Catechin Reduces Blood Pressure in Spontaneously Hypertensive Rats through Modulation of Arachidonic Acid Metabolism
Abstract
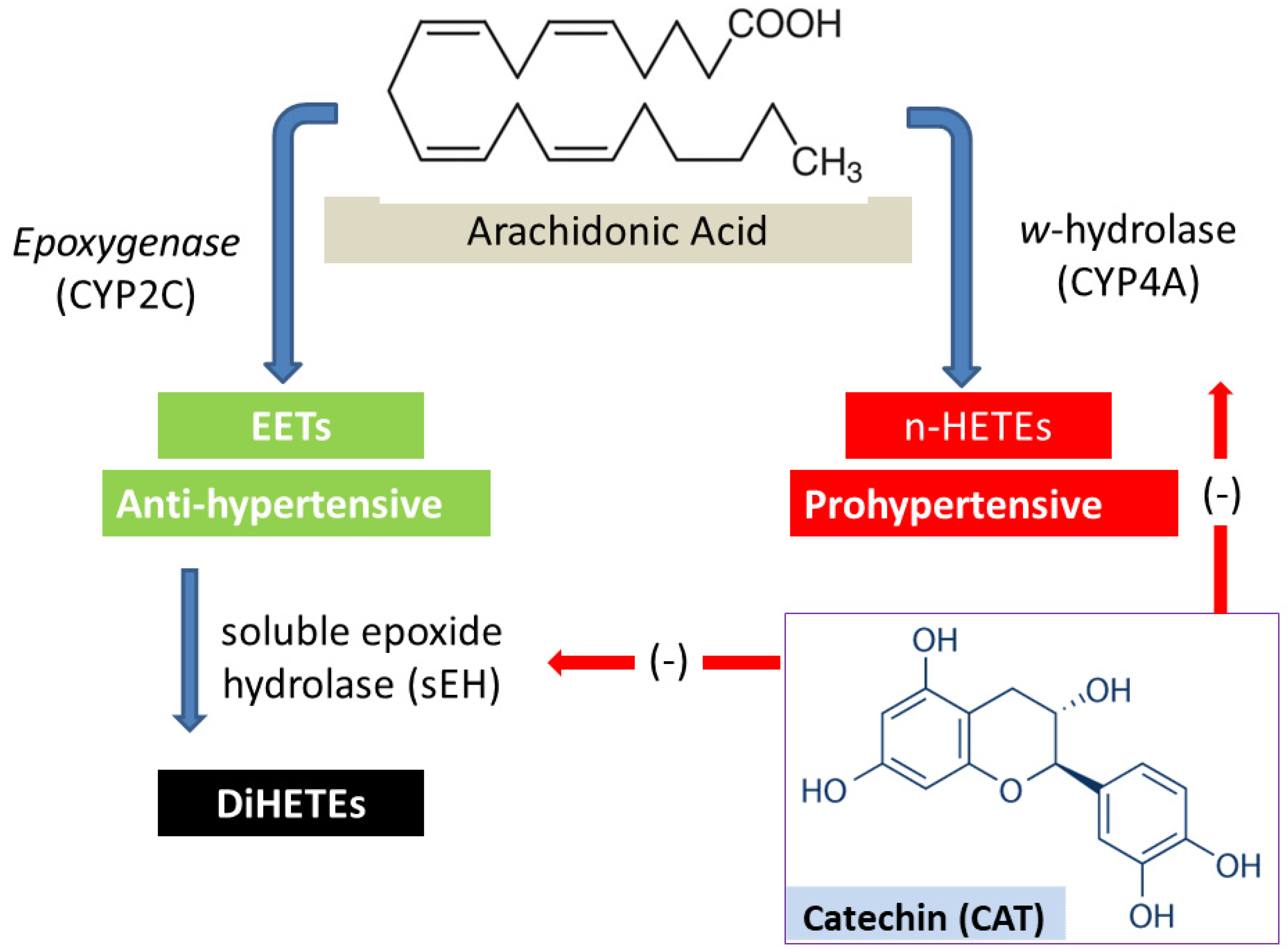
1. Introduction
2. Results
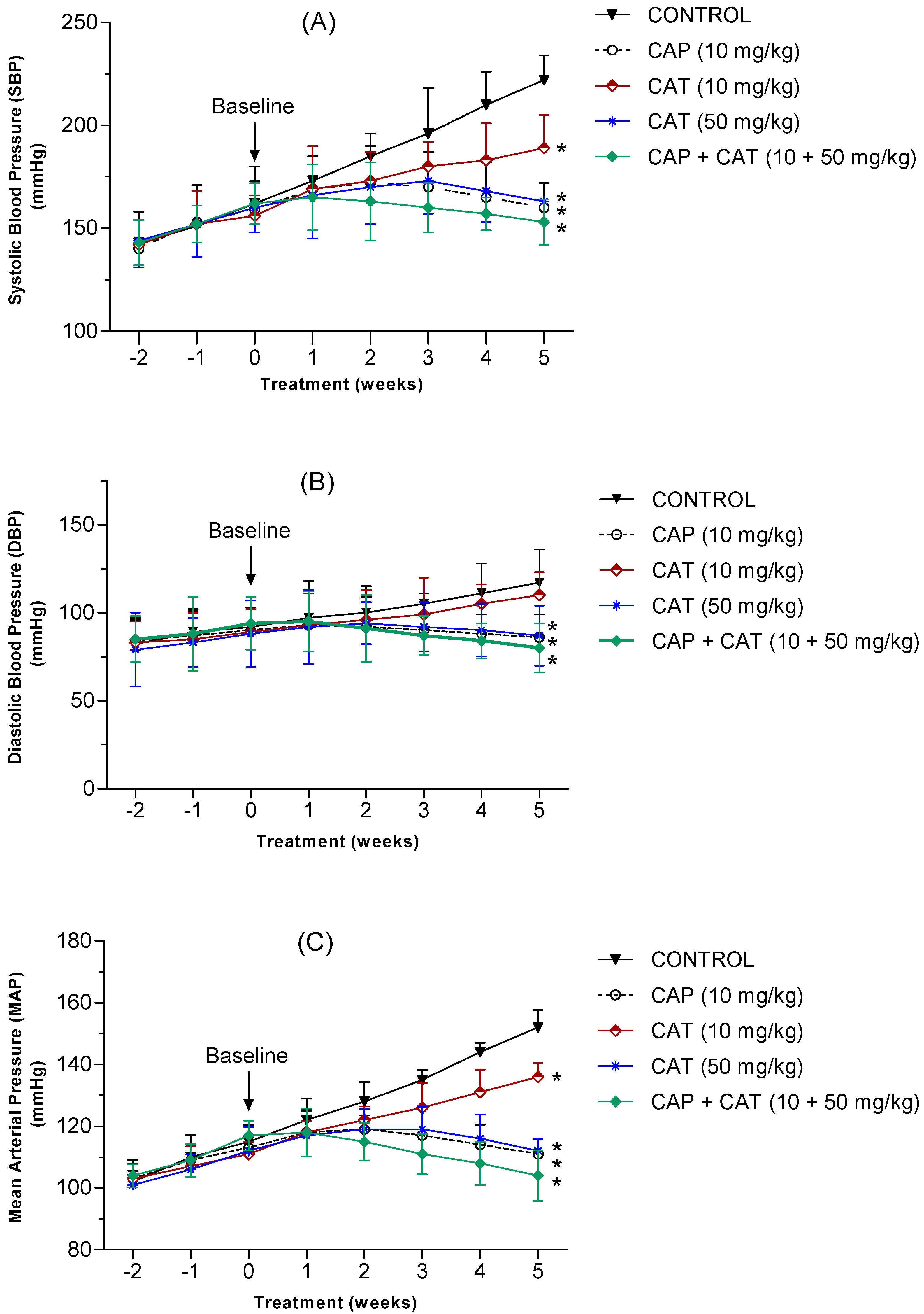
2.1. Effect of Catchin and Captopril Treatment on Blood Pressure
2.2. Effect of CAP and CAT Treatment on 20-HETE Formation in Renal Microsomes
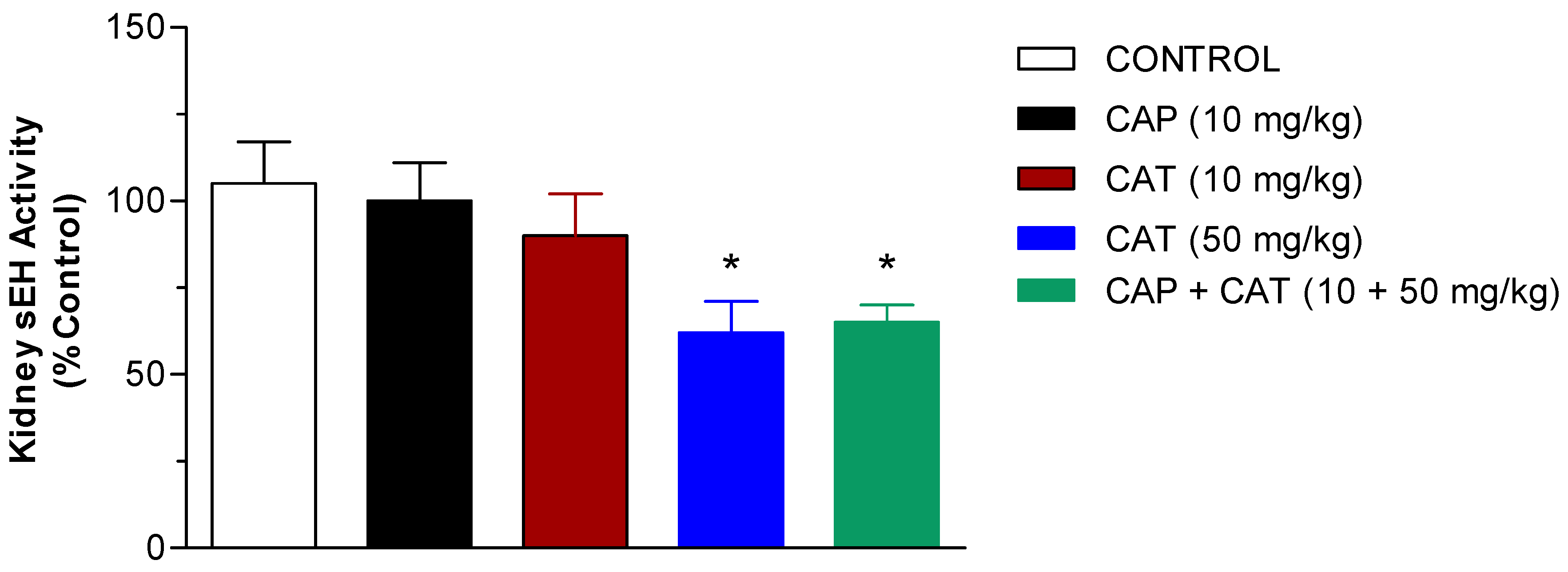
2.3. Effect of CAP and CAT Treatment on Soluble Epoxide Hydrolase (sEH) Activity
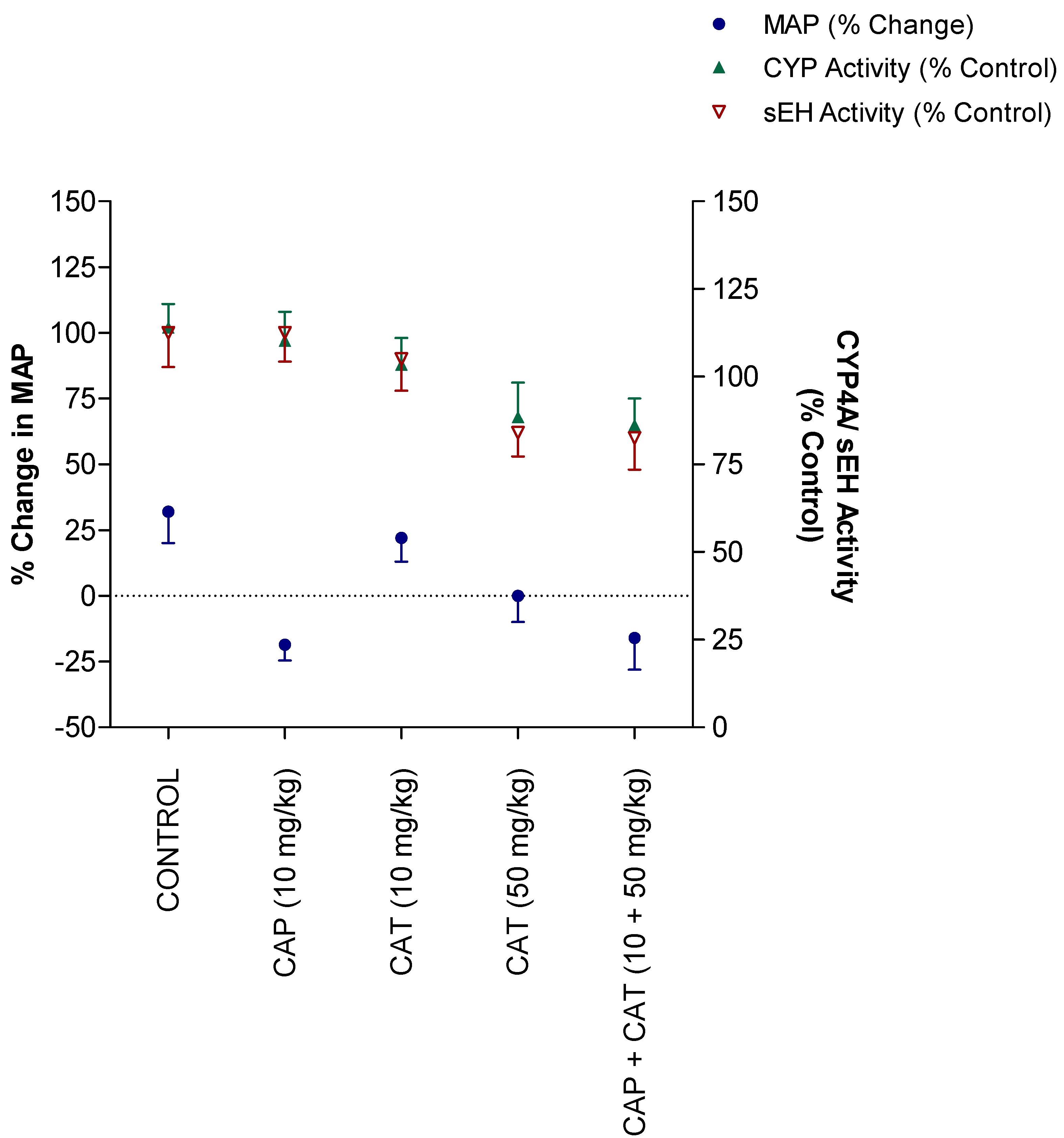
2.4. Correlation between Blood-Pressure Lowering Effect and Enzymatic Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Preparation of Catechin (CAT) and Captopril (CAP) Solutions
4.4. Catechin (CAT) and Captopril (CAP) Treatment
4.5. Blood Pressure Measurements
4.6. Tissue Collection
4.7. Quantification of 20-HETE Metabolite in Rat Kidney Microsomes
4.8. Measurement of Soluble Epoxide Hydrolase Activity Using Fluorescence Assay
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- CDC Facts About Hypertension|Cdc.Gov. Available online: https://www.cdc.gov/bloodpressure/facts.htm (accessed on 9 July 2020).
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Lasker, J.M.; Chen, W.B.; Wolf, I.; Bloswick, B.P.; Wilson, P.D.; Powell, P.K. Formation of 20-Hydroxyeicosatetraenoic Acid, a Vasoactive and Natriuretic Eicosanoid, in Human Kidney. Role of Cyp4F2 and Cyp4A11. J. Biol. Chem. 2000, 275, 4118–4126. [Google Scholar] [CrossRef] [PubMed]
- Makita, K.; Falck, J.R.; Capdevila, J.H. Cytochrome P450, the Arachidonic Acid Cascade, and Hypertension: New Vistas for an Old Enzyme System. FASEB J. 1996, 10, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Imig, J.D. Kidney CYP450 Enzymes: Biological Actions beyond Drug Metabolism. Curr. Drug Metab. 2003, 4, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Roman, R.J. P-450 Metabolites of Arachidonic Acid in the Control of Cardiovascular Function. Physiol. Rev. 2002, 82, 131–185. [Google Scholar] [CrossRef] [PubMed]
- Elbarbry, F.; Vermehren-Schmaedick, A.; Balkowiec, A. Modulation of Arachidonic Acid Metabolism in the Rat Kidney by Sulforaphane: Implications for Regulation of Blood Pressure. ISRN Pharmacol 2014, 2014, 683508. [Google Scholar] [CrossRef]
- Makita, K.; Takahashi, K.; Karara, A.; Jacobson, H.R.; Falck, J.R.; Capdevila, J.H. Experimental and/or Genetically Controlled Alterations of the Renal Microsomal Cytochrome P450 Epoxygenase Induce Hypertension in Rats Fed a High Salt Diet. J. Clin. Invest. 1994, 94, 2414–2420. [Google Scholar] [CrossRef]
- Campbell, W.B.; Falck, J.R. Arachidonic Acid Metabolites as Endothelium-Derived Hyperpolarizing Factors. Hypertension 2007, 49, 590–596. [Google Scholar] [CrossRef]
- Neckář, J.; Kopkan, L.; Husková, Z.; Kolář, F.; Papoušek, F.; Kramer, H.J.; Hwang, S.H.; Hammock, B.D.; Imig, J.D.; Malý, J.; et al. Inhibition of Soluble Epoxide Hydrolase by Cis-4-[4-(3-Adamantan-1-Ylureido)Cyclohexyl-Oxy]Benzoic Acid Exhibits Antihypertensive and Cardioprotective Actions in Transgenic Rats with Angiotensin II-Dependent Hypertension. Clin. Sci. 2012, 122, 513–527. [Google Scholar] [CrossRef]
- Wagner, K.M.; McReynolds, C.B.; Schmidt, W.K.; Hammock, B.D. Soluble Epoxide Hydrolase as a Therapeutic Target for Pain, Inflammatory and Neurodegenerative Diseases. Pharmacol. Ther. 2017, 180, 62–76. [Google Scholar] [CrossRef]
- Elbarbry, F.; Moshirian, N. The Modulation of Arachidonic Acid Metabolism and Blood Pressure-Lowering Effect of Honokiol in Spontaneously Hypertensive Rats. Molecules 2022, 27, 3396. [Google Scholar] [CrossRef] [PubMed]
- Elbarbry, F.; Abdelkawy, K.; Moshirian, N.; Abdel-Megied, A.M. The Antihypertensive Effect of Quercetin in Young Spontaneously Hypertensive Rats; Role of Arachidonic Acid Metabolism. Int. J. Mol. Sci. 2020, 21, 6554. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M. Green Tea Catechins: Nature’s Way of Preventing and Treating Cancer. Int. J. Mol. Sci. 2022, 23, 10713. [Google Scholar] [CrossRef] [PubMed]
- Del Seppia, C.; Federighi, G.; Lapi, D.; Gerosolimo, F.; Scuri, R. Effects of a Catechins-Enriched Diet Associated with Moderate Physical Exercise in the Prevention of Hypertension in Spontaneously Hypertensive Rats. Sci. Rep. 2022, 12, 17303. [Google Scholar] [CrossRef]
- Elbarbry, F.; Ung, A.; Abdelkawy, K. Studying the Inhibitory Effect of Quercetin and Thymoquinone on Human Cytochrome P450 Enzyme Activities. Pharmacogn Mag. 2018, 13, S895–S899. [Google Scholar] [CrossRef]
- Iyer, M.R.; Kundu, B.; Wood, C.M. Soluble Epoxide Hydrolase Inhibitors: An Overview and Patent Review from the Last Decade. Expert Opin Ther. Pat. 2022, 32, 629–647. [Google Scholar] [CrossRef]
- Yamamoto, H.; Okuzaki, D.; Yamanishi, K.; Xu, Y.; Watanabe, Y.; Yoshida, M.; Yamashita, A.; Goto, N.; Nishiguchi, S.; Shimada, K.; et al. Genetic Analysis of Genes Causing Hypertension and Stroke in Spontaneously Hypertensive Rats. Int. J. Mol. Med. 2013, 31, 1057–1065. [Google Scholar] [CrossRef][Green Version]
- Elbarbry, F.; Ung, A.; Rao, D.; Abdelkawy, K. Effect of Dietary Doses of Quercetin on Hepatic Drug Metabolizing Enzymes in Spontaneously Hypertensive Rats. Eur. J. Drug Metab. Pharm. 2019, 44, 761–770. [Google Scholar] [CrossRef]
- Amin, A.; CanGongora, M.; Elbarbry, F. Dietary Doses of Sulforaphane Affect Hepatic Drug Metabolizing Enzymes in Spontaneously Hypertensive Rats. Phytother Res. 2015, 29, 1412–1420. [Google Scholar] [CrossRef]
- Wienen, W.; Richard, S.; Champeroux, P.; Audeval-Gerard, C. Comparative Antihypertensive and Renoprotective Effects of Telmisartan and Lisinopril after Long-Term Treatment in Hypertensive Diabetic Rats. J. Renin Angiotensin Aldosterone Syst. 2001, 2, 31–36. [Google Scholar] [CrossRef]
- Harasawa, S.; Otsuka, Y.; Okubo, K.; Koike, M.; Fujita, H.; Kushiro, T.; Nagao, K.; Hirayama, A. Amlodipine Suppressed Cardiac Gene Expression of Brain Natriuretic Peptide, Transforming Growth Factor-Β₁ and Fibronectin Mediated by Aldosterone in Male Stroke-Prone Spontaneously Hypertensive Rats. J. Pharm. Pharmacol. 2010, 62, 1740–1745. [Google Scholar] [CrossRef]
- Wienen, W.; Schierok, H.J. Effects of Telmisartan, Hydrochlorothiazide and Their Combination on Blood Pressure and Renal Excretory Parameters in Spontaneously Hypertensive Rats. J Renin Angiotensin Aldosterone Syst. 2001, 2, 123–128. [Google Scholar] [CrossRef]
- Wu, C.-C.; Gupta, T.; Garcia, V.; Ding, Y.; Schwartzman, M.L. 20-HETE and Blood Pressure Regulation. Cardiol Rev. 2014, 22, 1. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Pavlov, T.S.; Christain, S.V.; Neckář, J.; Staruschenko, A.; Gauthier, K.M.; Capdevila, J.H.; Falck, J.R.; Campbell, W.B.; Imig, J.D. Epoxyeicosatrienoic Acid Analogue Lowers Blood Pressure through Vasodilation and Sodium Channel Inhibition. Clin. Sci. 2014, 127, 463–474. [Google Scholar] [CrossRef]
- Wang, M.H.; Zhang, F.; Marji, J.; Zand, B.A.; Nasjletti, A.; Laniado-Schwartzman, M. CYP4A1 Antisense Oligonucleotide Reduces Mesenteric Vascular Reactivity and Blood Pressure in SHR. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R255–R261. [Google Scholar] [CrossRef]
- Mullin, C.A.; Hammock, B.D. Chalcone Oxides--Potent Selective Inhibitors of Cytosolic Epoxide Hydrolase. Arch. Biochem. Biophys. 1982, 216, 423–439. [Google Scholar] [CrossRef]
- Morisseau, C.; Goodrow, M.H.; Newman, J.W.; Wheelock, C.E.; Dowdy, D.L.; Hammock, B.D. Structural Refinement of Inhibitors of Urea-Based Soluble Epoxide Hydrolases. Biochem. Pharmacol. 2002, 63, 1599–1608. [Google Scholar] [CrossRef]
- Su, P.; Kaushal, K.M.; Kroetz, D.L. Inhibition of Renal Arachidonic Acid Omega-Hydroxylase Activity with ABT Reduces Blood Pressure in the SHR. Am. J. Physiol. 1998, 275, R426–R438. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, G.-Z.; Wang, Y.; Wang, D.-W. Role of Cytochrome P450 Epoxygenase-Dependent Arachidonic Acid Metabolites in Kidney Physiology and Diseases. Sheng Li Xue Bao 2018, 70, 591–599. [Google Scholar]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The Safety of Green Tea and Green Tea Extract Consumption in Adults - Results of a Systematic Review. Regul Toxicol Pharm. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific Opinion on the Safety of Green Tea Catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef]
- Pradhan, A.; Sengupta, S.; Sengupta, R.; Chatterjee, M. Attenuation of Methotrexate Induced Hepatotoxicity by Epigallocatechin 3-Gallate. Drug Chem Toxicol 2022, 1–9. [Google Scholar] [CrossRef]
- Yue, H.; Jansen, S.A.; Strauss, K.I.; Borenstein, M.R.; Barbe, M.F.; Rossi, L.J.; Murphy, E. A Liquid Chromatography/Mass Spectrometric Method for Simultaneous Analysis of Arachidonic Acid and Its Endogenous Eicosanoid Metabolites Prostaglandins, Dihydroxyeicosatrienoic Acids, Hydroxyeicosatetraenoic Acids, and Epoxyeicosatrienoic Acids in Rat Brain Tissue. J. Pharm. Biomed. Anal. 2007, 43, 1122–1134. [Google Scholar] [CrossRef]
- Nelson, J.W.; Subrahmanyan, R.M.; Summers, S.A.; Xiao, X.; Alkayed, N.J. Soluble Epoxide Hydrolase Dimerization Is Required for Hydrolase Activity. J. Biol. Chem. 2013, 288, 7697–7703. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbarbry, F.; Jones, G.; Ung, A. Catechin Reduces Blood Pressure in Spontaneously Hypertensive Rats through Modulation of Arachidonic Acid Metabolism. Molecules 2022, 27, 8432. https://doi.org/10.3390/molecules27238432
Elbarbry F, Jones G, Ung A. Catechin Reduces Blood Pressure in Spontaneously Hypertensive Rats through Modulation of Arachidonic Acid Metabolism. Molecules. 2022; 27(23):8432. https://doi.org/10.3390/molecules27238432
Chicago/Turabian StyleElbarbry, Fawzy, Gabriel Jones, and Aimy Ung. 2022. "Catechin Reduces Blood Pressure in Spontaneously Hypertensive Rats through Modulation of Arachidonic Acid Metabolism" Molecules 27, no. 23: 8432. https://doi.org/10.3390/molecules27238432
APA StyleElbarbry, F., Jones, G., & Ung, A. (2022). Catechin Reduces Blood Pressure in Spontaneously Hypertensive Rats through Modulation of Arachidonic Acid Metabolism. Molecules, 27(23), 8432. https://doi.org/10.3390/molecules27238432






