Effect of IL-27, Teriflunomide and Retinoic Acid and Their Combinations on CD4+ T Regulatory T Cells—An In Vitro Study
Abstract
1. Introduction
2. Results
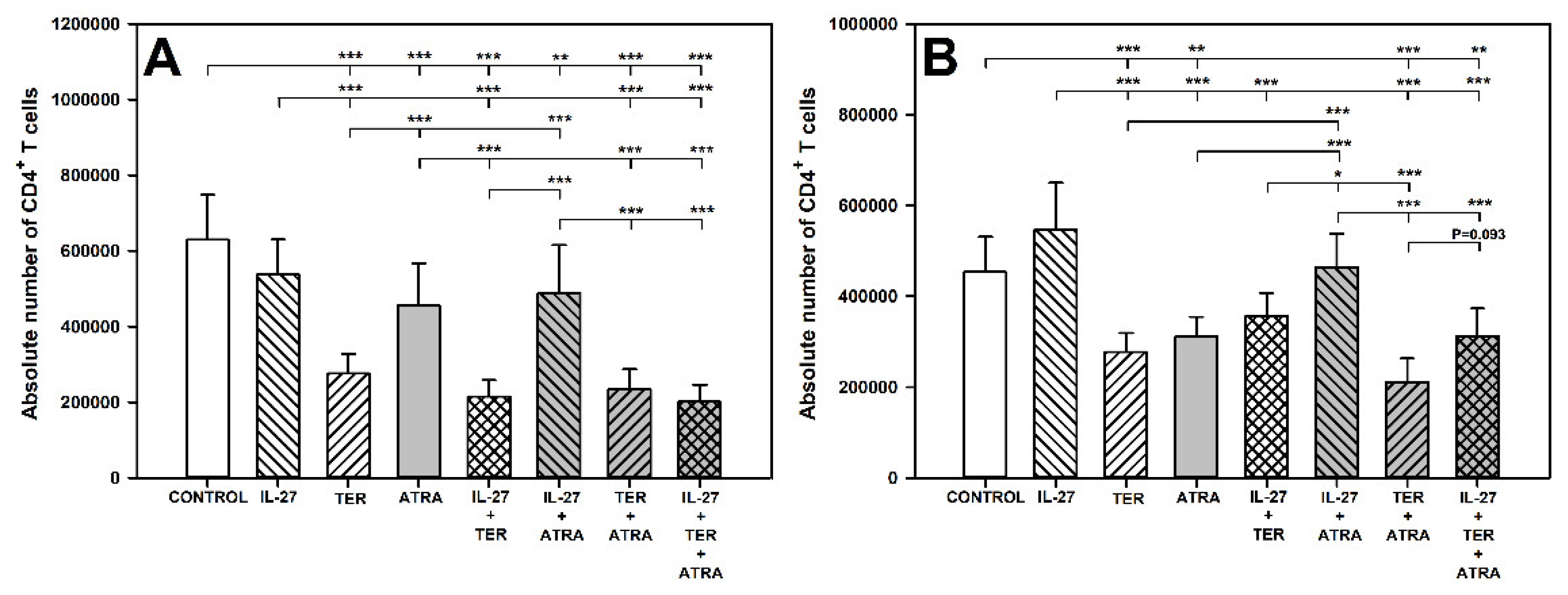
2.1. TER and ATRA Deplete CD4+ T Cells
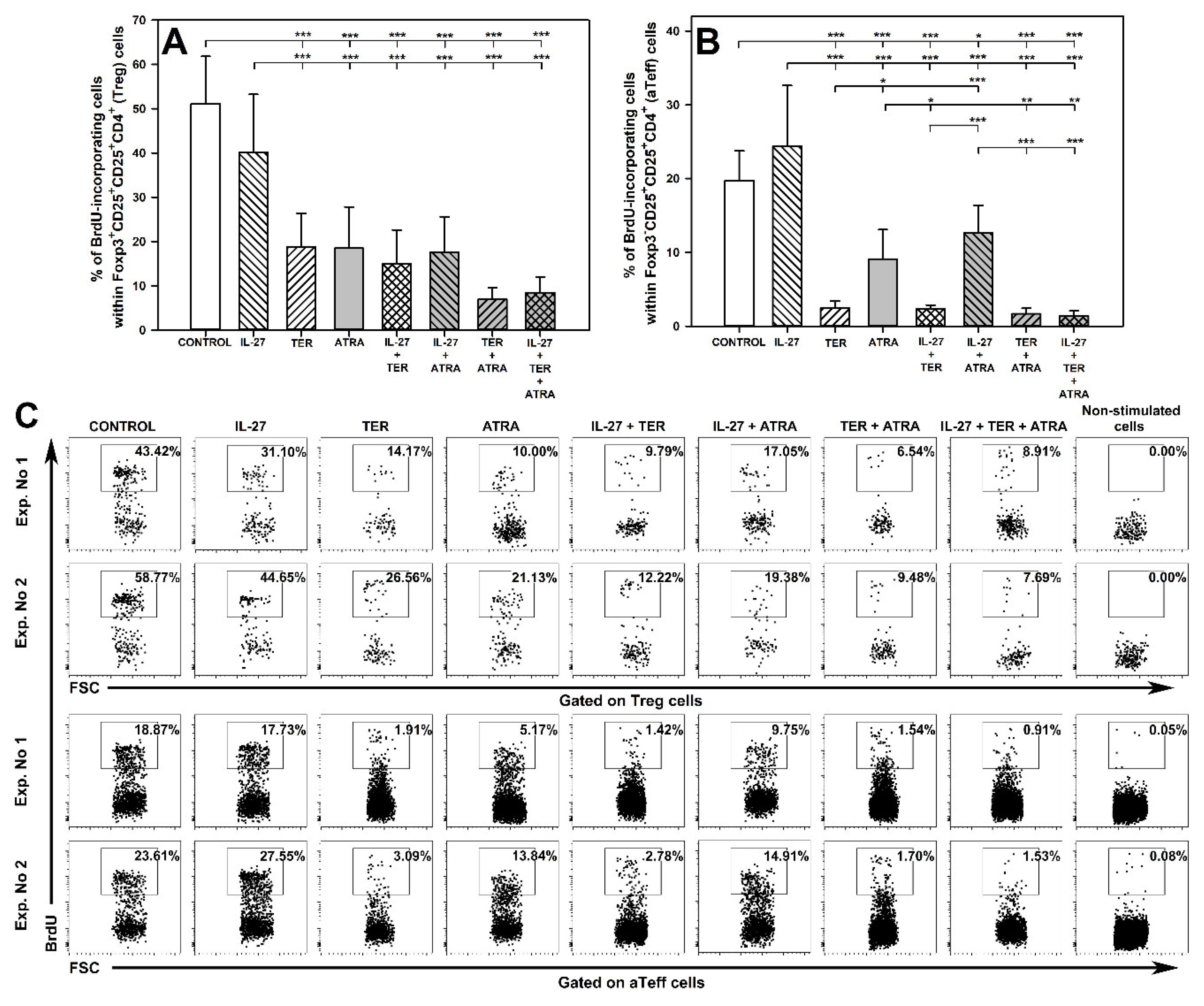
2.2. TER and ATRA, but Not IL-27, Reduce the Number of Proliferating Treg and Teff Cells
2.3. TER Increases the Percentage and Absolute Count of Foxp3+CD25very highCD4+ T Cells under Activation Conditions
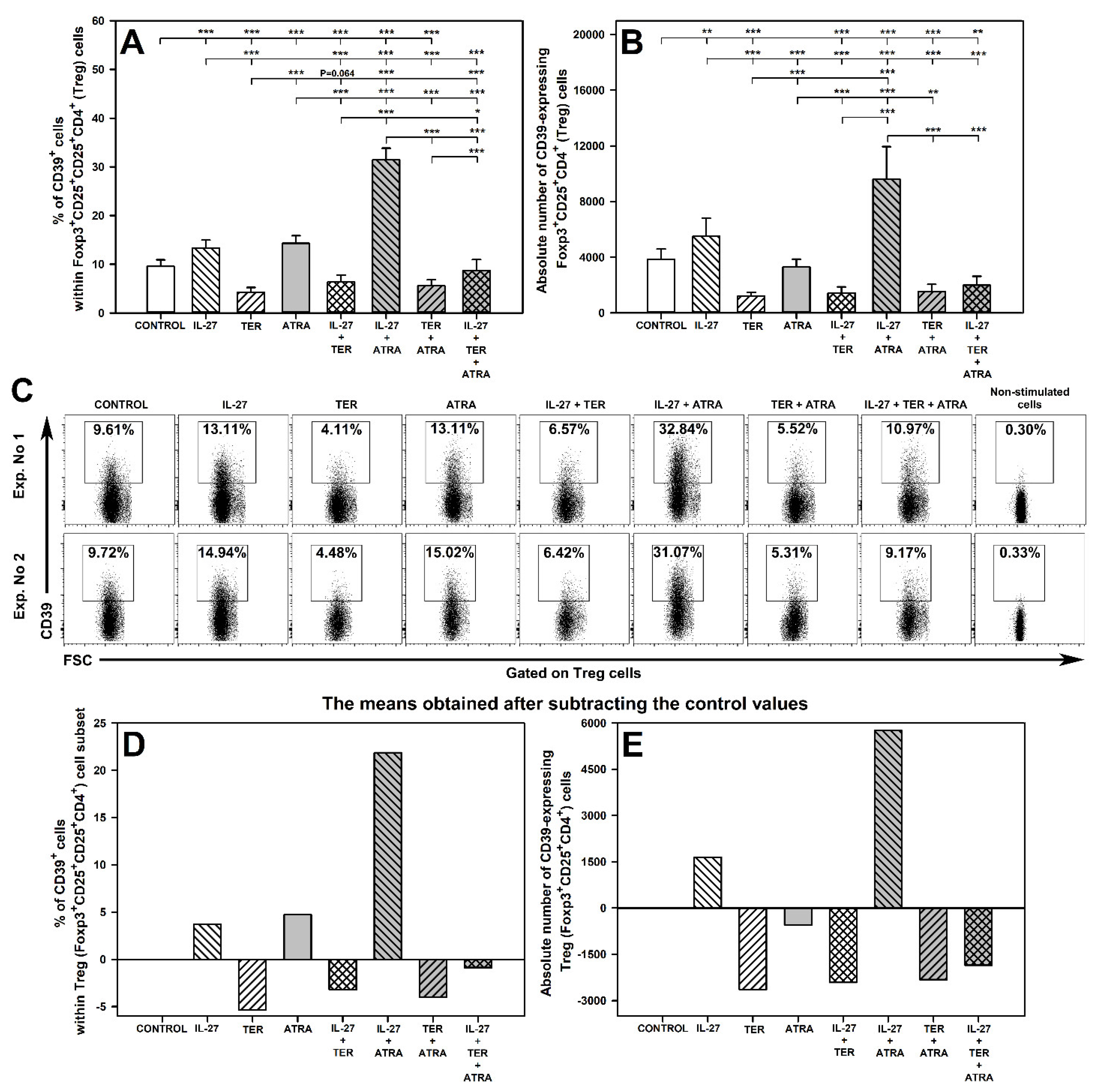
2.4. IL-27 and ATRA Exert a Synergistic Effect on Induction of CD39 Expression on Treg Cells under Activation Conditions
2.5. Under Unstimulated Conditions IL-27 Decreases and TER Increases the Percentage of Treg Cells and IL-27 Increases the Percentage and Absolute Count of CD39-Expressing Treg Cells
2.6. IL-27- and ATRA-Induced Superadditive Effect on the Induction of CD39 Expression on Treg Cells Is Dose-Dependent
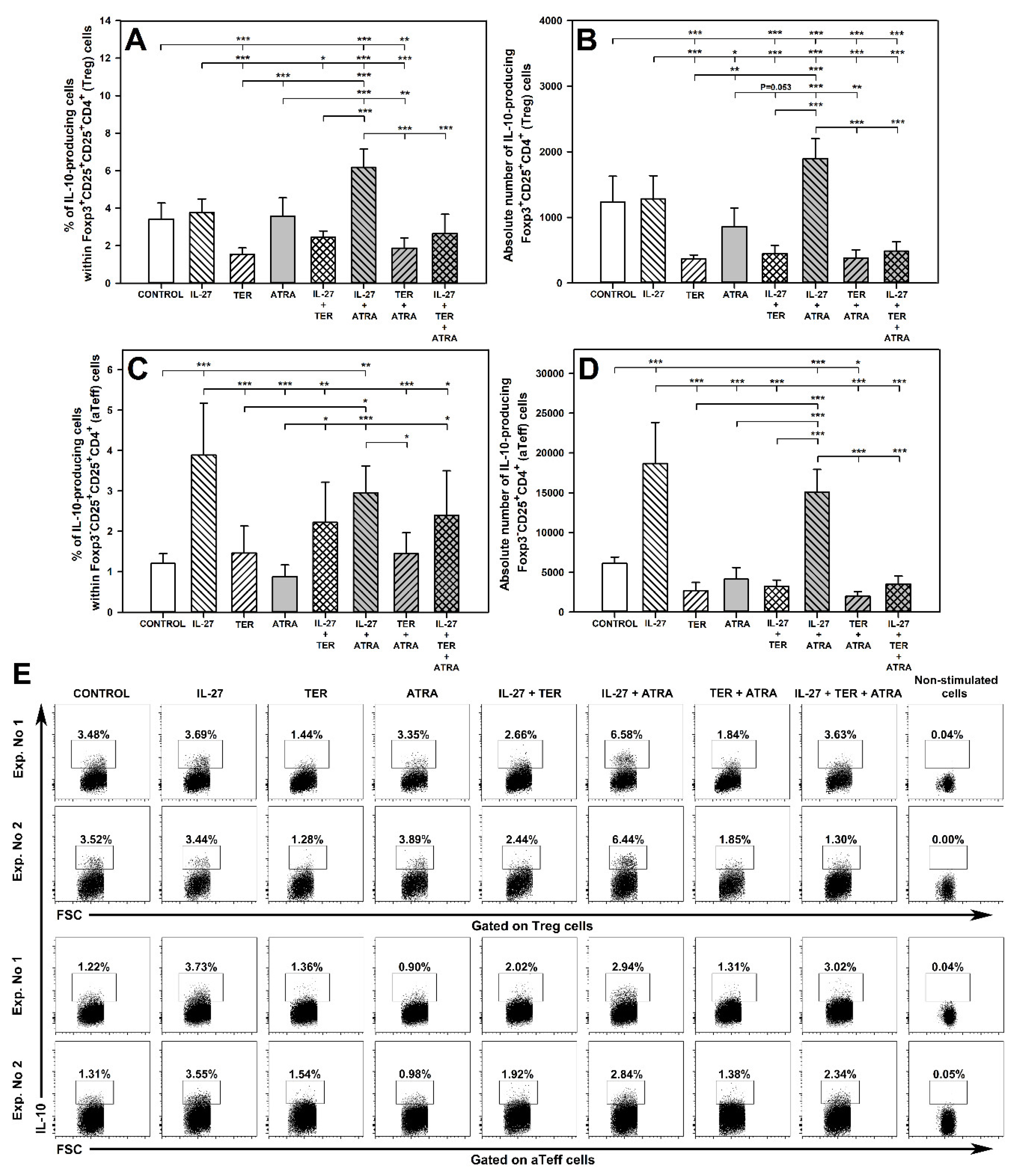
2.7. IL-27 and ATRA in Combination, but Not Alone, Increase the Percentage and Absolute Count of IL-10-Producing Treg Cells and TER Decreases the Percentage and Absolute Count of IL-10-Producing Treg Cells
2.8. IL-27 Induces an Increase in the Percentage and Absolute Number of IL-10-Producing aTeff Cells and TER Antagonizes This Effect
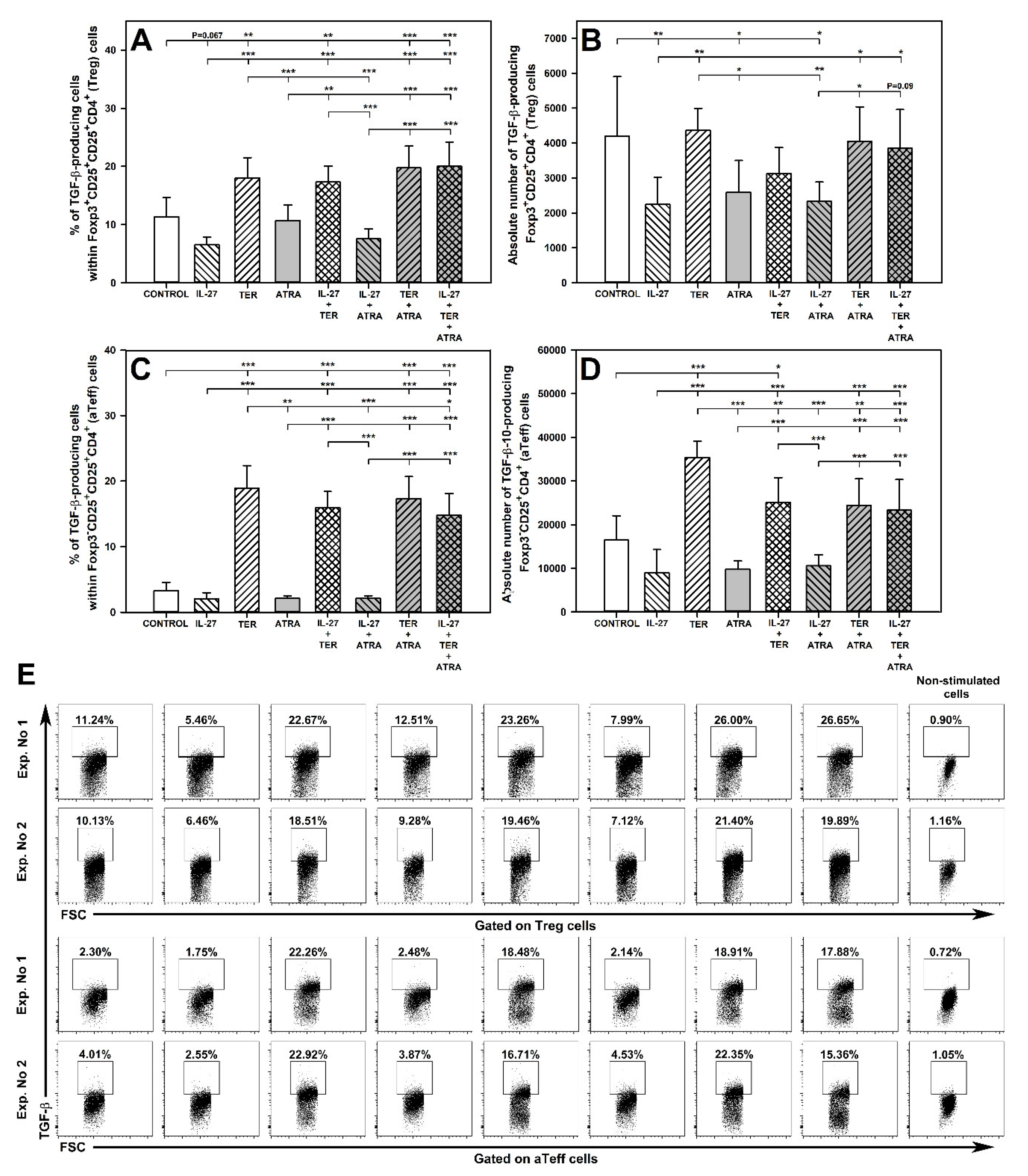
2.9. TER Increases the Percentage of TGF-β-Producing Treg Cells
2.10. TER Induces an Increase in the Percentage and Absolute Number of TGF-β-Producing aTeff Cells
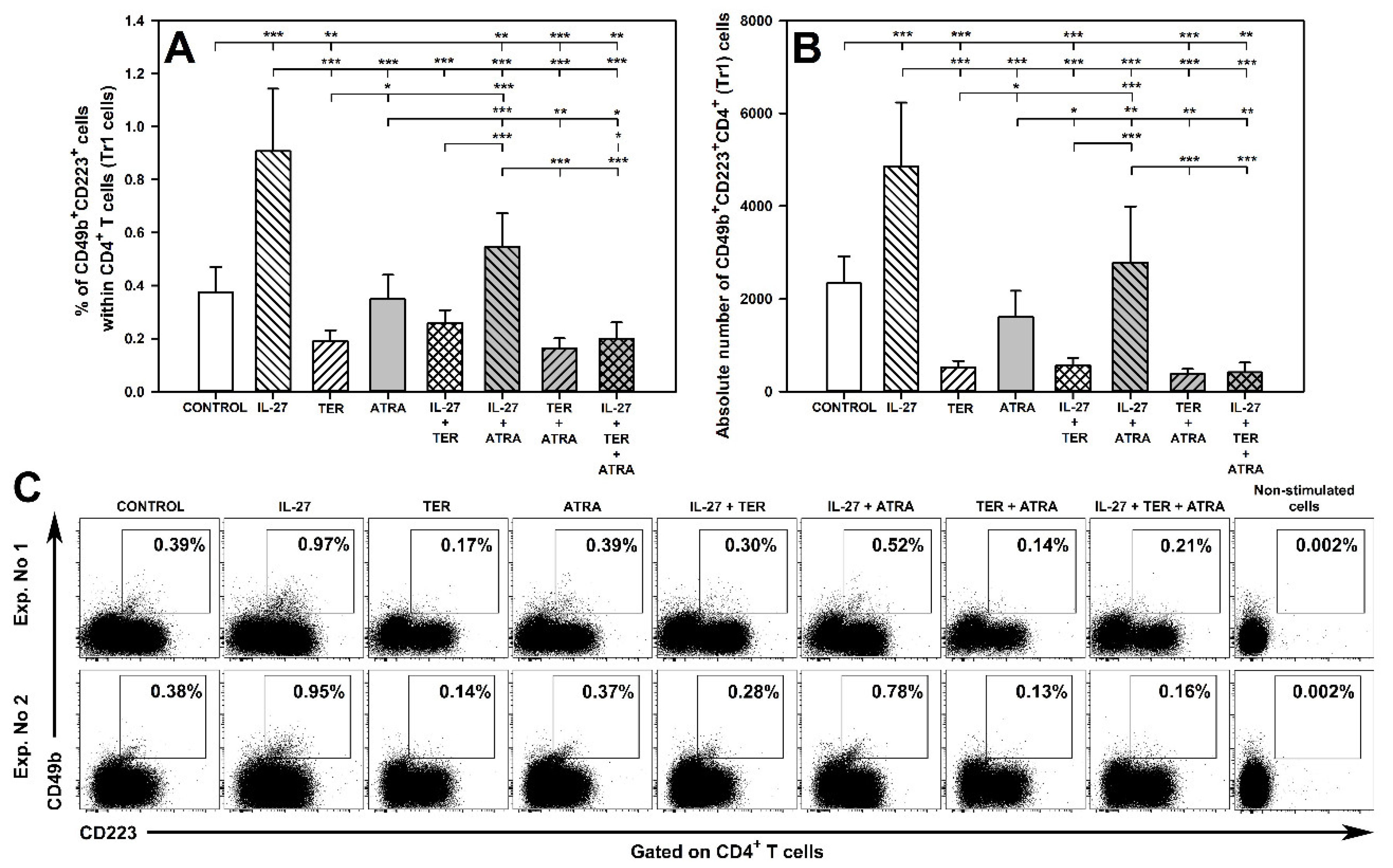
2.11. TER Decreases the Induction of Tr1 Cells as Well as Abrogates IL-27-Mediated Induction of These Cells
3. Discussion
- IL-27: (a) induced CD39 expression on Treg cells; (b) induced the ability of aTeff cells to IL-10 production; (c) induced the generation of Tr1 cells.
- TER: (a) induced Foxp3-expressing CD4+ T cells characterized by more dense expression of CD25; (b) induced the ability of Treg and aTeff cells to TGF-β production.
- ATRA: induced CD39 expression on Treg cells.
- In turn, the undesired effects of each substance can be summarized as follows:
- IL-27: down-regulated Foxp3 expression on Treg cells under unstimulated conditions.
- TER: (a) down-regulated CD39 expression on Treg cells; (b) suppressed the capacity of Treg cells to produce IL-10; (c) counteracted the induction of Tr1 cells.
4. Materials and Methods
4.1. Animals
4.2. Isolation of Lymphocytes
4.3. In Vitro Stimulation and Culture Conditions
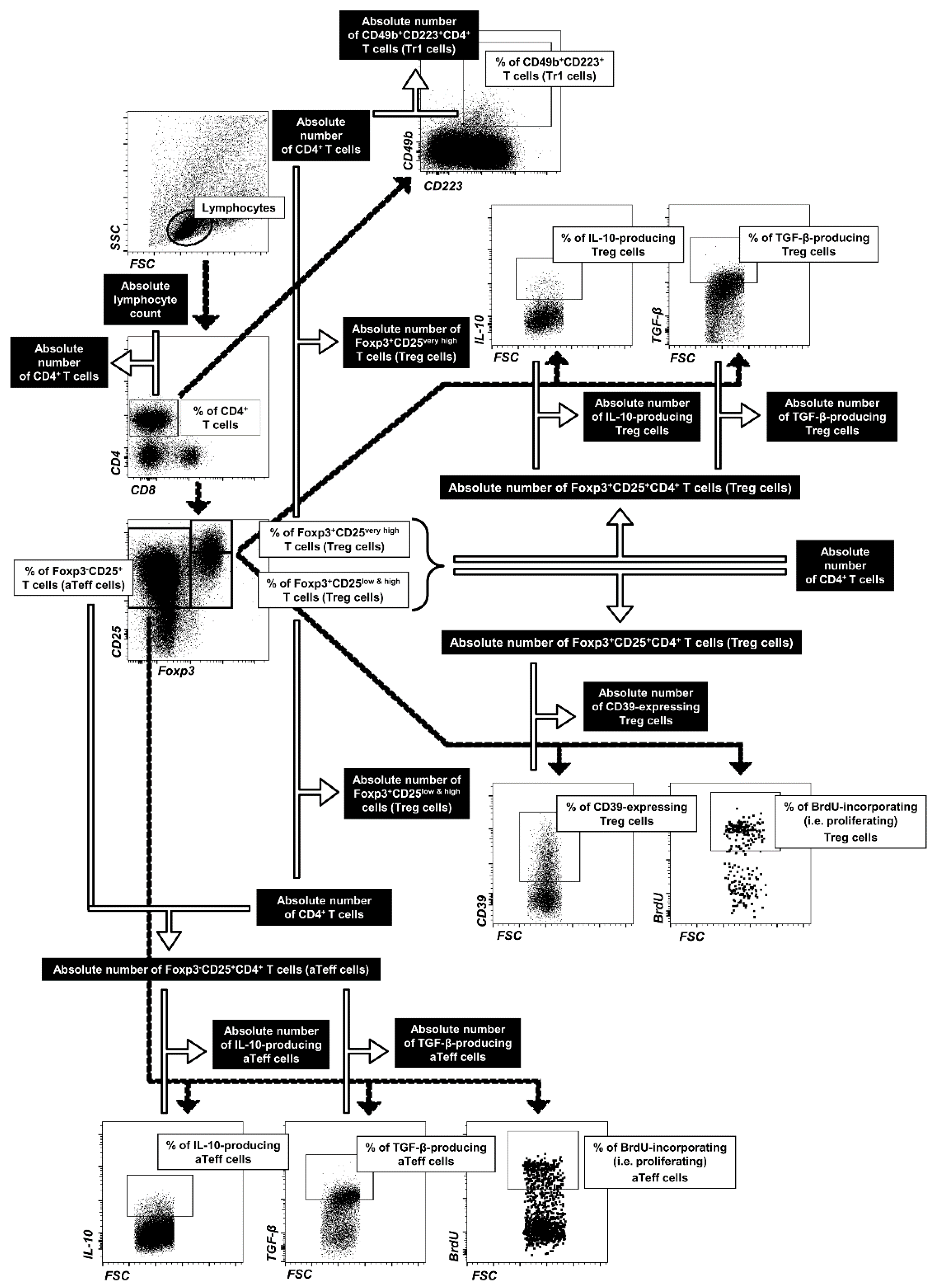
4.4. Flow Cytometry
4.4.1. Extracellular Staining
4.4.2. Intracellular Staining for Determination of Treg and Tr1 Cells
4.4.3. Intracellular Staining for Determination of IL-10- and TGF-β-Producing Treg and aTeff
4.4.4. Intracellular Staining for Determination of Proliferating Treg and aTeff Cells
4.5. FACS Acquisition and Data Analysis
4.6. Statistical Analyses
4.7. The Approach to Interpreting the Absence/Presence of Interactions between the Evaluated Agents and Types of These Interactions
5. Conclusions
6. Limitation of the Study
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fontenot, J.D.; Rudensky, A.Y. A well adapted regulatory contrivance: Regulatory T cell development and the forkhead family transcription factor Foxp3. Nat. Immunol. 2005, 6, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Trinath, J.; Hegde, P.; Sharma, M.; Maddur, M.S.; Rabin, M.; Vallat, J.M.; Magy, L.; Balaji, K.N.; Kaveri, S.V.; Bayry, J. Intravenous immunoglobulin expands regulatory T cells via induction of cyclooxygenase-2-dependent prostaglandin E2 in human dendritic cells. Blood 2013, 122, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.G.; Williams, C.B. Generation and function of induced regulatory T cells. Front. Immunol. 2013, 4, 152. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.F. FOXP3: Of mice and men. Annu. Rev. Immunol. 2006, 24, 209–226. [Google Scholar] [CrossRef]
- Bailey-Bucktrout, S.L.; Martinez-Llordella, M.; Zhou, X.; Anthony, B.; Rosenthal, W.; Luche, H.; Fehling, H.J.; Bluestone, J.A. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 2013, 39, 949–962. [Google Scholar] [CrossRef]
- Komatsu, N.; Okamoto, K.; Sawa, S.; Nakashima, T.; Oh-hora, M.; Kodama, T.; Tanaka, S.; Bluestone, J.A.; Takayanagi, H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 2014, 20, 62–68. [Google Scholar] [CrossRef]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef]
- Cobbold, S.P.; Castejon, R.; Adams, E.; Zelenika, D.; Graca, L.; Humm, S.; Waldmann, H. Induction of FoxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J. Immunol. 2004, 172, 6003–6010. [Google Scholar] [CrossRef]
- Park, H.B.; Paik, D.J.; Jang, E.; Hong, S.; Youn, J. Acquisition of anergic and suppressive activities in transforming growth factor-beta-costimulated CD4+CD25− T cells. Int. Immunol. 2004, 16, 1203–1213. [Google Scholar] [CrossRef]
- Shevach, E.M. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity 2009, 30, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Bastid, J.; Regairaz, A.; Bonnefoy, N.; Déjou, C.; Giustiniani, J.; Laheurte, C.; Cochaud, S.; Laprevotte, E.; Funck-Brentano, E.; Hemon, P.; et al. Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity. Cancer Immunol. Res. 2015, 3, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Zarek, P.E.; Huang, C.T.; Lutz, E.R.; Kowalski, J.; Horton, M.R.; Linden, J.; Drake, C.G.; Powell, J.D. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 2008, 111, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Gerner, M.C.; Ziegler, L.S.; Schmidt, R.L.J.; Krenn, M.; Zimprich, F.; Uyanik-Ünal, K.; Konstantopoulou, V.; Derdak, S.; Del Favero, G.; Schwarzinger, I.; et al. The TGF-b/SOX4 axis and ROS-driven autophagy co-mediate CD39 expression in regulatory T-cells. FASEB J. 2020, 34, 8367–8384. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal, M.R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Palomares, O.; Martín-Fontecha, M.; Lauener, R.; Traidl-Hoffmann, C.; Cavkaytar, O.; Akdis, M.; Akdis, C.A. Regulatory T cells and immune regulation of allergic diseases: Roles of IL-10 and TGF-β. Genes Immun. 2014, 15, 511–520. [Google Scholar] [CrossRef]
- Nakamura, K.; Kitani, A.; Strober, W. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J. Exp. Med. 2001, 194, 629–644. [Google Scholar] [CrossRef]
- Levings, M.K.; Sangregorio, R.; Sartirana, C.; Moschin, A.L.; Battaglia, M.; Orban, P.C.; Roncarolo, M.G. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J. Exp. Med. 2002, 196, 1335–1346. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Flavell, R.A. T Cell-Produced Transforming Growth Factor-Beta1 Controls T Cell Tolerance and Regulates Th1- and Th17-Cell Differentiation. Immunity 2007, 26, 579–591. [Google Scholar] [CrossRef]
- Wang, J.; Li, T.; Wang, J.L.; Xu, Z.; Meng, W.; Wu, Q.F. Talpid3-Mediated Centrosome Integrity Restrains Neural Progenitor Delamination to Sustain Neurogenesis by Stabilizing Adherens Junctions. Cell. Rep. 2020, 33, 108495. [Google Scholar] [CrossRef]
- Cosovanu, C.; Neumann, C. The Many Functions of Foxp3+ Regulatory T Cells in the Intestine. Front. Immunol. 2020, 11, 600973. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Gregori, S.; Bacchetta, R.; Roncarolo, M.G. Tr1 cells: From discovery to their clinical application. Semin. Immunol. 2006, 18, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Gagliani, N.; Magnani, C.F.; Huber, S.; Gianolini, M.E.; Pala, M.; Licona-Limon, P.; Guo, B.; Herbert, D.R.; Bulfone, A.; Trentini, F.; et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat. Med. 2013, 19, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Roncarolo, M.G.; Gregori, S.; Battaglia, M.; Bacchetta, R.; Fleischhauer, K.; Levings, M.K. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 2006, 212, 28–50. [Google Scholar] [CrossRef]
- Eggenhuizen, P.J.; Ng, B.H.; Ooi, J.D. Treg Enhancing Therapies to Treat Autoimmune Diseases. Int. J. Mol. Sci. 2020, 21, 7015. [Google Scholar] [CrossRef]
- Bayati, F.; Mohammadi, M.; Valadi, M.; Jamshidi, S.; Foma, A.M.; Sharif-Paghaleh, E. The Therapeutic Potential of Regulatory T Cells: Challenges and Opportunities. Front. Immunol. 2021, 11, 585819. [Google Scholar] [CrossRef]
- Goswami, T.K.; Singh, M.; Dhawan, M.; Mitra, S.; Emran, T.B.; Rabaan, A.A.; Mutair, A.A.; Alawi, Z.A.; Alhumaid, S.; Dhama, K. Regulatory T cells (Tregs) and their therapeutic potential against autoimmune disorders—Advances and challenges. Hum. Vaccin. Immunother. 2022, 18, 2035117. [Google Scholar] [CrossRef]
- Karagiannidis, C.; Akdis, M.; Holopainen, P.; Woolley, N.J.; Hence, G.; Rückert, B.; Mantel, P.Y.; Menz, G.; Akdis, C.A.; Blaser, K.; et al. Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J. Allergy Clin. Immunol. 2004, 114, 1425–1433. [Google Scholar] [CrossRef]
- Dao Nguyen, X.; Robinson, D.S. Fluticasone propionate increases CD4+CD25+ T regulatory cell suppression of allergen-stimulated CD4+CD25− T cells by an IL-10-dependent mechanism. J. Allergy Clin. Immunol. 2004, 114, 296–301. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Z.; Zhu, B.; Ma, Z. Peroxisome proliferator-activated receptor-gamma agonist induces regulatory T cells in a murine model of allergic rhinitis. Otolaryngol. Head Neck Surg. 2011, 144, 506–513. [Google Scholar] [CrossRef]
- Min, S.Y.; Yan, M.; Du, Y.; Wu, T.; Khobahy, E.; Kwon, S.R.; Taneja, V.; Bashmakov, A.; Nukala, S.; Ye, Y.; et al. Intra-articular nuclear factor-κB blockade ameliorates collagen-induced arthritis in mice by eliciting regulatory T cells and macrophages. Clin. Exp. Immunol. 2013, 172, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Wang, Y.; Lu, Y.; Jiang, Y.H.; Zhao, D.Y. Effects of sublingual immunotherapy for Dermatophagoides farinae on Th17 cells and CD4+CD25+ regulatory T cells in peripheral blood of children with allergic asthma. Int. Forum Allergy Rhinol. 2014, 4, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Repáraz, J.; Colpitts, S.L.; Kircher, C.; Kasper, E.J.; Telesford, K.M.; Begum-Haque, S.; Pant, A.; Kasper, L.H. Induction of gut regulatory CD39+ T cells by teriflunomide protects against EAE. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e291. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Siegmund, S.; Garbers, C. The biology of interleukin-27 reveals unique pro- and anti-inflammatory functions in immunity. Cytokine Growth Factor Rev. 2015, 26, 579–586. [Google Scholar] [CrossRef]
- Hanson, M.L.; Hixon, J.A.; Li, W.; Felber, B.K.; Anver, M.R.; Stewart, C.A.; Janelsins, B.M.; Datta, S.K.; Shen, W.; McLean, M.H.; et al. Oral delivery of IL-27 recombinant bacteria attenuates immune colitis in mice. Gastroenterology 2014, 146, 210–221. [Google Scholar] [CrossRef]
- Hirahara, K.; Ghoreschi, K.; Yang, X.P.; Takahashi, H.; Laurence, A.; Vahedi, G.; Sciumè, G.; Hall, A.O.; Dupont, C.D.; Francisco, L.M.; et al. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity 2012, 36, 1017–1030. [Google Scholar] [CrossRef]
- Niedbala, W.; Cai, B.; Wei, X.; Patakas, A.; Leung, B.P.; McInnes, I.B.; Liew, F.Y. Interleukin 27 attenuates collagen-induced arthritis. Ann. Rheum. Dis. 2008, 67, 1474–1479. [Google Scholar] [CrossRef]
- Cherwinski, H.M.; Cohn, R.G.; Cheung, P.; Webster, D.J.; Xu, Y.Z.; Caulfield, J.P.; Young, J.M.; Nakano, G.; Ransom, J.T. The immunosuppressant leflunomide inhibits lymphocyte proliferation by inhibiting pyrimidine biosynthesis. J. Pharmacol. Exp. Ther. 1995, 275, 1043–1049. [Google Scholar]
- Nwankwo, E.; Allington, D.R.; Rivey, M.P. Emerging oral immunomodulating agents–focus on teriflunomide for the treatment of multiple sclerosis. Degener. Neurol. Neuromuscul. Dis. 2012, 2, 15–28. [Google Scholar]
- Kaltwasser, J.P.; Nash, P.; Gladman, D.; Rosen, C.F.; Behrens, F.; Jones, P.; Wollenhaupt, J.; Falk, F.G.; Mease, P. Treatment of Psoriatic Arthritis Study Group. Efficacy and safety of leflunomide in the treatment of psoriatic arthritis and psoriasis: A multinational, double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum. 2004, 50, 1939–1950. [Google Scholar] [CrossRef]
- Osiri, M.; Deesomchok, U.; Tugwell, P. Disease activity and functional changes of RA patients receiving different DMARDs in clinical practice. Clin. Rheumatol. 2006, 25, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, Z.; Zhang, P.; Chen, W. Antisense oligonucleotides and all-trans retinoic acid have a synergistic anti-tumor effect on oral squamous cell carcinoma. BMC Cancer 2008, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Chambon, P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996, 10, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Siddikuzzaman; Guruvayoorappan, C.; Berlin Grace, V.M. All trans retinoic acid and cancer. Immunopharmacol. Immunotoxicol. 2011, 33, 241–249. [Google Scholar] [CrossRef]
- Awasthi, A.; Carrier, Y.; Peron, J.P.; Bettelli, E.; Kamanaka, M.; Flavell, R.A.; Kuchroo, V.K.; Oukka, M.; Howard, L.; Weiner, H.L. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 2007, 8, 1380–1389. [Google Scholar] [CrossRef]
- Fitzgerald, D.C.; Zhang, G.X.; El-Behi, M.; Fonseca-Kelly, Z.; Li, H.; Yu, S.; Saris, C.J.; Gran, B.; Ciric, B.; Rostami, A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat. Immunol. 2007, 8, 1372–1379. [Google Scholar] [CrossRef]
- Stumhofer, J.S.; Silver, J.S.; Laurence, A.; Porrett, P.M.; Harris, T.H.; Turka, L.A.; Ernst, M.; Saris, C.J.; O’Shea, J.J.; Hunter, C.A. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 2007, 8, 1363–1371. [Google Scholar] [CrossRef]
- Batten, M.; Kljavin, N.M.; Li, J.; Walter, M.J.; de Sauvage, F.J.; Ghilardi, N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J. Immunol. 2008, 180, 2752–2756. [Google Scholar] [CrossRef]
- Jasiecka-Mikołajczyk, A.; Socha, P. Teriflunomide inhibits activation-induced CD25 expression on T cells and may affect Foxp3-expressing regulatory T cells. Res. Vet. Sci. 2020, 132, 17–27. [Google Scholar] [CrossRef]
- Klotz, L.; Eschborn, M.; Lindner, M.; Liebmann, M.; Herold, M.; Janoschka, C.; Torres Garrido, B.; Schulte-Mecklenbeck, A.; Gross, C.C.; Breuer, J.; et al. Teriflunomide Treatment for Multiple Sclerosis Modulates T Cell Mitochondrial Respiration with Affinity-Dependent Effects. Sci. Transl. Med. 2019, 11, eaao5563. [Google Scholar] [CrossRef]
- Cao, W.W.; Kao, P.N.; Aoki, Y.; Xu, J.C.; Shorthouse, R.A.; Morris, R.E. A novel mechanism of action of the immunomodulatory drug, leflunomide: Augmentation of the immunosuppressive cytokine, TGF-β1, and suppression of the immunostimulatory cytokine, IL-2. Transplant. Proc. 1996, 28, 3079–3080. [Google Scholar] [PubMed]
- Benson, M.J.; Pino-Lagos, K.; Rosemblatt, M.; Noelle, R.J. All-trans retinoic acid mediates enhanced Treg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 2007, 204, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.L.; Siddiqui, K.R.; Arancibia-Cárcamo, C.V.; Hall, J.; Sun, C.M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Jin, H.; Korn, T.; Liu, S.M.; Oukka, M.; Lim, B.; Kuchroo, V.K. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J. Immunol. 2008, 181, 2277–2284. [Google Scholar] [CrossRef]
- Ringshausen, I.; Oelsner, M.; Bogner, C.; Peschel, C.; Decker, T. The immunomodulatory drug Leflunomide inhibits cell cycle progression of B-CLL cells. Leukemia 2008, 22, 635–638. [Google Scholar] [CrossRef]
- Szondy, Z.; Reichert, U.; Fésüs, L. Retinoic acids regulate apoptosis of T lymphocytes through an interplay between RAR and RXR receptors. Cell Death Differ. 1998, 5, 4–10. [Google Scholar] [CrossRef]
- Wiendl, H.; Gross, C.C.; Lindner, M.; Eschborn, M.; Weisser, L.; Posevitz-Fejfar, A.; Schulte-Mecklenbeck, A.; Brette, S.; Turner, T.J.; Jagerschmidt, A.; et al. T- and B-Lymphocyte Modulation Associated with Teriflunomide Treatment in Patients with Relapsing-Remitting MS: Analysis of the Phase 3b Teri-DYNAMIC Study. In Proceedings of the Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC), National Harbor, MD, USA, 1–4 June 2016; Volume SC03. [Google Scholar]
- Gandoglia, I.; Ivaldi, F.; Laroni, A.; Benvenuto, F.; Solaro, C.; Mancardi, G.; Kerlero de Rosbo, N.; Uccelli, A. Teriflunomide treatment reduces B cells in patients with MS. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e403. [Google Scholar] [CrossRef]
- Tang, D.; Liu, S.; Sun, H.; Qin, X.; Zhou, N.; Zheng, W.; Zhang, M.; Zhou, H.; Tuersunayi, A.; Duan, C.; et al. All-trans-retinoic acid shifts Th1 towards Th2 cell differentiation by targeting NFAT1 signalling to ameliorate immune-mediated aplastic anaemia. Br. J. Haematol. 2020, 191, 906–919. [Google Scholar] [CrossRef]
- Jacobsen, S.E.; Fahlman, C.; Blomhoff, H.K.; Okkenhaug, C.; Rusten, L.S.; Smeland, E.B. All-trans- and 9-cis-retinoic acid: Potent direct inhibitors of primitive murine hematopoietic progenitors in vitro. J. Exp. Med. 1994, 179, 1665–1670. [Google Scholar] [CrossRef]
- Nonnecke, B.J.; Franklin, S.T.; Horst, R.L.; Reinhardt, T.A. In vitro modulation of function, proliferation, and phenotype of bovine mononuclear leukocytes by 13-cis retinoic acid. J. Nutr. Immunol. 1994, 2, 39–59. [Google Scholar] [CrossRef]
- Tang, Q.; Bluestone, J.A. The Foxp3+ regulatory T cell: A jack of all trades, master of regulation. Nat. Immunol. 2008, 9, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Shevach, E.M. Foxp3+ T Regulatory Cells: Still Many Unanswered Questions-A Perspective after 20 Years of Study. Front. Immunol. 2018, 15, 1048. [Google Scholar] [CrossRef] [PubMed]
- Pandiyan, P.; Zheng, L.; Ishihara, S.; Reed, J.; Lenardo, M.J. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 2007, 8, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- McNally, A.; Hill, G.R.; Sparwasser, T.; Thomas, R.; Steptoe, R.J. CD4+CD25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasis. Proc. Natl. Acad. Sci. USA 2011, 108, 7529–7534. [Google Scholar] [CrossRef]
- Chinen, T.; Kannan, A.K.; Levine, A.G.; Fan, X.; Klein, U.; Zheng, Y.; Gasteiger, G.; Feng, Y.; Fontenot, J.D.; Rudensky, A.Y. An essential role for the IL-2 receptor in Treg cell function. Nat. Immunol. 2016, 17, 1322–1333. [Google Scholar] [CrossRef]
- Do, J.S.; Visperas, A.; Sanogo, Y.O.; Bechtel, J.J.; Dvorina, N.; Kim, S.; Jang, E.; Stohlman, S.A.; Shen, B.; Fairchild, R.L.; et al. An IL-27/Lag3 axis enhances Foxp3+ regulatory T cell-suppressive function and therapeutic efficacy. Mucosal. Immunol. 2016, 9, 137–145. [Google Scholar] [CrossRef]
- Batten, M.; Li, J.; Yi, S.; Kljavin, N.M.; Danilenko, D.M.; Lucas, S.; Lee, J.; de Sauvage, F.J.; Ghilardi, N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 2006, 7, 929–936. [Google Scholar] [CrossRef]
- Diveu, C.; McGeachy, M.J.; Boniface, K.; Stumhofer, J.S.; Sathe, M.; Joyce-Shaikh, B.; Chen, Y.; Tato, C.M.; McClanahan, T.K.; de Waal Malefyt, R.; et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J. Immunol. 2009, 182, 5748–5756. [Google Scholar] [CrossRef]
- Park, Y.J.; Ryu, H.; Choi, G.; Kim, B.S.; Hwang, E.S.; Kim, H.S.; Chung, Y. IL-27 confers a protumorigenic activity of regulatory T cells via CD39. Proc. Natl. Acad. Sci. USA 2019, 116, 3106–3111. [Google Scholar] [CrossRef]
- Tilly, G.; Cadoux, M.; Garcia, A.; Morille, J.; Wiertlewski, S.; Pecqueur, C.; Brouard, S.; Laplaud, D.A.; Degauque, N. Teriflunomide treatment of multiple sclerosis selectively modulates CD8 memory T cells. Front. Immunol. 2021, 12, 730342. [Google Scholar] [CrossRef]
- Saeed, M.H.; Kurosh, K.; Zahra, A.; Hossein, D.M.; Davood, R.; Ataollahi, M.R. Decreased serum levels of IL-27and IL-35 in patients with Graves disease. Arch. Endocrinol. Metab. 2021, 64, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Aubagio®, Assessment Report. International Non-Proprietary Name: Teriflunomide. Procedure No. EMEA/H/C/002514/0000. 27 June 2013 EMA/529295/2013. Available online: https://www.ema.europa.eu/en/documents/assessment-report/aubagio-epar-public-assessment-report_en.pdf (accessed on 27 November 2022).
- Ponthan, F.; Kogner, P.; Bjellerup, P.; Klevenvall, L.; Hassan, M. Bioavailability and dose-dependent anti-tumour effects of 9-cis retinoic acid on human neuroblastoma xenografts in rat. Br. J. Cancer 2001, 85, 2004–2009. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jing, J.; Nelson, C.; Paik, J.; Shirasaka, Y.; Amory, J.K.; Isoherranen, N. Physiologically Based Pharmacokinetic Model of All- trans-Retinoic Acid with Application to Cancer Populations and Drug Interactions. J. Pharmacol. Exp. Ther. 2017, 361, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, G.; Mittal, A.; Weiner, H.L. Identification of an IL-27/osteopontin axis in dendritic cells and its modulation by IFN-gamma limits IL-17-mediated autoimmune inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 11495–11500. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Kishida, T.; Nakano, H.; Yoshimoto, K.; Shin-Ya, M.; Shimada, T.; Nakai, S.; Imanishi, J.; Yoshimoto, T.; Hisa, Y.; et al. Interleukin-27 activates natural killer cells and suppresses NK-resistant head and neck squamous cell carcinoma through inducing antibody-dependent cellular cytotoxicity. Cancer Res. 2009, 69, 2523–2530. [Google Scholar] [CrossRef]
- Gregorczyk, I.; Jasiecka-Mikołajczyk, A.; Maślanka, T. Blockade of NF-κB translocation and of RANKL/RANK interaction decreases the frequency of Th2 and Th17 cells capable of IL-4 and IL-17 production, respectively, in a mouse model of allergic asthma. Molecules 2021, 26, 3117. [Google Scholar] [CrossRef]
- Jasiecka-Mikołajczyk, A.; Jaroszewski, J.J.; Maślanka, T. Oclacitinib, a Janus Kinase Inhibitor, Reduces the Frequency of IL-4- and IL-10-, but Not IFN-γ-, Producing Murine CD4+ and CD8+ T Cells and Counteracts the Induction of Type 1 Regulatory T Cells. Molecules 2021, 26, 5655. [Google Scholar] [CrossRef]
- Bouguermouh, S.; Fortin, G.; Baba, N.; Rubio, M.; Sarfati, M. CD28 co-stimulation down regulates Th17 development. PLoS ONE 2009, 4, e5087. [Google Scholar] [CrossRef]
- Bughani, U.; Saha, A.; Kuriakose, A.; Nair, R.; Sadashivarao, R.B.; Venkataraman, R.; Patel, S.; Deshchougule, A.T.; Satish, K.S.; Montero, E.; et al. T cell activation and differentiation is modulated by a CD6 domain 1 antibody Itolizumab. PLoS ONE 2017, 12, e0180088. [Google Scholar] [CrossRef]
- Burns, L.A.; Maroof, A.; Marshall, D.; Steel, K.J.A.; Lalnunhlimi, S.; Cole, S.; Catrina, A.; Kirkham, B.; Taams, L.S. Presence, function, and regulation of IL-17F-expressing human CD4+Tcells. Eur. J. Immunol. 2020, 50, 568–580. [Google Scholar] [CrossRef]
- Toews, M.L.; Bylund, D.B. Pharmacologic principles for combination therapy. Proc. Am. Thorac. Soc. 2005, 2, 282–289. [Google Scholar] [CrossRef] [PubMed]



| Marker | Fluorochrome | Clone | Isotype | Evaluated Parameters |
|---|---|---|---|---|
| CD4 | PerCP-Cy 5.5 | clone RM4-5 | IgG2a, κ | The percentage and absolute count of:
|
| CD25 | PE-Cy7 | clone PC61 | IgG1, λ | |
| Foxp3 | Alexa Fluor 488 | MF23 | IgG2b | |
| CD39 | PE | Duha59 | IgG2a, κ | |
| CD49b | PE-CF594 | DX5 | IgM, κ | |
| CD223 | APC | C9B7W | IgG1, κ | |
| CD4 | PerCP-Cy 5.5 | RM4-5 | IgG2a, κ | The percentage and absolute count of:
|
| CD25 | PE-Cy7 | PC61 | IgG1, λ | |
| Foxp3 | Alexa Fluor 488 | MF23 | IgG2b | |
| IL-10 | APC | JES5-16E3 | IgG2b | |
| TGF-β | PE | TW7-16B4 | IgG1, λ | |
| CD4 | FITC | H129.19 | IgG2a, κ | The percentage of:
|
| CD25 | PE-Cy7 | PC61 | IgG1, λ | |
| Foxp3 | PE | MF23 | IgG2b | |
| BrdU | APC | IgG2a | BU1/75 |
| Description of the Effect of Two Drugs on Evaluated Parameter | Interpretation and Comment |
|---|---|
| (1) Agents A and B applied alone have unidirectional action, i.e., change the value of a given parameter in the same direction: | |
| Superadditivity: synergism (1 + 1 > 2) |
| Additivity (1 + 1 = 2) |
| Subadditivity (1 + 1 > 1 but <2) |
| Absence of an interaction (1 + 2 = 2) |
| Absence of an interaction (1 + 1 = 1) |
| (2) Agent A applied alone acts in a given direction on a given parameter while agent B applied alone does not affect this parameter: | |
| Absence of an interaction (1 + 0 = 1) |
| Superadditivity: enhancement (1 + 0 > 1) |
| Antagonism (1 + 0 < 1) |
| (3) Agents A and B applied alone do not affect the value of a given parameter: | |
| Absence of an interaction (0 + 0 = 0) |
| Induction of an effect (0 + 0 > 0) |
| (4) Agents A and B applied alone have opposite actions, i.e., change the value of a given parameter in the opposite direction: | |
| Antagonism (1 + −1 = 0) |
| Antagonism (1 + −1 > 0) |
| Antagonism (1 + −1 = −1 or 1 + −1 = 1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maślanka, T. Effect of IL-27, Teriflunomide and Retinoic Acid and Their Combinations on CD4+ T Regulatory T Cells—An In Vitro Study. Molecules 2022, 27, 8471. https://doi.org/10.3390/molecules27238471
Maślanka T. Effect of IL-27, Teriflunomide and Retinoic Acid and Their Combinations on CD4+ T Regulatory T Cells—An In Vitro Study. Molecules. 2022; 27(23):8471. https://doi.org/10.3390/molecules27238471
Chicago/Turabian StyleMaślanka, Tomasz. 2022. "Effect of IL-27, Teriflunomide and Retinoic Acid and Their Combinations on CD4+ T Regulatory T Cells—An In Vitro Study" Molecules 27, no. 23: 8471. https://doi.org/10.3390/molecules27238471
APA StyleMaślanka, T. (2022). Effect of IL-27, Teriflunomide and Retinoic Acid and Their Combinations on CD4+ T Regulatory T Cells—An In Vitro Study. Molecules, 27(23), 8471. https://doi.org/10.3390/molecules27238471






