Influence of Block Ratio on Thermal, Optical, and Photovoltaic Properties of Poly(3-hexylthiophene)-b-poly(3-butylthiophene)-b-poly(3-octylthiophene)
Abstract
1. Introduction
2. Results
2.1. Synthesis
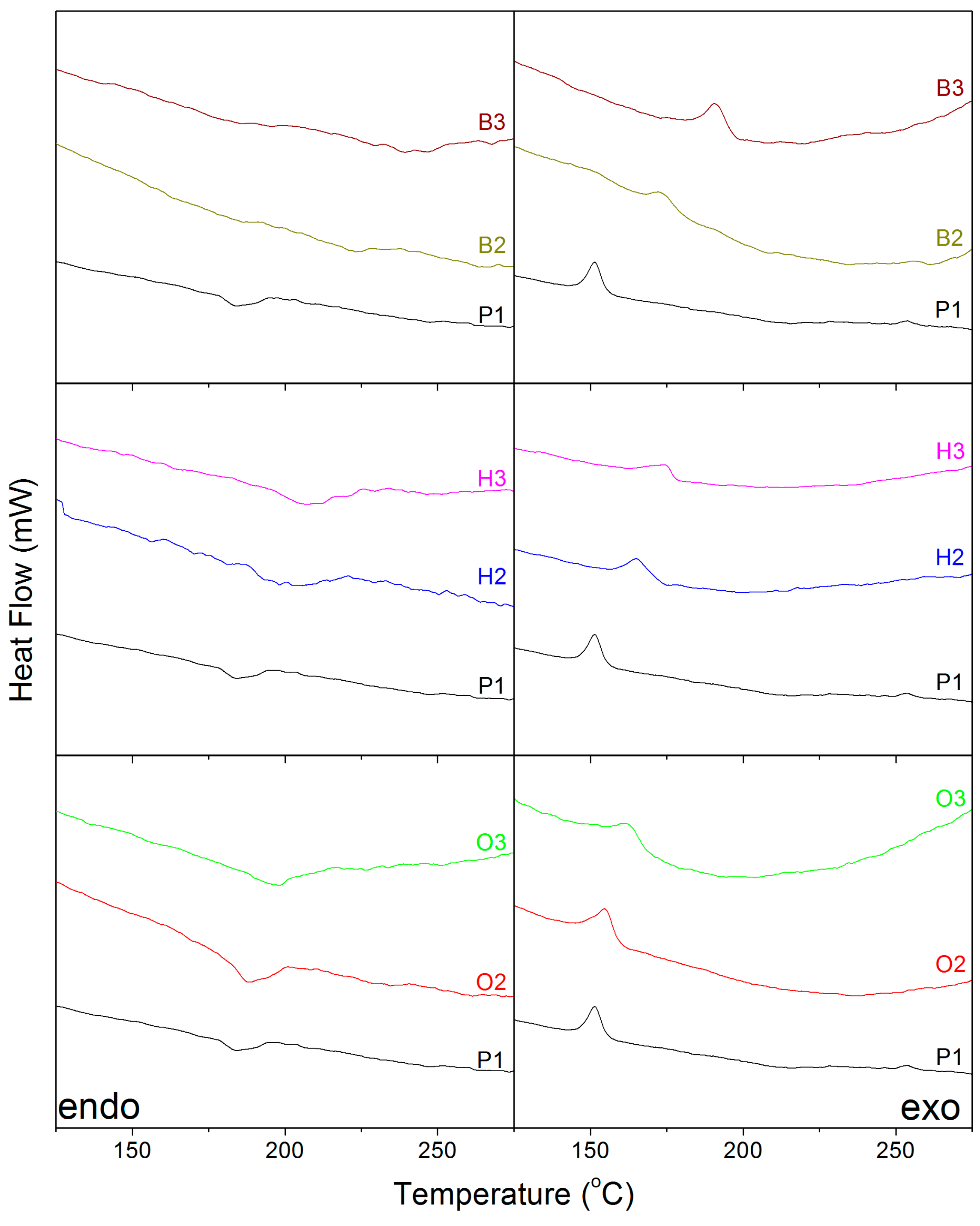
2.2. Thermal Analysis
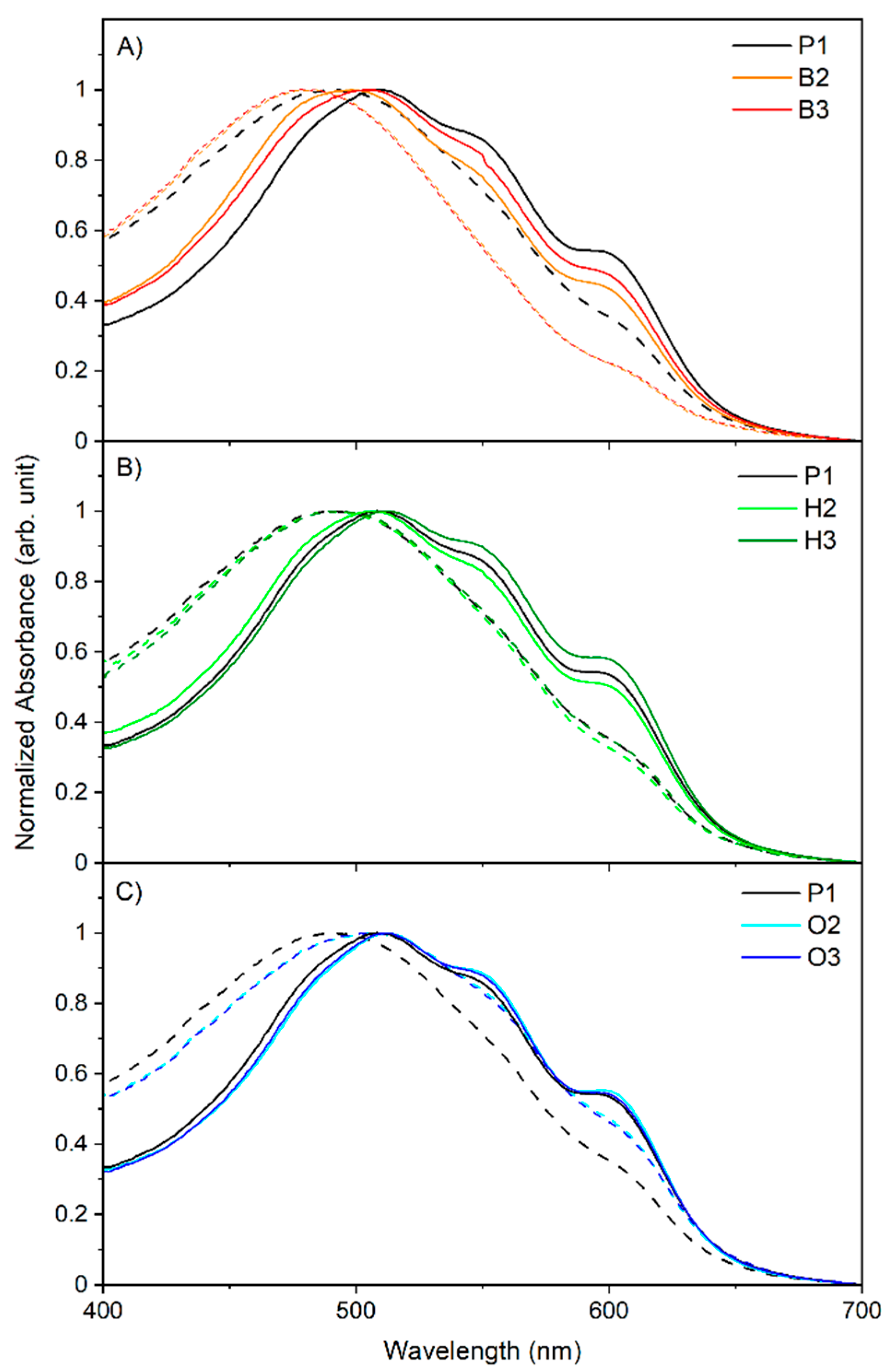
2.3. Optical Properties
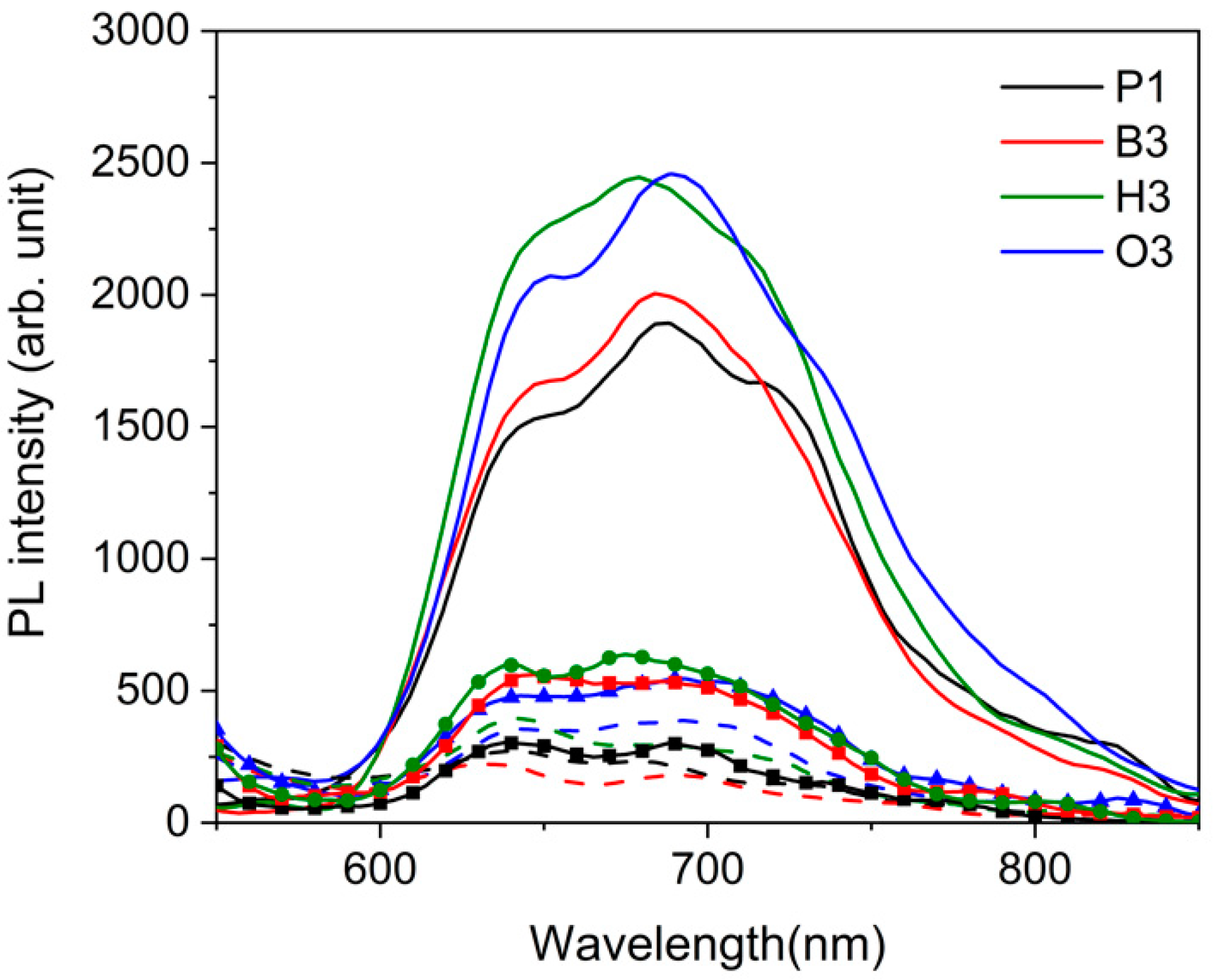
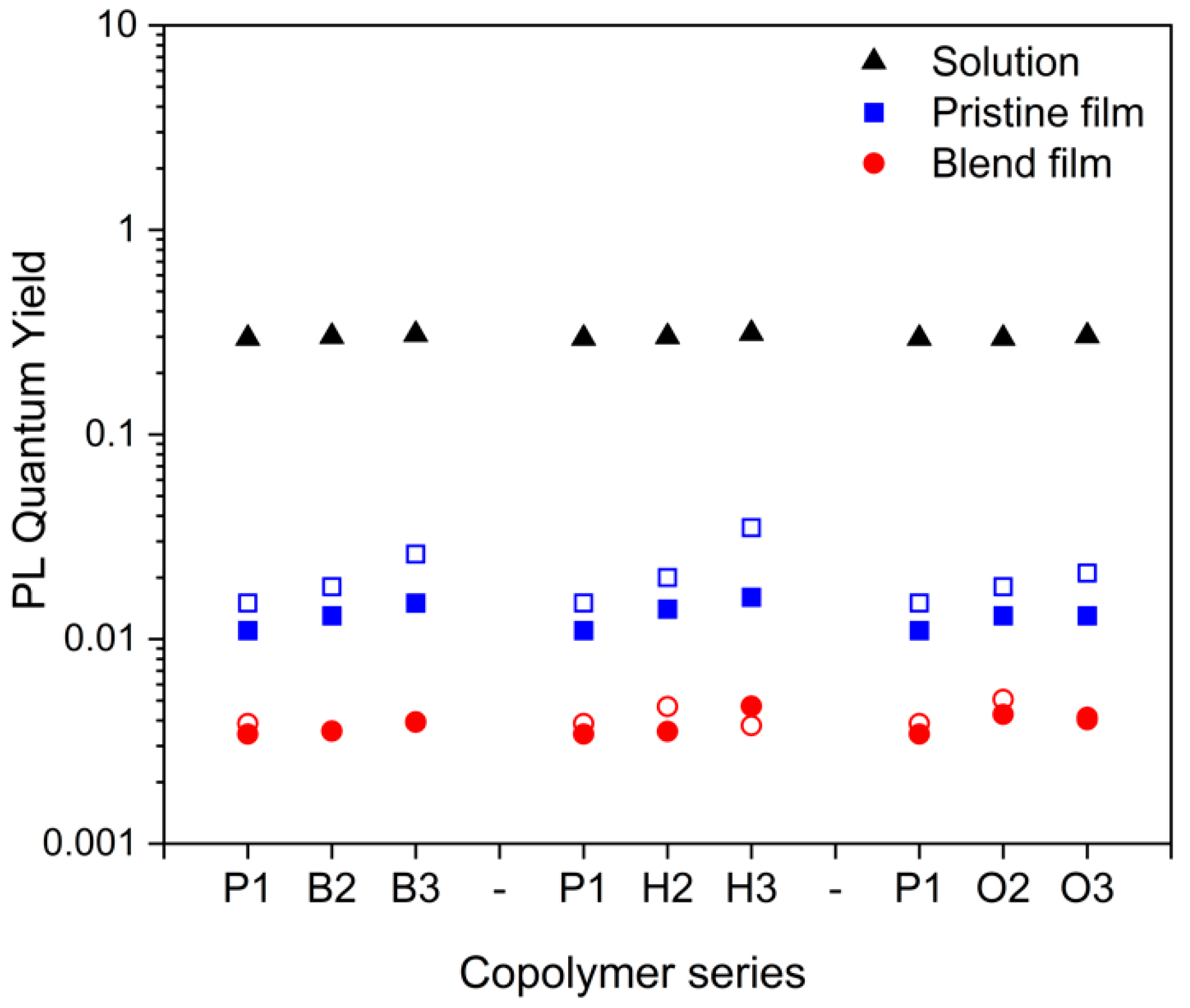
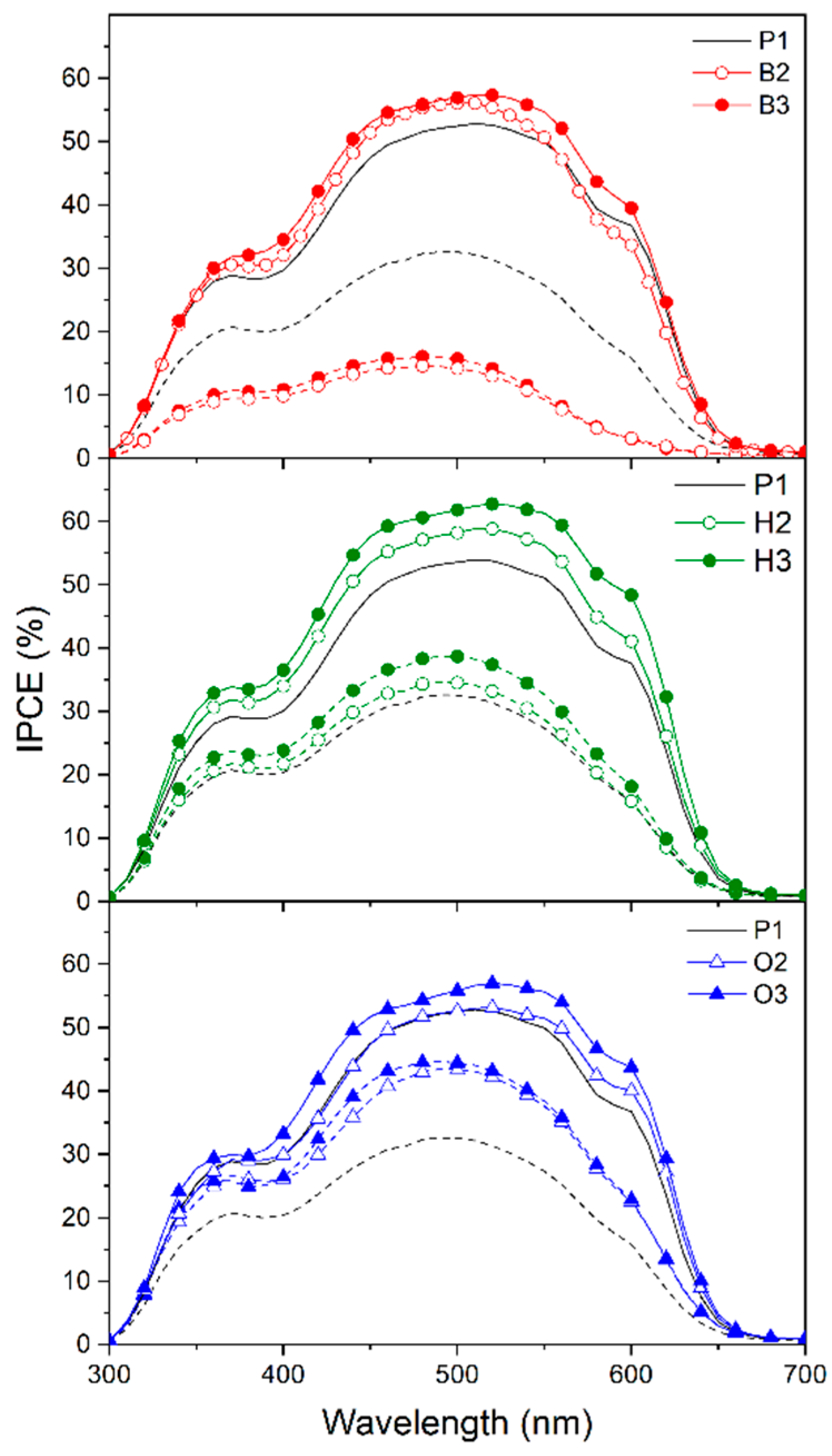
2.3.1. Pristine Films of Copolymers
2.3.2. Blend Films of Copolymers
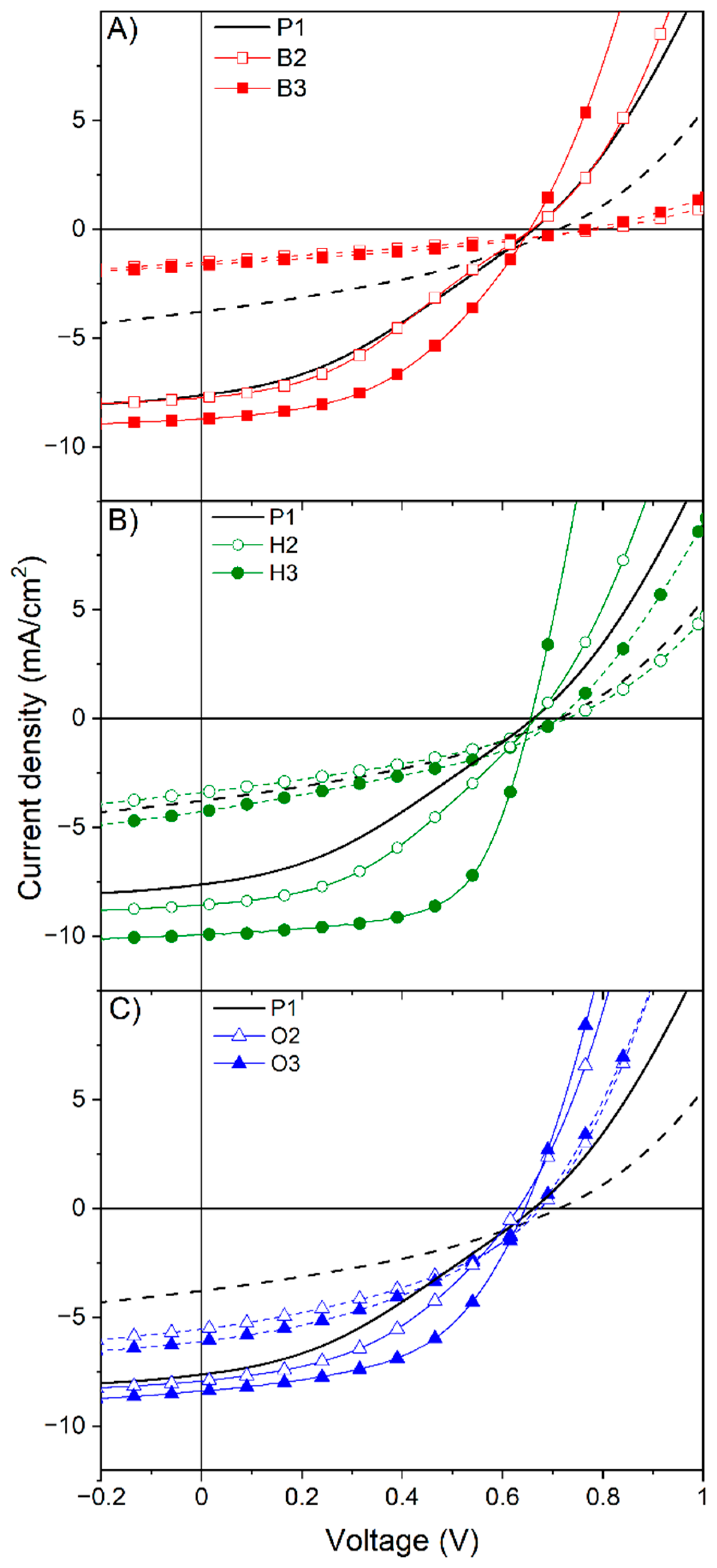
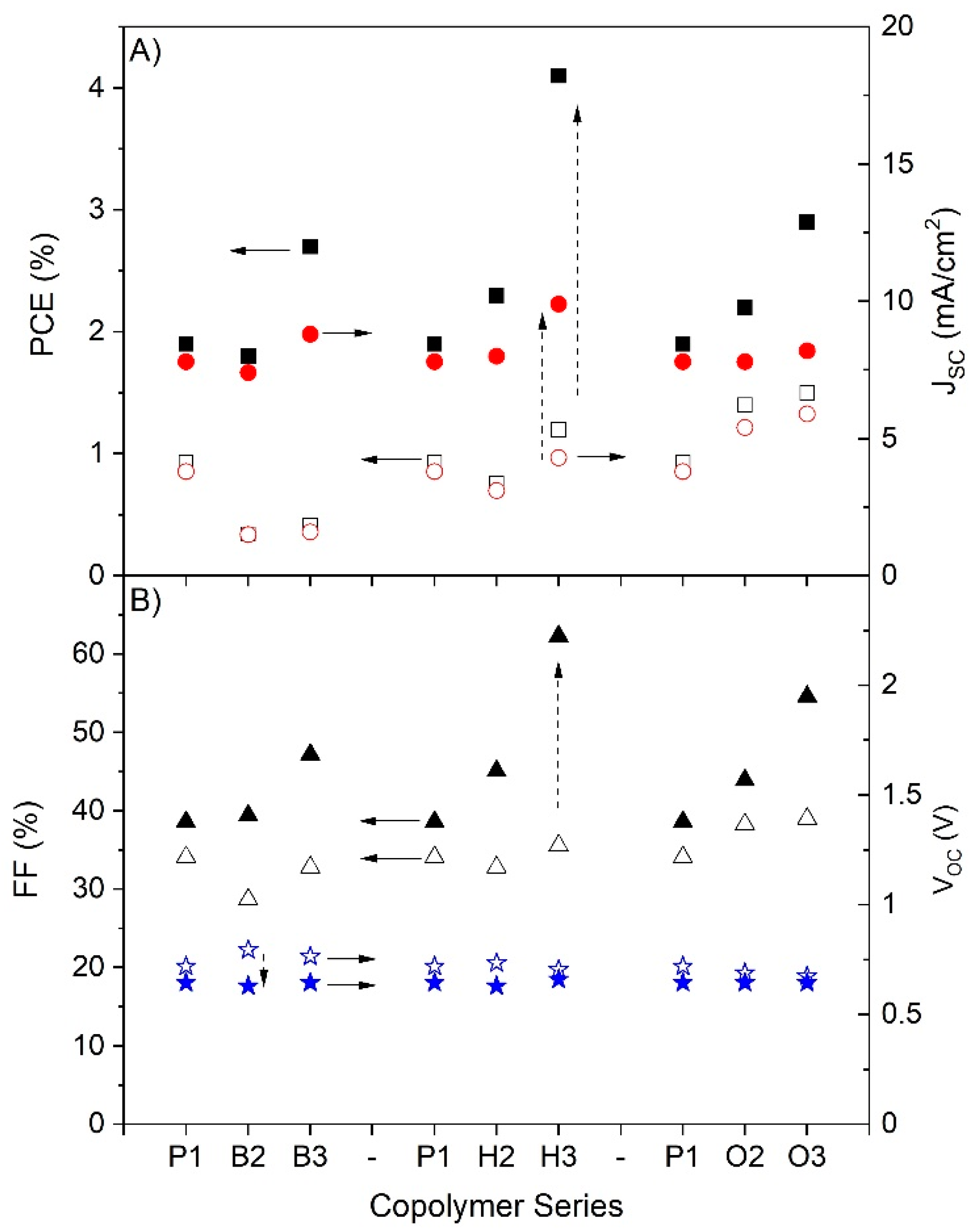
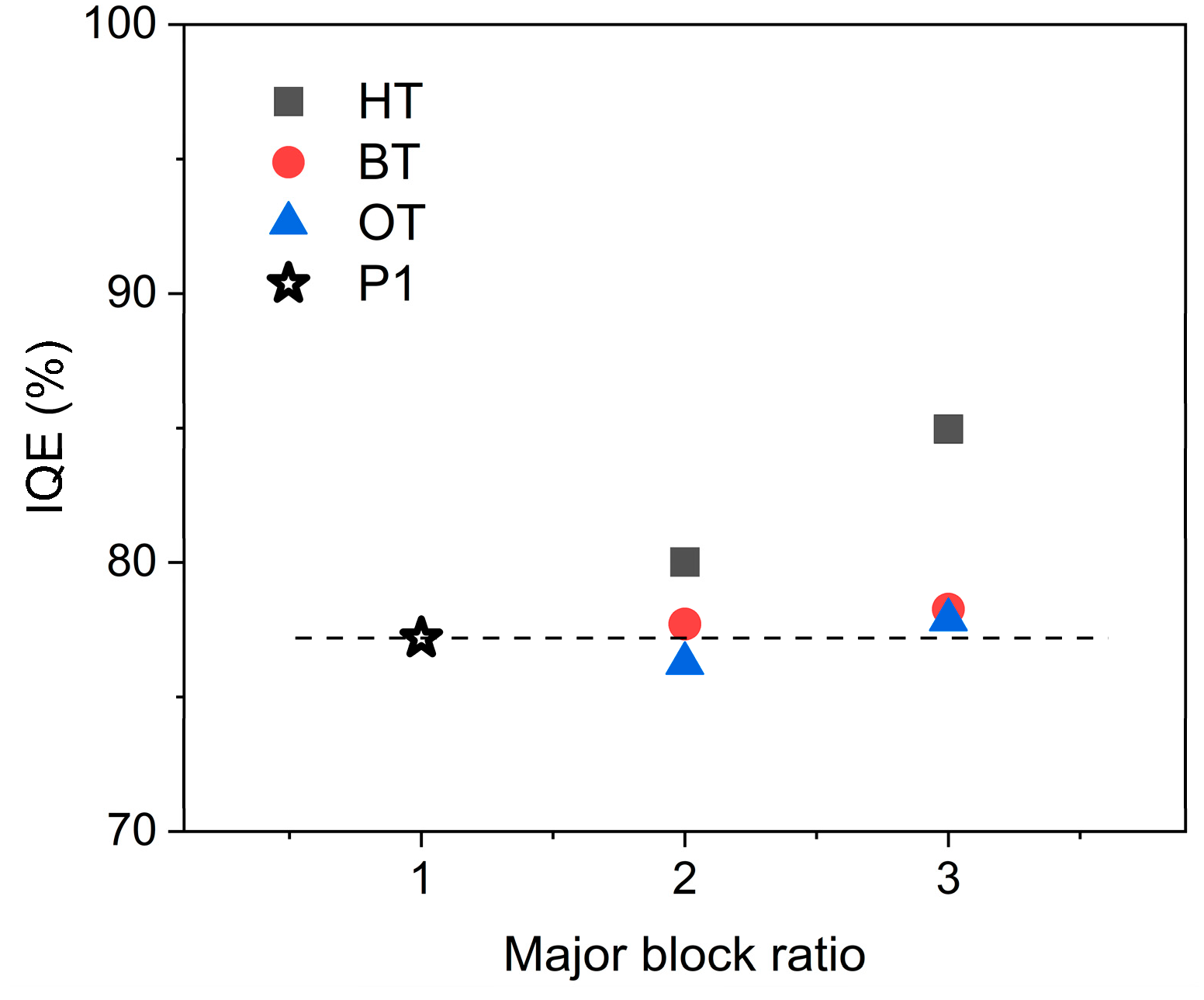
2.4. Device Characteristics
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oka, K.; Kamimori, K.; Winther-Jensen, B.; Nishide, H. Poly (3-alkylthiophene) Films as Solvent-Processable Photoelectrocatalysts for Efficient Oxygen Reduction to Hydrogen Peroxide. Adv. Energy Sustain. Res. 2021, 2, 2100103. [Google Scholar] [CrossRef]
- Saifuddin, M.; Roy, S.; Mandal, S.; Hazra, S. Vibronic States and Edge-On Oriented π-Stacking in Poly (3-alkylthiophene) Thin Films. ACS Appl. Polym. Mater. 2022, 4, 1377–1386. [Google Scholar] [CrossRef]
- Agbolaghi, S.; Zenoozi, S. A comprehensive review on poly (3-alkylthiophene)-based crystalline structures, protocols and electronic applications. Org. Electron. 2017, 51, 362–403. [Google Scholar] [CrossRef]
- Sheina, E.E.; Liu, J.; Iovu, M.C.; Laird, D.W.; McCullough, R.D. Chain growth mechanism for regioregular nickel-initiated cross-coupling polymerizations. Macromolecules 2004, 37, 3526–3528. [Google Scholar] [CrossRef]
- Xie, Z.; Wei, Q.; Shan, T.; Zheng, X.; Zhang, Y.; Zhong, H. Preparing polythiophene derivative with alternating alkyl and thioalkyl side chains via Kumada coupling for efficient organic solar cells. Polym. Chem. 2021, 12, 6456–6464. [Google Scholar] [CrossRef]
- Loewe, R.S.; Khersonsky, S.M.; McCullough, R.D. A simple method to prepare head-to-tail coupled, regioregular poly (3-alkylthiophenes) using Grignard metathesis. Adv. Mater. 1999, 11, 250–253. [Google Scholar] [CrossRef]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Abdullah, S.N.; Brza, M.A. Conducting polymers for optoelectronic devices and organic solar cells: A review. Polymers 2020, 12, 2627. [Google Scholar] [CrossRef]
- Iovu, M.C.; Sheina, E.E.; Gil, R.R.; McCullough, R.D. Experimental evidence for the quasi-“living” nature of the grignard metathesis method for the synthesis of regioregular poly (3-alkylthiophenes). Macromolecules 2005, 38, 8649–8656. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Hoppe, H.; Erb, T.; Guenes, S.; Gobsch, G.; Sariciftci, N.S. Effects of annealing on the nanomorphology and performance of poly (alkylthiophene): Fullerene bulk-heterojunction solar cells. Adv. Funct. Mater. 2007, 17, 1071–1078. [Google Scholar] [CrossRef]
- Nguyen, T.-D.; Nguyen, V.-H.; Song, J.; An, J.; Truong, N.-T.; Dang, C.-H.; Im, C. Molecular Weight-Dependent Physical and Photovoltaic Properties of Poly (3-alkylthiophene) s with Butyl, Hexyl, and Octyl Side-Chains. Polymers 2021, 13, 3440. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Zhang, B.; Ye, F.; Tang, H.; Chen, Z.; Yang, X. Synergism of molecular weight, crystallization and morphology of poly (3-butylthiophene) for photovoltaic applications. Org. Electron. 2014, 15, 414–427. [Google Scholar] [CrossRef]
- Savagatrup, S.; Printz, A.D.; Rodriquez, D.; Lipomi, D.J. Best of both worlds: Conjugated polymers exhibiting good photovoltaic behavior and high tensile elasticity. Macromolecules 2014, 47, 1981–1992. [Google Scholar] [CrossRef]
- Kudret, S.; Van den Brande, N.; Defour, M.; Van Mele, B.; Lutsen, L.; Vanderzande, D.; Maes, W. Synthesis of ester side chain functionalized all-conjugated diblock copolythiophenes via the Rieke method. Polym. Chem. 2014, 5, 1832–1837. [Google Scholar] [CrossRef]
- Wu, P.-T.; Ren, G.; Li, C.; Mezzenga, R. Crystalline diblock conjugated copolymers: Synthesis, self-assembly, and microphase separation of poly (3-butylthiophene)-b-poly (3-octylthiophene). Macromolecules 2009, 42, 2317–2320. [Google Scholar] [CrossRef]
- Hollinger, J.; Jahnke, A.A.; Coombs, N.; Seferos, D.S. Controlling phase separation and optical properties in conjugated polymers through selenophene− thiophene copolymerization. J. Am. Chem. Soc. 2010, 132, 8546–8547. [Google Scholar] [CrossRef]
- Palermo, E.F.; McNeil, A.J. Impact of copolymer sequence on solid-state properties for random, gradient and block copolymers containing thiophene and selenophene. Macromolecules 2012, 45, 5948–5955. [Google Scholar] [CrossRef]
- Ge, J.; He, M.; Qiu, F.; Yang, Y. Synthesis, cocrystallization, and microphase separation of all-conjugated diblock copoly(3-alkylthiophene)s. Macromolecules 2010, 43, 6422–6428. [Google Scholar] [CrossRef]
- Babel, A.; Jenekhe, S.A. Alkyl chain length dependence of the field-effect carrier mobility in regioregular poly(3-alkylthiophene)s. Synth. Met. 2005, 148, 169–173. [Google Scholar] [CrossRef]
- Miyane, S.; Mori, H.; Higashihara, T. Synthesis and characterization of all-conjugated hard-soft-hard ABA triblock copolythiophenes. Microsyst. Technol. 2016, 22, 3–10. [Google Scholar] [CrossRef]
- Zhai, D.; Zhu, M.; Chen, S.; Yin, Y.; Shang, X.; Li, L.; Zhou, G.; Peng, J. Effect of block sequence in all-conjugated triblock copoly(3-alkylthiophene)s on control of the crystallization and field-effect mobility. Macromolecules 2020, 53, 5775–5786. [Google Scholar] [CrossRef]
- Yokoyama, A.; Miyakoshi, R.; Yokozawa, T. Chain-growth polymerization for poly (3-hexylthiophene) with a defined molecular weight and a low polydispersity. Macromolecules 2004, 37, 1169–1171. [Google Scholar] [CrossRef]
- Malz, F.; Jancke, H. Validation of quantitative NMR. J. Pharm. Biomed. Anal. 2005, 38, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR spectroscopy. TrAC Trends Anal. Chem. 2012, 35, 5–26. [Google Scholar] [CrossRef]
- Wong, M.; Hollinger, J.; Kozycz, L.M.; McCormick, T.M.; Lu, Y.; Burns, D.C.; Seferos, D.S. An apparent size-exclusion quantification limit reveals a molecular weight limit in the synthesis of externally initiated polythiophenes. ACS Macro Lett. 2012, 1, 1266–1269. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, M.; Morin, J.-F. Design and Synthesis of Conjugated Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Yi, H.; Im, C.; An, J.; Lee, S.; Park, H. Acceptor blending ratio dependence of bulk heterojunction organic photovoltaic devices. J. Korean Phys. Soc. 2014, 64, 910–916. [Google Scholar] [CrossRef]
- Cook, S.; Katoh, R.; Furube, A. Ultrafast studies of charge generation in PCBM: P3HT blend films following excitation of the fullerene PCBM. J. Phys. Chem. C 2009, 113, 2547–2552. [Google Scholar] [CrossRef]
- Jiang, X.M.; Österbacka, R.; Korovyanko, O.; An, C.P.; Horovitz, B.; Janssen, R.A.; Vardeny, Z.V. Spectroscopic studies of photoexcitations in regioregular and regiorandom polythiophene films. Adv. Funct. Mater. 2002, 12, 587–597. [Google Scholar] [CrossRef]
- Dante, M.; Peet, J.; Nguyen, T.-Q. Nanoscale charge transport and internal structure of bulk heterojunction conjugated polymer/fullerene solar cells by scanning probe microscopy. J. Phys. Chem. C 2008, 112, 7241–7249. [Google Scholar] [CrossRef]
- Im, C.; Kang, S.W.; Choi, J.Y.; An, J. Comparing donor- and acceptor-originated excitons of non-fullerene acceptor blend polymeric system. Polymers 2021, 13, 1770. [Google Scholar] [CrossRef]
- Cates, N.C.; Gysel, R.; Beiley, Z.; Miller, C.E.; Toney, M.F.; Heeney, M.; McCulloch, I.; McGehee, M.D. Tuning the properties of polymer bulk heterojunction solar cells by adjusting fullerene size to control intercalation. Nano Lett. 2009, 9, 4153–4157. [Google Scholar] [CrossRef]
- Kim, Y.; Liu, H.; Liu, Y.; Jin, B.; Zhang, H.; Tian, W.; Im, C. Long-lasting photoluminescence quantum yield of cesium lead halide perovskite-type quantum dots. Front. Chem. Sci. Eng. 2020, 15, 187–197. [Google Scholar] [CrossRef]
- Jin, B.; Park, H.; Liu, Y.; Liu, L.; An, J.; Tian, W.; Im, C. Charge-carrier photogeneration and extraction dynamics of polymer solar cells probed by a transient photocurrent nearby the regime of the space charge-limited current. Front. Chem. Sci. Eng. 2020, 15, 164–179. [Google Scholar] [CrossRef]
- Park, H.; An, J.; Song, J.; Lee, M.; Ahn, H.; Jahnel, M.; Im, C. Thickness-dependent internal quantum efficiency of narrow band-gap polymer-based solar cells. Sol. Energy Mater. Sol. Cells 2015, 143, 242–249. [Google Scholar] [CrossRef]
- Hoppe, H.; Niggemann, M.; Winder, C.; Kraut, J.; Hiesgen, R.; Hinsch, A.; Meissner, D.; Sariciftci, N.S. Nanoscale morphology of conjugated polymer/fullerene-based bulk-heterojunction solar cells. Adv. Funct. Mater. 2004, 14, 1005–1011. [Google Scholar] [CrossRef]
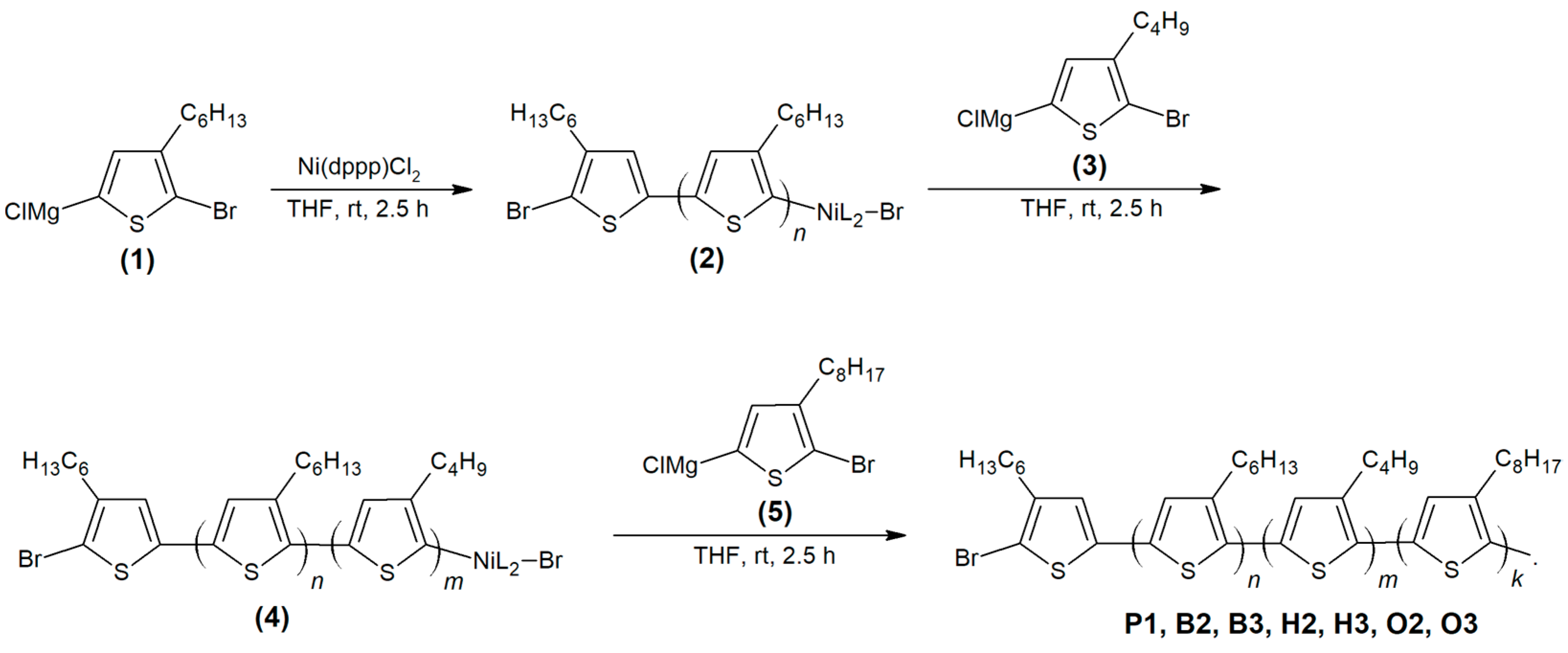
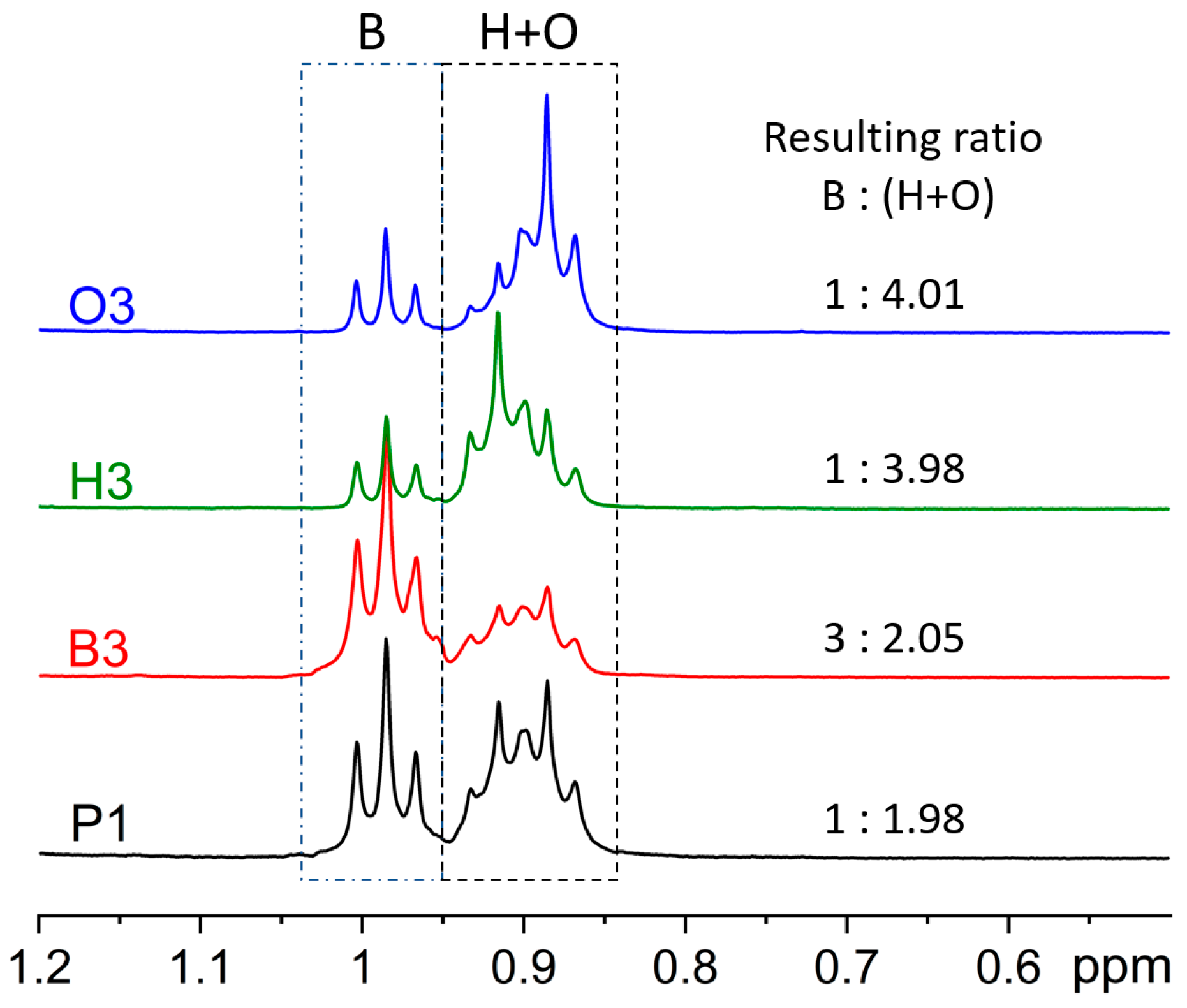

| Copolymers | Ratios (n: m: k) | Mn (Da) | MW (Da) | PDI | RR (%) |
|---|---|---|---|---|---|
| P1 | 1:1:1 | 13,000 | 17,000 | 1.297 | 98 |
| B2 | 1:2:1 | 11,000 | 16,000 | 1.440 | 98 |
| B3 | 1:3:1 | 12,500 | 19,000 | 1.503 | 98 |
| H2 | 2:1:1 | 12,000 | 17,500 | 1.461 | 98 |
| H3 | 3:1:1 | 15,000 | 21,500 | 1.443 | 99 |
| O2 | 1:1:2 | 17,000 | 25,000 | 1.476 | 99 |
| O3 | 1:1:3 | 21,000 | 31,000 | 1.491 | 98 |
| Copolymers | Tm (°C) | Tc (°C) |
|---|---|---|
| P1 | 184 | 151 |
| B2 | 222 | 173 |
| B3 | 239 | 190 |
| H2 | 196 | 165 |
| H3 | 206 | 173 |
| O2 | 188 | 188 |
| O3 | 197 | 196 |
| Copolymer | JSC (mA/cm2) | VOC (V) | FF | PCE (%) |
|---|---|---|---|---|
| P1 | 7.6 | 0.66 | 34.8 | 1.8 |
| B2 | 7.7 | 0.66 | 35.9 | 1.9 |
| B3 | 8.7 | 0.66 | 45.3 | 2.6 |
| H2 | 8.6 | 0.66 | 41.0 | 2.3 |
| H3 | 9.9 | 0.66 | 62.3 | 4.1 |
| O2 | 7.8 | 0.65 | 41.1 | 2.2 |
| O3 | 8.4 | 0.65 | 51.0 | 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.H.; Nguyen, T.D.; Song, J.; An, J.; Im, C. Influence of Block Ratio on Thermal, Optical, and Photovoltaic Properties of Poly(3-hexylthiophene)-b-poly(3-butylthiophene)-b-poly(3-octylthiophene). Molecules 2022, 27, 8469. https://doi.org/10.3390/molecules27238469
Nguyen VH, Nguyen TD, Song J, An J, Im C. Influence of Block Ratio on Thermal, Optical, and Photovoltaic Properties of Poly(3-hexylthiophene)-b-poly(3-butylthiophene)-b-poly(3-octylthiophene). Molecules. 2022; 27(23):8469. https://doi.org/10.3390/molecules27238469
Chicago/Turabian StyleNguyen, Van Hai, Thanh Danh Nguyen, Jongwoo Song, Jongdeok An, and Chan Im. 2022. "Influence of Block Ratio on Thermal, Optical, and Photovoltaic Properties of Poly(3-hexylthiophene)-b-poly(3-butylthiophene)-b-poly(3-octylthiophene)" Molecules 27, no. 23: 8469. https://doi.org/10.3390/molecules27238469
APA StyleNguyen, V. H., Nguyen, T. D., Song, J., An, J., & Im, C. (2022). Influence of Block Ratio on Thermal, Optical, and Photovoltaic Properties of Poly(3-hexylthiophene)-b-poly(3-butylthiophene)-b-poly(3-octylthiophene). Molecules, 27(23), 8469. https://doi.org/10.3390/molecules27238469








