.; Sato, O.; Gao, S. First Fe(II)-based cyano-bridged single molecule magnet [CrIIIFeII] with a large anisotropy. Chem. Commun. 2010, 46, 6959–6961. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Gómes-Coca, S.; Cremades, E.; Aliaga-Alcaldeand, N.; Ruiz, E. Mononuclear single-molecule magnets: Tailoring the magnetic anisotropy of first-row transition-metal complexes. J. Am. Chem. Soc. 2013, 135, 7010–7018. [Google Scholar] [CrossRef]
- Meng, Y.-S.; Jiang, S.D.; Wang, B.-W.; Gao, S. Understanding the magnetic anisotropy toward single-ion magnets. Acc. Chem. Res. 2016, 49, 2381–2389. [Google Scholar] [CrossRef]
- Saberand, M.R.; Dunbar, K.R. Ligands effects on the magnetic anisotropy of tetrahedral cobalt complexes. Chem. Commun. 2014, 50, 12266–12269. [Google Scholar]
- Huang, X.-C.; Zhou, C.; Shao, D.; Wang, X.-Y. Field-induced slow magnetic relaxation in cobalt(II) compounds with pentagonal bipyramid geometry. Inorg. Chem. 2014, 53, 12671–12673. [Google Scholar] [CrossRef]
- Bar, A.K.; Gogoi, N.; Pichon, C.; Goli, D.P.; Thlijeni, M.; Duhayon, C.; Suaud, N.; Guihéry, N.; Barra, A.L.; Ramasesha, S.; et al. Pentagonal bipyramid FeII complexes: Robust Ising-spin units towards heteropolynuclear nanomagnets. Chem. Eur. J. 2017, 23, 4380–4396. [Google Scholar] [CrossRef]
- Gogoi, N.; Thlijeni, M.; Duhayon, C.; Sutter, J.-P. Heptacoordinated nickel(II) as an Ising-type anisotropic building unit: Illustration with a pentanuclear [(NiL)3{W(CN)8}2] complex. Inorg. Chem. 2013, 52, 2283–2285. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Liu, J.-L.; Ungur, L.; Liu, J.; Li, Q.-W.; Wang, L.-F.; Ni, Z.-P.; Chibotaru, L.F.; Chen, X.-M.; Tong, M.-L. Symmetry-supported magnetic blocking at 20 K in pentagonal bipyramidal Dy(III) single-ion magnets. J. Am. Chem. Soc. 2016, 138, 2829–2837. [Google Scholar] [CrossRef] [PubMed]
- Ruamps, R.; Batchelor, L.J.; Maurice, R.; Gogoi, N.; Jiménez-Lozano, P.; Guihéry, N.; de Graaf, C.; Barra, A.L.; Sutter, J.-P.; Mallah, T. Origin of the magnetic anisotropy in heptacoordinate NiII and CoII complexes. Chem. Eur. J. 2013, 19, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Gavey, E.L.; Beldjoudi, Y.; Rawson, J.M.; Stamatatosand, T.C.; Pilkington, M. Slow relaxation in the first penta-aza Dy(iii) macrocyclic complex. Chem. Commun. 2014, 50, 3741–3743. [Google Scholar] [CrossRef]
- Unger, L.; Chibotaru, L.F. Strategies toward high-temperature lantanide-based single-molecule magnets. Inorg. Chem. 2016, 55, 10043–10056. [Google Scholar] [CrossRef] [PubMed]
- Palenik, G.J.; Wester, D.W.; Rychlewska, U.; Palenik, R.C. Pentagonal-bipyramidal complexes. Synthesis and crystal structures of diaqua [2,6-diacetylpyridine bis(semicarbazone)]chromium(III) hydroxide dinitrate hydrate and dichloro[2,6-diacetylpyridine bis(semicarbazone)]iron(III) chloride dihydrate. Inorg. Chem. 1976, 15, 1814–1819. [Google Scholar] [CrossRef]
- Palenik, G.J.; Wester, D.W. Pentagonal-bipyramidal complexes. Crystal and molecular structures of chloroaqua(2,6-diacetylpyridine bis(semicarbazone))manganese(II), -iron(II), -cobalt(II), and -zinc(II) chloride dihydrates. Inorg. Chem. 1978, 17, 864–870. [Google Scholar] [CrossRef]
- Giordano, T.J.; Palenik, G.J.; Palenik, R.C.; Sullivan, D.A. Pentagonal-bipyramidal complexes. Synthesis and characterization of aqua(nitrato)[2,6-diacetylpyridine bis(benzoyl hydrazone)]cobalt(II) nitrate and diaqua[2,6-diacetylpyridine bis(benzoyl hydrazone)]nickel(II) nitrate dihydrate. Inorg. Chem. 1979, 18, 2445–2450. [Google Scholar] [CrossRef]
- Gerloch, M.; Morgenstern-Badarau, I. Magnetic and spectral properties of chloroaqua[2,6-diacetylpyridinebis(semicarbazone)]iron(II) and diaqua[2,6-diacetylpyridinebis(semicarbazone)]nickel(II): Ligand fields and bonding in pentagonal-bipyramidal complexes. Inorg. Chem. 1979, 18, 3225–3229. [Google Scholar] [CrossRef]
- Lorenzini, C.; Pelizzi, C.; Pelizzi, G.; Predieri, G. Investigation into aroylhydrazones as chelating agents. Part 3. Synthesis and spectroscopic characterization of complexes of MnII, CoII, NiII, CuII, and ZnII with 2,6-diacetylpyridine bis(benzoylhydrazone) and X-ray structure of aquachloro[2,6-diacetylpyridine bis(benzoylhydrazone)]manganese(II) chloride. J. Chem. Soc. Dalton Trans. 1983, 721–727. [Google Scholar] [CrossRef]
- Ianelli, S.; Pelizzi, C.; Pelizzi, G.; Tarasconi, P. Heptacoordination in MnII, NiII, and CuII complexes of 2,6-diacetylpyridine bis(acetylhydrazone). J. Chem. Crystallogr. 1996, 26, 195–201. [Google Scholar] [CrossRef]
- Carcelli, M.; Ianelli, S.; Pelagatti, P.; Pelizzi, G. Structural characterization of a new ligand mode of 2,6-diacetylpyridine bis(semicarbazone), H2daps. Inorg. Chim. Acta 1999, 292, 121–126. [Google Scholar] [CrossRef]
- Ivanović-Burmazović, I.; Andjelković, K. Transition metal complexes with bis(hydrazone) ligands of 2,6-diacetylpyridine. Hepta-coordination of 3d metals. Adv. Inorg. Chem. 2004, 55, 315–360. [Google Scholar]
- Pichon, C.; Elrez, B.; Béreau, V.; Duhayon, C.; Sutter, J.-P. From heptacoordinated CrIII complexes with cyanide or isothiocyanate apical groups to 1D heterometallic assemblages with all-pentagonal-bipyramid coordination geometries. Eur. J. Inorg. Chem. 2018, 2018, 340–348. [Google Scholar] [CrossRef]
- Bazhenova, T.A.; Zorina, L.V.; Simonov, S.V.; Mironov, V.S.; Maximova, O.V.; Spillecke, L.; Koo, C.; Klingeler, R.; Manakin, Y.V.; Vasiliev, A.N.; et al. The first pentagonal-bipyramidal vanadium(III) complexes with a Schiff-base N3O2 pentadentate ligand: Synthesis, structure and magnetic properties. Dalton Trans. 2020, 49, 15287–15298. [Google Scholar] [CrossRef]
- Bazhenova, T.A.; Zorina, L.V.; Simonov, S.V.; Manakin, Y.V.; Kornev, A.B.; Lyssenko, K.A.; Mironov, V.S.; Gilmutdinov, I.F.; Yagubskii, E.B. A novel family of hepta-coordinated Cr(III) complexes with a planar pentadentate N3O2 Schiff base ligand: Synthesis, structure and magnetism. Inorg. Chim. Acta 2021, 522, 120358. [Google Scholar] [CrossRef]
- Bar, A.K.; Kalita, P.; Sutter, J.-P.; Chandrasekhar, V. Pentagonal-bipyramid Ln(III) complexes exhibiting single-ion-magnet behavior: A rational synthetic approach for a rigid equatorial plane. Inorg. Chem. 2018, 57, 2398–2401. [Google Scholar] [CrossRef]
- Mironov, V.S.; Bazhenova, T.A.; Manakin, Y.V.; Lyssenko, K.A.; Talantsev, A.D.; Yagubskii, E.B. A new Mo(IV) complex with the pentadentate (N3O2) Schiff-base ligand: The first non-cyanide pentagonal-bipyramidal paramagnetic 4d complex. Dalton Trans. 2017, 46, 14083–14087. [Google Scholar] [CrossRef]
- Manakin, Y.V.; Mironov, V.S.; Bazhenova, T.A.; Lyssenko, K.A.; Gilmutdinov, I.F.; Bikbaev, K.S.; Masitov, A.A.; Yagubskii, E.B. (Et4N)[MoIII(DAPBH)Cl2], the first pentagonal-bipyramidal Mo(III) complex with a N3O2-type Schiff-base ligand: Manifestation of unquenched orbital momentum and Ising-type magnetic anisotropy. Chem. Commun. 2018, 54, 10084–10087. [Google Scholar] [CrossRef]
- Bazhenova, T.A.; Kopotkov, V.A.; Korchagin, D.V.; Manakin, Y.V.; Zorina, L.V.; Simonov, S.V.; Yakushev, I.A.; Mironov, V.S.; Vasiliev, A.N.; Maximova, O.V.; et al. A series of novel pentagonal-bipyramidal erbium(III) complexes with acyclic chelating N3O2 Schiff-base ligands: Synthesis, structure, and magnetism. Molecules 2021, 26, 6908. [Google Scholar] [CrossRef]
- Spillecke, L.; Koo, C.; Maximova, O.; Mironov, V.S.; Kopotkov, V.A.; Korchagin, D.V.; Vasiliev, A.N.; Yagubskii, E.B.; Klingeler, R. Magnetic behavior of the novel pentagonal-bipyramidal erbium(III) complex (Et3NH)[Er(H2DAPS)Cl2]: High-frequency EPR study and crystal-field analysis. Dalton Trans. 2021, 50, 18143–18154. [Google Scholar] [CrossRef]
- Kalita, P.; Ahmed, N.; Bar, A.K.; Dey, S.; Jana, A.; Rajaraman, G.; Sutter, J.-P.; Chandrasekhar, V. Pentagonal bipyramidal Ln(III) complexes containing an axial phosphine oxide ligand: Field-induced single-ion magnetism behavior of the Dy(III) analogues. Inorg. Chem. 2020, 59, 6603–6612. [Google Scholar] [CrossRef]
- Mondal, A.K.; Mondal, A.; Dey, B.; Konar, S. Influence of the coordination environment on easy-plane magnetic anisotropy of pentagonal bipyramidal cobalt(II) complexes. Inorg. Chem. 2018, 57, 9999–10008. [Google Scholar] [CrossRef] [PubMed]
- Kopotkov, V.A.; Korchagin, D.V.; Sasnovskaya, V.D.; Gilmutdinov, I.F.; Yagubskii, E.B. A series of field-induced single-ion magnets based on the seven-coordinate Co(II) complexes with pentadentate (N3O2) H2dapsc ligand. Magnetochemistry 2019, 5, 58. [Google Scholar] [CrossRef]
- Mondal, A.K.; Mondal, A.; Konar, S. Slow magnetic relaxation in a one-dimensional coordination polymer constructed from hepta-coordinate cobalt(II) nodes. Magnetochemistry 2020, 6, 45. [Google Scholar] [CrossRef]
- Batchelor, L.J.; Sangalli, M.; Guillot, R.; Guihéry, N.; Maurice, R.; Tuna, F.; Mallah, T. Pentanuclear cyanide-bridged complexes based on highly anisotropic CoII seven-coordinate building blocks: Synthesis, structure, and magnetic behavior. Inorg. Chem. 2011, 50, 12045–12052. [Google Scholar] [CrossRef]
- Sasnovskaya, V.D.; Kopotkov, V.A.; Talantsev, A.D.; Morgunov, R.B.; Yagubskii, E.B.; Simonov, S.V.; Zorina, L.V.; Mironov, V.S. Synthesis, structure, and magnetic properties of 1D {[MnIII(CN)6][MnII(dapsc)]}n coordination polymers: Origin of unconventional single-chain magnet behavior. Inorg. Chem. 2017, 56, 8926–8943. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Li, Z.-Y.; Yamashita, M.; Bu, X.-H. Recent progress on cyano-bridged transition-metal-based single-molecule magnets and single-chain magnets. Coord. Chem. Rev. 2021, 428, 213617. [Google Scholar] [CrossRef]
- Zorina, L.V.; Simonov, S.V.; Sasnovskaya, V.D.; Talantsev, A.D.; Morgunov, R.B.; Mironov, V.S.; Yagubskii, E.B. Slow magnetic relaxation, antiferromagnetic ordering, and metamagnetism in MnII(H2dapsc)-FeIII(CN)6 chain complex with highly anisotropic Fe-CN-Mn spin coupling. Chem. Eur. J. 2019, 25, 14583–14597. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.; Sarma, B.; Gogoi, N. Cyano bridged heterometallic Mn(II)-Fe(III) aggregates: Synthesis, structure and magnetic properties. Inorg. Chim. Acta 2018, 469, 20–24. [Google Scholar] [CrossRef]
- Pichon, C.; Suaud, N.; Duhayon, C.; Guihéry, N.; Sutter, J.-P. Cyano-bridged Fe(II)−Cr(III) single-chain magnet based on pentagonal bipyramid units: On the added value of aligned axial anisotropy. J. Am. Chem. Soc. 2018, 140, 7698–7704. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Pichon, C.; Sutter, J.-P.; Pamu, D.; Sarma, B.; Mudoia, P.P.; Gogoi, N. Accessing water processable cyanido bridged chiral heterobimetallic Co(II)–Fe(III) one dimensional network. Chem. Commun. 2021, 57, 207. [Google Scholar] [CrossRef]
- Bretosh, K.; Béreau, V.; Duhayon, C.; Pichon, C.; Sutter, J.-P. A ferromagnetic Ni(II)–Cr(III) single-chain magnet based on pentagonal bipyramidal building units. Inorg. Chem. Front. 2020, 7, 1503–1511. [Google Scholar] [CrossRef]
- Mallah, T.; Auberger, C.; Verdaguer, M.; Veillet, P. A heptanuclear CrIIINiII6 complex with a low-lying S = 15/2 ground state. Chem. Commun. 1995, 61–62. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Sato, O. A cyano-bridged CrIIICoII ferromagnet with a chiral nanotubular structure constituted of interlocked single and double helices. Inorg. Chem. 2010, 49, 1271–1273. [Google Scholar] [CrossRef] [PubMed]
- Mabbs, F.; Machin, D. Magnetism and Transition Metal Complexes, 2nd ed.; Dover Publications Inc.: New York, NY, USA, 2008; pp. 1–206. [Google Scholar]
- Zheng, Y.-Z.; Xue, W.; Tong, M.-L.; Chen, X.-M.; Grandjean, F.; Long, G.J. A two-dimensional iron(II) carboxylate linear chain polymer that exhibits a metamagnetic spin-canted antiferromagnetic to single-chain magnetic transition. Inorg. Chem. 2008, 47, 4077–4087. [Google Scholar] [CrossRef] [PubMed]
- Wöhlert, S.; Boeckmann, J.; Wriedt, M.; Näther, C. Coexistence of metamagnetism and slow relaxation of the magnetization in a cobalt thiocyanate 2D coordination network. Angew. Chem. Int. Ed. 2011, 50, 6920–6923. [Google Scholar] [CrossRef]
- Wöhlert, S.; Tomkowicz, Z.; Rams, M.; Ebbinghaus, S.G.; Fink, L.; Schmidt, M.U.; Näther, C. Influence of the co-ligand on the magnetic and relaxation properties of layered cobalt(II) thiocyanato coordination polymers. Inorg. Chem. 2014, 53, 8298–8310. [Google Scholar] [CrossRef]
- Ni, W.-W.; Ni, Z.-H.; Cui, A.-L.; Liang, X.; Kou, H.-Z. Cyanide-bridged Mn(III)−Fe(III) bimetallic complexes based on the pentacyano(1-methylimidazole)ferrate(III) building block: structure and magnetic characterizations. Inorg. Chem. 2007, 46, 22–33. [Google Scholar] [CrossRef]
- Li, X.-B.; Ma, Y.; Gao, E.-Q. Random Co(II)–Ni(II) ferromagnetic chains showing coexistent antiferromagnetism, metamagnetism, and single-chain magnetism. Inorg. Chem. 2018, 57, 7446–7454. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhang, Y.; Jiao, C.; Zhao, L.; Hu, J.; Wang, J.; Liu, T. Coexistence of metamagnetism and single chain magnet behavior in a FeIII2CoII layer compound. Sci. China Chem. 2016, 59, 735–739. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Chorazy, S.; Takahashi, D.; Kinoshita, T.; Sieklucka, B.; Ohkoshi, S.-I. Cesium cyano-bridged CoII−MV (M = Mo and W) layered frameworks exhibiting high thermal durability and metamagnetism. Cryst. Growth Des. 2014, 14, 6093–6100. [Google Scholar] [CrossRef]
- Wen, H.-R.; Tang, Y.-Z.; Liu, C.-M.; Chen, J.-L.; Yu, C.-L. One-dimensional homochiral cyano-bridged heterometallic chain coordination polymers with metamagnetic or ferroelectric properties. Inorg. Chem. 2009, 48, 10177–10185. [Google Scholar] [CrossRef]
- Bonadio, F.; Senna, M.-C.; Ensling, J.; Sieber, A.; Neels, A.; Stoeckli-Evans, H.; Decurtins, S. Cyano-bridged structures based on [MnII(N3O2-macrocycle)]2+: a synthetic, structural, and magnetic study. Inorg. Chem. 2005, 44, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Mydosh, J.A. Spin Glasses: An Experimental Introduction; Taylor & Francis: Washington, DC, USA, 1993. [Google Scholar]
- Binder, K.; Young, A.P. Spin glasses: Experimental facts, theoretical concepts, and open questions. Rev. Mod. Phys. 1986, 58, 801–976. [Google Scholar] [CrossRef]
- Rebilly, J.-N.; Mallah, T. Synthesis of single-molecule magnets using metallocyanates. Struct. Bonding 2006, 122, 103–131. [Google Scholar]
- Kim, J.I.; Kwak, H.Y.; Yoon, J.H.; Ryu, D.W.; Yoo, I.Y.; Yang, N.; Cho, B.K.; Park, J.G.; Lee, H.; Hong, C.S. Cyanide-bridged FeIII−MnIII bimetallic complexes with dimeric and chain structures constructed from a newly made mer-Fe tricyanide: Structures and magnetic properties. Inorg. Chem. 2009, 48, 2956–2966. [Google Scholar] [CrossRef]
- Liu, X.; Cen, P.; Li, H.; Ke, H.; Zhang, S.; Wei, Q.; Xie, G.; Chen, S.; Gao, S. Solvent-induced syntheses, crystal structures, magnetic properties, and single-crystal-to-single-crystal tansformation of azido-Cu(II) coordination polymers with 2-naphthoic acid as co-ligand. Inorg. Chem. 2014, 53, 8088–8097. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.-J.; Hu, J.-X.; Jiao, C.-Q.; Wang, J.-L.; Duan, C.-Y.; Liu, T. Coexistence of the single chain magnet and spin-glass behavior in a cyano-bridged {FeIII2FeII} chain. Inorg. Chem. Commun. 2016, 66, 55–58. [Google Scholar] [CrossRef]
- Yao, M.-X.; Zheng, Q.; Cai, X.-M.; Li, Y.-Z.; Song, Y.; Zuo, J.-L. Chiral cyanide-bridged CrIII−MnIII heterobimetallic chains based on [(Tp)Cr(CN)3]−: Synthesis, structures, and magnetic properties. Inorg. Chem. 2012, 51, 2140–2149. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, R.; Zhuang, P.-F.; Zheng, H.; Jiao, C.-Q.; Wang, J.-L.; He, C.; Liu, T. 12-Metal 36-membered ring based WV–CoII layers showing spin-glass behavior. Dalton. Trans. 2015, 44, 12613–12617. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Shatruk, M.; Bertolasi, V.; Pramanik, K.; Ray, D. Self-assembled tetra- and pentanuclear nickel(II) aggregates from phenoxido-based ligand bound {Ni2} fragments: Carboxylate bridge controlled structures. Inorg. Chem. 2013, 52, 13894–13903. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Jyoti; Robinson, W.T.; Singh, K. Tetracyanoquinodimethane derivatives of pentagonal bipyramidal complexes of manganese(II), iron(II), nickel(II) and copper(II) with 2,6-diacetylpyridinebis(semicarbazone): Single crystal structure of dichloro [2,6-diacetylpyridinebis (semicarbazone)] manganese(II)monohydrate. J. Coord. Chem. 2002, 55, 281–285. [Google Scholar]
- Rigaku Oxford Diffraction Ltd. CrysAlisPro, Version 1.171.38; Rigaku Oxford Diffraction Ltd.: Oxford, UK, 2015.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Manakin, Y.V.; Mironov, V.S.; Bazhenova, T.A.; Yakushev, I.A.; Gilmutdinov, I.F.; Simonov, S.V.; Yagubskii, E.B. (Et4N)[WIII(DAPBH)(CN)2], the first pentagonal-bipyramidal W(III) complex with unquenched orbital angular momentum: A novel Ising-type magnetic building block for single-molecule magnets. Chem. Comm. 2022; in press. [Google Scholar]


| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Chemical formula | C18H25.4CrKMnN13O6.2 | C18H25.4CoCrKN13O6.2 | C28H36ClCrMn2N20O7 | C32H52ClCo2CrN20O11 |
| Formula weight | 669.15 | 673.14 | 962.10 | 1098.24 |
| Cell setting | monoclinic | monoclinic | monoclinic | triclinic |
| Space group, Z | P21, 4 | P21, 4 | P21/c, 2 | , 1 |
| Temperature (K) | 120(1) | 120(1) | 150(1) | 150(1) |
| a (Å) | 9.3525(2) | 9.4213(1) | 10.5837(4) | 9.7054(10) |
| b (Å) | 16.6438(3) | 16.5874(2) | 15.2401(5) | 11.2166(14) |
| c (Å) | 18.5601(3) | 18.1419(2) | 12.3806(4) | 11.4125(13) |
| α (o) | 90 | 90 | 90 | 71.915(11) |
| β (o) | 90.709(1) | 90.428(1) | 103.326(4) | 88.039(9) |
| γ (o) | 90 | 90 | 90 | 85.191(9) |
| Cell volume (Å3) | 2888.86(8) | 2835.05(6) | 1943.18(12) | 1176.8(2) |
| ρ (g/cm3) | 1.539 | 1.577 | 1.644 | 1.550 |
| μ, cm−1 | 10.15 | 11.74 | 10.56 | 10.56 |
| Refls colld/unique | 41,136/14,870 | 32,709/14,858 | 14,493/5499 | 9774/5136 |
| Rint | 0.0194 | 0.0280 | 0.0198 | 0.0924 |
| θmax (o) | 29.00 | 29.00 | 29.00 | 26.00 |
| Parameters refined | 814 | 826 | 312 | 341 |
| R1, wR2 [I > 2σ(I)] | 0.0285, 0.0695 | 0.0328, 0.0718 | 0.0292, 0.0671 | 0.0932, 0.2297 |
| Goodness-of-fit | 1.002 | 1.001 | 1.002 | 1.001 |
| CCDC number | 2219102 | 2219103 | 2219104 | 2219105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasnovskaya, V.D.; Zorina, L.V.; Simonov, S.V.; Talantsev, A.D.; Yagubskii, E.B. [MII(H2dapsc)]-[Cr(CN)6] (M = Mn, Co) Chain and Trimer Complexes: Synthesis, Crystal Structure, Non-Covalent Interactions and Magnetic Properties. Molecules 2022, 27, 8518. https://doi.org/10.3390/molecules27238518
Sasnovskaya VD, Zorina LV, Simonov SV, Talantsev AD, Yagubskii EB. [MII(H2dapsc)]-[Cr(CN)6] (M = Mn, Co) Chain and Trimer Complexes: Synthesis, Crystal Structure, Non-Covalent Interactions and Magnetic Properties. Molecules. 2022; 27(23):8518. https://doi.org/10.3390/molecules27238518
Chicago/Turabian StyleSasnovskaya, Valentina D., Leokadiya V. Zorina, Sergey V. Simonov, Artem D. Talantsev, and Eduard B. Yagubskii. 2022. "[MII(H2dapsc)]-[Cr(CN)6] (M = Mn, Co) Chain and Trimer Complexes: Synthesis, Crystal Structure, Non-Covalent Interactions and Magnetic Properties" Molecules 27, no. 23: 8518. https://doi.org/10.3390/molecules27238518
APA StyleSasnovskaya, V. D., Zorina, L. V., Simonov, S. V., Talantsev, A. D., & Yagubskii, E. B. (2022). [MII(H2dapsc)]-[Cr(CN)6] (M = Mn, Co) Chain and Trimer Complexes: Synthesis, Crystal Structure, Non-Covalent Interactions and Magnetic Properties. Molecules, 27(23), 8518. https://doi.org/10.3390/molecules27238518







