In Silico Evaluation and In Vitro Determination of Neuroprotective and MAO-B Inhibitory Effects of Pyrrole-Based Hydrazones: A Therapeutic Approach to Parkinson’s Disease
Abstract
1. Introduction
2. Results
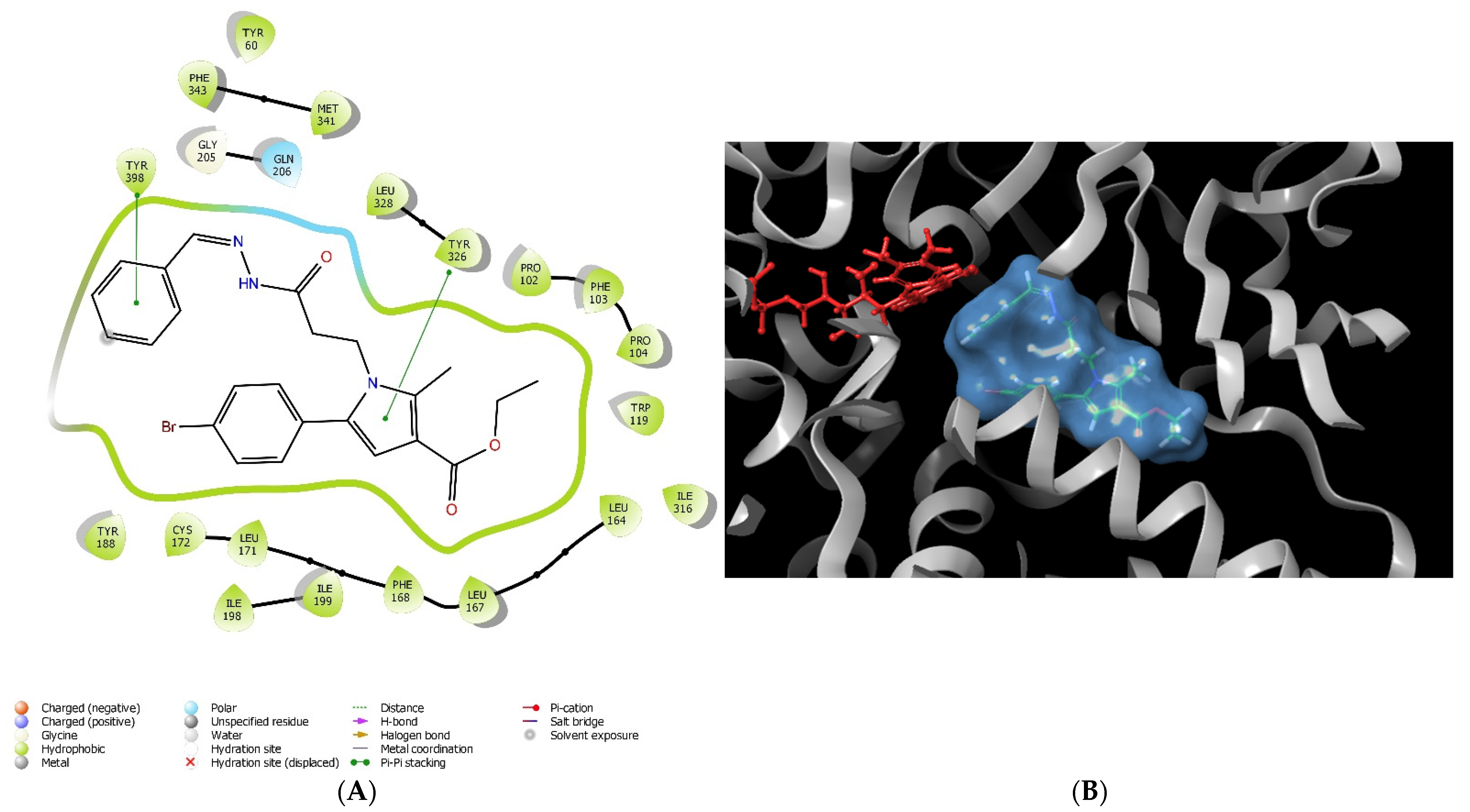
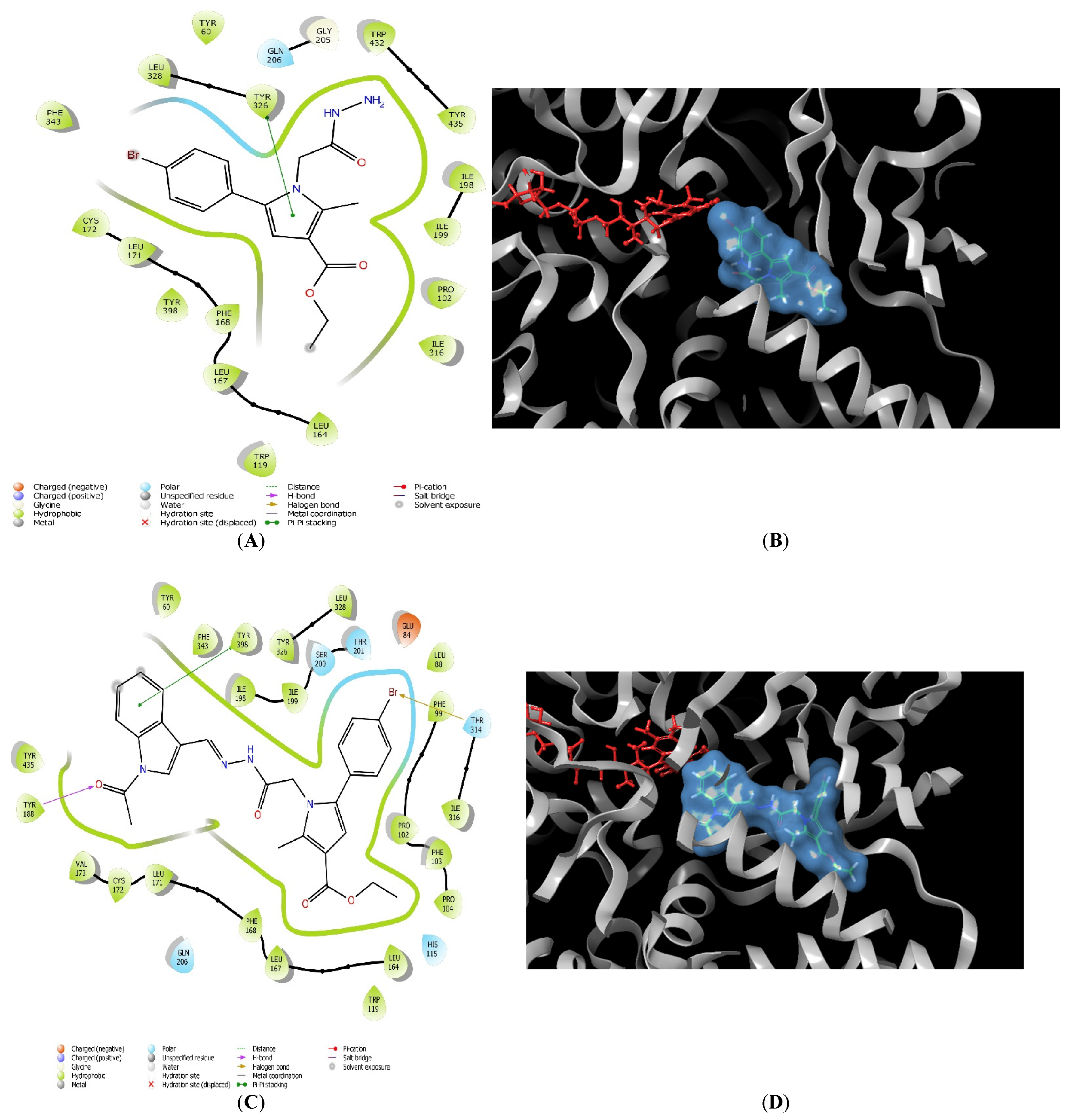
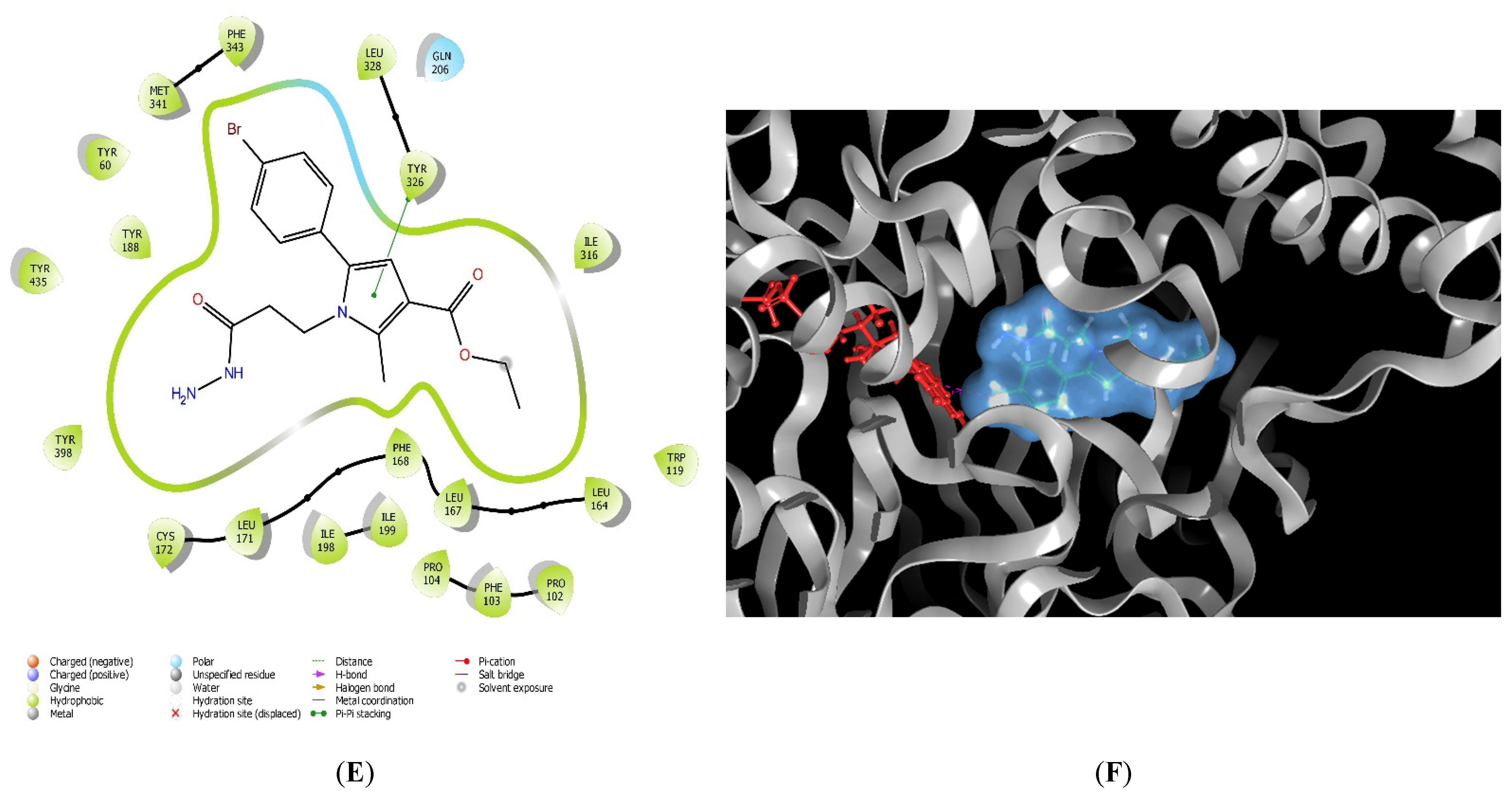
2.1. Molecular Docking
2.2. In Vitro Neurotoxicity Evaluations
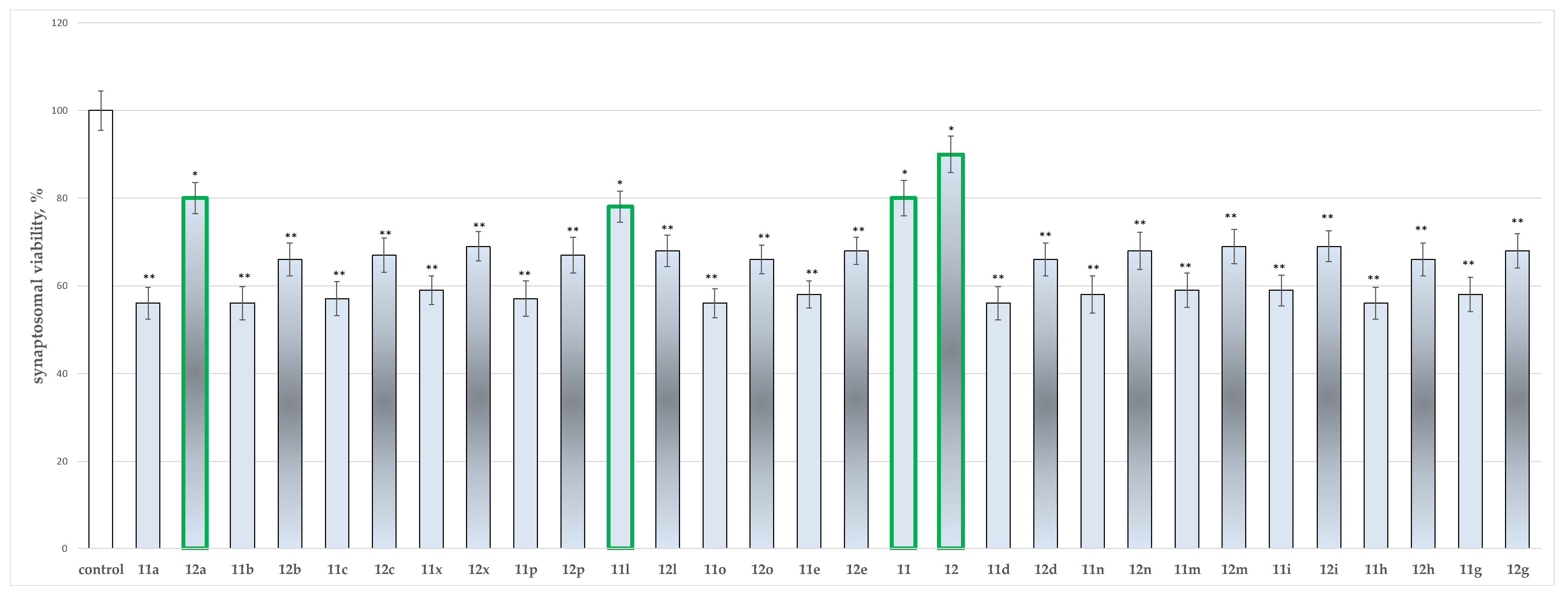
2.2.1. Effects of the Tested Compounds 11, 11a–11x, 12, and 12a–12x on the Synaptosomal Viability of Isolated Rat Brain Synaptosomes
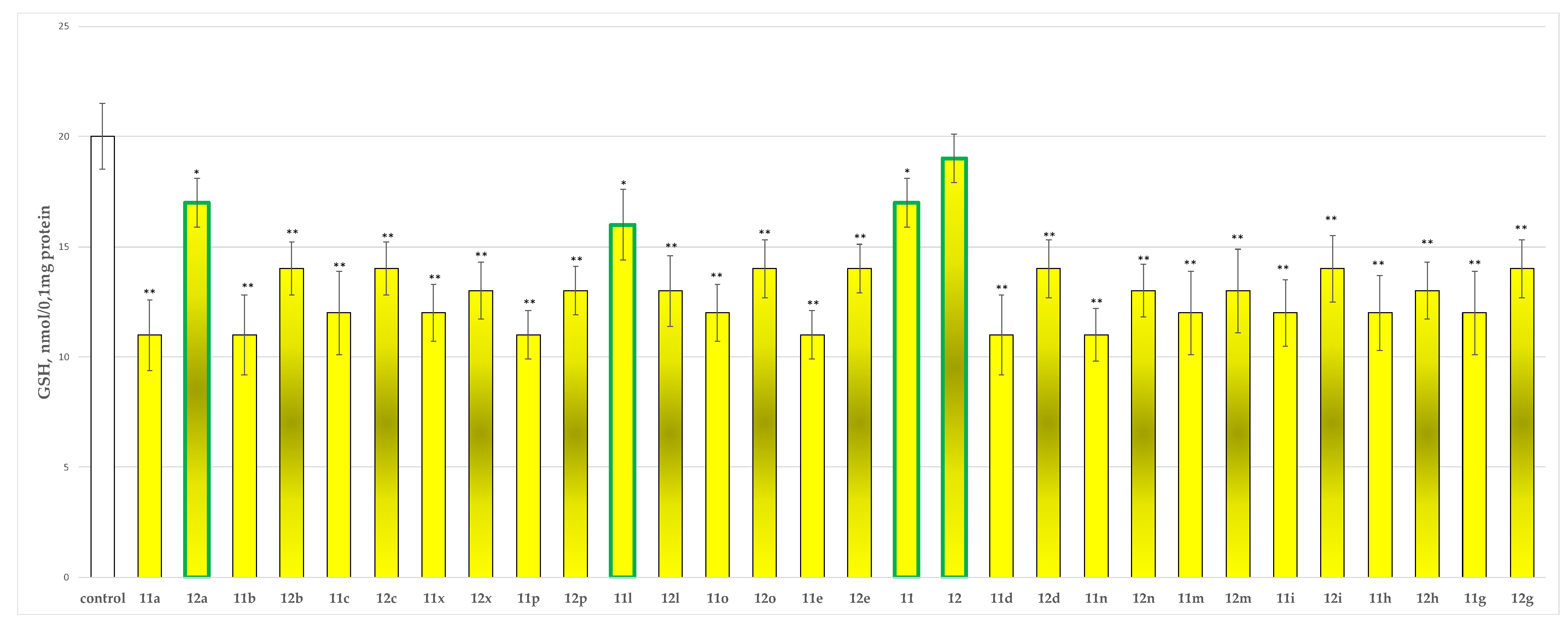
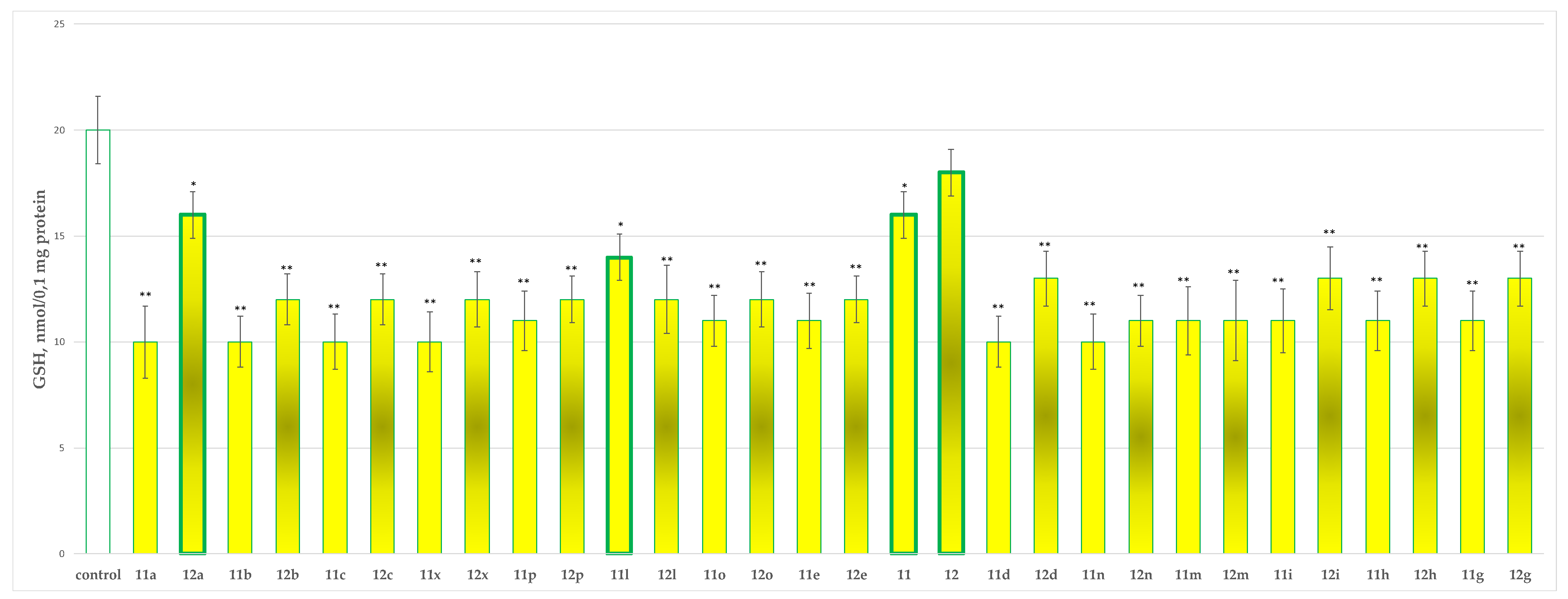
2.2.2. Effects of the Tested Compounds 11, 11a–11x, 12 and 12a–12x on the GSH Level in Isolated Rat Brain Synaptosomes
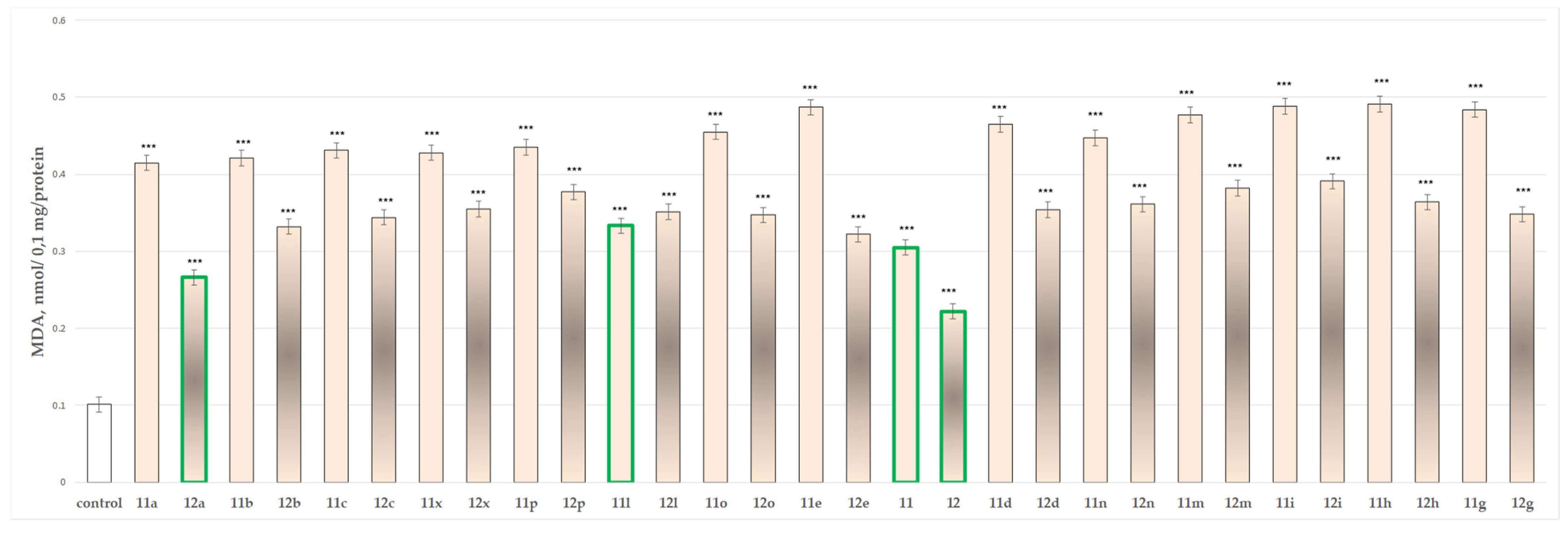
2.2.3. Effects of the Tested Compounds 11, 11a–11x, 12 and 12a–12x on the MDA Production and GSH Level in Isolated Rat Brain Mitochondria
2.2.4. Effects of the Tested Compounds 11, 11a–11x, 12 and 12a–12x on the Isolated Rat Brain Microsomes
2.3. In Vitro Evaluations of Neuroprotective Effects of the Tested N-Pyrrolyl Hydrazide–Hydrazones
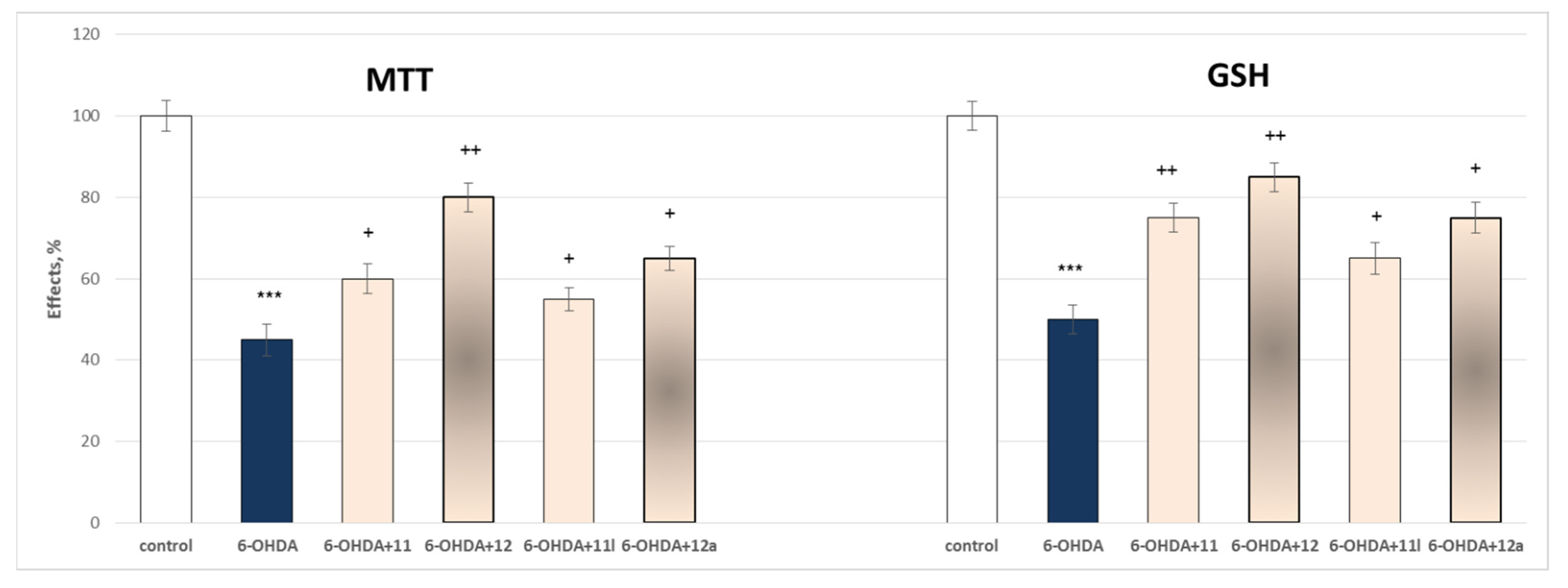
2.3.1. Protective Effects of the Tested Compounds 11, 11a–11x, 12 and 12a–12x in a Model of 6-OHDA-Induced Oxidative Stress of Isolated Rat Brain Synaptosomes
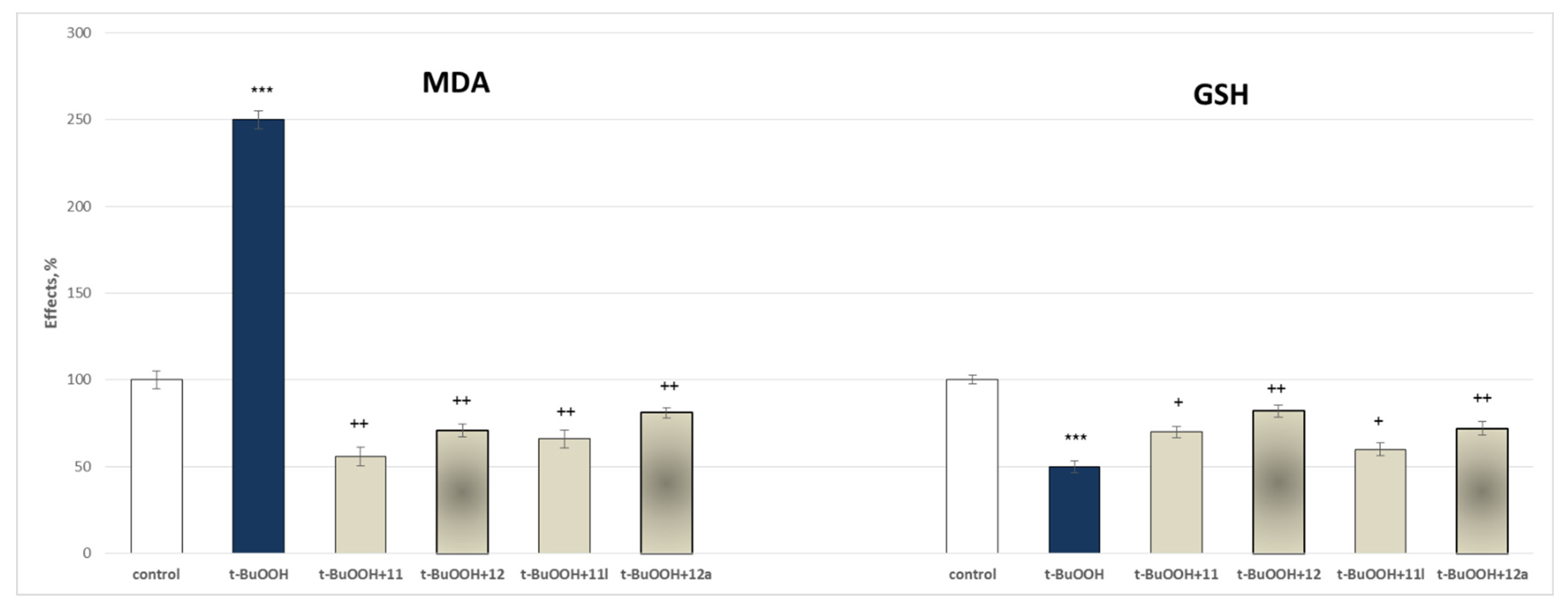
2.3.2. Protective Effects of the Tested Compounds 11, 11a–11x, 12 and 12a–12x in a Model of Tert-Butyl Hydroperoxide (t-BuOOH) Induced Oxidative Stress of Isolated Rat Brain Mitochondria
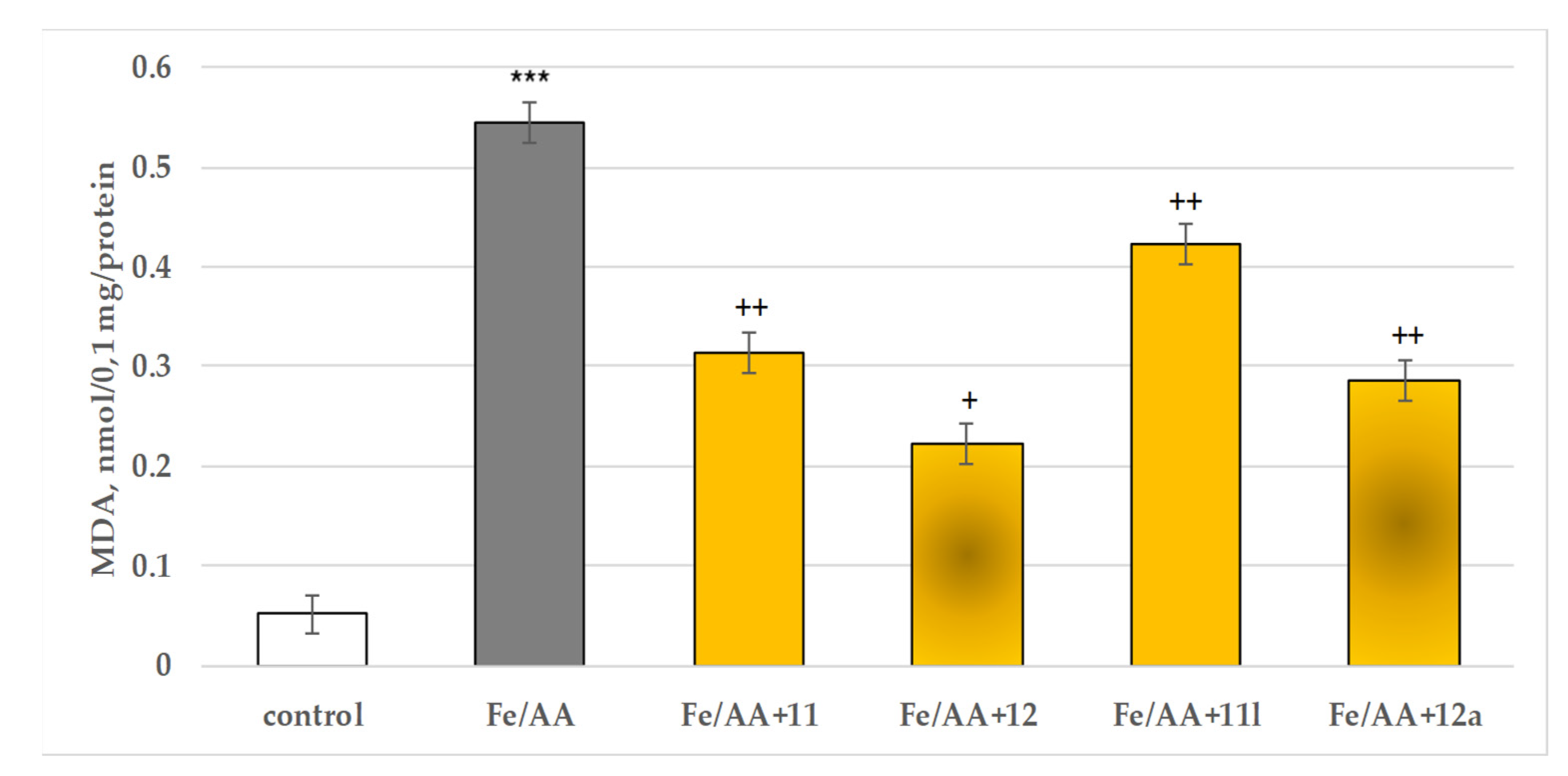
2.3.3. Protective Effects of the Tested Compounds 11, 11a–11x, 12 and 12a–12x in a Model of Iron Ascorbate (Fe2+/AA) Induced Lipid Peroxidation in Isolated Rat Brain Microsomes
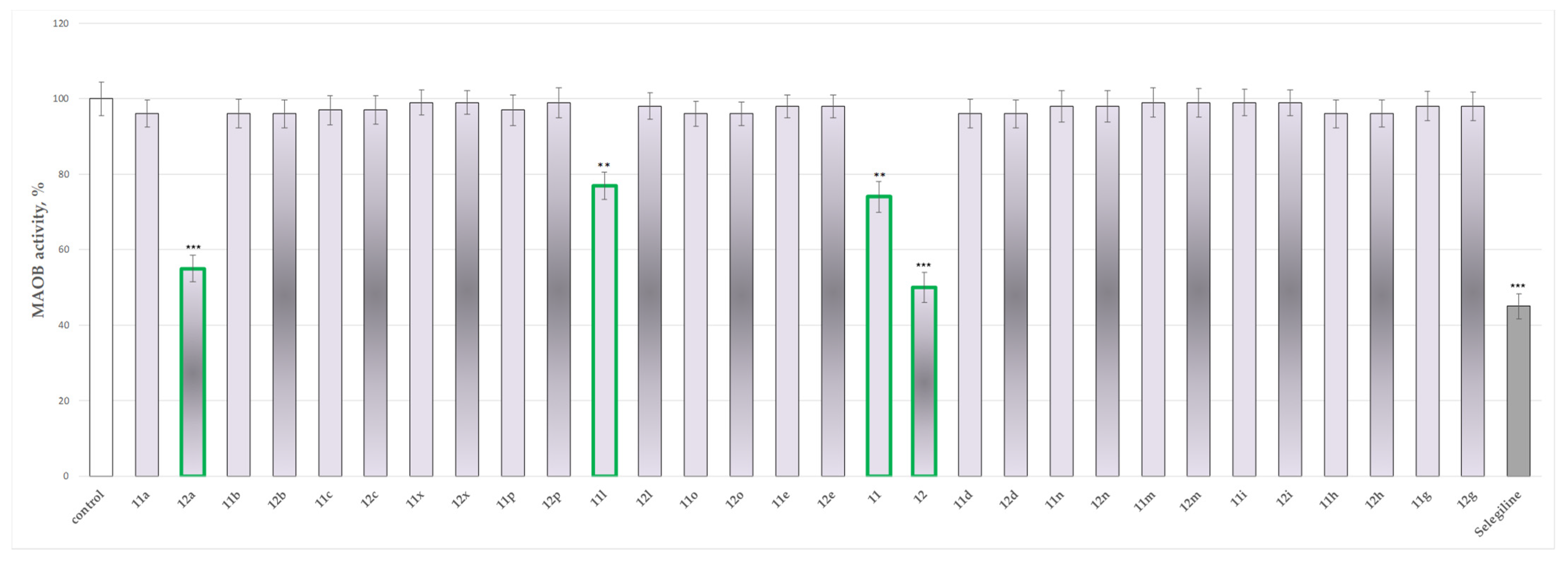
2.4. In Vitro Evaluation of the Effects of the Tested 11, 11a–11x, 12 and 12a–12x on the Activity of Human Recombinant MAO-B (hMAOB) Enzyme
3. Discussion
3.1. In Silico Molecular Docking Assessment
3.2. In Vitro Assessment of Neurotoxicity and Neuroprotection of the Evaluated Derivatives
3.3. In Vitro Evaluation of the Targeted N-Pyrrolyl Hydrazide–Hydrazones on the Activity of Human Recombinant MAO-B Enzyme
4. Materials and Methods
4.1. Chemistry
4.2. Molecular Docking
4.2.1. Hardware
4.2.2. Selection and Preparation of Proteins
4.2.3. Preparation of Ligands
4.2.4. Docking Protocol
4.3. Biological Evaluation
4.3.1. Animals
4.3.2. Isolation and Incubation of Rat Brain Synaptosomes and Mitochondria
4.3.3. Model of 6-OHDA-Induced Neurotoxicity in Synaptosomes
4.3.4. Tert-Butyl Hydroperoxide (t-BuOOH)-Induced Oxidative Stress in Isolated Brain Mitochondria
4.3.5. Determination of Synaptosomal Viability and Reduced Glutathione (GSH) Level
4.3.6. Determination of Malondialdehyde (MDA) Production and Reduced GSH Level in Rat Brain Mitochondria
4.3.7. Isolation of Rat Brain Microsomes
4.3.8. Development of a Model of Non-Enzyme-Induced Lipid Peroxidation and Determination of MDA in Isolated Rat Brain Microsomes
4.3.9. Evaluation of Human Monoamine Oxidase B (hMAOB) Activity
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Teive, H.A.G.; Meneses, M.S. Histórico. In Doença de Parkinson: Aspectos Clínicos e Cirúrgicos; Meneses, M.S., Teive, H.A.G., Eds.; Guanabara Koogan: Rio de Janeiro, Brazil, 1996; pp. 4–14. [Google Scholar]
- Percário, S.; da Silva Barbosa, A.; Varela, E.L.P.; Gomes, A.R.Q.; Ferreira, M.E.S.; Moreira, T.N.A.; Dolabela, M.F. Oxidative stress in Parkinson’s disease: Potential benefits of antioxidant supplementation. Oxidative Med. Cell. Longev. 2020, 2020, 23608721–23608723. [Google Scholar] [CrossRef] [PubMed]
- Savica, R.; Grossardt, B.R.; Rocca, W.A.; Bower, J.H. Parkinson disease with and without dementia: A prevalence study and future projections. Mov. Disord. 2018, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lana, R.C.; Álvares, L.M.R.S.; Nasciutti-Prudente, C.; Goulart, F.R.P.; Teixeira-Salmela, L.F.; Cardoso, F.E. Percepção da qualidade de vida de indivíduos com doença de Parkinson através do PDQ-39. Rev. Bras. Fisioter. 2007, 11, 397–402. [Google Scholar] [CrossRef][Green Version]
- Schildknecht, S.; Pape, R.; Meiser, J.; Karreman, C.; Strittmatter, T.; Odermatt, M.; Cirri, E.; Friemel, A.; Ringwald, M.; Pasquarelli, N.; et al. Preferential extracellular generation of the active parkinsonian toxin MPP+ by transporter-independent export of the intermediate MPDP+. Antioxid. Redox Signal. 2015, 23, 1001–1016. [Google Scholar] [CrossRef]
- Schildknecht, S.; Di Monte, D.A.; Pape, R.; Tieu, K.; Leist, M. Tipping points and endogenous determinants of nigrostriatal degeneration by MPTP. Trends Pharmacol. Sci. 2017, 38, 541–555. [Google Scholar] [CrossRef]
- Langston, J.W. The MPTP story. J. Park. Dis. 2017, 7, S11–S19. [Google Scholar] [CrossRef]
- Leathem, A.; Ortiz-Cerda, T.; Dennis, J.M.; Witting, P.K. Evidence for Oxidative Pathways in the Pathogenesis of PD: Are Antioxidants Candidate Drugs to Ameliorate Disease Progression? Int. J. Mol. Sci. 2022, 23, 6923. [Google Scholar] [CrossRef]
- Dionísio, P.A.; Amaral, J.D.; Rodrigues, C.M.P. Oxidative stress and regulated cell death in Parkinson’s disease. Ageing Res. Rev. 2021, 67, 101263. [Google Scholar] [CrossRef]
- Dorszewska, J.; Kowalska, M.; Prendecki, M.; Piekut, T.; Kozłowska, J.; Kozubski, W. Oxidative stress factors in Parkinson’s disease. Neural Regen. Res. 2021, 16, 1383–1391. [Google Scholar] [CrossRef]
- Nam, M.-H.; Sa, M.; Ju, Y.H.; Park, M.G.; Lee, C.J. Revisiting the Role of Astrocytic MAOB in Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 4453. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Y.; Jenner, P.; Chen, S.D. Monoamine Oxidase-B Inhibitors for the Treatment of Parkinson’s Disease: Past, Present, and Future. Review. J. Park. Dis. 2022, 12, 477–493. [Google Scholar] [CrossRef]
- Ahmad, S.; Alam, O.; Naim, M.J.; Shaquiquzzaman, M.M.; Alam, M.; Iqbal, M. Pyrrole: An insight into recent pharmacological advances with structure activity relationship. Eur. J. Med. Chem. 2018, 157, 527–561. [Google Scholar] [CrossRef]
- Fatahala, S.S.; Mohamed, M.S.; Sabry, J.Y.; El-Deen Mansour, Y.E. Synthesis strategies and medicinal value of pyrrole and its fused heterocyclic compounds. Med. Chem. 2022, 18, 1013–1043. [Google Scholar] [PubMed]
- Tzankova, D.; Aluani, D.; Kondeva-Burdina, M.; Georgieva, M.; Vladimirova, S.; Peikova, L.; Tzankova, V. Antioxidant properties, neuroprotective effects and in vitro safety evaluation of new pyrrole derivatives. Pharm. Chem. J. 2022, 55, 1310–1319. [Google Scholar] [CrossRef]
- Mateev, E.; Valkova, I.; Angelov, B.; Georgieva, M.; Zlatkov, A. Validation through re-docking, cross-docking and ligand enrichment in various well-resoluted MAO-B receptors. Int. J. Pharm. Sci. Res. 2022, 13, 1099–1107. [Google Scholar]
- Wang, E.; Sun, H.; Wang, J.; Wang, Z.; Liu, H.; Zhang, J.Z.H.; Hou, T. End-point binding free energy calculation with MM/PBSA and MM/GBSA: Strategies and applications in drug design. Chem. Rev. 2019, 119, 9478–9508. [Google Scholar] [CrossRef] [PubMed]
- Saikia, S.; Bordoloi, M. Molecular docking: Challenges, advances and its use in drug discovery perspective. Curr. Drug Targets 2019, 20, 501–521. [Google Scholar] [CrossRef]
- Binda, C.; Newton-Vinson, P.; Hubálek, F.; Edmondson, D.E.; Mattevi, A. Structure of human monoamine oxidase B, a drug target for the treatment of neurological disorders. Nat. Struct. Biol. 2002, 9, 22–28. [Google Scholar] [CrossRef]
- Geha, R.M.; Chen, K.; Wouters, J.; Ooms, F.; Shih, J.C. Analysis of conserved active site residues in monoamine oxidase A and B and their three-dimensional molecular modeling. J. Biol. Chem. 2002, 277, 17209–17225. [Google Scholar] [CrossRef]
- Spencer, P.S.; Lein, P.J. Encyclopedia of Toxicology, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2014; ISBN 978-0-12-386455-0. [Google Scholar]
- Schapira, A.H.; Jenner, P. Etiology and pathogenesis of Parkinson’s disease. Mov. Disord. 2011, 26, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chu, C.T. Mitochondrial dysfunction in Parkinson’s disease. J. Alzheimers Dis. 2010, 20 (Suppl. 2), S325–S334. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.D.; Parks, J.K.; Swerdlow, R.H. Complex I deficiency in Parkinson’s disease frontal cortex. Brain Res. 2008, 16, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P.; Olanow, W. The pathogenesis of cell death in Parkinson’s disease. Neurology 2006, 66, S24–S36. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Park. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef]
- Richardson, J.R.; Quan, Y.; Sherer, T.B.; Greenamyre, J.T.; Miller, G.W. Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicol. Sci. 2005, 88, 193–201. [Google Scholar] [CrossRef]
- Callio, J.; Oury, T.; Chu, C. Manganese superoxide dismutase protects against 6-hydroxydopamine injury in mouse brains. J. Biol. Chem. 2005, 6, 18356–18542. [Google Scholar] [CrossRef]
- Perier, C.; Bovè, J.; Vila, M.; Przedborski, S. The rotenone model of Parkinson’s disease. Trends Neurosci. 2003, 26, 345–346. [Google Scholar] [CrossRef]
- Reed, D.J. Glutathione: Toxicological implications. Annu. Rev. Pharmacol. Toxicol. 1990, 30, 603–631. [Google Scholar] [CrossRef]
- Ran, Q.; Liang, H.; Gu, M.; Qi, W.; Walter, C.A.; Roberts, L.J., II; Herman, B.; Richardson, A.; Van Remmen, H. Transgenic mice overexpressing glutathione peroxidase 4 are protected against oxidative stress-induced apoptosis. J. Biol. Chem. 2004, 279, 55137–55146. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–121. [Google Scholar] [CrossRef]
- Pre, J. Lipid peroxidation. Pathol. Biol. 1991, 39, 716–736. [Google Scholar] [PubMed]
- Abdel-Monem, Y.K.; Abou El-Enein, S.A.; El-Sheikh-Amer, M.M. Design of new metal complexes of 2-(3-amino-4,6-dimethyl-1H-pyrazolo[3,4-b]pyridin-1-yl)aceto-hydrazide: Synthesis, characterization, modelling and antioxidant activity. J. Mol. Struct. 2017, 1127, 386–396. [Google Scholar] [CrossRef]
- Bär, F.; Hopf, H.; Knorr, M.; Krahl, J. Synthesis, characterization and antioxidant properties of 2,4,6-tris-isopropylbenzoic acid hydrazide in biodiesel. Fuel 2018, 215, 249–257. [Google Scholar] [CrossRef]
- Boulebd, H.; Zine, Y.; Khodja, I.A.; Mermer, A.; Demir, A.; Debache, A. Synthesis and radical scavenging activity of new phenolic hydrazone/hydrazide derivatives: Experimental and theoretical studies. J. Mol. Struct. 2022, 1249, 131546–131551. [Google Scholar] [CrossRef]
- Bijev, A.; Georgieva, M. Pyrrole-based hydrazones synthesized and evaluated in vitro as potential tuberculostatics. Lett. Drug Des. Discov. 2010, 7, 430–437. [Google Scholar] [CrossRef]
- Georgieva, M.; Bijev, A.; Prodanova, P. Synthesis and comparative stud of tuberculostatic activity of pyrrole-based hydrazones related to structural variations. Pharmacia 2010, 57, 3–14. [Google Scholar]
- Mateev, E.; Valkova, I.; Tzankova, D.; Georgieva, M.; Zlatkov, A. Optimizing molecular docking protocols of pyrrole containing MAO-B inhibitors through correlation coefficients. Proc. CBU Med. Pharm. 2021, 2, 92–98. [Google Scholar] [CrossRef]
- Sahakyan, H. Improving virtual screening results with MM/GBSA and MM/PBSA rescoring. J. Comput. Aided Mol. Des. 2021, 35, 731–736. [Google Scholar] [CrossRef]
- Taupin, P.; Zini, S.; Cesselin, F.; Ben-Ari, Y.; Roisin, M.P. Subcellular fractionation on Percoll gradient of mossy fiber synaptosomes: Morphological and biochemical characterization in control and degranulated rat hippocampus. J. Neurochem. 1994, 62, 1586–1595. [Google Scholar] [CrossRef]
- Parmentier, Y.; Bossant, M.-J.; Bertrand, M.; Walther, B. In Vitro studies of drug metabolism. In Comprehensive Medicinal Chemistry II. 5; Taylor, J.B., Triggle, D.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 231–257. [Google Scholar]
- Pourahmad, J.; Hosseini, M.J. Application of isolated mitochondria in toxicological and clinical studies. Iran. J. Pharm. Res. 2012, 11, 703–704. [Google Scholar] [PubMed]
- Karlsson, J.; Emgard, M.; Brundin, P.; Burkitt, M. Trans-Resveratrol protects embryonic mesencephalic cells from tert-butyl hydroperoxide: Electron paramagnetic resonance spin trapping evidence for a rdical scavenging mechanism. J. Neurochim. 2000, 75, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Mungarro-Menchaca, X.; Ferrera, P.; Moran, J.; Arias, C. Beta-Amyloid peptide induces ultrastructural changes in synaptosomes and potentiates mitochondrial dysfunction in the presence of ryanodine. J. Neurosci. Res. 2002, 68, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Robyt, J.F.; Ackerman, R.J.; Chittenden, C.G. Reaction of protein disulfide groups with Ellman’s reagent: A case study of the number of sulfhydryl and disulfide groups in Aspergillus oryzae-amylase, papain, and lysozyme. Arch. Biochem. Biophys. 1971, 147, 262–269. [Google Scholar] [CrossRef]
- Maryam, S.; Saeid, A.; Masoud, M.; Amin, D.M. The ameliorative effect of quercetin on bisphenol A- induced toxicity in mitochondria isolated from rats. Environ. Sci. Pollition Res. 2019, 26, 7688–7696. [Google Scholar] [CrossRef]
- Ravindranath, V.; Anandatheerthavarada, H.K. Preparation of brain microsomes with cytochrome P450 activity using calcium aggregation method. Anal. Biochem. 1990, 187, 310–313. [Google Scholar] [CrossRef]
- Mansuy, D.; Sassi, A.; Dansette, P.M.; Plat, M. A new potent inhibitor of lipid peroxidation in vitro and in vivo, the hepatoprotective drug anisyldithiolthione. Biochem. Biophys. Res. Commun. 1986, 135, 1015–1021. [Google Scholar] [CrossRef]
- Bautista-Aguilera, O.M.; Esteban, G.; Bolea, I.; Nikolic, K.; Agbaba, D.; Moraleda, I.; Iriepa, I.; Samadi, A.; Soriano, E.; Unzeta, M.; et al. Design, synthesis, pharmacological evaluation, QSAR analysis, molecular modeling and ADMET of novel donepezil-indolyl hybrids as multipotent cholinesterase/monoamine oxidase inhibitors for the potential treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014, 75, 82–95. [Google Scholar] [CrossRef]
- Kasabova-Angelova, A.; Kondeva-Burdina, M.; Mitkov, J.; Georgieva, M.; Tzankova, V.; Zlatkov, A. Neuroprotective and MAOB inhibitory effects of a series of caffeine-8-thioglycolic acid amides. Br. J. Pharm. Sci. 2019, 55, 1–9. [Google Scholar] [CrossRef]


| Docked Compounds | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 12a | 12 | 11c | 11 | 11d | 11n | 11b | 11l | Selegiline | Safinamide | |
| ChemPLP fitness score | 124.38 | 132.97 | 129.15 | 134.40 | 109.22 | 123.85 | 115.70 | 96.88 | 140.28 | 166.40 |
| MM/GBSA | −56.21 | −54.46 | −46.44 | −44.15 | −41.04 | −33.85 | −12.31 | 57.59 | −50.80 | −79.72 |
| Compound | Hydrophobic Interaction | Polar Interaction | Hydrogen Bond | π–π Interaction | Steric Clashes |
|---|---|---|---|---|---|
| 11 | Tyr60, Pro102, Trp119, Leu164, Leu167, Phe168, Leu171, Cys172, Tyr188, Ile198, Ile199, Ile316, Leu328, Met341, Phe343, Tyr398, Trp432, Tyr435 | Gln206 | - | Tyr326 | - |
| 11l | Tyr60, Pro102, Trp119, Leu164, Leu167, Phe168, Leu171, Cys172, Val173, Tyr188, Ile198, Ile199, Ile316, Leu328, Met341, Phe343, Tyr398, Trp432, Tyr435 | His115, Ser200, Thr201, Gln206, Thr314 | Tyr188 | Tyr398 | Glu84, Leu164, Ile199 |
| 12 | Tyr60, Pro102, Phe103, Pro104, Trp119, Leu164, Leu167, Phe168, Leu171, Cys172, Val173, Tyr188, Ile198, Ile199, Ile316, Leu328, Met341, Phe343, Tyr398, Trp432, Tyr435 | Gln206 | - | Tyr326 | - |
| 12a | Tyr60, Pro102, Phe103, Pro104, Trp119, Leu164, Leu167, Phe168, Leu171, Cys172, Tyr188, Ile198, Ile199, Ile316, Leu328, Met341, Phe343, Tyr398, Tyr435 | Gln206 | - | Try326, Tyr398 | - |
| Selegiline | Tyr60, Phe168, Ile199, Gln206, Leu328, Met341, Phe343, Tyr326, Tyr398, Tyr435 | Gln206 | FAD | Leu171, Cys172, Ile198 | - |
| Safinamide | Tyr60, Phe103, Pro104, Leu171, Ile199, Gln206, Phe343, Tyr398, Tyr435 | Gln206 | Ile199, FAD | Leu171, Ile199, Ile316, Tyr326 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondeva-Burdina, M.; Mateev, E.; Angelov, B.; Tzankova, V.; Georgieva, M. In Silico Evaluation and In Vitro Determination of Neuroprotective and MAO-B Inhibitory Effects of Pyrrole-Based Hydrazones: A Therapeutic Approach to Parkinson’s Disease. Molecules 2022, 27, 8485. https://doi.org/10.3390/molecules27238485
Kondeva-Burdina M, Mateev E, Angelov B, Tzankova V, Georgieva M. In Silico Evaluation and In Vitro Determination of Neuroprotective and MAO-B Inhibitory Effects of Pyrrole-Based Hydrazones: A Therapeutic Approach to Parkinson’s Disease. Molecules. 2022; 27(23):8485. https://doi.org/10.3390/molecules27238485
Chicago/Turabian StyleKondeva-Burdina, Magdalena, Emilio Mateev, Borislav Angelov, Virginia Tzankova, and Maya Georgieva. 2022. "In Silico Evaluation and In Vitro Determination of Neuroprotective and MAO-B Inhibitory Effects of Pyrrole-Based Hydrazones: A Therapeutic Approach to Parkinson’s Disease" Molecules 27, no. 23: 8485. https://doi.org/10.3390/molecules27238485
APA StyleKondeva-Burdina, M., Mateev, E., Angelov, B., Tzankova, V., & Georgieva, M. (2022). In Silico Evaluation and In Vitro Determination of Neuroprotective and MAO-B Inhibitory Effects of Pyrrole-Based Hydrazones: A Therapeutic Approach to Parkinson’s Disease. Molecules, 27(23), 8485. https://doi.org/10.3390/molecules27238485








