Optimization of Wall Material of Freeze-Dried High-Bioactive Microcapsules with Yellow Onion Rejects Using Simplex Centroid Mixture Design Approach Based on Whey Protein Isolate, Pectin, and Sodium Caseinate as Incorporated Variables
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Onion Preparation
2.3. Extraction of Compounds from Yellow Onion Rejects Assisted by Pulsed Electric Fields
2.4. Preparation of Microencapsulated Powders
2.4.1. Total Phenolic Content (TPC)
2.4.2. Determination of Total Flavonoid Content (TFC)
2.4.3. Radical Scavenging Activity
2.4.4. Determination of Encapsulation Yield and Efficiency
2.4.5. Bulk Density of the Microcapsules
2.4.6. Determination of Glass Transition Temperature
2.4.7. Determination of Particle Size Distribution
2.4.8. Scanning Electron Microscopy
2.5. Antifungal Activity
2.6. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Encapsulated Powders
3.1.1. Moisture Content
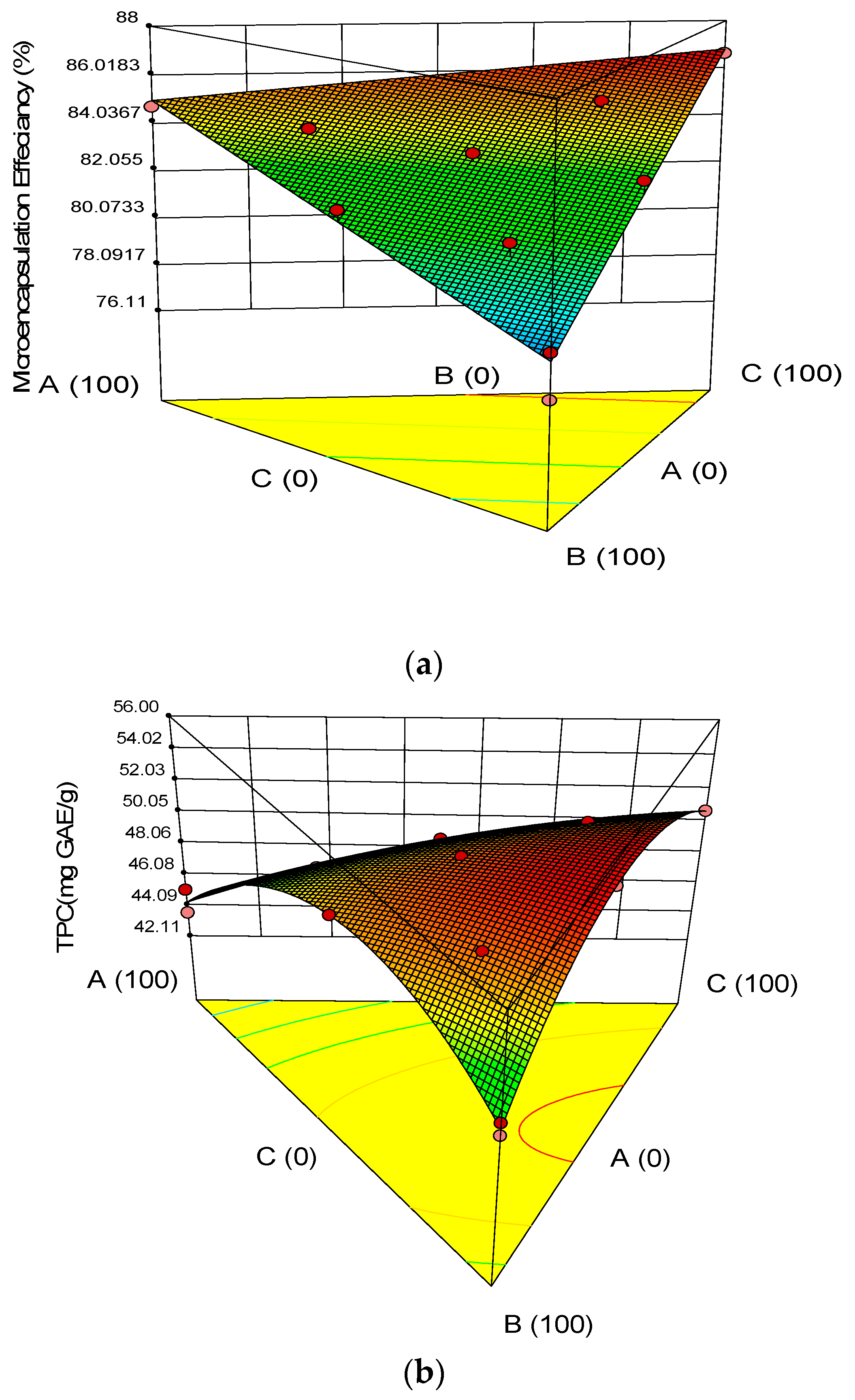
3.1.2. Encapsulation Yield
3.1.3. Bulk Density
3.1.4. Particle Size, Glass Transition, and the Scanning Electron Microscopy
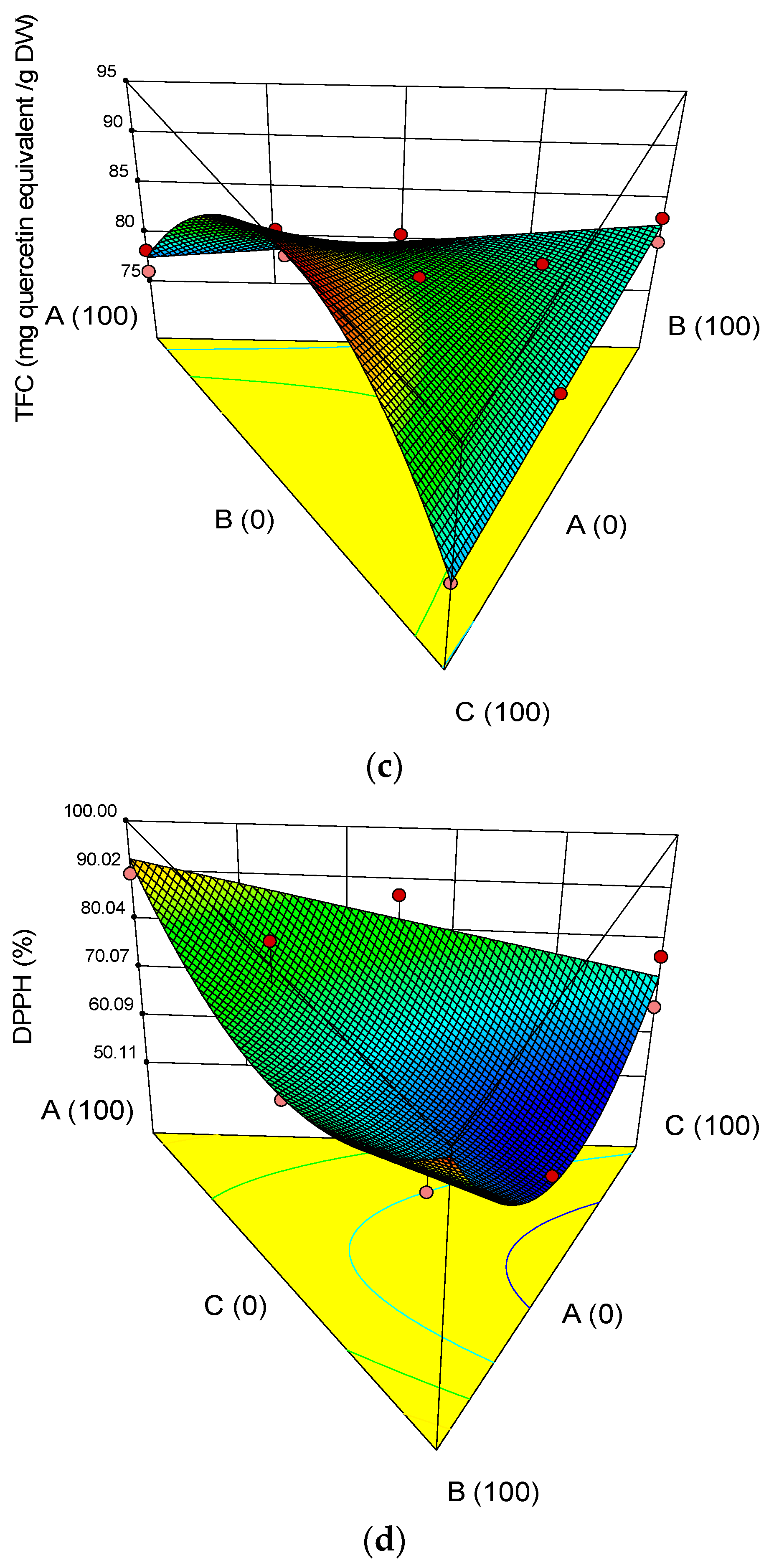
3.2. Chemical Properties of the Microcapsules
3.3. Optimization and Validation
3.4. Antifungal Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, G.; Jabbar, Z.; Athar, M.; Alam, M.S. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem. Toxicol. 2006, 44, 984–993. [Google Scholar] [CrossRef]
- Rodrigues, S.; Pinto, G.A.; Fernandes, F.A. Optimization of ultrasound extraction of phenolic compounds from coconut (Cocos nucifera) shell powder by response surface methodology. Ultrason. Sonochem. 2008, 15, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Abouzed, T.K.; Contreras, M.D.M.; Sadek, K.M.; Shukry, M.; Abdelhady, D.H.; Gouda, W.M.; Abdo, W.; Nasr, N.E.; Mekky, R.H.; Segura-Carretero, A.; et al. Red onion scales ameliorated streptozotocin-induced diabetes and diabetic nephropathy in Wistar rats in relation to their metabolite fingerprint. Diabetes Res. Clin. Pract. 2018, 140, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Milea, Ș.A.; Aprodu, I.; Enachi, E.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Whey protein isolate-xylose Maillard-based conjugates with tailored microencapsulation capacity of flavonoids from yellow onions skins. Antioxidants 2021, 10, 1708. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Plaza, L.; Elez-Martínez, P.; De Ancos, B.; Martín-Belloso, O.; Cano, M.P. Impact of high pressure and pulsed electric fields on bioactive compounds and antioxidant activity of orange juice in comparison with traditional thermal processing. J. Agric. Food Chem. 2005, 53, 4403–4409. [Google Scholar] [CrossRef]
- Fernandes, L.P.; Candido, R.C.; Oliveira, W.P. Spray drying microencapsulation of Lippia sidoides extracts in carbohydrate blends. Food Bioprod. Process. 2012, 90, 425–432. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.M.; Ghorbani, M.; Assadpoor, E. Spray-drying microencapsulation of anthocyanins by natural biopolymers: A review. Dry. Technol. 2014, 32, 509–518. [Google Scholar] [CrossRef]
- Stoica, F.; Condurache, N.N.; Horincar, G.; Constantin, O.E.; Turturică, M.; Stănciuc, N.; Aprodu, I.; Croitoru, C.; Râpeanu, G. Value-Added Crackers Enriched with Red Onion Skin Anthocyanins Entrapped in Different Combinations of Wall Materials. Antioxidants 2022, 11, 1048. [Google Scholar] [CrossRef]
- Khan, A.; Wang, C.; Sun, X.; Killpartrick, A.; Guo, M. Preparation and characterization of whey protein isolate–DIM nanoparticles. Int. J. Mol. Sci. 2019, 20, 3917. [Google Scholar] [CrossRef]
- Karrar, E.; Mahdi, A.A.; Sheth, S.; Ahmed, I.A.M.; Manzoor, M.F.; Wei, W.; Wang, X. Effect of maltodextrin combination with gum arabic and whey protein isolate on the microencapsulation of gurum seed oil using a spray-drying method. Int. J. Biol. Macromol. 2021, 171, 208–216. [Google Scholar] [CrossRef]
- Hogan, S.; O’riordan, E.; O’sullivan, M. Microencapsulation and oxidative stability of spray-dried fish oil emulsions. J. Microencapsul. 2003, 20, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, Q.; Zhou, M.; Xia, Y.; Nieh, M.-P.; Luo, Y. Development of “all natural” layer-by-layer redispersible solid lipid nanoparticles by nano spray drying technology. Eur. J. Pharm. Biopharm. 2016, 107, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.C.; Tan, C.P.; Nyam, K.L. Microencapsulation of refined kenaf (Hibiscus cannabinus L.) seed oil by spray drying using β-cyclodextrin/gum arabic/sodium caseinate. J. Food Eng. 2018, 237, 78–85. [Google Scholar] [CrossRef]
- Bai, X.; Li, C.; Yu, L.; Jiang, Y.; Wang, M.; Lang, S.; Liu, D. Development and characterization of soybean oil microcapsules employing kafirin and sodium caseinate as wall materials. LWT 2019, 111, 235–241. [Google Scholar] [CrossRef]
- Hosseinnia, M.; Khaledaba Rajabi d, M.A.; Almasi, H. Optimization of Ziziphora clinopodiodes essential oil microencapsulation by whey protein isolate and pectin: A comparative study. Int. J. Biol. Macromol. 2017, 101, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Malviya, R. Sources of pectin, extraction and its applications in pharmaceutical industry—An overview. Indian J. Nat. Prod. Resour. 2011, 2, 10–18. [Google Scholar]
- Liu, L.; Fishman, M.L.; Hicks, K.B. Pectin in controlled drug delivery—A review. Cellulose 2007, 14, 15–24. [Google Scholar] [CrossRef]
- Aberkane, L.; Roudaut, G.; Saurel, R. Encapsulation and oxidative stability of PUFA-rich oil microencapsulated by spray drying using pea protein and pectin. Food Bioprocess Technol. 2014, 7, 1505–1517. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef]
- Mahalleh, A.A.; Sharayei, P.; Mortazavi, S.A.; Azarpazhooh, E.; Niazmand, R. Optimization of the pulsed electric field-assisted extraction of functional compounds from Nepeta binaludensis. Agric. Eng. Int. CIGR J. 2019, 21, 184–194. [Google Scholar]
- Dastangoo, S.; Hamed Mosavian, M.T.; Yeganehzad, S. Optimization of pulsed electric field conditions for sugar extraction from carrots. Food Sci. Nutr. 2020, 8, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, H.; Ghorbani, M.; Jafari, S.M.; Mahoonak, A.S.; Rajabzadeh, G. Retention of saffron bioactive components by spray drying encapsulation using maltodextrin, gum Arabic and gelatin as wall materials. Food Hydrocoll. 2015, 51, 327–337. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Antioxidative phenolic constituents of skins of onion varieties and their activities. J. Funct. Foods 2013, 5, 1191–1203. [Google Scholar] [CrossRef]
- Siger, A.; Nogala-kalucka, M.; Lampart-szczapa, E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipids 2008, 15, 137–149. [Google Scholar] [CrossRef]
- Azarpazhooh, E.; Sharayei, P.; Zomorodi, S.; Ramaswamy, H.S. Physicochemical and phytochemical characterization and storage stability of freeze-dried encapsulated pomegranate peel anthocyanin and in vitro evaluation of its antioxidant activity. Food Bioprocess Technol. 2019, 12, 199–210. [Google Scholar] [CrossRef]
- Idham, Z.; Muhamad, I.I.; Sarmidi, M.R. Degradation kinetics and color stability of spray-dried encapsulated anthocyanins from Hibiscus sabdariffa L. J. Food Process Eng. 2012, 35, 522–542. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Anthocyanin stability and antioxidant activity of spray-dried açai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Res. Int. 2010, 43, 907–914. [Google Scholar] [CrossRef]
- Mahalleh, A.A.; Sharayei, P.; Azarpazhooh, E. investigating the characteristics of the Nepeta binaludensis encapsulated extract and its release kinetics in laboratory conditions. Food Bioprocess Technol. 2021, 14, 164–176. [Google Scholar] [CrossRef]
- Porrarud, S.; Pranee, A. Microencapsulation of Zn-chlorophyll pigment from Pandan leaf by spray drying and its characteristic. Int. Food Res. J. 2010, 17, 1031–1042. [Google Scholar]
- Ahmed, M.; Akter, M.S.; Lee, J.-C.; Eun, J.-B. Encapsulation by spray drying of bioactive components, physicochemical and morphological properties from purple sweet potato. LWT-Food Sci. Technol. 2010, 43, 1307–1312. [Google Scholar] [CrossRef]
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Ramaswamy, H.S. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT 2019, 101, 342–350. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous optimization of several response variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Mohammed, J.K.; Mahdi, A.A.; Al-Ansi, W.; Zhang, M.; Al-Adeeb, A.; Wei, M.; Hsu, M.P.; Yao, W. Physicochemical properties, microstructure, and storage stability of Pulicaria jaubertii extract microencapsulated with different protein biopolymers and gum arabic as wall materials. Int. J. Biol. Macromol. 2021, 187, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.A.; Jafari, S.M.; Assadpoor, E.; Dehnad, D. Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Aidoo, R.P.; Afoakwa, E.O.; Dewettinck, K. Optimization of inulin and polydextrose mixtures as sucrose replacers during sugar-free chocolate manufacture–Rheological, microstructure and physical quality characteristics. J. Food Eng. 2014, 126, 35–42. [Google Scholar] [CrossRef]
- Kanakdande, D.; Bhosale, R.; Singhal, R.S. Stability of cumin oleoresin microencapsulated in different combination of gum arabic, maltodextrin and modified starch. Carbohydr. Polym. 2007, 67, 536–541. [Google Scholar] [CrossRef]
- Akdeniz, B.; Sumnu, G.; Sahin, S. Microencapsulation of phenolic compounds extracted from onion (Allium cepa) skin. J. Food Process. Preserv. 2018, 42, e13648. [Google Scholar] [CrossRef]
- Zuidam, N.J.; Shimoni, E. Overview of microencapsulates for use in food products or processes and methods to make them. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Zuidam, N.J., Nedovic, V.A., Eds.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Horiuchi, K. DSC studies on structural phase transitions and molecular motions in some A2MCl4 compounds. Phys. Status Solidi 2004, 210, 723–726. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation efficiency of food flavours and oils during spray drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Horincar, G.; Aprodu, I.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Interactions of flavonoids from yellow onion skins with whey proteins: Mechanisms of binding and microencapsulation with different combinations of polymers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 215, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Condurache, N.N.; Aprodu, I.; Crăciunescu, O.; Tatia, R.; Horincar, G.; Barbu, V.; Enachi, E.; Râpeanu, G.; Bahrim, G.E.; Oancea, A.; et al. Probing the functionality of bioactives from eggplant peel extracts through extraction and microencapsulation in different polymers and whey protein hydrolysates. Food Bioprocess Technol. 2019, 12, 1316–1329. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Klein, M.; Aserin, A.; Ishai, P.B.; Garti, N. Interactions between whey protein isolate and gum arabic. Colloids Surf. B Biointerfaces 2010, 79, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Milea, Ș.A.; Vasile, M.A.; Crăciunescu, O.; Prelipcean, A.M.; Bahrim, G.E.; Râpeanu, G.; Stănciuc, N. Co-microencapsulation of flavonoids from yellow onion skins and lactic acid bacteria lead to multifunctional ingredient for nutraceutical and pharmaceutics applications. Pharmaceutics 2020, 12, 1053. [Google Scholar] [CrossRef]
- Bourvellec, L.; Renard, C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Finney, J.; Buffo, R.; Reineccius, G.A. Effects of type ofatomization and processing temperatures on the physical properties and stability of spray-dried flavors. J. Food Sci. 2002, 67, 1108–1114. [Google Scholar] [CrossRef]
- Najaf Najafi, M.; Kadkhodaee, R.; Mortazavi, S.A. Effect of drying process and wall material on the properties of encapsulated cardamom oil. Food Biophys. 2011, 6, 68–76. [Google Scholar] [CrossRef]
- Buazzi, M.M.; Marth, E.H. Mechanisms in the inhibition ofListeria monocytogenes by potassium sorbate. Food Microbiol. 1991, 8, 249–256. [Google Scholar] [CrossRef]
- Ibrahium, M. Efficiency of pomegranate peel extract as antimicrobial, antioxidant and protective agents. World J. Agric. Sci. 2010, 6, 338–344. [Google Scholar]

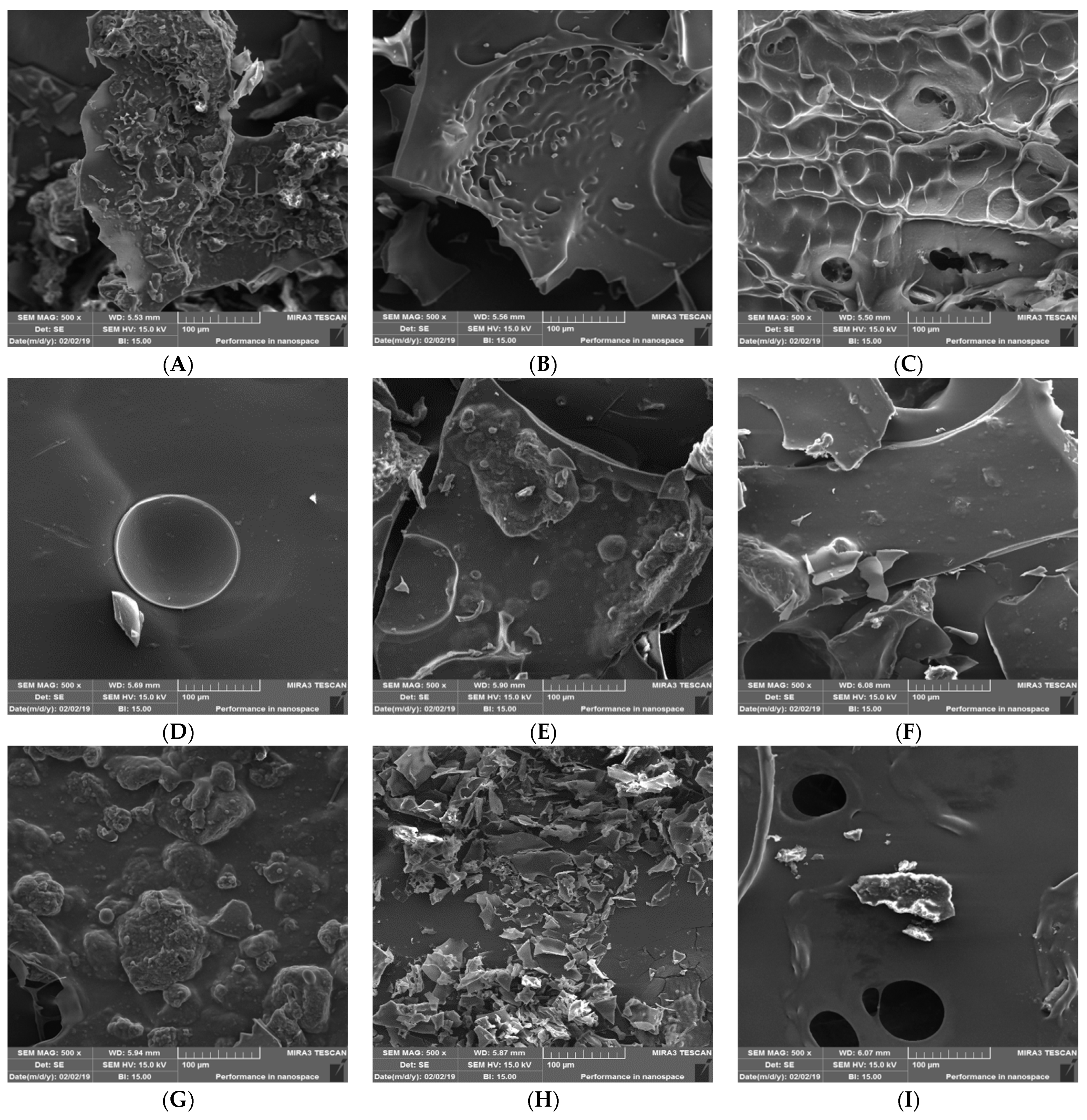
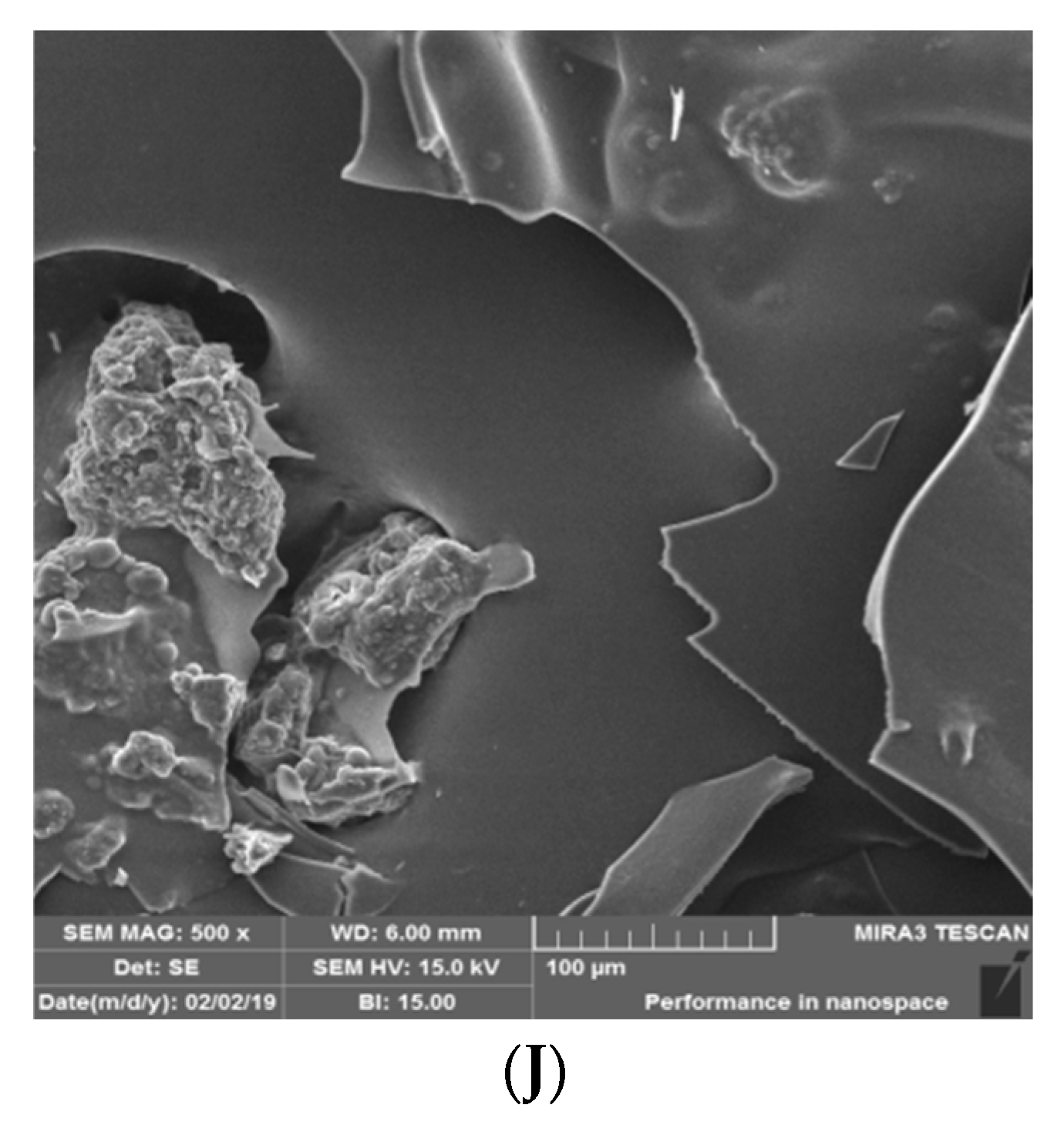

| Mixtures | Uncoded Values (Ingredient Proportions) | ||
|---|---|---|---|
| CS (%) | P (%) | WPI (%) | |
| 1 | 16.67 | 16.67 | 66.67 |
| 2 | 0 | 0 | 100 |
| 3 | 0 | 100 | 0 |
| 4 | 0 | 100 | 0 |
| 5 | 50 | 50 | 0 |
| 6 | 0 | 50 | 50 |
| 7 | 0 | 0 | 100 |
| 8 | 50 | 0 | 50 |
| 9 | 100 | 0 | 0 |
| 10 | 66.67 | 16.67 | 16.67 |
| 11 | 100 | 0 | 0 |
| 12 | 50 | 50 | 0 |
| 13 | 33.33 | 33.33 | 33.33 |
| 14 | 16.67 | 66.67 | 16.67 |
| Mixtures | Physical Properties of Encapsulated Powders | ||||
|---|---|---|---|---|---|
| M (%) | EY (%) | BD (kg/m3) | PS (μm) | Tg (°C) | |
| 1 | 10.2 ± 0.31 | 96.5 ± 0.76 | 215.1 ± 2.35 | 33.2 ± 0.35 | 28.1 ± 0.11 |
| 2 | 9.7 ± 0.24 | 96.1 ± 0.46 | 214.5 ± 3.58 | - | - |
| 3 | 11.5 ± 0.15 | 96.8 ± 0.67 | 221.7 ± 6.57 | - | - |
| 4 | 11.5 ± 0.38 | 98.2 ± 0.49 | 223.0 ± 5.92 | 31.2 ± 0.54 | 41.1 ± 0.02 |
| 5 | 10.2 ± 0.12 | 84.7 ± 0.42 | 210.3 ± 4.42 | - | - |
| 6 | 10.8 ± 0.42 | 99.7 ± 1.01 | 217.8 ± 8.56 | 43.5 ± 0.67 | 27.1 ± 0.32 |
| 7 | 10.2 ± 0.54 | 95.0 ± 1.42 | 214.5 ± 5.47 | - | - |
| 8 | 9.6 ± 0.64 | 96.8 ± 0.96 | 219.3 ± 6.54 | - | - |
| 9 | 11.5 ± 0.33 | 97.2 ± 0.95 | 229.9 ± 0.92 | - | - |
| 10 | 9.6 ± 0.52 | 91.5 ± 1.09 | 215.0 ± 9.36 | 35.4 ± 0.66 | 27.2 ± 0.12 |
| 11 | 11.9 ± 0.16 | 93.9 ± 0.82 | 229.9 ± 9.65 | 36.4 ± 0.47 | 41.2 ± 0.52 |
| 12 | 10.3 ± 0.22 | 86.0 ± 0.69 | 210.3 ± 10.48 | 34.6 ± 0.65 | 28.8 ± 0.65 |
| 13 | 10.2 ± 0.14 | 88.0 ± 0.88 | 213.7 ± 8.46 | - | - |
| 14 | 10.8 ± 0.12 | 88.4 ± 0.83 | 214.5 ± 10.66 | - | - |
| Fitted Model | Moisture (M) | Encapsulated Yield (EY) | Bulk Density (BD) |
|---|---|---|---|
| Quadratic (p ≤ 0.001) | Linear (p ≤ 0.0001) | Quadratic (p ≤ 0.03) | |
| Lack of fit | p = 0.191 | p = 0.972 | p = 0.060 |
| R Squared (%) | 0.89 | 0.99 | 0.98 |
| Adjusted R Squared (%) | 0.84 | 0.99 | 0.97 |
| Adeq Precision | 11.0 | 282 | 36.9 |
| Mixtures No. | Chemical Properties of Encapsulated Powders | |||
|---|---|---|---|---|
| Encapsulation Efficiency (%) | TPC (mg Gallic Acid Equivalent/g DW) | TFC (mg Quercetin Equivalent/g DW) | DPPH (%) | |
| Onion Extract | - | 65.34 ± 4.43 | 98.45 ± 4.438 | 99.89 ± 3.45 |
| 1 | 85.25 ± 1.23 | 52.48 ± 0.43 | 86.87 ± 1.33 | 63.64 ± 2.44 |
| 2 | 86.65 ± 3.43 | 50.56 ± 0.68 | 79.58 ± 0.85 | 65.37 ± 1.34 |
| 3 | 78.90 ± 0.45 | 49.06 ± 0.33 | 82.73 ± 0.96 | 98.26 ± 2.64 |
| 4 | 77.12 ± 0.75 | 48.23 ± 0.75 | 80.23 ± 1.17 | 99.33 ± 1.89 |
| 5 | 82.23 ± 1.15 | 52.37 ± 0.69 | 80.52 ± 2.75 | 74.79 ± 0.95 |
| 6 | 83.20 ± 0.37 | 54.24 ± 0.65 | 81.15 ± 4.35 | 60.12 ± 0.64 |
| 7 | 86.65 ± 0.67 | 50.56 ± 0.38 | 79.58 ± 2.47 | 75.98 ± 0.95 |
| 8 | 85.62 ± 0.46 | 48.66 ± 0.53 | 94.65 ± 1.76 | 87.00 ± 1.66 |
| 9 | 84.71 ± 0.63 | 45.23 ± 0.27 | 78.31 ± 1.66 | 89.82 ± 0.94 |
| 10 | 84.27 ± 0.45 | 49.57 ± 0.62 | 86.24 ± 0.18 | 86.56 ± 1.66 |
| 11 | 84.71 ± 0.87 | 43.69 ± 0.87 | 76.00 ± 0.69 | 89.82 ± 1.54 |
| 12 | 82.23 ± 0.63 | 52.37 ± 1.15 | 80.52 ± 0.85 | 74.79 ± 0.84 |
| 13 | 83.77 ± 0.37 | 53.08 ± 2.11 | 87.18 ± 1.45 | 66.78 ± 1.38 |
| 14 | 81.53 ± 0.54 | 53.22 ± 1.18 | 83.21 ± 1.18 | 67.40 ± 1.65 |
| Encapsulation Efficiency | TPC | TFC | DPPH | |
|---|---|---|---|---|
| Fitted Model | Linear (p ≤ 0.0001) | Quadratic (p ≤ 0.0001) | Quadratic (p ≤ 0.0001) | Quadratic (p ≤ 0.0002) |
| Lack of fit | p = 0.672 | p = 0.994 | p = 0.995 | p = 0.263 |
| R Squared (%) | 0.96 | 0.98 | 0.917 | 0.91 |
| Adjusted R Squared (%) | 0.95 | 0. 97 | 0.96 | 0.87 |
| Adeq Precision | 31.32 | 33.72 | 35.15 | 14.21 |
| Samples | The Mean Diameter of Inhibition Zone |
|---|---|
| Onion Extract (mg/L) | |
| 1000 | 10.18 ± 0.12c |
| 2000 | 11.76 ±0.04b |
| 3000 | 18.63 ± 0.19a |
| Sodium Nitrate(120 ppm) | 18.78 ± 0.04a |
| Ethanol 70% (negative control) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azarpazhooh, E.; Sharayei, P.; Rui, X.; Gharibi-Tehrani, M.; Ramaswamy, H.S. Optimization of Wall Material of Freeze-Dried High-Bioactive Microcapsules with Yellow Onion Rejects Using Simplex Centroid Mixture Design Approach Based on Whey Protein Isolate, Pectin, and Sodium Caseinate as Incorporated Variables. Molecules 2022, 27, 8509. https://doi.org/10.3390/molecules27238509
Azarpazhooh E, Sharayei P, Rui X, Gharibi-Tehrani M, Ramaswamy HS. Optimization of Wall Material of Freeze-Dried High-Bioactive Microcapsules with Yellow Onion Rejects Using Simplex Centroid Mixture Design Approach Based on Whey Protein Isolate, Pectin, and Sodium Caseinate as Incorporated Variables. Molecules. 2022; 27(23):8509. https://doi.org/10.3390/molecules27238509
Chicago/Turabian StyleAzarpazhooh, Elham, Parvin Sharayei, Xin Rui, Mehranoosh Gharibi-Tehrani, and Hosahalli S. Ramaswamy. 2022. "Optimization of Wall Material of Freeze-Dried High-Bioactive Microcapsules with Yellow Onion Rejects Using Simplex Centroid Mixture Design Approach Based on Whey Protein Isolate, Pectin, and Sodium Caseinate as Incorporated Variables" Molecules 27, no. 23: 8509. https://doi.org/10.3390/molecules27238509
APA StyleAzarpazhooh, E., Sharayei, P., Rui, X., Gharibi-Tehrani, M., & Ramaswamy, H. S. (2022). Optimization of Wall Material of Freeze-Dried High-Bioactive Microcapsules with Yellow Onion Rejects Using Simplex Centroid Mixture Design Approach Based on Whey Protein Isolate, Pectin, and Sodium Caseinate as Incorporated Variables. Molecules, 27(23), 8509. https://doi.org/10.3390/molecules27238509








